Abstract
The adaptive immune systems of all vertebrates rely on self-DNA mutating enzymes to assemble their antigen receptors in lymphocytes of their two principal lineages. In jawed vertebrates, the RAG1/2 recombinase directs V(D)J recombination of B cell and T cell receptor genes, whereas the activation-induced cytidine deaminase AID engages in their secondary modification. The recombination activating genes (RAG) 1 and 2 evolved from an ancient transposon-encoded genome modifier into a self-DNA mutator serving adaptive immunity; this was possible as a result of domestication, involving several changes in RAG1 and RAG2 proteins suppressing transposition and instead facilitating-coupled cleavage and recombination. By contrast, recent evidence supports the notion that the antigen receptors of T-like and B-like cells of jawless vertebrates, designated variable lymphocyte receptors (VLRs), are somatically assembled through a process akin to gene conversion that is believed to be dependent on the activities of distant relatives of AID, the cytidine deaminases CDA1 and CDA2, respectively. It appears, therefore, that the precursors of AID and CDAs underwent a domestication process that changed their target range from foreign nucleic acids to self-DNA; this multi-step evolutionary process ensured that the threat to host genome integrity was minimized. Here, we review recent findings illuminating the evolutionary steps associated with the domestication of the two groups of genome editors, RAG1/2 and cytidine deaminases, indicating how they became the driving forces underlying the emergence of vertebrate adaptive immune systems.
Current Opinion in Immunology 2020, 65:32–41
This review comes from a themed issue on Evolutionary and systems immunology
Edited by Petter Brodin and Lluis Quintana-Murci
For a complete overview see the Issue and the Editorial
Available online 27th April 2020
https://doi.org/10.1016/j.coi.2020.03.001
0952-7915/© 2020 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
RNA and DNA modifying enzymes constitute a large family of structurally and functionally diverse proteins that participate in a bewildering array of biochemical reactions in organisms from all branches of the evolutionary tree. Notably, nucleic acid editors are also key drivers of the evolution of immune defense mechanisms of uni- and multicellular organisms [1,2]. Examples include prokaryotic restriction/modification [3, 4, 5, 6] and CRISPR/Cas systems [7, 8, 9, 10], anti-viral proteins of the AID/APOBEC-Deaminase (AAD) class and their institutionalized descendants [11, 12, 13,14•], and the products of the recombination activating genes (RAG) [15••,16••], underlying the somatic rearrangement and assembly of antigen receptor loci in lymphocytes of jawed vertebrates [17].
A small subset of self-DNA mutating enzymes plays key roles in adaptive immune systems of vertebrates. RAG1/2 genes [15••,16••,17] and cytidine deaminases [18••,19••,20••] are required for the somatic diversification of the two molecularly distinct classes of antigen receptors of vertebrates. Jawed vertebrates (ranging from cartilaginous fishes to humans) deploy immunoglobulin domain-based antigen receptors for self/nonself discrimination [21••], whereas jawless vertebrates (lampreys, hagfishes) rely on leucine rich repeat-based multi-domain proteins for antigen-specific immune responses [22••,23•,24••,25•]. While the Ig-type antigen receptors require the RAG proteins for their functional assembly in lymphocytes [26], evidence is mounting that the LRR-type of receptors are assembled by institutionalized cytidine deaminases [19••,20••,24••,27•].
Despite their apparent advantage for vertebrate adaptive immunity, self-DNA mutating enzymes are potentially harmful and hence their activities must be strictly controlled in order to avoid collateral damage to the host genome [28, 29, 30, 31, 32, 33, 34, 35, 36]. Because of their inherent proliferative capacity, lymphocytes are particularly susceptible to such off-target effects. As will be discussed in detail below, illegitimate chromosomal rearrangements such as translocations or inversions caused by RAG proteins can result in ectopic or unscheduled expression of proto-oncogenes [37], whereas the uncontrolled activity of AID has been shown to introduce oncogenic mutations in a number of genes that are physiologically expressed in lymphocytes [31,32]. Thus, in a process collectively referred to as domestication [38•,39], mechanisms evolved to restrict the activities of these mutators not only to specific loci in the genome, but also to specific immune-related cell lineages at certain stages of development and/or differentiation [40,41••,42••].
Evolution of vertebrates and their lymphocyte lineages
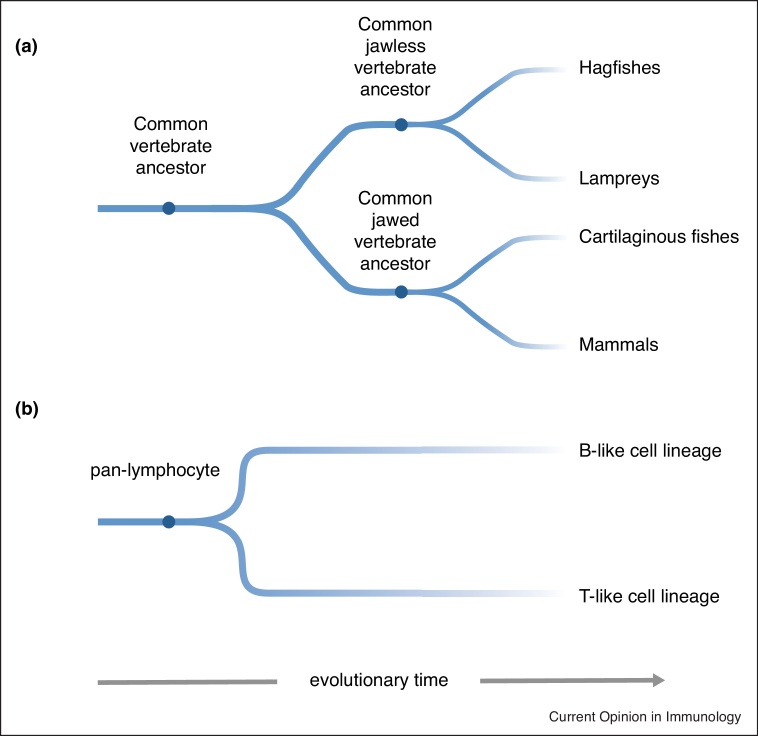
About 500 million years ago, the common vertebrate ancestor emerged [43,44]. The two extant groups of vertebrates are represented by ∼200 species of jawless vertebrates (lampreys and hagfishes), and the ∼60 000 species of jawed vertebrates that encompass groups as diverse as cartilaginous fishes and mammals (Figure 1a) [43,44]. Immune cells with phagocytic activities (originally discovered in starfish larvae by E. Metchnikov at the end of the 19th century [45]) are present in many metazoan species, including vertebrates. By contrast, lymphocytes expressing somatically diversified antigen-specific receptors are only found in vertebrates; they may have had their evolutionary origin in lymphocyte-like granular cells that were recently described in tunicates [46]. With respect to the evolutionary trajectory of antigen recognition, it is conceivable that this facility emerged in a step-wise fashion. Lymphocytes may have initially expressed different kinds of germline-encoded (and hence invariant) antigen-specific receptors, akin to pattern recognition receptors. However, a variegated expression mode of such ‘sensors’, in combination with the astounding proliferative potential of lymphocytes, may have afforded early vertebrates with the capability of primordial antigen-related responses and memory formation through proliferation of a subset of clones. Once somatic diversification of antigen receptors was invented, and their clonal representation implemented, the immune system was provided with an exquisite facility of fine-tuning immune responses and memory formation. In concert with the evolution of thymus-derived T and bone marrow-derived B lymphocytes, the stage was set for co-evolution of innate and adaptive facilities of vertebrate immune systems [47]. All vertebrates so far examined possess B-like and T-like lymphocytes [48•,49,50], indicating that the dichotomy of the vertebrate immune system likely was established in the common vertebrate ancestor (Figure 1b).
Figure 1.
Evolutionary trajectory of vertebrates and their adaptive immune systems.
(a) Cladogram depicting the evolutionary split between and within jawless and jawed vertebrates. About 500 million years ago, the hypothetical common vertebrate ancestor gave rise to both jawless and jawed vertebrate ancestors, and subsequently to their diversified descendants. In jawless vertebrates, extant species are restricted to the clades of hagfishes and lampreys, whereas jawed vertebrates comprise a more diverse group, here exemplified by cartilaginous fishes and mammals. (b) Cladogram representing the emergence of B-like and T-like cells during vertebrate evolution. Following the evolutionary timeline of (a), the two arms of the adaptive immune system originated from the hypothetical pan-lymphocyte, a primordial lymphoid cell type present in the common vertebrate ancestor. B-like and T-like cell lineages were specified at a later stage, however most likely before the emergence of the distinct ancestors of jawless and jawed vertebrates. This evolutionary scenario explains why both jawless and jawed vertebrates possess B-like and T-like cell types.
The origins of the Rag recombinase and split antigen receptor genes
In jawed vertebrates, the heterodimeric endonuclease formed by the products of the RAG1 and RAG2 genes initiate and coordinate the so-called V(D)J recombination process, by which individual gene segments of the incomplete B-cell receptor (immunoglobulin) and T cell receptor (TCR) germline genes are rearranged and assembled into functional units in developing lymphocytes [17,51••,52•]. Soon after the discovery of the RAG genes, it was speculated that they might have originated from a transposon of the Transib family [53•,54•,55••]. It is thought that the insertion of such a transposon into the gene encoding a primordial cell surface receptor established the prototype of a split antigen receptor gene (split AgR) [56]. After excision, the transposon left behind characteristic tandem inverted repeats that evolved into the recombination signal sequences (RSSs); they are composed of conserved heptamer and nonamer sequences, and are recognized by RAG proteins (Figure 2a) [56]. Since RSSs flank variable (V), diversity (D) and joining (J) gene segments of Ig and TCR genes, they serve as anchor points for the cleavage/ligation processes required for the somatic assembly of functional antigen receptor genes [17].
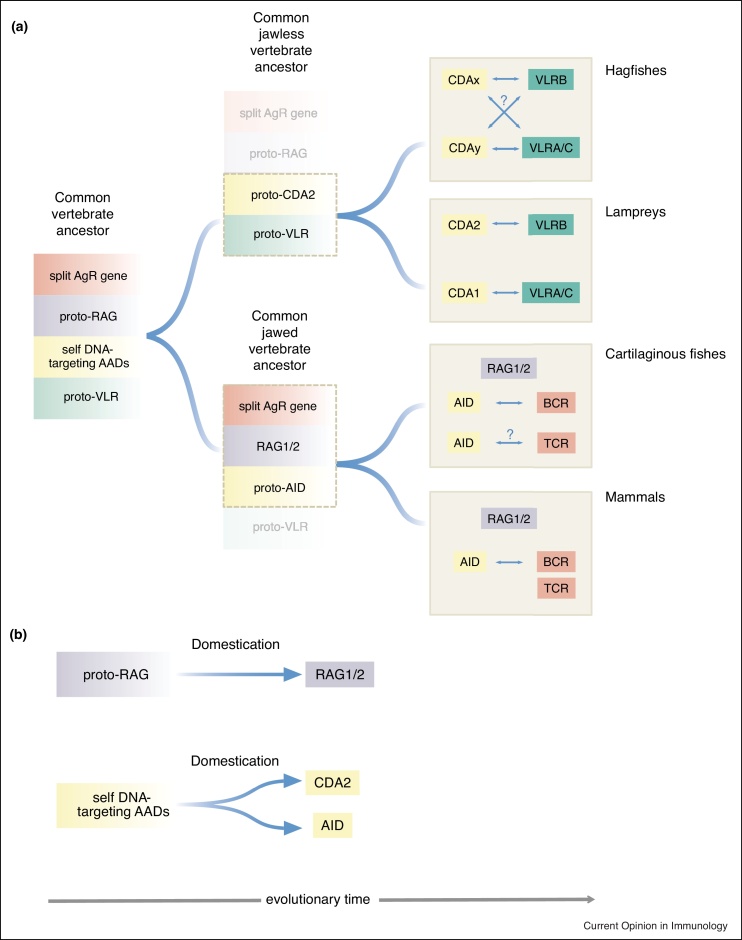
Figure 2.
Evolution of key components of vertebrate adaptive immune systems.
(a) Evolution of key genetic features that characterize the vertebrate adaptive immune systems. Four sets of immune-related genes were initially present in the common vertebrate ancestor: the split antigen receptor (split AgR) gene and its companion, proto-RAG (a vertebrate-specific descendant of the ProtoRAG present in amphioxus); the proto-variable lymphocyte receptor (proto-VLR) and its self-DNA targeting AID/APOBEC deaminase (AAD) companion(s). The genes evolving from each of the four sets are indicated in the same colored box as the one of origin (red, purple, yellow and green).
The indicated genetic building blocks of adaptive immunity evolved along different evolutionary trajectories in jawless and jawed vertebrates. It appears likely that the genomes of jawless and jawed vertebrate ancestors retained only parts of the original gene sets. Jawless vertebrates lost the split AgR gene and proto-RAG (represented by faded boxes); by contrast, a proto-VLR gene(s) was retained alongside a proto-CDA2 gene that emerged from a self DNA-targeting AAD. The dashed box around proto-CDA2 and proto-VLR indicate that these were subsequently exapted to underpin the adaptive immune systems of jawless fishes, comprising hagfishes and lampreys (upper brown boxes). In lampreys, it has now been demonstrated that CDA2 is required for VLRB receptor gene assembly [20••]; likewise, it is assumed but not yet proven that CDA1 assembles VLRA/C receptor genes. In hagfishes, the VLR/CDA system might operate in a similar fashion; however, since the repertoire of AAD genes in this group of animals remains uncharacterized, their potential roles in the assembly of VLRA/C are unknown.
The proto-RAG gene originates from the transposon of the Transib family that later gave rise to the domesticated RAG1/2 variants in the jawed vertebrate ancestor. Concomitantly, the insertion (and subsequent excision) of the transposon into a gene encoding a primordial cell surface receptor established the prototype of a split AgR in the common vertebrate ancestor. The common jawed vertebrate ancestor lost the proto-VLR gene, here represented by the faded box, possessing instead three of the four sets of genes, the split AgR, RAG1/2 and proto-AID, the latter derived from a self DNA-targeting AAD. These genes (outlined by a dashed box) were later exapted to support the adaptive immune systems of jawed vertebrates, here exemplified by cartilaginous fishes and mammals (lower brown boxes). The process of V(D)J recombination, the antigen receptor (BCR and TCR) genes, and the domesticated forms of the recombinase (RAG1/2) are shared by both groups; the same is true for AID, derived from proto-AID, which is involved in the affinity maturation of the BCR. Note, however, that in cartilaginous fishes, recent evidence points to an additional (perhaps ancestral) role of AID in the somatic hypermutation of the TCR, a function that seems to have been lost in other jawed vertebrates.
RAG-like genes can also be found in species other than jawed vertebrates [41••,53•,54•,57, 58, 59,60••]. For instance, the genome of the purple sea urchin Strongylocentrotus purpuratus also contains RAG1-like and RAG2-like sequences [53•,54•]; moreover, the basal chordate amphioxus Brachiostoma belcheri possesses a transposable element superfamily, ProtoRAG, encoding both RAG1-like and RAG2-like proteins, strongly reminiscent of the configuration of the presumptive ancestral RAG transposon [41••,53•,58,59,60••] (Figure 2a). Collectively, these observations suggest a vertical rather than horizontal transmission mode of RAG-like sequences in the deuterostome and chordate lineages. Hence, it will be interesting to examine in a more systematic fashion whether the convergently transcribed RAG-like genes that are arranged in the characteristic closely juxtaposed configuration [16••] can also be found in other branches of the animal tree. This information would help establishing the time point of the original lateral gene transfer event(s) in the metazoan lineage. Notably, RAG-like sequences have not been found in the genomes of jawless vertebrates [41••,61•,62, 63, 64], suggesting that they might have been lost after the split between jawless and jawed vertebrates (Figure 2a).
Reconstructing the domestication process of RAG proteins
The conversion of the ancestral Transib transposase into the vertebrate heterodimeric RAG recombinase driving V(D)J recombination appears to have been a step-wise process. The only known active RAG1-like Transib transposase is found in the moth Helicoverpa zea and possesses unique structural characteristics that compensate for the apparent absence of a RAG2-like protein in the transposition process [55••]. Based on comparative analyses, it was proposed that a Transib transposon at some point acquired a RAG2-like sequence to form the RAG1-RAG2 transposon [41••,65•]. This event initiated a co-evolutionary process during which RAG1 likely acquired an interaction surface for RAG2, replacing its own original target-site interaction surface [55••]. Additional changes in both proteins served to eventually suppress transposition in favor of coupled cleavage and recombination that is required for antigen receptor gene assembly [66,67,68••]. Thus, the recent studies [42••,55••] document in exquisite detail how the domestication process converted an ancient selfish genome modifier [69,70••] into a self-DNA mutator serving a crucial function in adaptive immunity of jawed vertebrates [42••,55••].
Even after domestication, erroneous utilization by the RAG proteins of orphan RSS still occurs [71,72], despite depletion of such sequences in the vicinity of genes that are expressed in lymphocytes [40,73]. Many of such somatic events are likely to be innocuous. However, chromosomal repositioning next to transcriptionally active antigen receptor gene loci may result in uncontrolled expression of proto-oncogenes [29]. This possibly endows the afflicted cell with a selective growth advantage, and finally may lead to malignant transformation. Illegitimate chromosomal rearrangements may occur purely as a consequence of accidental alignment of chromosomal regions in trans rather than the usual cis configuration in the antigen receptor loci, or as a result of somatic mutations in RAG proteins that relax their stringent transposition suppression sequence signatures.
Base editors as mediators of antigen receptor diversification
It has long been recognized that several members of the cytidine deaminases of the AID/APOBEC family (AADs) may evolve in response to the continual genetic conflicts between genetic parasites and their eukaryotic hosts [74]. For instance, the rapid expansion of APOBEC3 genes in the genomes of diverse mammals is thought to be the result of ongoing pressure exerted by viruses, retroviruses and (retro)transposons threatening the integrity of the host genome [13]. Most interestingly, however, in a radical deviation from this ‘classical’ innate immune-related trajectory, some AADs have evolved the ability to target self-DNA rather than foreign DNA. These events laid the foundations for an entirely new type of immune defense that is based on the somatic diversification of antigen receptor genes [12]. Hence, in a remarkable change of evolutionary strategy of immune defense, AADs evolved away from a direct executing role to assume an indirect facilitating role of much broader specificity.
About 20 years ago, activation-induced cytidine deaminase (AID) was identified as the key enzyme contributing to somatic diversification of immunoglobulin genes in jawed vertebrates by hypermutation, gene conversion, and class switch recombination [18••,75•,76•]. More recently, two additional members of the AAD clade with strong sequence homology to AID were discovered in the genome of the sea lamprey Petromyzon marinus, designated cytidine deaminases 1 and 2 (CDA1 and CDA2) [19••,77]. Subsequent work associated their expression with the two principal B-like and T-like lymphocyte lineages identified in lampreys [24••,78,79•]. These findings supported the hypothesis [25•] that CDA1 and CDA2 might be involved in the assembly of the genes encoding the leucine-rich repeat (LRR)-containing variable lymphocyte receptors (VLRs) [79•]. Detailed analysis of assembly intermediates of VLR genes suggested a process akin to gene conversion [19••,27•,80], in analogy to the function of AID in the generation of the immunoglobulin repertoire in birds and certain mammalian species [76•,81•]. The expression analyses of CDA genes [24••] and subsequent structural and functional in vitro studies [82••] were highly suggestive of their roles in VLR gene assembly. However, clear-cut experimental evidence has only recently been provided, as will be discussed below. The evolutionary precursor of VLR genes likely existed already in the genome of the common vertebrate ancestor, but might have been lost in the jawed vertebrate lineage (Figure 2a).
Function of the CDA2 gene
Of the two principal types of cytidine deaminases identified in lampreys, the CDA2 gene appears to be the most conserved [82••]. The sea lamprey CDA2 gene has clear orthologues in many other lamprey species, such as the Japanese lamprey Lethenteron camtschaticum and the European brook lamprey Lampetra planeri [82••]. This stability suggests that the gene is no longer part of ongoing genetic conflicts with extraneous parasites, but has become ‘institutionalized’ to carry out functions in the context of lamprey development and/or physiology [14•]. Indeed, it is transcribed — albeit at low levels — in cells expressing the VLRB antibody genes of lamprey, and in cells situated in hematopoietic tissues in lamprey larvae (kidney and typhlosole), compatible with a role in the assembly of functional VLRB genes from non-functional germline precursors [24••,83••].
Recently, following improvements in the methodology to rear in vitro-fertilized lampreys in the laboratory, in combination with highly efficient CRISPR/Cas9-mediated gene disruption, we have been able to directly examine a potential role of CDA2 in the VLRB assembly process in L. planeri [20••]. We generated a large series of CDA2-deficient L. planeri CRISPR/Cas9-induced mutants (crispants), exhibiting bi-allelic deleterious mutations of the CDA2 gene. In L. planeri larvae lacking intact CDA2 genes, VLRB assemblies were found to be entirely absent. By contrast, VLRA or VLRC assemblies characteristic of the T lineages of lampreys proceeded normally, confirming a lineage-specific function of CDA2. Because AADs of hagfishes have not yet been characterized, it is unclear whether CDA1-like and CDA2-like genes exist and whether they are involved in VLR gene assembly (Figure 2a).
The complexity of CDA1 and CDA1-like gene families
In addition to the CDA2 gene, initial studies identified a second cytidine deaminase gene in the genome of sea lamprey, designated CDA1 [19••]. Whereas the coding sequence of the CDA2 gene is distributed across six exons, CDA1 is composed of a single exon [19••,82••]. Subsequently, genes closely resembling the sea lamprey CDA1 were found in the genomes of other lamprey species (L. planeri, L. japonicum, and L. fluviatilis) [82••]. However, in contrast to the extremely high degree of protein sequence identities among CDA2 orthologues, the CDA1 genes encode proteins of much greater sequence divergence [14•,82••]. Moreover, when studying the genome of L. planeri, we found the presence of several additional CDA1-like genes (designated CDA1Ls); however, the number of such genes varied among individuals of the same species [82••]. Structural studies of the predicted proteins encoded by CDA1L genes suggested the presence of two clades (CDA1L1 and CDA1L2); only one of the genes identified in L. planeri so far, CDA1L1_4, encodes a protein that exhibits a surface charge distribution similar to P. marinus CDA1 and AID of jawed vertebrates and is therefore predicted to represent their functional homolog. Interestingly, both CDA1 and CDA1L1_4 expression is found in VLRA+ and VLRC+ lymphocytes [24••,25•], and in cells situated in the thymoid, representing the presumptive thymus equivalent of lampreys that is located at the tips of the larval gill filaments [83••]. Based on the role of CDA2 in VLRB gene assembly [20••], we hypothesize that CDA1 or CDA1L1_4 genes are required for the somatic assembly of VLRA and VLRC genes in developing T-like lymphocytes. Of note, the greater variability of CDA1 and CDA1L proteins (as compared to the highly conserved CDA2 sequences) may have precluded the identification of members of this clade of AADs in sequence collections of hagfishes; alternatively, the CDA2 gene might assume a more general role in VLR gene assembly in these species (Figure 2a).
Several AID/APOBEC-like genes are present in the genome of the echinoderm S. purpuratus and the brachiopod Lingula anatina [14•,84]. In phylogenetic analyses of these AID/APOBEC family members, SpAID-like proteins cluster with the sea lamprey CDA2 protein, suggesting that CDA2 is more ancient and/or evolves much slower than the proteins encoded by CDA1 and CDA1L genes. Interestingly, the expression of the invertebrate enzymes is enriched in tissues of the digestive tracts where continuous interactions with foreign pathogens and symbiotic microbiota occur; moreover, it appears that their expression is induced upon pathogen exposure. The existence of numerous alleles of AID/APOBEC genes in S. purpuratus individuals is indicative of their involvement in innate immune mechanisms, a feature that they may have in common with CDA1L gene in lampreys [82••] and APOBEC3 genes in mammals [13,85, 86, 87].
Domestication of AADs for self-DNA mutagenic activity
The mechanism(s) of domestication that were associated with the ‘institutionalization’ of AID and CDAs are unknown. However, it is conceivable that a number of mechanisms were involved. Transcriptional control mechanisms could have restricted expression of AADs initially to immunocytes and later to lymphocytes, and finally to certain differentiation stages only; this scenario would be similar to that of RAG genes that are subject to tight transcriptional control during lymphocyte differentiation in jawed vertebrates [88,89]. Other mechanisms of taming the mutator activities of AID/CDA enzymes might have involved-specific protein modification(s) to target their activity to certain subcellular compartments, and/or the recruitment of specific enzymes/co-factors to achieve the required locus-specific targeting in the genome of lymphocytes. As is the case with RAG-induced chromosomal modifications, AADs can still inflict collateral damage to self-DNA [90]. For AID, it is conceivable that a trade-off may have emerged, balancing the short-term advantage of an efficient immune response (for instance, by affinity maturation of antibodies) against the long-term risk of initiating tumor development. The latter likely occurs in B cells after activating mutations in proto-oncogenes but may require additional insult in the genome for the development of full-blown malignancy. In evolutionarily ancient jawed vertebrates, such as cartilaginous fishes, where AID-induced mutations are found in TCRs [91•], the trade-off is less clear. However, in this group of jawed vertebrates, it is possible that instead of post-selection affinity maturation, AID might be employed for repertoire diversification after initial RAG-mediated assembly but before selection for self-compatibility.
Evolutionary implications of CDA phylogeny in jawless vertebrates
In contrast to the lymphocyte lineage-independent expression pattern and function of RAG1 and RAG2 genes in jawed vertebrates, CDA genes in lampreys exhibit a lineage-specific signature and function, as demonstrated for CDA2 in the B-like lineage [20••]. These findings suggest an evolutionary scenario for the CDA gene family that has wider implications for our understanding of the emergence of lymphocyte lineages in jawless vertebrates.
Because RAG1 and RAG2 proteins function in the assembly of both Ig and TCR genes in all jawed vertebrates [17], the RAG1-RAG2 transposon might have invaded the primordial antigen receptor gene (split-AgR gene) in an ancient jawed vertebrate possessing only one type of lymphocyte lineage (Figure 2a). It is conceivable that this pan-lymphocyte lineage subsequently diversified into antibody-producing cells and those mediating cellular immune functions. Excision of the original transposon sequence then generated a primordial antigen receptor gene of the V(D)J type, which after duplication/expansion evolved into the diversified Ig and TCR gene classes present in all extant jawed vertebrates. Alternatively, the split-AgR gene might have been present in the ancestor common to all vertebrates; if so, it must have been lost, together with the RAG-like transposon in the lineage that gave rise to extant jawless vertebrates (Figure 2a). In any case, at some point during this evolutionary process (most likely in the common ancestor of jawed vertebrates), the ProtoRAG proteins evolved into domesticated forms (Figure 2b) that are present in all extant jawed vertebrates [42••].
Likewise, primordial self DNA-targeting AAD(s) might have already been present in the ancestor common to all vertebrates (Figure 2a), and then evolved into proto-CDA2 and proto-AID genes in the ancestors of jawless and jawed vertebrates, respectively. This was followed by the emergence of the ‘domesticated’ versions of CDA2 and AID in the two extant vertebrate lineages (Figure 2b). Interestingly, AID appears to be involved in somatic hypermutation of both Ig and TCR genes in the most basal group of jawed vertebrates, the cartilaginous fishes [91•,92•]; this dual function may have been lost in other jawed vertebrates. By analogy, it is possible that the VLR system, and the associated CDA-mediated assembly process, initially was dependent on CDA2 in the two principal lymphocyte lineages of the common ancestor of jawless vertebrates (Figure 2a). Subsequently, retrotransposed copies of the primordial CDA2 gene may have become expressed in T-like cells, eventually leading to the silencing of the CDA2 gene in that lineage. The recruitment of the primordial CDA1 genes for the assembly of the emerging VLRA/C genes would then correspond to the lineage-specific ‘division of labor’ between CDA genes that is found in extant lampreys. Moreover, it is possible that only the CDA1 clade became associated with VLRA/C gene assembly in T-like cells, whereas other CDA1L genes assumed more innate immune defense-related functions by targeting non-self DNA, perhaps also in non-hematopoietic cell types. The latter hypothesis is supported by conspicuous structural characteristics of CDA1L enzymes distinguishing them from CDA1 and CDA1L1_4 [82••]. The optimal in vitro activity of some CDA1L enzymes at acidic pH suggests that they may be involved in direct antiviral function in acidic cellular compartments, such as endosomes [82••].
Conclusions and outlook
Technological advances in rearing in vitro fertilized lampreys have opened up unprecedented opportunities to interrogate the function of alternative adaptive immune systems of jawless vertebrates. This development will foster in-depth studies on the ontogeny of the immune system and may in the future provide the basis for comparative studies among different lamprey species and perhaps even hagfish. The fact that lampreys have now become genetically tractable will make this animal model amenable to functional studies. Through gene ablation and transgenesis combined with conventional molecular analyses, these studies will address the functional roles of the key components of immune facilities so far identified. Key questions that should be addressed in the near future are: (1) Are CDA1L genes required for VLR gene assembly or do they function in innate-type antiviral defenses only? (2) What is the molecular basis of deployment of CDAs to the T and B lineages? (3) Is the development of lymphoid organs and of separate lymphocyte lineages in jawless vertebrates governed by the same transcriptional circuitries that are found in hematopoietic and associated stromal cells of jawed vertebrates? (4) Is it possible to extend the similarities between the adaptive immune systems of jawed and jawless vertebrates to mechanisms of antigen presentation and tolerance induction?
An intriguing outcome of the recent comparative studies of adaptive immunity in jawless and jawed vertebrates is the astounding convergence of immune functionalities. The new opportunities of lamprey immunogenetics will make it possible to study in precise detail both commonalities and similarities in immune response and memory formation. One aspect of particular interest in this regard concerns the principles by which ancient innate and more recent adaptive facilities became integrated into the coherent whole of vertebrate immunity. In addition, these studies will be of great value for understanding the fundamental rules underlying pathophysiological states of the innate and/or adaptive aspects of immunity, such as immunodeficiency and autoimmunity. The answers to these questions will provide evolutionary perspectives to interconnect the functions of different components of the two adaptive immune systems.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
These studies received funding from the Max Planck Society, the Jung Foundation for Science and Research, and the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013), ERC Grant agreement 323126 (all to T.B.). R.M. was supported by a JSPS Overseas Research Fellowship.
References
- 1.Liaw S.H., Chang Y.J., Lai C.T., Chang H.C., Chang G.G. Crystal structure of Bacillus subtilis guanine deaminase: the first domain-swapped structure in the cytidine deaminase superfamily. J Biol Chem. 2004;279:35479–35485. doi: 10.1074/jbc.M405304200. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton C.E., Papavasiliou F.N., Rosenberg B.R. Diverse functions for DNA and RNA editing in the immune system. RNA Biol. 2010;7:220–228. doi: 10.4161/rna.7.2.11344. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 4.Makarova K.S., Wolf Y.I., Koonin E.V. Comparative genomics of defense systems in archaea and bacteria. Nucleic Acids Res. 2013;41:4360–4377. doi: 10.1093/nar/gkt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasu K., Nagaraja V. Diverse functions of restriction-modification systems in addition to cellular defense. Microbiol Mol Biol Rev. 2013;77:53–72. doi: 10.1128/MMBR.00044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Houte S., Buckling A., Westra E.R. Evolutionary ecology of prokaryotic immune mechanisms. Microbiol Mol Biol Rev. 2016;80:745–763. doi: 10.1128/MMBR.00011-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makarova K.S., Wolf Y.I., Alkhnbashi O.S., Costa F., Shah S.A., Saunders S.J., Barrangou R., Brouns S.J., Charpentier E., Haft D.H. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanraju P., Makarova K.S., Zetsche B., Zhang F., Koonin E.V., van der Oost J. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science. 2016;353 doi: 10.1126/science.aad5147. [DOI] [PubMed] [Google Scholar]
- 9.Koonin E.V., Makarova K.S., Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78. doi: 10.1016/j.mib.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shmakov S., Smargon A., Scott D., Cox D., Pyzocha N., Yan W., Abudayyeh O.O., Gootenberg J.S., Makarova K.S., Wolf Y.I. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15:169–182. doi: 10.1038/nrmicro.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris R.S., Liddament M.T. Retroviral restriction by APOBEC proteins. Nat Rev Immunol. 2004;4:868–877. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- 12.Moris A., Murray S., Cardinaud S. AID and APOBECs span the gap between innate and adaptive immunity. Front Microbiol. 2014;5:534. doi: 10.3389/fmicb.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salter J.D., Bennett R.P., Smith H.C. The APOBEC protein family: united by structure, divergent in function. Trends Biochem Sci. 2016;41:578–594. doi: 10.1016/j.tibs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Krishnan A., Iyer L.M., Holland S.J., Boehm T., Aravind L. Diversification of AID/APOBEC-like deaminases in metazoa: multiplicity of clades and widespread roles in immunity. Proc Natl Acad Sci U S A. 2018;115:E3201–E3210. doi: 10.1073/pnas.1720897115. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic analysis of the AAD gene family in multicellular organisms.
- 15••.Schatz D.G., Oettinger M.A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]; Discovery of the RAG1 gene.
- 16••.Oettinger M.A., Schatz D.G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]; Discovery of the RAG2 gene adjacent to RAG1.
- 17.Schatz D.G., Swanson P.C. V(D)J recombination: mechanisms of initiation. Annu Rev Genet. 2011;45:167–202. doi: 10.1146/annurev-genet-110410-132552. [DOI] [PubMed] [Google Scholar]
- 18••.Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]; Together with Ref. [75•], discovery of AID and its requirement for somatic hypermutation and class switch recombination.
- 19••.Rogozin I.B., Iyer L.M., Liang L., Glazko G.V., Liston V.G., Pavlov Y.I., Aravind L., Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]; First speculation on the association of CDAs and assembly of VLR genes.
- 20••.Morimoto R., O´Meara C.P., Holland S.J., Trancoso I., Souissi A., Schorpp M., Vassaux D., Iwanami N., Giorgetti O.B., Evanno G. Cytidine deaminase 2 is required for VLRB antibody gene assembly in lampreys. Sci Immunol. 2020 doi: 10.1126/sciimmunol.aba0925. [DOI] [PubMed] [Google Scholar]; First evidence that CDAs are required for VLR assembly in jawless vertebrates.
- 21••.Williams A.F., Barclay A.N. The immunoglobulin superfamily—domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]; Description of the Ig family of proteins.
- 22••.Pancer Z., Amemiya C.T., Ehrhardt G.R., Ceitlin J., Gartland G.L., Cooper M.D. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]; Discovery of the VLR system in lamprey.
- 23•.Pancer Z., Saha N.R., Kasamatsu J., Suzuki T., Amemiya C.T., Kasahara M., Cooper M.D. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci U S A. 2005;102:9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of VLR genes in hagfish, the sister group of lampreys.
- 24••.Guo P., Hirano M., Herrin B.R., Li J., Yu C., Sadlonova A., Cooper M.D. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of the dichotomy of T-like and B-like lymphoid cells in lamprey.
- 25•.Hirano M., Guo P., McCurley N., Schorpp M., Das S., Boehm T., Cooper M.D. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of an additional T cell lineage in lampreys.
- 26.Schatz D.G., Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol. 2011;11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 27•.Nagawa F., Kishishita N., Shimizu K., Hirose S., Miyoshi M., Nezu J., Nishimura T., Nishizumi H., Takahashi Y., Hashimoto S. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]; Description of a molecular mechanism of the VLR gene assembly process.
- 28.Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- 29.Roth D.B. Restraining the V(D)J recombinase. Nat Rev Immunol. 2003;3:656–666. doi: 10.1038/nri1152. [DOI] [PubMed] [Google Scholar]
- 30.Matthews A.G., Oettinger M.A. Regulation of RAG transposition. Adv Exp Med Biol. 2009;650:16–31. doi: 10.1007/978-1-4419-0296-2_2. [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka H., Tran T.H., Kobayashi M., Aida M., Honjo T. Preventing AID, a physiological mutator, from deleterious activation: regulation of the genomic instability that is associated with antibody diversity. Int Immunol. 2010;22:227–235. doi: 10.1093/intimm/dxq023. [DOI] [PubMed] [Google Scholar]
- 32.Alt F.W., Zhang Y., Meng F.L., Guo C., Schwer B. Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell. 2013;152:417–429. doi: 10.1016/j.cell.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodgers W., Byrum J.N., Sapkota H., Rahman N.S., Cail R.C., Zhao S., Schatz D.G., Rodgers K.K. Spatio-temporal regulation of RAG2 following genotoxic stress. DNA Repair (Amst) 2015;27:19–27. doi: 10.1016/j.dnarep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieber M.R. Mechanisms of human lymphoid chromosomal translocations. Nat Rev Cancer. 2016;16:387–398. doi: 10.1038/nrc.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lescale C., Deriano L. The RAG recombinase: beyond breaking. Mech Ageing Dev. 2017;165:3–9. doi: 10.1016/j.mad.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Bonnet M., Sarmento L.M., Martins A.C., Sobral D., Silva J., Demengeot J. iRAGu: a novel inducible and reversible mouse model for ubiquitous recombinase activity. Front Immunol. 2017;8:1525. doi: 10.3389/fimmu.2017.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg J.M., Boehm T., Sofroniew M.V., Keynes R.J., Barton S.C., Norris M.L., Surani M.A., Spillantini M.G., Rabbitts T.H. Segmental and developmental regulation of a presumptive T-cell oncogene in the central nervous system. Nature. 1990;344:158–160. doi: 10.1038/344158a0. [DOI] [PubMed] [Google Scholar]
- 38•.Miller W.J., Hagemann S., Reiter E., Pinsker W. P-element homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc Natl Acad Sci U S A. 1992;89:4018–4022. doi: 10.1073/pnas.89.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Introduction of the concept of repurposing laterally transferred exogenous genetic material for the benefit of the host.
- 39.Miller W.J., McDonald J.F., Pinsker W. Molecular domestication of mobile elements. Genetica. 1997;100:261–270. [PubMed] [Google Scholar]
- 40.Passagem-Santos D., Bonnet M., Sobral D., Trancoso I., Silva J.G., Barreto V.M., Athanasiadis A., Demengeot J., Pereira-Leal J.B. RAG recombinase as a selective pressure for genome evolution. Genome Biol Evol. 2016;8:3364–3376. doi: 10.1093/gbe/evw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Carmona L.M., Schatz D.G. New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination. FEBS J. 2017;284:1590–1605. doi: 10.1111/febs.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent perspective on the evolution of RAG transposon domestication.
- 42••.Zhang Y., Cheng T.C., Huang G., Lu Q., Surleac M.D., Mandell J.D., Pontarotti P., Petrescu A.J., Xu A., Xiong Y. Transposon molecular domestication and the evolution of the RAG recombinase. Nature. 2019;569:79–84. doi: 10.1038/s41586-019-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [55••], description of the molecular basis of domestication of the ProtoRAG-like transposon.
- 43.Kumar S., Hedges S.B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 44.Janvier P. Facts and fancies about early fossil chordates and vertebrates. Nature. 2015;520:483–489. doi: 10.1038/nature14437. [DOI] [PubMed] [Google Scholar]
- 45.Metchnikoff E. Pипол Классик; 1893. Lectures on the Comparative Pathology of Inflammation: Delivered at the Pasteur Institute in 1891. [Google Scholar]
- 46.Rosental B., Kowarsky M., Seita J., Corey D.M., Ishizuka K.J., Palmeri K.J., Chen S.Y., Sinha R., Okamoto J., Mantalas G. Complex mammalian-like haematopoietic system found in a colonial chordate. Nature. 2018;564:425–429. doi: 10.1038/s41586-018-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011;11:307–317. doi: 10.1038/nri2944. [DOI] [PubMed] [Google Scholar]
- 48•.Cooper M.D., Alder M.N. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]; Description of the implication of an alternative immune system in jawless vertebrates.
- 49.Boehm T., Iwanami N., Hess I. Evolution of the immune system in the lower vertebrates. Annu Rev Genomics Hum Genet. 2012;13:127–149. doi: 10.1146/annurev-genom-090711-163747. [DOI] [PubMed] [Google Scholar]
- 50.Yuan S., Tao X., Huang S., Chen S., Xu A. Comparative immune systems in animals. Annu Rev Anim Biosci. 2014;2:235–258. doi: 10.1146/annurev-animal-031412-103634. [DOI] [PubMed] [Google Scholar]
- 51••.Tonegawa S., Steinberg C., Dube S., Bernardini A. Evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1974;71:4027–4031. doi: 10.1073/pnas.71.10.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of the V(D)J recombination process; also see Ref. [52].
- 52•.Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976;73:3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of the V(D)J recombination process; also see Ref. [51].
- 53•.Kapitonov V.V., Jurka J. RAG1 core and V(D)J recombination signal sequences were derived from Transib transposons. PLoS Biol. 2005;3:e181. doi: 10.1371/journal.pbio.0030181. [DOI] [PMC free article] [PubMed] [Google Scholar]; First suggestion that RAG originated from Transib transposons.
- 54•.Fugmann S.D., Messier C., Novack L.A., Cameron R.A., Rast J.P. An ancient evolutionary origin of the Rag1/2 gene locus. Proc Natl Acad Sci U S A. 2006;103:3728–3733. doi: 10.1073/pnas.0509720103. [DOI] [PMC free article] [PubMed] [Google Scholar]; First identification of RAG-like sequences outside vertebrates.
- 55••.Liu C., Yang Y., Schatz D.G. Structures of a RAG-like transposase during cut-and-paste transposition. Nature. 2019;575:540–544. doi: 10.1038/s41586-019-1753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [42••], description of the molecular basis of domestication of the ProtoRAG-like transposase.
- 56.Schatz D.G. Antigen receptor genes and the evolution of a recombinase. Semin Immunol. 2004;16:245–256. doi: 10.1016/j.smim.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Flajnik M.F. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol. 2002;2:688–698. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 58.Huang S., Chen Z., Yan X., Yu T., Huang G., Yan Q., Pontarotti P.A., Zhao H., Li J., Yang P. Decelerated genome evolution in modern vertebrates revealed by analysis of multiple lancelet genomes. Nat Commun. 2014;5:5896. doi: 10.1038/ncomms6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Xu K., Deng A., Fu X., Xu A., Liu X. An amphioxus RAG1-like DNA fragment encodes a functional central domain of vertebrate core RAG1. Proc Natl Acad Sci U S A. 2014;111:397–402. doi: 10.1073/pnas.1318843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Huang S., Tao X., Yuan S., Zhang Y., Li P., Beilinson H.A., Zhang Y., Yu W., Pontarotti P., Escriva H. Discovery of an active RAG transposon illuminates the origins of V(D)J recombination. Cell. 2016;166:102–114. doi: 10.1016/j.cell.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of the ProtoRAG gene in amphioxus.
- 61•.Mayer W.E., Uinuk-Ool T., Tichy H., Gartland L.A., Klein J., Cooper M.D. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci U S A. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of the molecular signature of lamprey lymphocytes.
- 62.Uinuk-Ool T., Mayer W.E., Sato A., Dongak R., Cooper M.D., Klein J. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc Natl Acad Sci U S A. 2002;99:14356–14361. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrin B.R., Cooper M.D. Alternative adaptive immunity in jawless vertebrates. J Immunol. 2010;185:1367–1374. doi: 10.4049/jimmunol.0903128. [DOI] [PubMed] [Google Scholar]
- 64.Hirano M., Das S., Guo P., Cooper M.D. The evolution of adaptive immunity in vertebrates. Adv Immunol. 2011;109:125–157. doi: 10.1016/B978-0-12-387664-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 65•.Kapitonov V.V., Koonin E.V. Evolution of the RAG1-RAG2 locus: both proteins came from the same transposon. Biol Direct. 2015;10:20. doi: 10.1186/s13062-015-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of the evolutionary origin of the RAG1/RAG2 gene pair.
- 66.Elkin S.K., Matthews A.G., Oettinger M.A. The C-terminal portion of RAG2 protects against transposition in vitro. EMBO J. 2003;22:1931–1938. doi: 10.1093/emboj/cdg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X., Cui Y., Best R.B., Wang H., Zhou Z.H., Yang W., Gellert M. Cutting antiparallel DNA strands in a single active site. Nat Struct Mol Biol. 2020;27:119–126. doi: 10.1038/s41594-019-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Chen X., Cui Y., Wang H., Zhou Z.H., Gellert M., Yang W. How mouse RAG recombinase avoids DNA transposition. Nat Struct Mol Biol. 2020;27:127–133. doi: 10.1038/s41594-019-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Description of the molecular mechanism underlying RAG activity.
- 69.Hiom K., Melek M., Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 70••.Agrawal A., Eastman Q.M., Schatz D.G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]; Experimental evidence for transposition activity of the RAG recombinase.
- 71.Finger L.R., Harvey R.C., Moore R.C., Showe L.C., Croce C.M. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science. 1986;234:982–985. doi: 10.1126/science.3490692. [DOI] [PubMed] [Google Scholar]
- 72.Boehm T., Buluwela L., Williams D., White L., Rabbitts T.H. A cluster of chromosome 11p translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988;7:2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teng G., Maman Y., Resch W., Kim M., Yamane A., Qian J., Kieffer-Kwon K.R., Mandal M., Ji Y., Meffre E. RAG represents a widespread threat to the lymphocyte genome. Cell. 2015;162:751–765. doi: 10.1016/j.cell.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Compton A.A., Hirsch V.M., Emerman M. The host restriction factor APOBEC3G and retroviral Vif protein coevolve due to ongoing genetic conflict. Cell Host Microbe. 2012;11:91–98. doi: 10.1016/j.chom.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O., Catalan N., Forveille M., Dufourcq-Labelouse R., Gennery A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]; Together with Ref. [18••], discovery of AID and its requirement for somatic hypermutation and class switch recombination.
- 76•.Arakawa H., Hauschild J., Buerstedde J.M. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]; Discovery of the requirement of AID for immunoglobulin gene conversion in chicken; see also Ref. [81].
- 77.Hirano M. Evolution of vertebrate adaptive immunity: immune cells and tissues, and AID/APOBEC cytidine deaminases. Bioessays. 2015;37:877–887. doi: 10.1002/bies.201400178. [DOI] [PubMed] [Google Scholar]
- 78.Alder M.N., Herrin B.R., Sadlonova A., Stockard C.R., Grizzle W.E., Gartland L.A., Gartland G.L., Boydston J.A., Turnbough C.L., Jr., Cooper M.D. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 79•.Boehm T., Hirano M., Holland S.J., Das S., Schorpp M., Cooper M.D. Evolution of alternative adaptive immune systems in vertebrates. Annu Rev Immunol. 2018;36:19–42. doi: 10.1146/annurev-immunol-042617-053028. [DOI] [PubMed] [Google Scholar]; Recent overview of the LRR-based adaptive immune systems in jawless vertebrates.
- 80.Alder M.N., Rogozin I.B., Iyer L.M., Glazko G.V., Cooper M.D., Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 81•.Reynaud C.A., Anquez V., Grimal H., Weill J.C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987;48:379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]; Discovery of the requirement of gene conversion for the generation of the primary immunoglobulin gene repertoire; see also Ref. [76].
- 82••.Holland S.J., Berghuis L.M., King J.J., Iyer L.M., Sikora K., Fifield H., Peter S., Quinlan E.M., Sugahara F., Shingate P. Expansions, diversification, and interindividual copy number variations of AID/APOBEC family cytidine deaminase genes in lampreys. Proc Natl Acad Sci U S A. 2018;115:E3211–E3220. doi: 10.1073/pnas.1720871115. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of the expansion of CDA1-like genes in European brook lamprey, L. planeri.
- 83••.Bajoghli B., Guo P., Aghaallaei N., Hirano M., Strohmeier C., McCurley N., Bockman D.E., Schorpp M., Cooper M.D., Boehm T. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]; Discovery of the thymus equivalent in lampreys.
- 84.Liu M.C., Liao W.Y., Buckley K.M., Yang S.Y., Rast J.P., Fugmann S.D. AID/APOBEC-like cytidine deaminases are ancient innate immune mediators in invertebrates. Nat Commun. 2018;9:1948. doi: 10.1038/s41467-018-04273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conticello S.G., Thomas C.J., Petersen-Mahrt S.K., Neuberger M.S. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22:367–377. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- 86.Kidd J.M., Newman T.L., Tuzun E., Kaul R., Eichler E.E. Population stratification of a common APOBEC gene deletion polymorphism. PLoS Genet. 2007;3:e63. doi: 10.1371/journal.pgen.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.OhAinle M., Kerns J.A., Li M.M., Malik H.S., Emerman M. Antiretroelement activity of APOBEC3H was lost twice in recent human evolution. Cell Host Microbe. 2008;4:249–259. doi: 10.1016/j.chom.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuo T.C., Schlissel M.S. Mechanisms controlling expression of the RAG locus during lymphocyte development. Curr Opin Immunol. 2009;21:173–178. doi: 10.1016/j.coi.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gostissa M., Alt F.W., Chiarle R. Mechanisms that promote and suppress chromosomal translocations in lymphocytes. Annu Rev Immunol. 2011;29:319–350. doi: 10.1146/annurev-immunol-031210-101329. [DOI] [PubMed] [Google Scholar]
- 90.Meng F.L., Du Z., Federation A., Hu J., Wang Q., Kieffer-Kwon K.R., Meyers R.M., Amor C., Wasserman C.R., Neuberg D. Convergent transcription at intragenic super-enhancers targets AID-initiated genomic instability. Cell. 2014;159:1538–1548. doi: 10.1016/j.cell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Ott J.A., Castro C.D., Deiss T.C., Ohta Y., Flajnik M.F., Criscitiello M.F. Somatic hypermutation of T cell receptor alpha chain contributes to selection in nurse shark thymus. eLife. 2018;7 doi: 10.7554/eLife.28477. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of somatic hypermutation in TCRs; also see Ref. [92].
- 92•.Chen H., Bernstein H., Ranganathan P., Schluter S.F. Somatic hypermutation of TCR gamma V genes in the sandbar shark. Dev Comp Immunol. 2012;37:176–183. doi: 10.1016/j.dci.2011.08.018. [DOI] [PubMed] [Google Scholar]; Discovery of somatic hypermutation in TCRs; also see Ref. [91].