Abstract
This is a prospective observational cohort study aiming to include 4000 patients with stages I to III colon cancer treated at 35 specialist institutions in Japan, South Korea, Germany, Russia, Lithuania and Taiwan. The anatomical distribution of lymph nodes and feeding arteries are investigated using surgical specimens according to pre-specified categorizing methods using intraoperative anatomical markings. Primary analyses are performed to identify the general principles of metastatic lymph node distribution in terms of its relation to the location of the primary tumor and feeding arteries. Secondary analyses will be used to estimate prognostic outcomes according to bowel resection length and central radicality and will be used to evaluate the quality of resected surgical specimens. Through in-depth lymph node mapping, standardized criteria for the definite area of ‘regional’ lymph node resection in routine surgical procedures can be identified, which is expected to contribute to international standardization in colon cancer surgery (ClinicalTrials.gov NCT02938481).
Keywords: lymphadenectomy, bowel resection margin, lymph node mapping, D3 dissection, complete mesocolic excision
The T-REX study is an international initiative to determine standardized criteria for regional lymph node resection in colon cancer surgery using in-depth lymph node mapping.
Introduction
Although standardization of surgical procedures is expected to improve oncological long-term outcomes (1), there is a great deal of international variation in colon cancer surgery, especially in terms of the length of bowel resection and central radicality. For example, studies have reported that the length of resected bowel specimens in Japan is approximately half that of resected specimens from specialist institutions in Western countries such as Germany (2).
In addition, from an international viewpoint, there are various anatomical landmarks of central radicality that vary by country; these include the middle of the colic arteries (3), the superior mesenteric vein/artery (4), and the extramesenteric lymph nodes over the head of the pancreas and along the gastroepiploic arcade (1). The resection margin of the bowel and central radicality are significantly associated with patients’ oncologic outcomes after surgery. There are a number of possible reasons for such noticeable international differences in surgical procedures.
First, although the optimal extent of bowel resection in colon cancer surgery is largely determined by how one defines regional pericolic lymph nodes, which should be removed in routine practice because of the risk of harboring metastatic disease in the pericolic area, there are no standardized criteria for regional pericolic lymph nodes. Second, central lymph node dissection, a surgical procedure that characterizes both complete mesocolic excision (CME) and Japanese D3 dissection, has recently attracted the attention of colorectal surgeons as a high-quality surgery to treat colon cancer; however, its surgical indication has not been confirmed. Third, although ethnic differences in physique are widely recognized, whether there are ethnic differences in the distribution of lymph nodes and feeding arteries has not been studied. This information should be clarified to estimate whether globally standardized surgical procedures for colon cancer can be successfully achieved.
An international symposium ‘Standardization of Surgical Treatment of Colon Cancer’ was organized by Prof. Sugihara with support by Translational Research Center for Medical Innovation (TRI) in Tokyo, Japan, in 2013. At this symposium, researchers from the USA, Europe and Asia presented their surgical procedures and treatment outcomes to discuss standardization of surgical procedures. As a result, it was recognized that an international collaborative study with a common study protocol was to be made in order to establish scientific evidence for the optimal resection margin of the bowel and anatomical landmarks of central radicality in colon cancer surgery.
Based on such clinical situations in the field of colon cancer, the International Prospective Observational Cohort Study for Optimal Bowel Resection Extent and Central Radicality for Colon Cancer (T-REX study) was conducted by the Japanese Society for Cancer of the Colon and Rectum (JSCCR). In this study, the actual distribution of the metastatic nodes in the pericolic, intermediate, and main lymph nodes (Fig. 1) and its association with the location of the primary tumor and feeding artery will be elucidated. The primary purpose of the study is to establish an international consensus on the extent of bowel resection and appropriate central lymphadenectomy in colon cancer surgery based on an in-depth analysis of lymph node mapping.
Figure 1.
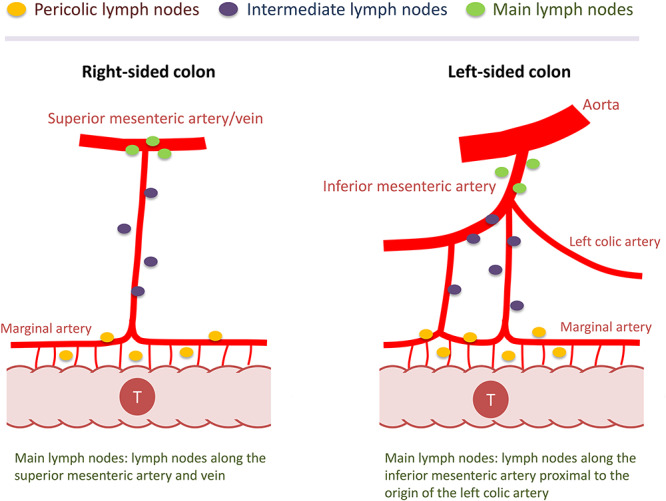
Lymph node classification in colon cancer. According to the Japanese Classification of Colorectal Carcinoma (2nd English edition), lymph nodes were classified into pericolic, intermediate and main lymph nodes. Note that different anatomical landmarks are used to determine main lymph nodes depending on the primary tumor location (i.e. main lymph nodes are those along the superior mesenteric artery/vein for tumors in the right-sided colon or those located along the inferior mesenteric artery proximal to the origin of the left colic artery in the left-sided colon). (Reprinted with permission from ``Japanese D3 Dissection'' by Hideki Ueno and Kenichi Sugihara in Surgical Treatment of Colorectal Cancer (ISBN 978-981-10-5142-5; Springer Nature Singapore Pte Ltd. 2018))
Patients and methods
Study type and ethical issues
The T-REX study is an international, multicenter, prospective, observational, cohort study that collects clinical and pathological data to assess the metastatic lymph node distribution and identify the optimal length of bowel resection and central radicality for colon cancer management.
This study was conducted in accordance with the Declaration of Helsinki, as amended on October 22, 2008, by the 59th World Medical Association General Assembly in Seoul (https://www.wma.net/wp-content/uploads/2018/07/DoH-Oct2008.pdf).
The final study protocol, including the final version of the subject information and consent forms, were approved in writing by the Ethics Committee of the JSCCR and the Investigational Review Board of each institution. Patients are included in the T-REX study only after written informed consent has been obtained.
Sample size setting
One of the important investigations in the T-REX study is the sub-population analysis, including the analysis of metastatic lymph node distribution according to T stage, for which stages T1, T2, T3, T4a and T4b, respectively, account for 17%, 13%, 56%, 8% and 6% in patients with colon cancer (5). The sample size calculation depended on the number of patients that should be included in the smallest population sub-set, i.e. the T4b population. On the basis of the consensus of opinion among the investigators, the number of patients included as the smallest population was determined to be ~200. Consequently, the target sample size of the T-REX study is 4000, which allows for a margin of error of 1% in the T4b population.
Patients included
Patients with colon cancer of preoperative stage I, II or III, who received potentially curative surgery at participating institutions (Table 1), are recruited between 30 May 2013, and 31 December 2018. The patient enrollment was initially started on 30 May 2013, only in Japan and then started internationally on 22 January 2015. The data of patients registered until 30 May2013, which include only those of Japanese patients, will be analyzed together with the international data. The inclusion criteria are as follows: (1) histologically proven colon adenocarcinoma; (2) pathological stage I, II or III; (3) potentially curative surgery; and (4) informed consent for observational data collection. Based on the protocol, the following patients are excluded from the study: (1) patients with Tis (mucosal cancer), (2) patients with multiple colon cancers, and (3) patients with preoperative adjuvant therapy.
Table 1.
Collaborating institutions in the T-REX study
| Japan (from north to south)
1. Keiyukai Sapporo Hospital 2. Yamagata Prefectural Central Hospital 3. Tochigi Cancer Center 4. National Defense Medical College 5. Saitama Cancer Center 6. National Cancer Center Hospital East 7. Teikyo University Chiba Medical Center 8. National Cancer Center Central Hospital 9. Tokyo Medical and Dental University 10. Teikyo University School of Medicine 11. Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital 12. Kyorin University School of Medicine 13. Tokyo Women’s Medical University 14. Kanagawa Cancer Center Hospital 15. Yokohama City University 16. Yokohama City University Medical Center 17. Saiseikai Yokohamashi Nanbu Hospital 18. Niigata Cancer Center 19. Shizuoka Cancer Center Hospital 20. Aichi Cancer Center Hospital 21. Fujita Health University 22. Mie University Graduate School of Medicine 23. Shiga University of Medical Science 24. Osaka International Cancer Institute 25. Wakayama Medical University 26. Kurume University School of Medicine 27. Takano Hospital |
| South Korea
1. Yonsei University 2. Asan Medical Center |
| Germany
1. University Hospital Erlangen |
| Russia
1. First Moscow State Medical University |
| Lithuania
1. Klaipeda University Hospital 2. National Cancer Institute |
| Taiwan
1. National Taiwan University Hospital 2. China Medical University Hospital |
| UK
1. Leeds Universitya |
aProtocol development and evaluation of the photographs of resected surgical specimens.
Parameters collected
All clinicopathological information is collected via the electronic data capture system, eClinical Base (eCB®) administered by the TRI. The data collected included patient characteristics (age, sex, height, body weight and tumor location); surgery-related factors (year and month of surgery, operation type, intraoperative marking method, length of bowel resected, level of central radicality [Fig. 2], operation time and estimated blood loss and morbidity and mortality); feeding artery information (the location of the first and second feeding arteries, distance from the primary tumor [Fig. 3] and pattern of feeding artery distribution [Fig. 4]); pathological information (timing of lymph node retrieval, depth of tumor invasion (pT), tumor grade, residual tumor, circumferential margin involvement and lymph node distribution status [Fig. 5]); and adjuvant chemotherapy information (Table 2). Prognostic information (year and month of last follow-up, prognostic outcome, year and month of recurrence and recurrence site) will be collected at 4 years after the last patient registration (January 2023). Photographs of resected surgical specimens are collected, and their quality will be assessed by central review as described below.
Figure 2.
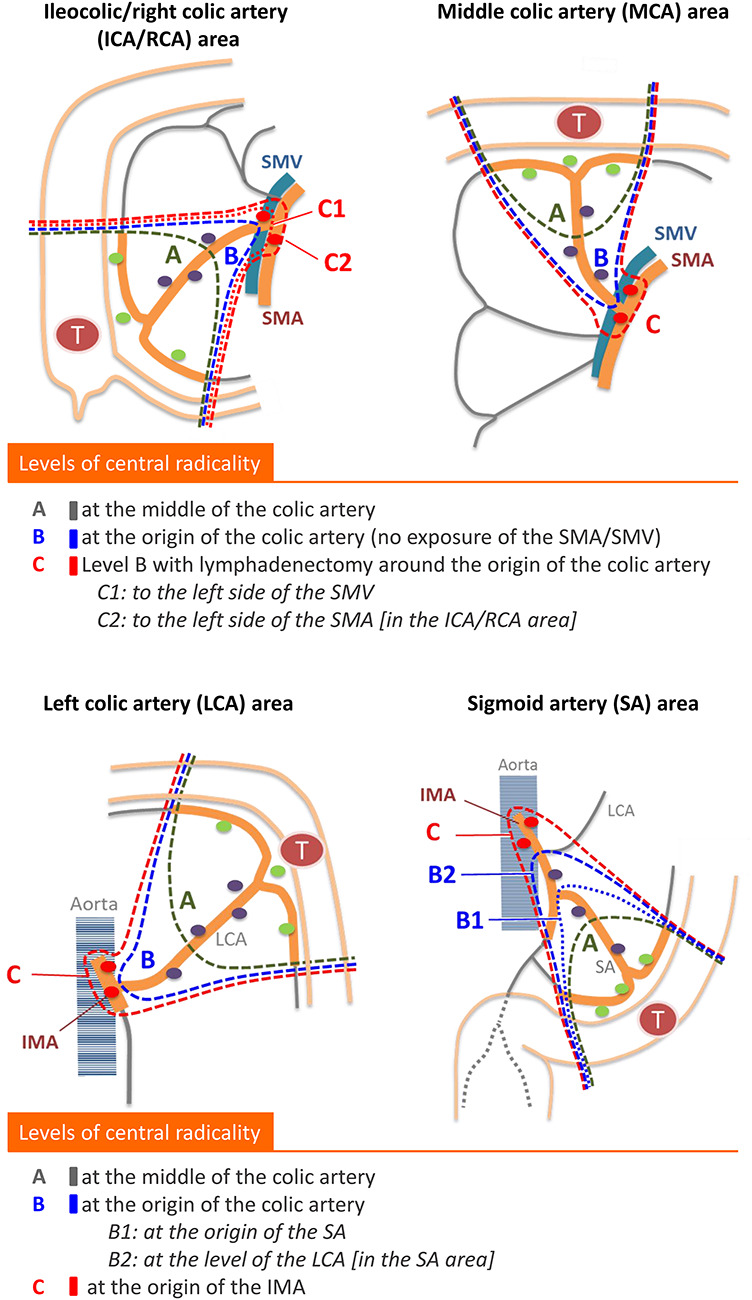
Schematic of the levels of central radicality in the T-REX study. Three categories of lymphadenectomy are provided for central radicality according to pre-specified anatomical landmarks. Subclassifications are provided for level C in tumors located in the ileocolic and right colic artery area and for level B in tumors located in the sigmoid artery area.
Figure 3.
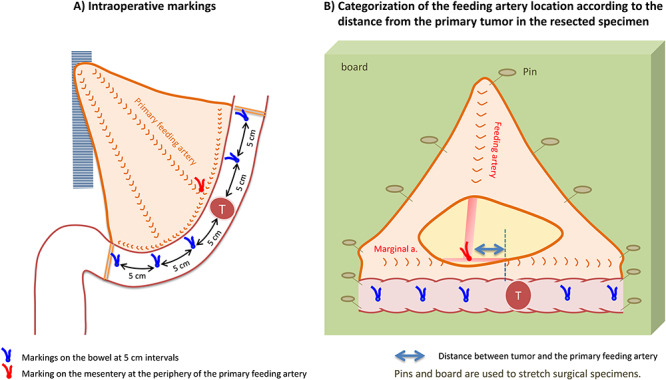
Intraoperative markings and identification of feeding arteries in resected specimens. Intraoperative markings on the bowel at 5 cm intervals make 11 pericolic segments: i.e. primary tumor area; 0 < D ≤ 5 cm (proximal/distal sides); 5 < D ≤ 10 cm (proximal/distal sides); 10 < D ≤ 15 cm (proximal/distal sides); 15 < D ≤ 20 cm (proximal/distal sides) and 20 cm < D (proximal/distal sides). The location of feeding arteries and pericolic lymph nodes are classified according to these segments.
Figure 4.
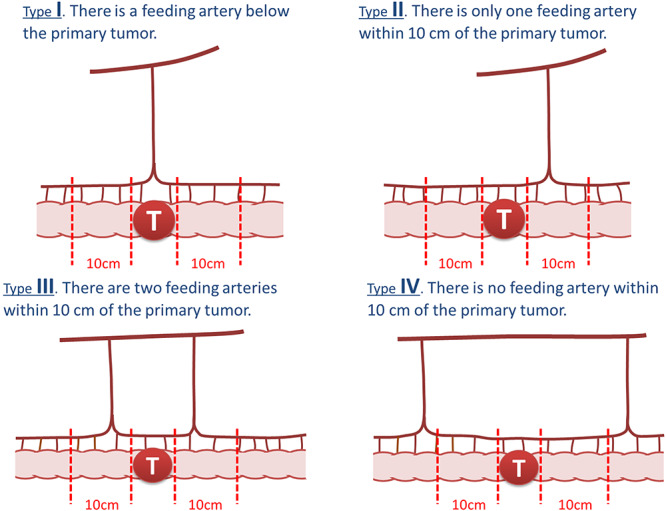
Patterns of feeding artery distribution. According to the Japanese Classification of Colorectal Carcinoma (2nd English edition), the pattern of feeding artery distribution is classified into four based on the distance of the first and second feeding arteries from the primary tumor.
Figure 5.
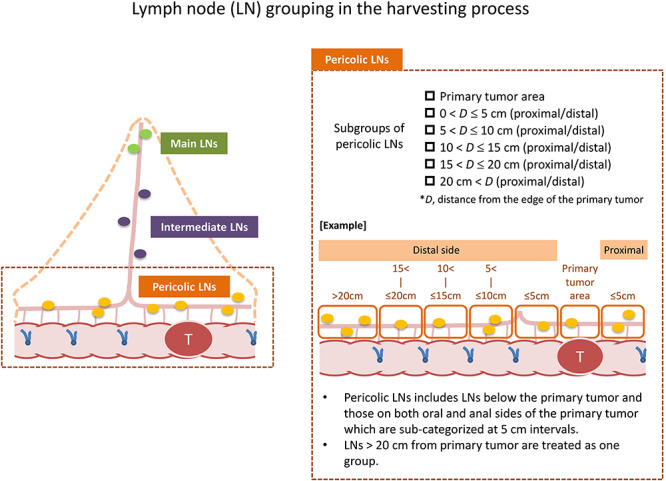
Groupings of retrieved lymph nodes in the T-REX study. Retrieved lymph nodes from the resected specimens were classified into pericolic, intermediate and main lymph nodes. The T-REX study provides subgroups of pericolic lymph nodes according to the pericolic segments determined by intraoperative markings at 5 cm intervals.
Table 2.
Clinical and pathological data collected from the T-REX study
| Clinical and pathological data |
| Patient characteristics − Age and sex − Height and body weight − Tumor location |
| Surgery-related factors − Year and month of surgery − Operation type (open/laparoscopic) − Intraoperative marking method (under direct vision/laparoscopic) − Length of the bowel resected (proximal/distal to the tumor) − Level of central radicality (Fig. 2) − Operation time and blood loss − Morbidity (Clavien–Dindo grade) and 30-day mortality |
| Feeding artery information − Name of first and second feeding arteries − Location of first and second feeding arteries (distal/proximal/within the tumor area) − Distance from the primary tumor (cm) (Fig. 3) − Pattern of feeding artery distribution (Fig. 4) |
| Pathological information − Timing of lymph node retrieval (before fixation/after fixation) − pT stage − Tumor grade − Residual tumor (R0/R1) − Circumferential resection margin − Number of lymph nodes retrieved and involved (a) Pericolic lymph nodes according to the 5 cm interval (Fig. 5) (b) Intermediate lymph nodes (around first feeding artery/around other feeding arteries) (c) Main lymph nodes |
| Postoperative treatment − Adjuvant chemotherapy |
| Prognostic outcomes − Year and month of final follow-up − Prognostic outcomes and cause of death − Year and month of recurrence − Recurrence site (remnant mesenteric lymph nodes/anastomosis/liver/lung/peritoneum/non-mesenteric lymph nodes/other) |
| Other Photographs of resected specimen (Fig. 6) |
Intraoperative procedure (surgical process)
This study does not regulate the level of lymph node dissection or the bowel resection length. Retrieved lymph nodes are grouped as pericolic, intermediate or main lymph nodes (Fig. 1). This study provides the original classification for the level of central radicality, which is categorized into three levels (A, B and C) according to the extent of lymphadenectomy of intermediate and main lymph nodes (Fig. 2).
Prior to bowel resection, the distance from the closest tumor edge is measured in the natural state, and the points at 5, 10, 15 and 20 cm from the proximal and distal edge of the tumor are respectively marked on the bowel wall with a stitch (Fig. 3). This procedure yields 11 pericolic segments (primary tumor area; 0 < distance from the edge of the primary tumor (D) ≤ 5 cm; 5 < D ≤ 10 cm; 10 < D ≤ 15 cm; 15 < D ≤ 20 cm; and 20 cm < D), which are used to categorize the location of pericolic lymph nodes and feeding arteries.
Postoperative procedure (pathological process)
Photographs of resected surgical specimens
High-resolution digital color photographs are taken on fresh specimens immediately after resection, including anterior and posterior views adjacent to a metric scale for calibration (Fig. 6). All photographs are submitted to the University of Leeds where morphometric analysis and surgical specimen grading of the dissection plane based on the presence and extent of any identifiable mesocolic defects will be made by one of the authors (N.W.) according to a method previously reported (6, 7).
Figure 6.
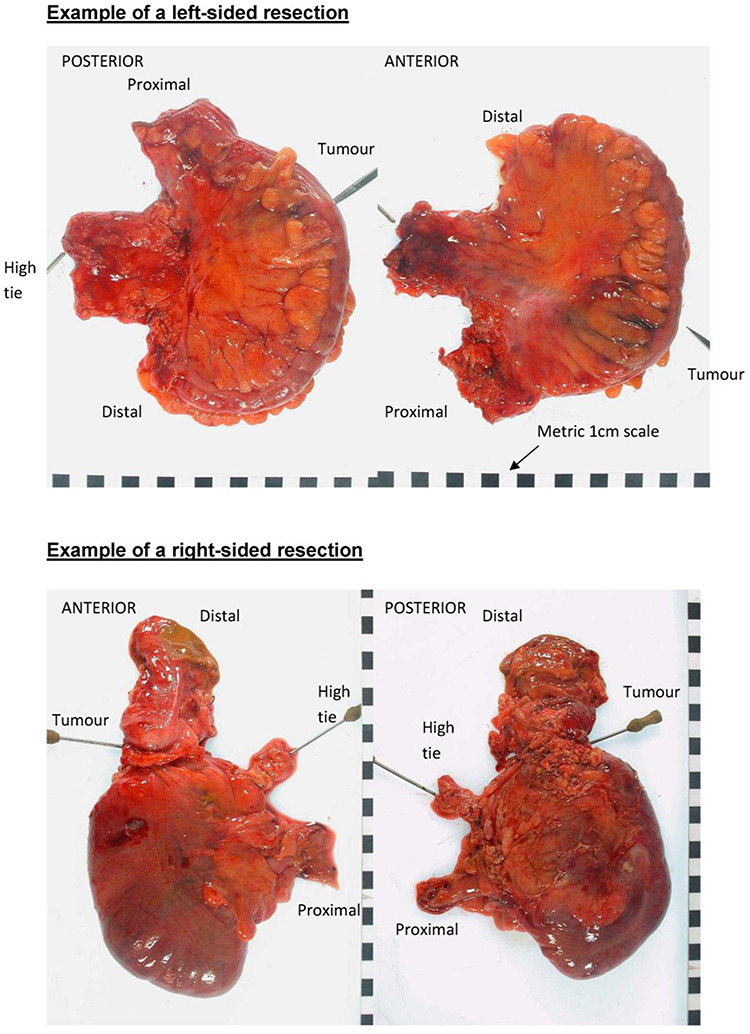
Photography protocol for of colon cancer specimens. General principles. (1) Photographs should be taken with a high-resolution digital camera; (2) the camera should be mounted on a fixed stand to minimize movement artifacts; (3) images should not contain any direct patient identifiers but should be identified by the unique study number and preferably a second indirect identifier; (4) all images must include a metric scale to allow calibration when using image analysis software and (5) a white background is ideal, although any plain color is acceptable. Whole-specimen photographs: (1) ideally, the whole specimen should be photographed before it is opened; (2) the mesentery should be laid out flat but not under tension; (3) the site of the tumor and the site of the vascular ties must be clearly visible on the photographs; (4) the whole specimen should be visible in the image; (5) photographs should be taken from both the front and the back of the specimen; (6) ideally, the distal and proximal aspects should be labeled (mainly for left-sided and wedge excisions where orientation may be difficult); (7) photographs should be taken directly above the specimen to reduce distortion artifact and (8) additional close-up pictures if any mesocolic defects or perforations exist.
Evaluation of the anatomical location of feeding arteries (Fig. 3)
The anatomical location of the primary feeding artery (a branch of the superior or inferior mesenteric artery located closest to the primary tumor) and the secondary feeding artery (artery arcade next to the primary feeding artery) are evaluated on either fresh surgical specimens or formalin-fixed specimens, depending on the pathological practice adopted in individual institutions. Similar to the location of the feeding arteries, the bowel segment that these feeding arteries flow into is recorded using the abovementioned method of bowel segmentation with intraoperative markings.
Grouping of the retrieved lymph nodes (Fig. 5)
The timing of the lymph node retrieval process (i.e. either before or after formalin fixation) is recorded. Specimens retrieved as lymph nodes are examined by routine pathological practice. In this study, tumor nodules with no pathological evidence of lymph node structure are considered as lymph node metastases, irrespective of their size and contour morphology.
Lymph nodes retrieved from resected surgical specimens are categorized as follows: pericolic lymph nodes (tumor area; 0 < D ≤ 5 cm; 5 < D ≤ 10 cm; 10 < D ≤ 15 cm; 15 < D ≤ 20 cm; and 20 cm < D), intermediate lymph nodes (lymph nodes around the primary feeding artery and lymph nodes around the other feeding arteries) and main lymph nodes.
Analysis
All data, except prognostic data, will be fixed for analyses at the end of June 2020. Primary analyses will be performed to elucidate the distribution of metastatic lymph nodes. We will analyze not only lymph node status in the resected specimen but also the status of lymph node recurrence for the purpose of elucidating the actual distribution of metastatic lymph node. Sub-group analyses for primary outcomes will be based on various essential clinicopathological factors, including pT, types of feeding artery, tumor location and populations. The results of these outcomes will be presented as the frequency of metastasis and the percentage of accuracy with a measure of precision (95% confidence intervals).
The T-REX study will also estimate prognostic outcomes according to the bowel resection length and central radicality as a secondary outcome. As another secondary outcome, this study will focus on the morphometric data of resected specimens, as well as on the plane of surgery evaluated by the quality of surgical specimens. Various statistical methods, including the chi-squared test and t-test, will be used to evaluate the differences. To analyze the survival durations, the Kaplan–Meier survival curves and the log-rank test will be used.
Discussion
The oncological outcomes of colon cancer surgery are greatly influenced by the quality of surgery, particularly with lymph node dissection. The pericolic lymph nodes located along the bowel and marginal artery are the most common site of metastasis in colon cancer (8). Currently, there are no standardized criteria for regional lymph nodes in the pericolic region, which should be resected in routine practice because of the possibility of harboring metastasis. For example, the tumor–node–metastasis classification regards all pericolic lymph nodes as regional lymph nodes, with no sub-classification based on their clinical implications. The T-REX study is expected to identify the anatomical distribution of metastatic pericolic lymph nodes, particularly in terms of their association with the location of the primary tumor and feeding arteries, which determines regional pericolic lymph nodes.
In Western countries, the bowel resection length is generally thought to depend on the area of the arterial supply to the colon (9). In addition, the vascular arcade next to the primary feeding artery is routinely resected (2). Consequently, wide bowel resection is conventionally applied in routine practice (2, 3). In Japan, in contrast, bowel resection 10 cm from the tumor edge (the 10 cm rule) has long been used in routine clinical practice on the assumption that the 10 cm rule ensures that no regional pericolic lymph nodes remain (10, 11).
Recently, in Japan, the definition of pericolic regional lymph nodes was revised in the Japanese Classification of Colorectal Carcinoma (second English edition, 2009) published by the JSCCR (4). In this definition, the distribution of the feeding artery is classified into four patterns (Fig. 4), and in addition to an anatomical landmark of 10 cm from the primary tumor, another landmark (5 cm from the feeding artery) was adopted to define regional lymph nodes depending on feeding artery distribution (the feeding artery-oriented rule) (4).
Currently, we have no robust scientific evidence to support the appropriateness of the extent of bowel resection based on the Western-type wide resection, the 10 cm rule or the feeding artery-oriented rule. With both CME with central vascular ligation and Japanese D3 dissection, which have reported good surgical outcomes (1, 6, 12), radical central lymph node dissection, including removal of the main nodes (lymph nodes located around the superior mesenteric artery/vein or the inferior mesenteric artery/vein), is applied. However, the questions of whether the resection of main nodes should be included in central lymph node dissection and who should receive this kind of extended operation, if it should be included, have not been solved.
It is well recognized that among populations, there are great differences in physical characteristics, including body size and body habitus. We should consider the possible differences in the anatomical structures in the bowel, including the anatomical distribution of feeding arteries to the colon, which can affect the distribution of metastatic lymph nodes and the optimal anatomical area for lymphadenectomy (2). The T-REX study is expected to elucidate whether an international standard for regional lymph nodes is possible in colon cancer.
In conclusion, the T-REX study aims to clarify the metastatic lymph node distribution in colon cancer. This study would enable establishment of standardized criteria for the definite area of regional lymph nodes in colon cancer, thereby providing reliable evidence about the optimal length of bowel resection and the extent of central lymphadenectomy in colon cancer surgery.
Acknowledgement
This study is supported by the Translational Research Center for Medical Innovation (TRI). The authors thank Professor Masanori Fukushima for his contributions to this trial as a director of the TRI. We also thank Dr. Tsutomu Nishimura (TRI) for his efforts at the launch of this project.
This study is also supported by the Translational Research Program; Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) (Japan Agency for Medical Research and Development; 2017–2018).
Appendix
Co-authors other than those listed in the title page
Masaaki Ito, MD (Department of Colorectal Surgery, National Cancer Center Hospital East, Chiba, Japan); Kazuo Hase, MD (Department of Surgery, National Defense Medical College, Saitama, Japan); Koji Komori, MD (Department of Gastroenterological Surgery, Aichi Cancer Center Hospital, Aichi, Japan); Kenji Matsuda, MD (Second Department of Surgery, Wakayama Medical University, Wakayama, Japan); Yusuke Kinugasa, MD (Department of Gastrointestinal Surgery, Tokyo Medical and Dental University, Tokyo, Japan); Toshihiko Sato, MD (Department of Gastroenterological Surgery, Yamagata Prefectural Central Hospital, Yamagata, Japan); Kazutaka Yamada, MD (Department of Surgery, Coloproctology Center Takano Hospital, Kumamoto, Japan); Yojiro Hashiguchi, MD (Department of Surgery, Teikyo University School of Medicine, Tokyo, Japan); Heita Ozawa, MD (Department of Surgery, Tochigi Cancer Center, Utsunomiya, Japan); Yukihide Kanemitsu, MD (Department of Colorectal Surgery, National Cancer Center, Tokyo, Japan); Takaya Kusumi, MD (Department of Surgery, Keiyukai Sapporo Hospital, Hokkaido, Japan); Hideyuki Ike, MD (Department of Surgery, Saiseikai Yokohamashi Nanbu Hospital, Kanagawa, Japan); Yasumasa Takii, MD (Department of Surgery, Niigata Cancer Center Hospital, Niigata, Japan); Tadahiko Masaki, MD (Department of Surgery, Kyorin University School of Medicine, Tokyo, Japan); Yuji Toiyama, MD (Department of Gastrointestinal and Pediatric Surgery, Division of Reparative Medicine, Institute of Life Sciences, Graduate School of Medicine, Mie University, Mie, Japan); Mitsuyoshi Ota, MD (Department of Surgery, Yokohama City University Medical Center, Kanagawa, Japan); Itaru Endo, MD (Department of Gastroenterological Surgery, Yokohama City University, Kanagawa, Japan); Hiromichi Sonoda, MD (Department of Surgery, Shiga University of Medical Science, Shiga, Japan); Keiji Koda, MD (Department of Surgery, Teikyo University Chiba Medical Center, Chiba); Yoshito Akagi, MD (Department of Surgery, Kurume University School of Medicine, Fukuoka, Japan); Masayuki Ohue, MD (Department of Surgery, Osaka International Cancer Institute, Osaka, Japan); Michio Itabashi, MD (Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, Tokyo, Japan); Philip Quirke, MD (Pathology and Data Analytics, Leeds Institute of Medical Research at St. James’s, University of Leeds, Leeds, UK); Werner Hohenberger, MD (Department of Surgery, University Hospital Erlangen, Erlangen, Germany).
Contributor Information
Manabu Shiozawa, Department of Gastrointestinal Surgery, Kanagawa Cancer Center, Kanagawa, Japan.
Hideki Ueno, Department of Surgery, National Defense Medical College, Saitama, Japan.
Akio Shiomi, Division of Colon and Rectal Surgery, Shizuoka Cancer Center, Shizuoka, Japan.
Nan Kyu Kim, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
Jin Cheon Kim, Division of Colon and Rectal Surger, Department of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
Petr Tsarkov, Department of Surgery, Faculty of Preventive Medicine, Clinic of Colorectal and Minimally Invasive Surgery, Sechenov First Moscow State Medical University, Moscow, Russia.
Robert Grützmann, Department of Surgery, University Hospital Erlangen, Friedrich-Alexander-University of Erlangen-Nuremberg, Erlangen, Germany.
Audrius Dulskas, Department of General and Abdominal Surgery and Oncology, National Cancer Institute, Vilnius, Lithuania.
Jin-Tung Liang, Division of Colorectal Surgery, Department of Surgery, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan.
Narimantas Samalavičius, Department of Surgery, Klaipeda University Hospital, Klaipeda, Lithuania; Health Research and Innovation Science Center, Faculty of Health Sciences, Klaipeda University, Klaipeda, Lithuania.
Nick West, Pathology and Data Analytics, Leeds Institute of Medical Research at St. James’s, University of Leeds, Leeds, UK.
Kenichi Sugihara, Tokyo Medical and Dental University, Tokyo, Japan.
Funding
The study is primarily conducted by the Japanese Society for Cancer of the Colon and Rectum.
This work is supported by the Translational Research Center for Medical Innovation, under grants as described below.
Japan Initiated Global Clinical Trials System Development Project (2013–2017): Ministry of Health, Labor and Welfare in Japan/Japan Agency for Medical Research and Development (JP16lk0703001).
Funds for the Research and Development Promotion (2019): Foundation for Biomedical Research and Innovation at Kobe.
Central pathological review at the University of Leeds is supported by Yorkshire Cancer Research.
Abbreviations
CME, complete mesocolic excision; D, distance from the edge of the primary tumor; JSCCR, Japanese Society for Cancer of the Colon and Rectum; T-REX, International Prospective Observational Cohort Study for Optimal Bowel Resection Extent and Central Radicality for Colon Cancer
Conflict of interest
The authors declare no conflict of interests.
References
- 1. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation-technical notes and outcome. Colorectal Dis 2009;11:354–64. [DOI] [PubMed] [Google Scholar]
- 2. West NP, Kobayashi H, Takahashi K et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 2012;30:1763–9. [DOI] [PubMed] [Google Scholar]
- 3. Corman ML. Corman's Colon and Rectal Surgery (Six Edition). New York: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 4. Japanese Society for Cancer of the Colon and Rectum Japanese Classification of Colorectal Carcinoma (Second English Edition). Tokyo: Kanehara&Co., Ltd, 2009. [Google Scholar]
- 5. Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010;28:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010;28:272–8. [DOI] [PubMed] [Google Scholar]
- 7. West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 2008;9:857–65. [DOI] [PubMed] [Google Scholar]
- 8. Kim NK, Kim YW, Han YD et al. Complete mesocolic excision and central vascular ligation for colon cancer: principle anatomy, surgical technique, and outcomes. Surg Oncol 2016;25:252–62. [DOI] [PubMed] [Google Scholar]
- 9. Nelson H, Petrelli N, Carlin A et al. Guideline 2000 for colon and rectal cancer surgery. J National Cancer Institute 2001;93:583–96. [DOI] [PubMed] [Google Scholar]
- 10. Hida J, Yasutomi M, Maruyama T, Fujimoto K, Uchida T, Okuno K. The extent of lymph node dissection for colon carcinoma. The potential impact on laparoscopic surgery. Cancer 1997;80:188–92. [PubMed] [Google Scholar]
- 11. Hashiguchi Y, Hase K, Ueno H, Mochizuki H, Shinto E, Yamamoto J. Optimal margins and lymphadenectomy in colonic cancer surgery. Br J Surg 2011;98:1171–8. [DOI] [PubMed] [Google Scholar]
- 12. Kanemitsu Y, Komori K, Kimura K, Kato T. D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 2013;56:815–24. [DOI] [PubMed] [Google Scholar]


