Abstract
The prognosis of elderly patients with acute myeloid leukemia (AML) and high-grade myelodysplastic syndrome (MDS) is limited due to the lack of therapy options and high relapse rates. Dendritic cell (DC)-based immunotherapy seems to be a promising treatment tool. DC are potent antigen-presenting cells and play a pivotal role on the interface of the innate and the adaptive immune system. Myeloid leukemia blasts can be converted to DC of leukemic origin (DC<sub>leu</sub>), expressing costimulatory molecules along with the whole leukemic antigen repertoire of individual patients. These generated DC<sub>leu</sub> are potent stimulators of various immune reactive cells and increase antileukemic immunity ex vivo. Here we review the generating process of DC/DC<sub>leu</sub> from leukemic peripheral blood mononuclear cells as well as directly from leukemic whole blood with “minimized” Kits to simulate physiological conditions ex vivo. The purpose of adoptive cell transfer of DC/DC<sub>leu</sub> as a vaccination strategy is discussed. A new potential therapy option with Kits for patients with myeloid leukemia, which would render an adoptive DC/DC<sub>leu</sub> transfer unnecessary, is presented. In summary, DC/DC<sub>leu</sub>-based therapies seem to be promising treatment tools for patients with AML or MDS but ongoing research including trials in animals and humans have to be performed.
Keywords: Acute myeloid leukemia, Leukemia-derived dendritic cells, Immunotherapy
State of the Art: Current Prognosis and Therapy Options for Patients with Acute Myeloid Leukemia
Acute myeloid leukemia (AML) and (high-grade) myelodysplastic syndromes (MDS) are hematopoietic disorders characterized by uncontrolled proliferation and impaired differentiation of leukemic blasts in the bone marrow (BM). The blasts replace the physiological hematopoiesis and cause typical symptoms such as anemia, bleeding, and infections [1]. The prognosis of AML patients, especially among the elderly (age >65 years), is unsatisfactory, with 5-year overall survival rates of 5–15% in older cohorts and 30% in younger cohorts. The outcomes of (most of the time elderly) MDS patients, characterized by hematopoietic failure and increasing blasts, are even worse [2, 3, 4].
High-dose induction chemotherapy with cytarabin ± anthracycline (e.g., 7 + 3 regimen) followed by allogeneic hematopoietic stem cell transplantation (HSCT) is the only potential curative treatment and it is the standard therapy, especially for young AML/MDS patients with fewer comorbidities [5, 6, 7]. Different side effects such as graft-versus-host disease (GVHD), which mainly effects the skin, the liver, and the gastrointestinal tract characterize and limit the therapy [8, 9]. Due to high morbidity and mortality rates, HSCT is no therapy option for patients older than 65 years, who represent the main cohort of patients affected by AML or MDS [2, 5]. Measurable residual disease (MRD), previously called minimal residual disease, i.e., a small reservoir of cells in the BM far below the 5% leukemic blast threshold or in tissues, resistant against chemotherapy, is one of the main reasons for relapse [10, 11]. HSCT and donor lymphocyte infusion can affect MRD via the graft versus leukemia effect mainly mediated by allogeneic effector T and natural killer (NK) cells [9, 12]. However, relapse of AML remains the main treatment failure [13]. Low-dose cytarabin or hypomethylating agents − with or without combined Venetoclax, a drug influencing apoptosis of leukemic blasts − are therapy options for patients with a low tolerance for intensive induction therapy or refractoriness to hypomethylating agents [3, 14].
In the last few years new (non-immunotherapeutic), treatment strategies for AML patients, i.e., antileukemic protein kinase inhibitors (e.g., FLT3 inhibitors), epigenetic modulators, new cytotoxic agents, and mitochondrial inhibitors including apoptosis-based therapies and therapies that target specific oncogenic proteins, have been in development. Their clinical value still has to be demonstrated for myeloid malignant diseases [15].
Dendritic Cell Based Immunotherapy for Patients with AML or MDS
Due to the high rates of relapse or persisting disease after treatment and the limited therapy options, especially for elderly patients, there is a need for alternatives such as immunotherapy to maintain stable remissions and/or stable disease. Due to the potential induction of an antileukemia immunity against MRD, different work groups have tried to utilize dendritic cells (DC) as a treatment tool for patients with AML [16]. DC are major antigen-presenting cells (APC) that internalize and process antigens (e.g., microbe fragments and necrotic tumor cell products) and present these fragments via major histocompatibility complex I and II (MHC I and II) molecules in lymphoid organs to cells of the innate and adaptive immune system [17, 18]. On the one hand, DC activate cells of the innate immune system, e.g., NK cells or invariant natural kill cells to avoid pathogen spread until cells of the adaptive immune system are activated [19]. On the other hand, DC form immunological synapses with T cells, resulting in a clonally restricted and potent T-cell activation against the presented antigens [20, 21, 22]. In that context, DC play a crucial role on the interface and crosstalk of the innate and the adaptive immune system [23, 24].
Monocyte-Derived DC
Ex vivo DC can be generated from autologous or allogeneic CD14+ monocytes (monocyte-derived DC; mo-DC) [25]. Generated mo-DC have to be loaded with leukemia-associated antigens (LAA) which are overexpressed peptides or proteins on leukemic blasts compared to healthy tissue [26]. Wilms tumor antigen 1 (WT1), preferentially expressed antigen in melanoma (PRAME), and human telomerase reverse transcriptase (hTERT) are widely used LAA for the loading process of mo-DC [25, 27, 28]. Mo-DC can be pulsed with apoptotic leukemic cells, whole leukemic cell lysates or RNA electroporation of whole leukemic cell-derived RNA as well as messenger ribonucleic acid (mRNA) encoding one or even more defined LAA [16, 25, 27, 29, 30, 31, 32]. After manufacture of mo-DC under Good Manufacturing Practice (GMP), they are re-administered to the patient as an intradermal or intravenous vaccination.
Leukemia-Derived DC
In AML, MDS and chronic myeloid leukemia (CML) DC can be generated directly from the malignant leukemic cells (leukemia-derived DC; DCleu) with different DC/DCleu-generating protocols due to the fact that leukemic blasts and DC originate from the same precursor cells [30]. For the generation of DCleu, peripheral blood mononuclear cells (PBMNC) from patients with AML or MDS are cultured in the presence of different combinations of response modifiers [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51]. Leukemic blasts thereby gain a typical DC morphology with an increased expression of costimulatory molecules as well as a higher antigen presentation capacity.
Proof of Leukemic Derivation of DCleu and Characterization of Subtypes
DCleu are characterized by the expression of individual patients' whole leukemic antigen repertoire including known as well as unknown leukemic antigens [35, 40, 52, 53, 54]. The leukemic origin of DCleu can be confirmed by Western blot as well as fluorescence in situ hybridization (FISH) analyses by the detection of leukemia-specific numeric or structural chromosomal aberrations in the nucleolus of the generated DCleu [35, 44, 55].
It has to be taken into account that leukemic blasts, CD14+ monocytes, or CD34+ stem cells in PBMNC obtained from peripheral blood (PB) or BM from patients with AML can be differentiated to DC/DCleu during cell culture [40, 56]. To quantify only generated DCleu and to differentiate them from nonleukemic DC or unconverted blasts, a special flow cytometric gating strategy has been developed [53]. Cells have to be stained with patient-specific blast-staining antibodies (e.g., CD15, CD34, CD65, and CD117) in combination with DC-staining antibodies (e.g., CD80, CD83, CD86, CD206, and CD209), which were not expressed on leukemic blasts before DC/DCleu culture [40, 53, 57, 58]. With that strategy DCleu can be quantified in the total cell fraction (DCleu/PBMNC), in the DC fraction (DCleu/DC+), or in the blast fraction to quantify the amount of blasts converted to DCleu (DCleu/bla+) [53].
Only mature DC/DCleu can activate immune reactive cells. Therefore, quantification of mature DC/DCleu is important. These cells are characterized by expression of the chemokine receptor 7 (CCR7), which acts as a lymph node-homing receptor and is crucial for the migratory capacity of DC/DCleu [59, 60]. Furthermore, expression of CD83, a member of the immunoglobulin superfamily, and secretion of IL 12 are also considered markers for maturation of DC/DCleu. Membrane-bound CD83 acts as a potent T-cell activating signal and plays an important role in the generation of thymocytes as well as in modulation of the immune response [61, 62, 63]. IL (interleukin)-12 is responsible for the induction of differentiation of CD8+ T cells toward cytotoxic T lymphocytes (CTL), activates NK cells, and mediates TH-1 development [64, 65, 66].
Protocols to Produce DC/DCleu from Blast-Containing PBMNC
Various attempts have been made to improve DC/DCleugeneration from isolated PBMNC from patients with AML, which might be used for an adoptive cell transfer. For generation of DC/DCleu from leukemic PBMNC, different protocols have been published so far and they are summarized in Table 1. The main differences in the protocols are caused by the use of different combinations of response modifiers, cell culture media (serum free or serum enriched), and culture times − resulting in different efficiencies of sufficient DC/DCleu generation − along with the lack of comparability of different studies.
Table 1.
DC/DCleu-generating protocols from leukemic PBMNC
| Response modifiers | Protocol | Sources of cells | Culture time, days | Results | Ref. |
|---|---|---|---|---|---|
| Cytokine-based DC/DCleu-generating protocols | |||||
| GM-CSF TNF-α SCF IL-6 |
Serum enriched | PB | n.g. | First published protocol for the generation of mature DCleu | 33a |
| GM-CSF | 80% of cases with a typical DC morphology | 34 | |||
| TNF-α | Generation of mature DCleu Potent antigen-presentation capacity Leukemic origin confirmed |
||||
| GM-CSF | Serum free | PB/BM | 10–15 | ↑ DC morphology of blasts | 35 |
| IL-4 | Serum enricheda | Generation of mature DCleu | 36a | ||
| TNF-α | ↑ lysis of autologous leukemic cells ↑ IL-12 production Leukemic origin confirmed |
37a | |||
| GM-CSF | Serum free | PB/B | 10–15 | ↑ DC morphology of blasts | 35 |
| IL-4 | Generation of mature DCleu | ||||
| CD40L | ↑ lysis of autologous leukemic cells Leukemic origin confirmed |
||||
| GM-CSF | Serum enriched | PB | 12–14 | 77% successful DCleu generation | 38a |
| IL-4 | Serum free | ↑ DC morphology of blasts | 109 | ||
| TNF-α | Generation of mature DCleu | 40 | |||
| FLT3 L | ↑ antileukemic immunity of T cells Leukemic origin confirmed |
||||
| GM-CSF | Serum enriched | PB/BM | 8 | 68% successful DCleu generation | 41a |
| TNF-α | ↑ DC morphology of blasts | ||||
| FLT3 L | Generation of mature DCleu | ||||
| TGF-β | ↑ antileukemic immunity of T cells | ||||
| SCF | Leukemic origin confirmed | ||||
| GM-CSF | Serum enriched | PB/BM | 3–5 | 100% successful DCleu generation | 42a |
| SCF | ↑ DC morphology of blasts | ||||
| FLT3 L | Generation of mature DCleu ↑ Stimulating potential on T cells Leukemic origin confirmed |
||||
| GM-CSF | Serum enriched | PB/BM | n.g. | ↑ DC morphology of blasts | 43a |
| SCF | Generation of mature DCleu | ||||
| TNF-α | ↑ Stimulating potential on T cells | ||||
| +/– IL-4 | Leukemic origin confirmed | ||||
| GM-CSF | Serum enriched | PB | 10 | ↑ DC morphology of blasts | 44a |
| IL-4 | Generation of mature DCleu | 45a | |||
| TNF-α | ↑ IL 12 production | ||||
| CD40L | ↑ Stimulating potential on T cells Leukemic origin confirmed |
||||
| GM-CSF | Serum enriched | PB | 8 | 80% successful DCleu generation | 46 a, 47 a |
| IL-4 | Serum free | Generation of mature DCleu | 40 | ||
| +/– TLR 3 A | ↑ IL 12 production (especially with TLR4 and TLR 7/8 agonists) | ||||
| +/– TLR 4 A | ↑ Stimulating potential on T cells | ||||
| +/– TLR 7/8 A | ↑ anti-leukemic immunity of T cells | ||||
| GM-CSF | Serum free | PB | 9–11 | Generation of mature DCleu | 40 |
| IL-4 | ↑ Stimulating potential on T cells | ||||
| Picibanil PGE2 |
↑ anti-leukemic immunity of T cells | ||||
| GM-CSF IL-4 Picibanil PGE1 |
Serum free | PB | 7–10 | Higher amounts as PGE2 containing protocols Generation of mature DCleu |
48 |
| GM-CSF IL-4 FLT3 L TNF-α IL-1β IL-6 PGE2 |
Serum free | PB | 10–12 | Generation of mature DCleu ↑ Stimulating potential on T cells ↑ anti-leukemic immunity of T cells |
40 |
| GM-CSF TNF-α IL-3 SCF FLT3 L IL-4 |
Serum free | PB/BM | 14 | 83% DCleu generation possible No induction of T-cell-mediated cytotoxicity |
49 |
| GM-CSF TNF-α IFN-α |
Serum free | PB | 8–11 | DC/DCleu less successful compared to other cytokine-based protocols ↑ antileukemic immunity of T cells |
50 |
| Cytokine free DC/DCleu-generating protocols | |||||
| IL-4 A23187 |
Serum free | PB/BM | 2 | 70% successful DCleu generation ↑ DC morphology of blasts ↑ mature DC compared to cytokine-based protocols ↑ antileukemic immunity of T cells |
49 40 |
| A23187 | Serum enriched | PB | 5–7 | ↑ DC morphology of blasts ↑ DCleu compared to cytokine-based protocols (GM-CSF, IL 4, TNF-α) I induction and proliferation of T cells |
51a |
↑, increase in cell amounts; +/–, with or without; n.g., not given.
Serum-enriched protocols.
For an adoptive cell transfer it is recommended to generate DC/DCleu in serum-free cell culture media to avoid unspecific immunoreactions and anaphylactic or immunoreactions against xenoantigens [67]. Fetal calf or bovine serum or healthy human serum contains a large number of identified and unidentified factors (including different growth factors) that might influence DC/DCleu generation in an unknown way [40, 67]. In general, DC/DCleu generation has been found to be comparable in serum-free as well as serum-enriched cell culture media [67].
DC/DCleu Differentiation and Maturation
In general, 3 steps are needed for successful generation of DC/DCleu from leukemic PBMNC, i.e., induction of hematopoietic differentiation, danger signaling, and DC/DCleu maturation [40, 48]. In a first step, substances (especially cytokines) such as granulocyte macrophage colony-stimulating factor (GM-CSF), ligand of the FLT3 receptor (FLT3 L), stem cell factor (SCF), interferon (IFN)-α, and IL-4 induce the differentiation of myeloid leukemia blasts to immature DC/DCleu. In a second step molecules such as the bacterial lysate Picibanil, toll-like receptor (TLR) agonists, nucleic acids, or lipopolysaccharides or transforming growth factor (TGF)-β mediate a danger signaling that is crucial for the activation of DC/DCleu. In the last step, response modifiers such as tumor necrosis factor (TNF)-α, prostaglandin (PG)E2, PGE1, and INF-α are used for the induction of maturation [35, 40, 48, 50]. The combination of response modifiers can have synergistic effects on danger signaling and DC/DCleu maturation.
The Most Relevant Response Modifiers, Their Combination, and Their Role in DC/DCleu Generation in Detail
Picibanil (OK-432) a lysis product of Streptococcus pyogenesis, acts as a TLR agonist, and induces danger signaling as well as maturation of generated mo-DC from CD14+ progenitor cells in cancer patients [68]. Picibanil-containing protocols have been shown to be usable for generation of DC/DCleu from PBMNC from patients with AML [40, 48]. The efficiency of sufficient DC/DCleu generation was higher with PGE1-containing protocols (Pici-PGE-1) in a direct comparison to PGE2-containing protocols (Pici-PGE-2), suggesting that PGE1 is not just responsible for the maturation of DC/DCleu but it is also involved in the induction of differentiation of leukemic blasts toward DC/DCleu [48]. We could already show that antileukemic activity is improved after stimulation of T-cell-enriched immune reactive cells with DC/DCleu generated with PGE1-containing protocols compared to PGE2-conaining ones [48]. This effect might be explained by the different mode of action of PGE2 and PGE1 on prostaglandin receptors (EP receptors) with different down streaming effects [69]. This effect might influence the expressions of indoleamin 2,3-dioxgenase-1 (IDO1; see below), which might not impair antigen presentation capacity of DC but could activate regulatory T cells [70].
Another parallel comparison of DC/DCleu generation showed that under serum-free conditions 3 out of 5 methods qualified most to generate DC/DCleu. With at least 1 of the 3 methods, i.e., MCM-Mimic (containing: GM-CSF, IL-4, FLT3 L, TNF-α, IL-1β, IL-6, and PGE2), Pici-PGE-2(containing: GM-CSF, IL 4, Picibanil, and PGE2), and Ca-Inophore (containing: IL 4 and calcium inophores [A23187]), it was possible in every given AML patient, whereas the protocols “Cytokines” (containing: GM-CSF, TNF-α IL-4, IL-3, SCF, and FLT3 L) or “Poly-I:C” (containing: GM-CSF, IL-4, and Poly I:C) were less efficient [40].
The combination of GM-CSF, IL-4, TNF-α, and FLT3 L generated significantly more DC/DCleu compared to cultures without added FLT3 L − as shown in serum-free and serum-enriched cell cultures [38, 39, 40, 71]. FLT3 L plays a crucial role as a growth and differentiation factor in the hematopoietic system [72]. Different TLR agonists, affecting TLR3 (e.g., Poly I:C), TLR4 (e.g., lipopolysaccharide), and TLR7/8 (e.g., R848), were used to increase the maturation of generated DC/DCleu from leukemic PBMNC. Maturation was increased especially after the addition of TLR7/8 [46]. The combination of TLR4 and TLR7/8 agonists seems to have a synergistic effect on the generation of mature DC/DCleu [47]. IFN-α, a protein with stimulating effects on the immune system was analyzed in a combination with GM-CSF +/– TNF-α for DC/DCleu generation from patients with AML. Sufficient DC/DCleu generation was possible, although amounts of generated DC/DCleu were decreased in some settings compared to other established protocols [50]. Furthermore, the CD40 ligand (CD40L, also known as CD154), an important costimulatory molecule for the activation and maturation of DC, and SCF, which regulates death, apoptosis, cell differentiation, and migration of hematopoietic cells, have been used in different combinations for DC/DCleu generation with various levels of efficiency [33, 35, 42, 43, 45, 73]. The addition of bryostatin-1, a substance that interferes with protein kinase C activity, increased the percentage of cases with sufficient DC/DCleu generation when combined with different cytokine-based DC/DCleu generating protocols [74].
To make the DC/DCleu generation process more cost efficient, reduction of the culture time is an important factor. In general, DC/DCleu cell cultures are conducted for 7–14 days. Two studies analyzed DC/DCleu generation in 2 days (using calcium ionophores plus IL-4), respectively days 3–5 (using GM-CSF, FLT3 L, SCF, and TNF-α). In both cases, DC/DCleu generation was possible in sufficient amounts, although there is a lack of comparability [42, 49].
As a cytokine-free generating protocol, calcium inophores (A23187) were analyzed with or without a combination of IL-4 for generation of DC/DCleu. It was shown that the culture time could be reduced to 2 days, maturation of DC/DCleu could be induced, and the T-cell stimulatory capacity could be preserved, although the viability of the generated DC/DCleu was reduced compared to other established cytokine-based DC/DCleu-generating protocols [49, 51].
DC/DCleu Generation in Leukemic Subtypes
The generated DC/DCleu are perfect APC due to the fact that they coexpress a variety of costimulatory molecules, MHC I and II complexes, and the whole leukemic antigen repertoire [75]. In general, ex vivo DC/DCleu generation is possible from AML and MDS samples, independently of the patient's subtype, age, sex, cytogenetic risk group, and blast counts [39, 40, 48, 71, 76]. The expression of CD14, CD34, CD120, as well as CD86 on leukemic blasts seems to be a preliminary predictor of a successful cytokine-based DC/DCleu generation [76, 77, 78]. Therefore, different protocols have been analyzed to increase the expression of CD14 on leukemic blasts before DC/DCleu culture to influence the efficiency of sufficient DC/DCleu generation [79]. Internal tandem duplication in the gene of the Fms-like tyrosin kinase 3 (FLT3) receptor, occurring in around 24 % of patients with AML, seems to impair differentiation of leukemic blasts towards DCleu [80].
Ex vivo Immune Stimulating Effect of Generated DC/DCleu
Stimulation of T-cell-enriched immune reactive cells with DC/DCleu, generated from leukemic PBMNC, in a mixed-lymphocyte culture (MLC) increased T-cell activation and shifted T-cell subsets to a higher activation status [81, 82]. Proliferating T cells (Tprol CD71+, CD69+), non-naive T cells (Tnon-naive, CD45RO+), β-integrin+ T cells, and T cells with an effector function such as central-memory T cells (Tcm, CD45RO+CCR7+) and effector (memory) T cells (Teff-em, CD45RO+CCR7−) increased while naive T cells (Tnaive CD45RO−) decreased during MLC [81, 82]. Furthermore, decreased amounts of regulatory T cells (Treg, CD25++CD127low) could be found after MLC with DC/DCleu compared to the control groups, although the general expression of CD25++CD127low molecules increased in both settings [82]. This might be explained as mentioned above by the potential induction of indoleamin 2,3-dioxgenase-1 (IDO1) [83, 84].
DC/DCleu stimulation was shown to induce regularly antileukemic activity against leukemic blasts after MLC, though not in every given case, pointing to a specific induction of antileukemic immunity [40, 85, 86].
These findings confirm that generated DC/DCleu can help to overcome anergy of immune reactive cells in AML. Due to the DC/DCleu concept, it can be postulated that DC/DCleu prime antileukemic T cells against several antigens [40]. This could be confirmed by the finding that increased frequencies of WT1 and PRAME-specific T cells could be detected after stimulation of T-cell-enriched immune reactive cells with generated DC/DCleu from leukemic PBMNC compared to the control [Klauer et al., pers. commun.].
Vaccination Strategies with ex vivo Generated DC/DCleu
As shown above, DC/DCleu generation from isolated leukemic PBMNC is regularly possible ex vivo. To go one step further, different working groups tried to use these generated leukemic APC as a potential treatment tool for patients with AML [52]. DC/DCleu were produced ex vivo and transferred to the patients as a subcutaneous vaccine. Irradiation of vaccinated cells is recommended to avoid transfer of not-to-DCleu-converted leukemic blasts. This strategy would render the complicated loading process of generated mo-DC with apoptotic leukemic cells or whole leukemic cells lysates or by RNA electroporation unnecessary. Furthermore, DC/DCleu have the advantage that they express the whole leukemic antigen repertoire in comparison to mo-DC (except mo-DC pulsed with apoptotic leukemic cells/lysates) and, therefore, the in vivo inducted antileukemic immunity is not just restricted to a single presented antigen [53]. The results of 3 preliminary phase 1/2 trials with autologous DC/DCleu are summarized in Table 2. Currently, there are no ongoing clinical trials with DC/DCleu for patients with AML [87]. In general, vaccinations with DC/DCleu are well tolerated (as also already shown in other trials). Only in 1 patient did extensive eczema with an increased antinuclear factor occur, possibly pointing to an induction of autoimmunity [88, 89, 90]. In the trial of Roddie et al. [90], 5 patients (after achieving complete remission with intensive chemotherapy) were vaccinated weekly with an escalating dose of generated DC/DCleu. It could be shown that IFN-γ-secreting antileukemic CTL increased and WT1-specific CTL could be detected. An (ongoing) clinical benefit of the DC/DCleu vaccinating strategy could not be ascertained due to the lack of a control group [90]. Li et al. showed comparable results. After biweekly vaccination of patients with a constant dose of DC/DCleu, 3 out of 5 patients achieved complete remission, whereas the other patients died due to rapid progression of the AML. As an immunological response PRAME-specific CD8+ T cells, a TH-1 cytokine release, and a higher intracellular IFN-γ concentration in CD4+ cells could be detected after the vaccination [89]. Dong et al. [88] combined vaccination of autologous DC with the administration of generated autologous cytokine-induced killer cells (CD3+CD56+ cells). In a direct comparison to the control group, treated with low-dose chemotherapy alone, significantly higher complete and partial remission rates could be achieved after DC/DCleu vaccination (although the effects mediated by CIK-cells or DC/DCleu could not be differentiated) [88]. In summary, the immunological phenomena found in the different trials point to a specific induction of antileukemic immunity after vaccination with DC/DCleu.
Table 2.
Clinical trials with autologous DC/DCeu generated from leukemic PBMNC
| Patients included in the study, n | Stage of disease | Source of DC | Cytokines used for DC generation | Vaccination protocol | Immunological effects | Clinical effects | Ref. |
|---|---|---|---|---|---|---|---|
| 5 | AD | Autologous DC/DCleu | GM-CSF | s.c. | ↑ PRAME (LAA)-specific CD8+ T cells | CR 3/5 | 89 |
| IL-4 | Four vaccinations | ↑ TH-1 cytokine release | PD 2/5 | ||||
| TNF-α | biweekly | ↑ IFN-γ CD4+ cells | No major side effects | ||||
| 5 × 106 DC/DCleu | |||||||
| 5 | After achieving CR | Autologous DC/DCleu | GM-CSF | s.c. | ↑ IFN-γ secreting antileukemic CTL | No evidence of a clinical benefit | 90 |
| IL-4 | 4 vaccinations | ↑ WT1-specfic CTL | (no control group) | ||||
| TNF-α | weekly | no change in amounts of Treg | Induction of autoimmunity (1/5) | ||||
| INF-γ | escalating doses: | ||||||
| poly I:C | 0.125 × 106 to 1 × 106 DC/DCleu | ||||||
| 21 | AD | Autologous DC/DCleu | GM-CSF | i.v. | ↑ CD3+, CD4+CD3+, CD8+CD3+ compared | CRa 6/21 | 88 |
| + | INF-α | 5 vaccinations | to before culture | PRa 9/21 | |||
| autologous CIK cells | FLT3 L | 7.36±0.48 ×107 DC/DCleu | f IL 12, IL 2, IL 7, INF-γ, and TNF-α | NR 6/21 | |||
| + | SCF | compared to before culture | Mild side effects | ||||
| low dose CTX | TGF-β | ||||||
AD, advanced disease; CR, complete remission; CIK cells, cytokine-induced killer cells (CD3+CD56+ cells); CTX, chemotherapy; s.c., subcutaneous; i.v., intravenous; CR, complete remission; PD, persisting disease; PR, partial remission; ↑, increase; CTL, cytotoxic T lymphocyte; Treg, regulatory T cells.
Significantly higher compared to the control group
Furthermore, an allogeneic DC/DCleu vaccine was developed from an AML cell line with expression of a wide range of different LAA (DCP-001). These generated DC/DCleu were analyzed in a phase 1 clinic trail as a post-HSCT therapy in 12 elderly AML patients and could show induction of cellular as well as humoral immunity in vivo with low side effects [91]. However, due to a lack of a control group the role of allogeneic effects alone could not be evaluated.
Different clinical trials with mo-DC vaccines (from autologous or allogeneic CD14+ monocytes) loaded with a variety of different antigens and methods for the treatment of AML have already been conducted and previously reviewed [16, 25]. Due to the “multi-antigen” concept mo-DC pulsed with apoptotic leukemic cells/lysates should be comparable with DC/DCleu generated from leukemic PBMNC. In preliminarily clinical trials with mo-DC pulsed with apoptotic leukemic cells/lysates the stimulatory capacity of T cells was increased and CD8+ T cell responses to WT1 and hTERT could be detected [92, 93].
Molecules, Cells, and Particles Influencing and Regulating DC/DCleu Functions
As demonstrated above, DC/DCleu generation is possible ex vivo. In principle, the same factors are necessary in vivo to induce successful (DC-based) activation of the immune system against different tumor cells and to avoid autoimmune reactivity. Regulatory mechanisms, i.e., regulatory T cells, regulatory cytokines (e.g., IL-10) as well as exosomes (various nanoparticles secreted by several cells, including DC and lymphocytes or even from tumor cells), which mediate cell-cell communication [81, 82, 94, 95, 96], are necessary and well known. Exosomes could qualify to mobilize the immune system against tumors, they could mediate tolerance, or they could be used for DC pulsing as demonstrated in preliminary experiments [97, 98]. Indoleamin 2,3-dioxgenase-1 (IDO1) is an immunoregulatory enzyme that is responsible for tumor-related immunosuppression, e.g., mediated by regulatory T cells [99]. It is currently being discussed whether certain response modifiers (e.g., PGE2) might be responsible for an induction of IDO1 expression leading to activation of immunosuppressive regulatory T cells or, vice versa, whether inhibition of IDO1 might increase antitumor reactions ex vivo or in vivo [99]. Monitoring of these molecules and cells under the influence of response modifiers and/or in the course of the leukemic disease (under treatment) is recommended.
From Bench to Bedside: from ex vivo to in vivo DC/DCleu Generation
Ex vivo production of mo-DC and generation of DC/DCleu from leukemic PBMNC to be used for vaccinations is a challenging process. The manufacturing process is time consuming, expensive, and has to be performed under GMP conditions. In summary, the whole vaccination process is logistically complicated. Cell products have to undergo quality (e.g., for infectious contamination) as well as quantity testing to control purity and amounts of generated DC/DCleu before re-administration to the patients.
Therefore, it would be highly preferable to activate an antileukemic immune response in vivo, thereby circumventing an adoptive cell transfer. DC/DCleu cultures with leukemic PBMNC are artificial cell culture models and do not represent the physiological situation in vivo. Therefore, our group established in a first step an ex vivo culture system thereby simulating physiological conditions, i.e., from individual AML or MDS patients' whole blood (WB), presenting all soluble and cellular components (including known and unknown activating or inhibitory factors) were cultured using a combination of at least 2 cytokines or response modifiers (“Kits”) to induce DC/DCleu generation. After an intensive comparative analysis of 12 different Kits, the best 3 Kits could be selected for further examination and are shown in Table 3 [Kugler et al., pers. commun.] (European patent No. EP 3 217 975 B1; MODIBLAST GmbH; (inventor Helga Schmetzer)). Kits represent a “minimalized” DC/DCleu-generating protocol (utilizing in addition patients' individual hematopoietic cellular and soluble blood background influencing immune reactions) and contain GM-CSF, responsible for the induction of differentiation of myeloid leukemia blasts and one of the response modifiers PGE1, PGE2, and Picibanil (OK-432) for a danger signaling effect and induction of maturation. It could be shown that generation of DC/DCleu is possible directly from leukemic WB with Kits in comparable amounts as with already established DC/DCleu-generating protocols [Kugler et al., pers. commun.] [48]. In a direct comparison of all 3 Kits, comparable amounts of DC/DCleu could be generated, although significantly more mature DC could be generated with PGE1-containing Kit M compared to PGE2-containing Kit K. Stimulation of leukemic WB with Kits did not induce proliferation of not-to-DC/DCleu-converted blasts [48, 100]. To imitate the in vivo situation even better, DC/DCleu culturesfrom leukemic WB were conducted in parallel under hypoxic conditions (10% oxygen) and normoxic conditions (21% oxygen). In both settings, the same amounts of DC/DCleu as well as the same functional features could be achieved [101].
Table 3.
Kits for the generation of DC/DCleu from leukemic WB
| Kit | Composition | Concentration | Culture Time, days | Reference |
|---|---|---|---|---|
| Ia | GM-CSF Picibanil |
800 U/mL 10 µg/mL | 7–10 | Kugler et al., pers. commun. |
| Ka | GM-CSF PGE2 |
800 U/mL 1 µg/mL | 7–10 | Kugler et al., pers. commun. |
| Ma | GM-CSF PGE1 |
800 U/mL 1 µg/mL | 7–10 | Kugler et al., pers. commun. |
European patent (No. EP 3 217 975 B1; developed by Helga Maria Schmetzer, MODIBLAST GmbH).
Kit-pretreated WB (containing DC/DCleu) improved the activation of autologous T-cell-enriched immune reactive cells in mixed MLC compared to the control; proliferating, nonnaive T cells, as well as CD8+ T cells, increased significantly during MLC [48][Ugur et al., pers. commun.]. Kit-generated DC/DCleu from leukemic WB might also influence cells on the interface of the innate and adoptive immune system and cells of the innate immune system. We could, moreover, show that NK cells (CD56+CD3−), CIK cells (CD56+CD3+), and invariant NK cells (6B11+) increased after stimulation with DC/DCleu [102] [Klauer et al., pers. commun.].
Due to the DC/DCleu concept induction of a specific immunological memory can be postulated. In preliminarily experiments significantly higher amounts of effector or central memory T cells could be found after stimulation of T-cell-enriched immune reactive cells in MLC with Kit-generated DC/DCleu compared to the control [48, 103].
One of the most important findings evaluated with functional assays is that the antileukemic activity could be significantly improved compared to the control. In a direct comparison of Kits, DC/DCleu generated with Kit M increased the antileukemic cytotoxicity of T cell-enriched immune reactive cells the most [Ugur et al., pers. commun.]. Studying the provision of leukemia-specific cells significantly increased the frequencies of IFN-y secreting T, NK, and CIK cells that could be detected with the cytokine secretion assay (CSA) after MLC [Klauer et al., pers. commun.].
Moreover, cytokine release profiles were shifted to a higher release of inflammatory cytokines and antitumor response-related cytokines after WB-DC/DCleu culture with Kits compared to patients' serum [48].
What Next? Kits as a Treatment Tool for Patients with AML?
Transforming the idea of ex vivo generation of DC/DCleu from leukemic WB from bench to bedside, our hypothesis is that the administration of Kits to patients with AML or MDS can convert leukemic blasts to DC/DCleu in vivo, thereby activating an antileukemic immunoreaction. This would render an adoptive transfer of ex vivo generated and manipulated mo-DC or DC/DCleu unnecessary. The concept of in vivo Kit therapy in comparison to an ex vivo adoptive cell transfer of DC/DCleu is illustrated in Figure 1. All Kit substances are approved for human treatment and they are already being used in the clinical routine; GM-CSF is used for the treatment of neutropenia in patients after chemotherapy or HSCT [104]. PGE1 is already being used for the reduction of gastrointestinal ulcers during treatment with nonsteroidal anti-inflammatory drugs, to treat veno-occlusive disease after HSCT, erectile dysfunction, or to maintain the patency of the ductus arteriosus in newborns with ductal-dependent cardiac lesions [105, 106, 107]. PGE2 is used for induction of labor in cases with a medical or obstetrical indication. Sclerotherapy of congenital lymphatic malformation in the head and neck is possible with Picibanil (OK 432) [108].
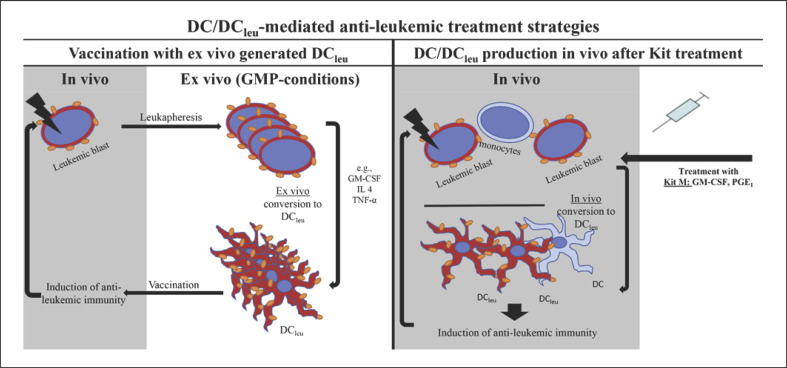
Fig. 1.
DC/DCleu-mediated antileukemic treatment strategies. Two potential DC/DCleu-generating strategies for the induction of antileukemic reactivity and immunity in vivo are shown. Left: vaccination with ex vivo generated DC/DCleu. The patient's blasts (enriched by leukapheresis) are cultured ex vivo in the presence of different combinations of “response modifiers” (e.g., GM-CSF, IL-4, and TNF-α) under GMP conditions. After conversion to DCleu and different quality as well as quantity analyses, generated cells are readministered to the patient as an intradermal vaccination. Irradiation of vaccinated cells is recommended to avoid transfer of not-to-DCleu-converted leukemic blasts. Right: Patients are treated directly with Kits (e.g., Kit M, containing GM-CSF, PGE1) that convert leukemic blasts to DC/DCleu in vivo. Also cells of nonleukemic origin such as monocytes could be converted to DC (these cells could also phagocyte, process, and present leukemic particles), thereby supporting the DCleu-based strategy. The Kit strategy would render the logistically complicated adoptive transfer of ex vivo generated DC/DCleu unnecessary. Both strategies (left and right) give rise to DC/DCleu that can migrate to tissues and induce antileukemic reactivity and immunity in vivo.
Our findings contribute to the development of a new potential therapy option for patients with AML or high-grade MDS, especially with the aim of stabilizing remissions or at least the disease. We could show that DC/DCleu generation is possible with Kits from leukemic WB, without the induction of blast proliferation, independently of the patient's age, gender, MHC or mutation status, or cytogenetic risk profile or any subtype of AML. The administration of Kits to patients with AML or MDS might generate DC/DCleu in vivo, which might activate the innate and adaptive immune system and especially leukemia-specific T cells followed by an immunoreaction against residual leukemic blasts. To prove our hypothesis, ongoing research with trials in animals and humans has to be performed.
Conflict of Interest Statement
All of the authors declare that there are no financial conflicts with regards to this work. “Use of immunomodulatory effective compositions for the immunotherapeutic treatment of patients suffering from myeloid leukemias” has a European patent (No. EP 3 217 975 B1) and was developed by Helga Maria Schmetzer, MODIBLAST GmbH.
Funding Sources
No funding was needed for this paper.
Author Contributions
D.C.A. and H.S.M. contributed to the writing, review and discussion of the manuscript.
References
- 1.Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999 Sep;341((14)):1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Sekeres MA. Treatment of older adults with acute myeloid leukemia: state of the art and current perspectives. Haematologica. 2008 Dec;93((12)):1769–72. doi: 10.3324/haematol.2008.000497. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Perez A, Montalban-Bravo G. Management of myelodysplastic syndromes after failure of response to hypomethylating agents. Ther Adv Hematol. 2019 May;10:2040620719847059. doi: 10.1177/2040620719847059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute Cancer stat facts: leukemia – acute myeloid leukemia (AML) Available from: https://seer.cancer.gov/statfacts/html/amyl.html.
- 5.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015 Sep;373((12)):1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 6.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan;129((4)):424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017 Jul;15((7)):926–57. doi: 10.6004/jnccn.2017.0116. [DOI] [PubMed] [Google Scholar]
- 8.Garrett WS. Enterococcus in Graft-versus-Host Disease. N Engl J Med. 2020 Mar;382((11)):1064–6. doi: 10.1056/NEJMcibr1915978. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson AM, Norden J, Li S, Hromadnikova I, Schmid C, Schmetzer H, et al. Graft-versus-Leukemia Effect Following Hematopoietic Stem Cell Transplantation for Leukemia. Front Immunol. 2017 Jun;8:496. doi: 10.3389/fimmu.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paietta E. Consensus on MRD in AML? Blood. 2018 Mar;131((12)):1265–6. doi: 10.1182/blood-2018-01-828145. [DOI] [PubMed] [Google Scholar]
- 11.Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018 Mar;131((12)):1275–91. doi: 10.1182/blood-2017-09-801498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004 Feb;103((3)):767–76. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 13.Dillon R, Hills R, Freeman S, Potter N, Jovanovic J, Ivey A, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood. 2020 Feb;135((9)):680–8. doi: 10.1182/blood.2019002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett AK. Treatment of Older Patients With Newly Diagnosed AML Unfit for Traditional Therapy. Clin Lymphoma Myeloma Leuk. 2018 Sep;18((9)):553–7. doi: 10.1016/j.clml.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Ansprenger C, Amberger DC, Schmetzer HM. Potential of immunotherapies in the mediation of antileukemic responses for patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) - With a focus on Dendritic cells of leukemic origin (DCleu) Clin Immunol. 2020 Aug;217:108467. doi: 10.1016/j.clim.2020.108467. [DOI] [PubMed] [Google Scholar]
- 16.Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy. 2012 Jul;14((6)):647–56. doi: 10.3109/14653249.2012.693744. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998 Mar;392((6673)):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001 Aug;106((3)):259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 19.Durai V, Murphy KM. Functions of Murine Dendritic Cells. Immunity. 2016 Oct;45((4)):719–36. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001 Aug;106((3)):263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 21.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999 Jul;285((5425)):221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 22.Reuther S, Schmetzer H, Schuster FR, Krell P, Grabrucker C, Liepert A, et al. In vitro-induced response patterns of antileukemic T cells: characterization by spectratyping and immunophenotyping. Clin Exp Med. 2013 Feb;13((1)):29–48. doi: 10.1007/s10238-012-0180-y. [DOI] [PubMed] [Google Scholar]
- 23.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999 Jan;19((1)):12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 24.Wan H, Dupasquier M. Dendritic cells in vivo and in vitro. Cell Mol Immunol. 2005 Feb;2((1)):28–35. [PubMed] [Google Scholar]
- 25.Van Acker HH, Versteven M, Lichtenegger FS, Roex G, Campillo-Davo D, Lion E, et al. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J Clin Med. 2019 Apr;8((5)):E579. doi: 10.3390/jcm8050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steger B, Floro L, Amberger DC, Kroell T, Tischer J, Kolb HJ, et al. WT1, PRAME, and PR3 mRNA Expression in Acute Myeloid Leukemia (AML) J Immunother. 2020 Jul-Aug;43((6)):204–15. doi: 10.1097/CJI.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 27.Smits EL, Berneman ZN, Van Tendeloo VF. Immunotherapy of acute myeloid leukemia: current approaches. Oncologist. 2009 Mar;14((3)):240–52. doi: 10.1634/theoncologist.2008-0165. [DOI] [PubMed] [Google Scholar]
- 28.Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017 Jan;27((1)):74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schürch CM, Riether C, Ochsenbein AF. Dendritic cell-based immunotherapy for myeloid leukemias. Front Immunol. 2013 Dec;4:496. doi: 10.3389/fimmu.2013.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westers TM, Ossenkoppele GJ, van de Loosdrecht AA. Dendritic cell-based immunotherapy in acute and chronic myeloid leukaemia. Biomed Pharmacother. 2007 Jul;61((6)):306–14. doi: 10.1016/j.biopha.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Xing D, Decker WK, Li S, Robinson SN, Yang H, Segal H, et al. AML-loaded DC generate Th1-type cellular immune responses in vitro. Cytotherapy. 2006;8((2)):95–104. doi: 10.1080/14653240600620093. [DOI] [PubMed] [Google Scholar]
- 32.Gong J, Koido S, Kato Y, Tanaka Y, Chen D, Jonas A, et al. Induction of anti-leukemic cytotoxic T lymphocytes by fusion of patient-derived dendritic cells with autologous myeloblasts. Leuk Res. 2004 Dec;28((12)):1303–12. doi: 10.1016/j.leukres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Santiago-Schwarz F, Coppock DL, Hindenburg AA, Kern J. Identification of a malignant counterpart of the monocyte-dendritic cell progenitor in an acute myeloid leukemia. Blood. 1994 Nov;84((9)):3054–62. [PubMed] [Google Scholar]
- 34.Robinson SP, English N, Jaju R, Kearney L, Knight SC, Reid CD. The in-vitro generation of dendritic cells from blast cells in acute leukaemia. Br J Haematol. 1998 Dec;103((3)):763–71. [PubMed] [Google Scholar]
- 35.Choudhury BA, Liang JC, Thomas EK, Flores-Romo L, Xie QS, Agusala K, et al. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood. 1999 Feb;93((3)):780–6. [PubMed] [Google Scholar]
- 36.Narita M, Takahashi M, Liu A, Ayres F, Satoh N, Abe T, et al. Generation of dendritic cells from leukaemia cells of a patient with acute promyelocytic leukaemia by culture with GM-CSF, IL-4 and TNF-alpha. Acta Haematol. 2001;106((3)):89–94. doi: 10.1159/000046595. [DOI] [PubMed] [Google Scholar]
- 37.Tong XM, Yao HP, Qian WB, Zhu LF, Fu ZH, Huang ZL, et al. The biological characteristics of dendritic cells derived in vitro from myelogeneous leukemia cells and healthy donor cells. Int J Lab Hematol. 2008 Oct;30((5)):372–81. doi: 10.1111/j.1751-553X.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 38.Woiciechowsky A, Regn S, Kolb HJ, Roskrow M. Leukemic dendritic cells generated in the presence of FLT3 ligand have the capacity to stimulate an autologous leukemia-specific cytotoxic T cell response from patients with acute myeloid leukemia. Leukemia. 2001 Feb;15((2)):246–55. doi: 10.1038/sj.leu.2402013. [DOI] [PubMed] [Google Scholar]
- 39.Kufner S, Zitzelsberger H, Kroell T, Pelka-Fleischer R, Salem A, de Valle F, et al. Leukemia-derived dendritic cells can be generated from blood or bone marrow cells from patients with acute myeloid leukaemia: a methodological approach under serum-free culture conditions. Scand J Immunol. 2005 Jul;62((1)):86–98. doi: 10.1111/j.1365-3083.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 40.Kremser A, Dressig J, Grabrucker C, Liepert A, Kroell T, Scholl N, et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: an evaluation of different methods. J Immunother. 2010 Feb-Mar;33((2)):185–99. doi: 10.1097/CJI.0b013e3181b8f4ce. [DOI] [PubMed] [Google Scholar]
- 41.Köhler T, Plettig R, Wetzstein W, Schmitz M, Ritter M, Mohr B, et al. Cytokine-driven differentiation of blasts from patients with acute myelogenous and lymphoblastic leukemia into dendritic cells. Stem Cells. 2000;18((2)):139–47. doi: 10.1634/stemcells.18-2-139. [DOI] [PubMed] [Google Scholar]
- 42.Panoskaltsis N, Belanger TJ, Liesveld JL, Abboud CN. Optimal cytokine stimulation for the enhanced generation of leukemic dendritic cells in short-term culture. Leuk Res. 2002 Feb;26((2)):191–201. doi: 10.1016/s0145-2126(01)00104-7. [DOI] [PubMed] [Google Scholar]
- 43.Brouwer RE, van der Hoorn M, Kluin-Nelemans HC, van Zelderen-Bhola S, Willemze R, Falkenburg JH. The generation of dendritic-like cells with increased allostimulatory function from acute myeloid leukemia cells of various FAB subclasses. Hum Immunol. 2000 Jun;61((6)):565–74. doi: 10.1016/s0198-8859(00)00111-7. [DOI] [PubMed] [Google Scholar]
- 44.Charbonnier A, Gaugler B, Sainty D, Lafage-Pochitaloff M, Olive D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur J Immunol. 1999 Aug;29((8)):2567–78. doi: 10.1002/(SICI)1521-4141(199908)29:08<2567::AID-IMMU2567>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 45.Lee JJ, Choi BH, Nam JH, Park MS, Song WH, Yang DH, et al. The generation of leukemic dendritic cells from acute myeloid leukemia cells is potentiated by the addition of CD40L at the terminal maturation stage. J Clin Apher. 2004;19((3)):130–6. doi: 10.1002/jca.20015. [DOI] [PubMed] [Google Scholar]
- 46.Nourizadeh M, Masoumi F, Memarian A, Alimoghaddam K, Moazzeni SM, Yaghmaie M, et al. In vitro induction of potent tumor-specific cytotoxic T lymphocytes using TLR agonist-activated AML-DC. Target Oncol. 2014 Sep;9((3)):225–37. doi: 10.1007/s11523-013-0285-6. [DOI] [PubMed] [Google Scholar]
- 47.Nourizadeh M, Masoumi F, Memarian A, Alimoghaddam K, Moazzeni SM, Hadjati J. Synergistic effect of Toll-like receptor 4 and 7/8 agonists is necessary to generate potent blast-derived dendritic cells in Acute Myeloid Leukemia. Leuk Res. 2012 Sep;36((9)):1193–9. doi: 10.1016/j.leukres.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Amberger DC, Doraneh-Gard F, Gunsilius C, Weinmann M, Möbius S, Kugler C, et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int J Mol Sci. 2019 Sep;20((18)):E4590. doi: 10.3390/ijms20184590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westers TM, Stam AG, Scheper RJ, Regelink JC, Nieuwint AW, Schuurhuis GJ, et al. Rapid generation of antigen-presenting cells from leukaemic blasts in acute myeloid leukaemia. Cancer Immunol Immunother. 2003 Jan;52((1)):17–27. doi: 10.1007/s00262-002-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirn Lopez A, Deen D, Fischer Z, Rabe A, Ansprenger C, Stein K, et al. Role of Interferon (IFN)α in “Cocktails” for the Generation of (Leukemia-derived) Dendritic Cells (DCleu) From Blasts in Blood From Patients (pts) With Acute Myeloid Leukemia (AML) and the Induction of Antileukemic Reactions. J Immunother. 2019 Jun;42((5)):143–61. doi: 10.1097/CJI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 51.Waclavicek M, Berer A, Oehler L, Stöckl J, Schloegl E, Majdic O, et al. Calcium ionophore: a single reagent for the differentiation of primary human acute myelogenous leukaemia cells towards dendritic cells. Br J Haematol. 2001 Aug;114((2)):466–73. doi: 10.1046/j.1365-2141.2001.02970.x. [DOI] [PubMed] [Google Scholar]
- 52.Pyzer AR, Avigan DE, Rosenblatt J. Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum Vaccin Immunother. 2014;10((11)):3125–31. doi: 10.4161/21645515.2014.982993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmetzer HM, Kremser A, Loibl J, Kroell T, Kolb HJ. Quantification of ex vivo generated dendritic cells (DC) and leukemia-derived DC contributes to estimate the quality of DC, to detect optimal DC-generating methods or to optimize DC-mediated T-cell-activation-procedures ex vivo or in vivo. Leukemia. 2007 Jun;21((6)):1338–41. doi: 10.1038/sj.leu.2404639. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Reinhardt P, Schmitt A, Barth TF, Greiner J, Ringhoffer M, et al. Dendritic cells generated from acute myeloid leukemia (AML) blasts maintain the expression of immunogenic leukemia associated antigens. Cancer Immunol Immunother. 2005 Jul;54((7)):685–93. doi: 10.1007/s00262-004-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kremser A, Kufner S, Konhaeuser E, Kroell T, Hausmann A, Tischer J, et al. Combined immunophenotyping and fluorescence in situ hybridization with chromosome-specific DNA probes allows quantification and differentiation of ex vivo generated dendritic cells, leukemia-derived dendritic cells and clonal leukemic cells in patients with acute myeloid leukemia. Leuk Lymphoma. 2013 Jun;54((6)):1297–308. doi: 10.3109/10428194.2012.751490. [DOI] [PubMed] [Google Scholar]
- 56.Mohty M, Isnardon D, Blaise D, Mozziconacci MJ, Lafage-Pochitaloff M, Brière F, et al. Identification of precursors of leukemic dendritic cells differentiated from patients with acute myeloid leukemia. Leukemia. 2002 Nov;16((11)):2267–74. doi: 10.1038/sj.leu.2402706. [DOI] [PubMed] [Google Scholar]
- 57.Dreyssig J, Kremser A, Liepert A, Grabrucker C, Freudenreich M, Schmid C, et al. Various ‘dendritic cell antigens’ are already expressed on uncultured blasts in acute myeloid leukemia and myelodysplastic syndromes. Immunotherapy. 2011 Sep;3((9)):1113–24. doi: 10.2217/imt.11.108. [DOI] [PubMed] [Google Scholar]
- 58.Graf M, Reif S, Hecht K, Pelka-Fleischer R, Pfister K, Schmetzer H. High expression of urokinase plasminogen activator receptor (UPA-R) in acute myeloid leukemia (AML) is associated with worse prognosis. Am J Hematol. 2005 May;79((1)):26–35. doi: 10.1002/ajh.20337. [DOI] [PubMed] [Google Scholar]
- 59.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 60.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005 Aug;5((8)):617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 61.Aerts-Toegaert C, Heirman C, Tuyaerts S, Corthals J, Aerts JL, Bonehill A, et al. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur J Immunol. 2007 Mar;37((3)):686–95. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 62.Prechtel AT, Turza NM, Theodoridis AA, Steinkasserer A. CD83 knockdown in monocyte-derived dendritic cells by small interfering RNA leads to a diminished T cell stimulation. J Immunol. 2007 May;178((9)):5454–64. doi: 10.4049/jimmunol.178.9.5454. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Ju X, Silveira PA, Abadir E, Hsu WH, Hart DN, et al. CD83: Activation Marker for Antigen Presenting Cells and Its Therapeutic Potential. Front Immunol. 2019 Jun;10:1312. doi: 10.3389/fimmu.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curti A, Pandolfi S, Aluigi M, Isidori A, Alessandrini I, Chiodoni C, et al. Interleukin-12 production by leukemia-derived dendritic cells counteracts the inhibitory effect of leukemic microenvironment on T cells. Exp Hematol. 2005 Dec;33((12)):1521–30. doi: 10.1016/j.exphem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kämpgen E, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996 Aug;184((2)):741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996 Mar;26((3)):659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 67.Houtenbos I, Westers TM, Stam AG, de Gruijl TD, Scheper RJ, Ossenkoppele GJ, et al. Serum-free generation of antigen presenting cells from acute myeloid leukaemic blasts for active specific immunisation. Cancer Immunol Immunother. 2003 Jul;52((7)):455–62. doi: 10.1007/s00262-003-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato M, Takayama T, Tanaka H, Konishi J, Suzuki T, Kaiga T, et al. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prostaglandin E(2) Cancer Sci. 2003 Dec;94((12)):1091–8. doi: 10.1111/j.1349-7006.2003.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araki Y, Suganami A, Endo S, Masuda Y, Fukushima K, Regan JW, et al. PGE1 and E3 show lower efficacies than E2 to β-catenin-mediated activity as biased ligands of EP4 prostanoid receptors. FEBS Lett. 2017 Nov;591((22)):3771–80. doi: 10.1002/1873-3468.12878. [DOI] [PubMed] [Google Scholar]
- 70.Trabanelli S, Lecciso M, Salvestrini V, Cavo M, Očadlíková D, Lemoli RM, et al. PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J Immunol Res. 2015;2015:253191. doi: 10.1155/2015/253191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kufner S, Fleischer RP, Kroell T, Schmid C, Zitzelsberger H, Salih H, et al. Serum-free generation and quantification of functionally active Leukemia-derived DC is possible from malignant blasts in acute myeloid leukemia and myelodysplastic syndromes. Cancer Immunol Immunother. 2005 Oct;54((10)):953–70. doi: 10.1007/s00262-004-0657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsapogas P, Mooney CJ, Brown G, Rolink A. The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis. Int J Mol Sci. 2017 May;18((6)):E1115. doi: 10.3390/ijms18061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000 Jan;67((1)):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 74.Roddie PH, Horton Y, Turner ML. Primary acute myeloid leukaemia blasts resistant to cytokine-induced differentiation to dendritic-like leukaemia cells can be forced to differentiate by the addition of bryostatin-1. Leukemia. 2002 Jan;16((1)):84–93. doi: 10.1038/sj.leu.2402335. [DOI] [PubMed] [Google Scholar]
- 75.Draube A, Beyer M, Wolf J. Activation of autologous leukemia-specific T cells in acute myeloid leukemia: monocyte-derived dendritic cells cocultured with leukemic blasts compared with leukemia-derived dendritic cells. Eur J Haematol. 2008 Oct;81((4)):281–8. doi: 10.1111/j.1600-0609.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 76.Houtenbos I., Westers T. M., Ossenkoppele G. J., van de Loosdrecht A. A. Identification of CD14 as a predictor for leukemic dendritic cell differentiation in acute myeloid leukemia. Leukemia. 2003;17((8)):1683–4. doi: 10.1038/sj.leu.2403014. author reply 1684; discussion 1685. [DOI] [PubMed] [Google Scholar]
- 77.Houtenbos I, Westers TM, Ossenkoppele GJ, van de Loosdrecht AA. Leukemia-derived dendritic cells: towards clinical vaccination protocols in acute myeloid leukemia. Haematologica. 2006 Mar;91((3)):348–55. [PubMed] [Google Scholar]
- 78.Houtenbos I, Westers TM, de Gruijl TD, Scheper RJ, Ossenkoppele GJ, van de Loosdrecht AA. TNF-alpha receptor 1 expression on acute myeloid leukemic blasts predicts differentiation into leukemic dendritic cells. Leukemia. 2004 Jun;18((6)):1149–53. doi: 10.1038/sj.leu.2403359. [DOI] [PubMed] [Google Scholar]
- 79.van den Ancker W, Wijnands PG, Ruben JM, Westers TM, Punt B, Bachas C, et al. Procedures for the expansion of CD14(+) precursors from acute myeloid leukemic cells to facilitate dendritic cell-based immunotherapy. Immunotherapy. 2013 Nov;5((11)):1183–90. doi: 10.2217/imt.13.125. [DOI] [PubMed] [Google Scholar]
- 80.Houtenbos I, Westers TM, Hess CJ, Waisfisz Q, Ossenkoppele GJ, van de Loosdrecht AA. Flt-3 internal tandem duplication hampers differentiation of AML blasts towards leukemic dendritic cells. Leukemia. 2006 Oct;20((10)):1892–5. doi: 10.1038/sj.leu.2404348. [DOI] [PubMed] [Google Scholar]
- 81.Vogt V, Schick J, Ansprenger C, Braeu M, Kroell T, Kraemer D, et al. Profiles of activation, differentiation-markers, or β-integrins on T cells contribute to predict T cells' antileukemic responses after stimulation with leukemia-derived dendritic cells. J Immunother. 2014 Jul-Aug;37((6)):331–47. doi: 10.1097/CJI.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 82.Schick J, Vogt V, Zerwes M, Kroell T, Kraemer D, Köhne CH, et al. Antileukemic T-cell responses can be predicted by the composition of specific regulatory T-cell subpopulations. J Immunother. 2013 May;36((4)):223–37. doi: 10.1097/CJI.0b013e31829180e7. [DOI] [PubMed] [Google Scholar]
- 83.Wobser M, Voigt H, Houben R, Eggert AO, Freiwald M, Kaemmerer U, et al. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother. 2007 Jul;56((7)):1017–24. doi: 10.1007/s00262-006-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005 Oct;106((7)):2375–81. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grabrucker C, Liepert A, Dreyig J, Kremser A, Kroell T, Freudenreich M, et al. The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive factor for the lytic potential of dendritic cells-primed leukemia-specific T cells. J Immunother. 2010 Jun;33((5)):523–37. doi: 10.1097/CJI.0b013e3181d87ffd. [DOI] [PubMed] [Google Scholar]
- 86.Schuster FR, Buhmann R, Reuther S, Hubner B, Grabrucker C, Liepert A, et al. Improved effector function of leukemia-specific T-lymphocyte clones trained with AML-derived dendritic cells. Cancer Genomics Proteomics. 2008 Sep-Oct;5((5)):275–86. [PubMed] [Google Scholar]
- 87.clinicaltrials.gov [Internet] Bethesda: US National Library of Medicine. 2020. Availabla from: https://clinicaltrials.gov/
- 88.Dong M, Liang D, Li Y, Kong D, Kang P, Li K, et al. Autologous dendritic cells combined with cytokine-induced killer cells synergize low-dose chemotherapy in elderly patients with acute myeloid leukaemia. J Int Med Res. 2012;40((4)):1265–74. doi: 10.1177/147323001204000405. [DOI] [PubMed] [Google Scholar]
- 89.Li L, Giannopoulos K, Reinhardt P, Tabarkiewicz J, Schmitt A, Greiner J, et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int J Oncol. 2006 Apr;28((4)):855–61. [PubMed] [Google Scholar]
- 90.Roddie H, Klammer M, Thomas C, Thomson R, Atkinson A, Sproul A, et al. Phase I/II study of vaccination with dendritic-like leukaemia cells for the immunotherapy of acute myeloid leukaemia. Br J Haematol. 2006 Apr;133((2)):152–7. doi: 10.1111/j.1365-2141.2006.05997.x. [DOI] [PubMed] [Google Scholar]
- 91.van de Loosdrecht AA, van Wetering S, Santegoets SJ, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol Immunother. 2018 Oct;67((10)):1505–18. doi: 10.1007/s00262-018-2198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JJ, Kook H, Park MS, Nam JH, Choi BH, Song WH, et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher. 2004;19((2)):66–70. doi: 10.1002/jca.10080. [DOI] [PubMed] [Google Scholar]
- 93.Kitawaki T, Kadowaki N, Fukunaga K, Kasai Y, Maekawa T, Ohmori K, et al. Cross-priming of CD8(+) T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in immunotherapy for elderly patients with acute myeloid leukemia. Exp Hematol. 2011 Apr;39((4)):424–433.e2. doi: 10.1016/j.exphem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998 May;4((5)):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 95.Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016 Apr;126((4)):1224–32. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischbacher D, Merle M, Liepert A, Grabrucker C, Kroell T, Kremser A, et al. Cytokine Release Patterns in Mixed Lymphocyte Culture (MLC) of T-Cells with Dendritic Cells (DC) Generated from AML Blasts Contribute to Predict anti-Leukaemic T-Cell Reactions and Patients' Response to Immunotherapy. Cell Commun Adhes. 2015 Apr-Dec;22((2-6)):49–65. doi: 10.1080/15419061.2016.1223634. [DOI] [PubMed] [Google Scholar]
- 97.Benites BD, da Silva Santos Duarte A, Longhini AL, Santos I, Alvarez MC, de Morais Ribeiro LN, et al. Exosomes in the serum of Acute Myeloid Leukemia patients induce dendritic cell tolerance: implications for immunotherapy. Vaccine. 2019 Mar;37((11)):1377–83. doi: 10.1016/j.vaccine.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 98.Yao Y, Wang C, Wei W, Shen C, Deng X, Chen L, et al. Dendritic cells pulsed with leukemia cell-derived exosomes more efficiently induce antileukemic immunities. PLoS One. 2014 Mar;9((3)):e91463. doi: 10.1371/journal.pone.0091463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010 Jul-Aug;16((4)):354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plett C, Amberger DC, Rabe A, Deen D, Stankova Z, Hirn Lopez A, et al. Immunomodulatory Kits do not induce AML-blasts' proliferation ex vivo. IPO-38 is an appropriate and reliable marker to detect and quantify proliferating blasts. Eur J Cancer. 2017;5((1)):3–4. [Google Scholar]
- 101.Doraneh-Gard F, Amberger DC, Weinman M, Boeck CL, Gunsilius C, Kugler C, et al. Standard-NORMOXIC versus physiological HYPOXIC culture of AML-patients' (pts) whole blood (WB) samples with immunomodulatory Kits yields comparable proportions of Dendritic cells and functional results. Eur J Cancer. 2018;92(supp1):10–1. [Google Scholar]
- 102.Boeck CL, Amberger DC, Doraneh-Gard F, Sutanto W, Guenther T, Schmohl J, et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J Immunother. 2017 Jul-Aug;40((6)):224–48. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 103.O'Brien LJ, Guillerey C, Radford KJ. Can Dendritic Cell Vaccination Prevent Leukemia Relapse? Cancers (Basel) 2019 Jun;11((6)):E875. doi: 10.3390/cancers11060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015 Aug;195((4)):1341–9. doi: 10.4049/jimmunol.1500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reiter M, Bucek RA, Stümpflen A, Minar E. Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2004;((1)):CD000986. doi: 10.1002/14651858.CD000986.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Akkinapally S, Hundalani SG, Kulkarni M, Fernandes CJ, Cabrera AG, Shivanna B, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev. 2018 Feb;2:CD011417. doi: 10.1002/14651858.CD011417.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reddy SC, Saxena A. Prostaglandin E1: first stage palliation in neonates with congenital cardiac defects. Indian J Pediatr. 1998 Mar-Apr;65((2)):211–6. doi: 10.1007/BF02752297. [DOI] [PubMed] [Google Scholar]
- 108.Sung MW, Lee DW, Kim DY, Lee SJ, Hwang CH, Park SW, et al. Sclerotherapy with picibanil (OK-432) for congenital lymphatic malformation in the head and neck. Laryngoscope. 2001 Aug;111((8)):1430–3. doi: 10.1097/00005537-200108000-00020. [DOI] [PubMed] [Google Scholar]
- 109.Kufner S, Zitzelsberger H, Kroell T, Pelka-Fleischer R, Salem A, de Valle F, et al. Leukaemia-derived dendritic cells can be generated from blood or bone marrow cells from patients with myelodysplasia: a methodological approach under serum-free culture conditions. Scand J Immunol. 2005 Jul;62((1)):75–85. doi: 10.1111/j.1365-3083.2005.01631.x. [DOI] [PubMed] [Google Scholar]