Abstract
Currently, the dichotomous definition of human epidermal growth factor receptor 2 (HER2)-positive versus HER2-negative disease undergoing a change through inclusion of the identification of the “HER2-low” category, for which new therapeutic compounds in the form of potent antibody drug conjugates (ADC) may be effective. In addition, resistance to HER2-directed targets has become a clinical challenge and, therefore, strategies to bypass the HER2 receptor are of high interest. These are new HER2 ADCs and tyrosine kinase inhibitors, such as tucatinib or neratinib. The underlying mechanisms of resistance to anti-HER2 therapies and compensatory pathways are complex and a wide range of mechanisms of resistance may coexist in the same cell. Therefore, the combined treatment with agents that interact with HER2-associated downstream signaling pathways like the phosphoinositide-3-kinase (PI3K) and the serine/threonine kinases AKT and mTOR might overcome HER2 resistance. In addition, targeting other members of the HER family is a promising approach to improve outcomes in breast cancer patients. This review gives an overview of treatment strategies in targeting HER2 and other members of the HER family, not only in HER2-positive breast cancer, but also in HER2-low expressing tumors, and of approaches to overcome HER2 resistance.
Keywords: Breast cancer, HER2, HER2 low expression, HER2 resistance, HER3, HER4
Introduction
The human epidermal growth factor receptor 2 (HER2) gene encodes a 185-kDa heavy transmembrane glycoprotein which is composed of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular receptor domain. There is no known ligand for the HER2 receptor but the other receptors of the HER2 family can be activated by different ligands. Ligand binding induces receptor dimerization. HER2 stabilizes homo- and heterodimerization with other receptors of the HER family (like the epidermal growth factor receptor [EGFR/HER1], HER3, or HER4). The heterodimerization between HER2 and HER3 leads to the most intense signaling. Downstream signaling via the phosphoinositide-3-kinase (PI3K)/AKT/mTOR pathway is then activated, which finally leads to proliferation, tumor growth, cell mobility, invasion, and angiogenesis. There are different cross-talks between the growth factor receptors and other pathways (Fig. 1). The development of the monoclonal antibody trastuzumab against HER2 was a milestone in the treatment of breast cancer. Further developments included agents targeting the intracellular tyrosine kinase of HER2 (lapatinib) and antibody-drug conjugates (T-DM1) which also use HER2 as a target. Despite the improvement of prognosis of HER2-positive breast cancer patients with anti-HER2 therapies, resistance to therapy is a relevant problem leading to disease progression. As a consequence of HER2 resistance, agents have been developed that not only target HER2 but also other receptors of the HER family or several tyrosine kinases. Some of the most promising agents will be introduced in this review (Table 1).
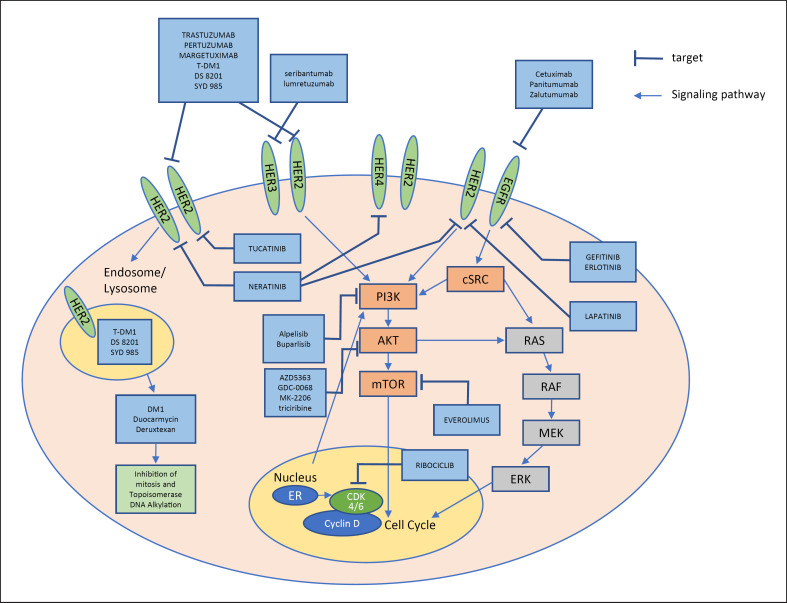
Fig. 1.
HER family and its cross-talk with other signaling pathways and agents targeting the HER family.
Table 1.
Overview of investigational drugs or agents in clinical development targeting receptors of the HER2 family or downstream signaling pathways
| Classification drug | Target | Approval/phase |
|---|---|---|
| Antibody-drug conjugates (ADCs) | ||
| TDM-1 | HER2 | Approved |
| Trastuzumab duocarmazine (SYD-985) | HER2 | Phase III |
| Trastuzumab deruxtecan (DS-8201) | HER2 | Approved (FDA) |
| TKIs | ||
| Lapatinib | HER2/EGFR (HER1) | Approved |
| Neratinib | EGFR/HER2/HER4 | Approved |
| Tucatinib | HER2 | Approved (FDA) |
| Gefitinib | EGFR | Approved |
| Erlotinib | EGFR | Approved |
| mAbs | ||
| Cetuximab | EGFR | Phase II |
| Panitumumab | EGFR | Phase II |
| Zalutumumab | EGFR | Phase II |
| Seribantumab | HER3 | Phase I |
| Lumretuzumab | HER3 | Phase I |
| PI3K inhibitors | ||
| Alpelisib | PIK3 | Approved (FDA) |
| Buparlisib | PIK3 | Phase II |
| AKT inhibitors | ||
| AZD5363 | AKT | Phase I/II |
| GDC-0068 | AKT | Phase II |
| MK-2206 | AKT | Phase II |
| Triciribine | AKT | Phase I |
| mTOR inhibitors | ||
| Everolimus | mTOR | Approved |
Targeting HER2 in HER2-Low Breast Carcinomas
Antibody-Drug Conjugates
ASCO/CAP guidelines aim to divide tumors into HER2-positive or HER2-negative categories by immunohistochemistry (IHC) or in situ hybridization (ISH) analysis. Hence, physicians have based their decision for anti-HER2 agents on a positive test result defined by HER2 overexpression and/or amplification.
In recent years, new antibody-drug conjugates (ADC) have been developed which are internalized into the cancer cell by certain targets and deliver chemotherapy inside the cells, thus reducing systemic side effects of the chemotherapeutic agent. The first approved ADC targeting HER2 was composed of trastuzumab and the cytotoxic agent emtansine (T-DM1). T-DM1 has shown superior efficacy and a favorable risk-benefit profile and was approved in HER2-positive breast cancer for the second-line metastatic setting or for use in patients with residual disease following neoadjuvant trastuzumab-containing chemotherapy [1, 2].
Other ADCs have been introduced, such as trastuzumab duocarmazine (SYD-985) and trastuzumab deruxtecan (DS-8201), which have demonstrated encouraging response rates not only in HER2-positive but also in the so-called HER2-low breast cancer patients.
Trastuzumab duocarmazine (SYD-985) is composed of trastuzumab and a drug containing duocarmycin. The linker drug contains a cleavable linker and the prodrug seco-DUBA. Following HER2 binding and internalization, the linker is cleaved in the lysosome by proteases that release the active toxin (DUBA), which alkylates DNA, thus resulting in DNA damage in both dividing and non-dividing cells and ultimately cell death. In a phase I trial that included patients with a variable HER2 status (including patients with HER2 IHC of at least 1+), treatment with trastuzumab duocarmazine led to a partial response in 28 and 40% of HER2-low estrogen receptor (ER)-positive and ER-negative breast cancer patients, respectively [3].
Trastuzumab deruxtecan (DS-8201) is an ADC composed of an anti-HER2 antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor. In a heavily pretreated metastatic breast cancer patient population (median number of prior lines equal to 6, ranging between 2 and 27), a study has shown an impressive objective response rate of 60.9% (95% CI 53.4–68.0), a clinical benefit rate at 6 months of 76.1% (95% CI 69.3–82.1), and a progression-free survival of 14.8 months (95% CI 13.8–16.9). The most common side effect was nausea of grade 1–2 in the majority of the cases. Notably, 13.6% of the patients developed interstitial lung disease, which was grade 5 in 4 (2.2%) of the patients [4]. Phase III trials with the use of this agent are already ongoing and may radically change the treatment algorithm for HER2-positive advanced breast cancer. Interestingly, the phase 1 study of DS-8201 also reported data on pretreated HER2-low metastatic breast cancer patients, demonstrating an overall response rate by independent central review of 37% with a median duration of response of 10.4 months in 54 extensively pretreated patients [5]. At present, a phase 3 study evaluating the safety and efficacy of DS-8201 in patients with HER2-low, unresectable, and/or metastatic breast cancer previously treated with standard chemotherapy is ongoing (DESTINY-Breast04; ClinicalTrials.gov identifier: NCT03734029). The HER2-low population in this trial is defined, including tumors with IHC 1+ and IHC 2+/ISH-negative HER2 expression.
Tyrosine Kinase Inhibitors
Another way of blocking the HER receptor family is on the intracellular domain performed by targeting tyrosine kinase inhibitors (TKIs). These HER-directed TKIs have a lower molecular weight compared with monoclonal antibodies, which theoretically allows them to penetrate more easily through the blood-brain barrier and make them superior for treatment of HER2 brain metastases. Lapatinib was the first approved pan-HER2 and EGFR/HER1 receptor affecting TKI but remains an agent for further line treatment in HER2-positive breast cancer due to its lower efficacy and also higher toxicity. There are, however, several novel TKIs in clinical development. Neratinib is an irreversible pan-HER kinase inhibitor (EGFR/HER1, HER2, and HER4) with activity as extended adjuvant therapy following standard trastuzumab-based adjuvant treatment in the phase III trial ExteNet [6]. In patients with HER2-positive breast cancer brain metastases, the combination of neratinib and capecitabine demonstrated a reduction of CNS lesions in 49% of patients in the phase II trial TBCRC 022 [7]. Neratinib might also be effective in a group of HER2-negative patients with HER2 mutations (see below). Another oral, pan-HER kinase inhibitor (EGFR/HER1, HER2, and HER4) is pyrotinib, which showed superiority in a phase II study as combination with capecitabine in HER2-positive metastatic or advanced breast cancer patients after anthracycline or taxane chemotherapy treatment in comparison with lapatinib and capecitabine (progression-free survival 18.1 vs. 7.0 months; HR 0.36; 95% CI 0.23–0.58; p = 0.001) [8]. The first results of the phase III trial PHOEBE seem to confirm the results in previously treated patients with HER2-positive metastatic breast cancer who had also received trastuzumab [9]. Nonetheless, targeting the EGFR/HER1 receptor is still elusive and treatment with lapatinib, neratinib, and pyrotinib should be handled with care due to serious toxicities, such as diarrhea, nausea, and rashes. Tucatinib is an investigational, oral, highly selective inhibitor of the HER2 tyrosine kinase and, thus, has fewer side effects than pan-HER kinase inhibitors. There are also data on the activation of receptor tyrosine kinases in HER2-negative breast cancer. The clinical translational challenge would be identifying cancers that are reliant on a specific kinase for growth and survival. The heterogeneity of breast cancer, however, and the potential for adaptive switching between tyrosine kinases after inhibition of a single kinase, present challenges to targeting individual tyrosine kinases in the clinic [10].
Overcoming HER2 Resistance
Several mechanisms leading to resistance in anti-HER2 therapy have been described, such as stimulating different pathways or reactivating the common HER2 pathway [11]. Comprising mechanisms are, for example, activation of alternative receptor tyrosine kinases and membrane receptors, such as insulin-like growth factor-1 receptor (IGF-1R), and upregulation of different HER receptors, such as HER3, and the PI3K/AKT/mTOR pathway by activating mutations and moderation of tumor suppressor genes [12]. Moreover, it has been found that different HER2 mRNA and protein levels lead to differences in anti-HER2 response rates [13]. In the CLEOPATRA trial, a phase III trial that led to the approval of pertuzumab in the first-line treatment of HER2-positive metastatic breast cancer, high HER2/HER3 mRNA levels and high HER2 protein levels were associated with a better outcome, whereas a PI3K catalytic subunit alpha (PIK3CA) mutation was a strong negative prognostic factor [14]. Moreover, in the phase III EMILIA trial, PIK3CA mutations showed shorter progression-free survival and overall survival when treated with capecitabine plus lapatinib but not T-DM1, and a high HER2 mRNA expression led to better outcomes in overall survival for T-DM1-treated HER2-positive patients [15]. It has been found that the hyperactivation of the PI3K/AKT/mTOR pathway in HER2-positive breast cancer is adjunctive with resistance to anti-HER2 therapy due to gain of function mutations in the tyrosine kinases of EGFR and HER2 or alternate compensatory mechanisms [16]. This led to the attempt at combining PI3K/AKT/mTOR inhibitors with anti-HER2-targeted agents in order to overcome HER2 resistance. However, two phase III trials that examined everolimus, an mTOR inhibitor, with trastuzumab and either paclitaxel (BOLERO-1) or vinorelbine (BOLERO-3) showed only a modest improvement in progression-free survival but higher toxicities [17, 18]. The NeoPHOEBE phase II trial randomized HER2-positive early breast cancer patients to either the PIK3 inhibitor buparlisib or placebo in association with paclitaxel and trastuzumab. Only a small proportion of patients presented a PIK3CA mutation (16% for each group). The authors reported lower pCR rates of 32% for the buparlisib arm versus 40% for the placebo arm [19]. Buparlisib administration was associated with a higher incidence of serious adverse events (36%) as compared with the placebo group (8%). A phase III study evaluating the PIK3 inhibitor alpelisib in combination with trastuzumab and pertuzumab as maintenance treatment for patients with HER2-positive advanced breast cancer whose tumor harbors a PIK3CA mutation following induction therapy with a taxane in combination with trastuzumab and pertuzumab is about to start (ClinicalTrials.gov Identifier: NCT04208178).
Currently, different direct AKT inhibitors such as AZD5363, GDC-0068 (catalytic), and the allosteric MK-2206 have been tested in different settings [16]. Those agents showed growth reduction and high activity in PIK3CA mutated cell models [20, 21]. In a trastuzumab-resistance model caused by PTEN deficiency, Lu et al. [22] discovered that not only the anti-HER2 sensitivity could be recovered by the AKT inhibitor triciribine and the mTOR inhibitor everolimus, but also growth of HER2-resistant cells could be reduced. Nonetheless, serious side effects such as neutropenia, gastrointestinal side effects, and mood disorders have been seen in AKT inhibitors, which could lead to the discontinuation of treatment due to safety reasons [16].
Early clinical data also supported the use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in HER2-driven breast cancers, especially in the subset of patients with ER-positive, HER2-positive disease. However, adding the CDK 4/6 inhibitor ribociclib to trastuzumab in HER2-positive metastatic breast cancer did not result in promising responses [23]. However, in the monarcHER trial, adding abemaciclib to the combination of fulvestrant and trastuzumab in patients with advanced hormone receptor-positive and HER2-positive breast cancer resulted in a 2.6-month longer progression-free survival compared with standard-of-care chemotherapy plus trastuzumab (8.3 vs. 5.7 months, p = 0.05) [24]. To date, despite our knowledge of the HER2 pathway and its cross-talks and mechanisms of resistance, no drug has been able to overcome HER2 resistance. This might change in the near future as many promising anti-HER2 therapies are being developed in this setting. Potential biomarkers of response or resistance might be helpful to better select patients for these strategies.
HER 2 Mutations
Somatic mutations in HER2 occur in approximately 3% of breast cancers, predominantly in the hormone receptor-positive HER2-negative subtype (lobular 7.8% vs. ductal 1.6%) [25]. The therapeutic relevance of HER2-directed therapy in HER2-mutant breast cancers is an area of ongoing investigation. In a multicenter phase II “basket” trial of single-agent neratinib in HER2-mutant advanced solid tumors (SUMMIT; NCT01953926) the greatest antitumor activity was observed in patients with breast cancer. Although some patients with HER2-mutant breast cancer showed good responses to neratinib, these responses were generally short and the median progression-free survival on neratinib was only 3.5 months [26]. Preexistent concurrent activating HER2 or HER3 alterations were associated with a poor treatment outcome. Similarly, acquisitions of multiple HER2-activating events as well as gatekeeper alterations were observed at disease progression in a high proportion of patients deriving clinical benefit from neratinib [27]. HER2 signaling activation has also been identified as a mechanism of endocrine therapy resistance. Indeed, in the ExteNet trial the greatest benefit of adding neratinib to the treatment of HER2-positive breast cancer patients was observed in the ER-positive subgroup [6].
Targeting EGFR/HER1
The EGFR/HER1 receptor works as an oncogenic driver in many different cancers, including breast cancer. In up to 14% of breast cancer patients EGFR/HER1 is overexpressed due to gene amplifications or missense mutations [28, 29]. Recently, it has been found that EGFR/HER1 and HER3 affect the therapeutic efficacy of anti-HER2-targeted therapy, that is trastuzumab, and worsen the prognostic outcome of HER2-positive breast cancer patients. The first pan-HER1/HER2 receptor affecting TKIs was lapatinib. Other small-molecule TKIs such as gefitinib, erlotinib, and neratinib have been investigated in clinical studies. Gefitinib and erlotinib bind reversibly to EGFR/HER1 and have shown modest efficacy in breast cancer patients [30]. The TKI neratinib binds irreversibly to EGFR/HER1 and also HER4 [28] and was approved by the FDA for adjuvant treatment of HER2-positive breast cancer after using trastuzumab (see above). Also, different monoclonal antibodies (mAbs) against EGFR/HER1, such as cetuximab, panitumumab, and zalutumumab, have been investigated in clinical studies in breast cancer, but no antibody has been approved for treatment up to now [28]. To date, EGFR/HER1 seems to be being further investigated as a therapeutic target, mainly in triple-negative breast cancer [31].
Targeting HER3
HER3 lacks or has little intrinsic tyrosine kinase activity. It frequently co-expresses and forms heterodimers with other receptor tyrosine kinases in cancer cells to activate oncogenic signaling, especially the PI3K/AKT pathway and Src kinase (acronym of cellular and sarcoma). Inhibition of HER3 is assumed to overcome resistance in HER2-positive breast cancer patients. HER3 constitutes a unique biomarker and molecular target for the treatment of human cancer. A human anti-HER3 monoclonal IgG2 antibody (MM-121, Seribantumab, Merrimack Pharmaceuticals, Cambridge, MA, USA) blocked ligand-induced HER2/HER3 dimerization and subsequently inhibited downstream signaling and has shown activity in breast cancer cell lines [32]. Since HER3 has to form heterodimer or heterotrimer complexes with other receptor tyrosine kinases in order to fully transduce signaling, anti-HER3 monotherapy is unlikely to show significant efficacy against human cancer. Recent studies offer new hope to develop epigenetic approaches, such as using a histone deacetylase inhibitor [33], specific miRNAs [34], or by targeting HER3 and its key downstream mediators. The HER3 ligand heregulin (HRG) can stimulate chemotaxis and invasion via HER2/HER3 heterodimers and it has been reported that HRG-induced activation of HER3 signaling is important in breast cancer brain metastasis [35]. Interestingly, recent studies suggest that higher HRG mRNA expression and low HER2 levels predict a clinical benefit from the addition of seribantumab (MM-121) to standard of care therapies in patients with hormone receptor-positive HER2-low breast cancer. The therapeutic window of lumretuzumab, another humanized anti-HER3 IgG1 monoclonal antibody (Roche Inc., Penzberg, Germany) in combination with the anti-HER2 antibody pertuzumab and paclitaxel for HER3-positive metastatic breast cancer was too narrow to warrant further clinical development [36]. Other anti-HER3 antibodies and also an HER1–3-neutralizing antibody mixture are under development and might be tested in clinical trials in the near future [37, 38]. Notably, an adenovirus encoding the full length human HER3 receptor was developed to be utilized as a cancer “vaccine.” HER3 vaccine-induced antibodies showed antiproliferative effects in HER2-resistant and also in triple-negative breast cancer cell lines in vivo [39].
Targeting HER4
The role of HER4 is less well understood in breast cancer. Unlike HER2, which cannot directly bind a ligand, and HER3, which does not have a functional kinase domain, HER4 is a fully functional receptor tyrosine kinase capable of signaling, both as a homo- as well as a heterodimer [40]. An advantageous impact of HER4 expression in breast cancer has been mechanistically attributed to ligand-dependent (i.e., HRG) receptor activation and the subsequent triggering of differentiation pathways that in turn antagonize oncogenic cellular features generated by other coexpressed HER receptor family members, above all HER2. It is assumed that via HER4-mediated signaling an interaction with the ER pathway occurs, leading to an improved outcome of ER-positive and tamoxifen-treated postmenopausal breast cancer patients in the absence of HER4 expression [41]. HER4 may also play a key role in the survival of HER2-positive cancer cells after they develop resistance to the anti-HER2 inhibitors lapatinib and trastuzumab [42]. No direct antibody against HER4 is under development and HER4 is only a target when pan-HER-targeted treatments, like TKIs, are investigated.
Conclusion
Several treatment strategies are being developed to target HER2 and other members of the HER family, not only in HER2-positive breast cancer but also in HER2-low expressing tumors, and different approaches are also being investigated to overcome HER2 resistance.
Conflict of Interest Statement
K.R. has no conflict of interest to declare. I.W. received speakers' honoraria from Amgen, Pfizer, Novartis, Roche, MSD, Daichi Sankyo, and Pierre Fabre Pharma, and institutional (non-personal) funding from MSD.
Funding Sources
No funding was received for this study.
Author Contributions
K.R. and I.W. drafted the manuscript.
Acknowledgements
We acknowledge Sabine Wuttke for drafting Figure 1.
References
- 1.Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015 Jan;26((1)):113–9. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. KATHERINE Investigators Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019 Feb;380((7)):617–28. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 3.Banerji U, van Herpen CM, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019 Aug;20((8)):1124–35. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 4.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. DESTINY-Breast01 Investigators Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020 Feb;382((7)):610–21. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol. 2020 Jun;38((17)):1887–96. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. ExteNET Study Group Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017 Dec;18((12)):1688–700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RA, Gelman RS, Melisko ME, Anders CK, Moy B, Blackwell KL, et al. TBCRC 022: Phase II trial of neratinib + capecitabine for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM) Journal of Clinical Oncology. 2017;35((15_suppl)):1005. [Google Scholar]
- 8.Blair HA. Pyrotinib: First Global Approval. Drugs. 2018 Nov;78((16)):1751–5. doi: 10.1007/s40265-018-0997-0. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Yan M, Ma F, Hu X-C, Feng JF, Ouyang Q, et al. Pyrotinib or lapatinib plus capecitabine for HER2+ metastatic breast cancer (PHOEBE): A randomized phase III trial. Journal of Clinical Oncology. 2020;38((15_suppl)):1003. [Google Scholar]
- 10.Anandappa G, Turner NC. Targeting receptor tyrosine kinases in HER2-negative breast cancer. Curr Opin Oncol. 2013 Nov;25((6)):594–601. doi: 10.1097/CCO.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 11.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66((1)):111–28. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 12.Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol. 2019 Mar;11:1758835919833519. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuciforo P, Thyparambil S, Aura C, Garrido-Castro A, Vilaro M, Peg V, et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol. 2016 Jan;10((1)):138–47. doi: 10.1016/j.molonc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Cortés J, Im SA, Clark E, Ross G, Kiermaier A, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014 Nov;32((33)):3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino AE, et al. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016 Aug;22((15)):3755–63. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona FJ, Montemurro F, Kannan S, Rossi V, Verma C, Baselga J, et al. AKT signaling in ERBB2-amplified breast cancer. Pharmacol Ther. 2016 Feb;158:63–70. doi: 10.1016/j.pharmthera.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015 Jul;16((7)):816–29. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 18.André F, Hurvitz S, Fasolo A, Tseng LM, Jerusalem G, Wilks S, et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J Clin Oncol. 2016 Jun;34((18)):2115–24. doi: 10.1200/JCO.2015.63.9161. [DOI] [PubMed] [Google Scholar]
- 19.Loibl S, de la Pena L, Nekljudova V, Zardavas D, Michiels S, Denkert C, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE) Eur J Cancer. 2017;85:133–45. doi: 10.1016/j.ejca.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012 Apr;11((4)):873–87. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 21.Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012 Oct;18((20)):5816–28. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007 Oct;13((19)):5883–8. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 23.Goel S, Pernas S, Tan-Wasielewski Z, Barry WT, Bardia A, Rees R, et al. Ribociclib Plus Trastuzumab in Advanced HER2-Positive Breast Cancer: Results of a Phase 1b/2 Trial. Clin Breast Cancer. 2019 Dec;19((6)):399–404. doi: 10.1016/j.clbc.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Tolaney SM, Wardley A, Zambelli S, Hilton J, Troso-Sandoval T, Ricci F, et al. monarcHER: A randomized Phase 2 study of abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with HR+, HER2+ advanced breast cancer (ABC) Ann Oncol. 2019;30:v851–934. [Google Scholar]
- 25.Ma CX, Bose R, Gao F, Freedman RA, Telli ML, Kimmick G, et al. Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin Cancer Res. 2017 Oct;23((19)):5687–95. doi: 10.1158/1078-0432.CCR-17-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018 Feb;554((7691)):189–94. doi: 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smyth LM, Piha-Paul SA, Won HH, Schram AM, Saura C, Loi S, et al. Efficacy and Determinants of Response to HER Kinase Inhibition in HER2-Mutant Metastatic Breast Cancer. Cancer Discov. 2020 Feb;10((2)):198–213. doi: 10.1158/2159-8290.CD-19-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maennling AE, Tur MK, Niebert M, Klockenbring T, Zeppernick F, Gattenlöhner S, et al. Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers (Basel) 2019 Nov;11((12)):E1826. doi: 10.3390/cancers11121826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Kuraya K, Schraml P, Torhorst J, Tapia C, Zaharieva B, Novotny H, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004 Dec;64((23)):8534–40. doi: 10.1158/0008-5472.CAN-04-1945. [DOI] [PubMed] [Google Scholar]
- 30.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012 Jan;16((1)):15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canonici A, Browne AL, Ibrahim MF, Fanning KP, Roche S, Conlon NT, et al. Combined targeting EGFR and SRC as a potential novel therapeutic approach for the treatment of triple negative breast cancer. Ther Adv Med Oncol. 2020 Jan;12:1758835919897546. doi: 10.1177/1758835919897546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoeberl B, Kudla A, Masson K, Kalra A, Curley M, Finn G, et al. Systems biology driving drug development: from design to the clinical testing of the anti-ErbB3 antibody seribantumab (MM-121) NPJ Syst Biol Appl. 2017 Jan;3((1)):16034. doi: 10.1038/npjsba.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res. 2009 Nov;69((21)):8403–11. doi: 10.1158/0008-5472.CAN-09-2146. [DOI] [PubMed] [Google Scholar]
- 34.Lyu H, Wang S, Huang J, Wang B, He Z, Liu B. Survivin-targeting miR-542-3p overcomes HER3 signaling-induced chemoresistance and enhances the antitumor activity of paclitaxel against HER2-overexpressing breast cancer. Cancer Lett. 2018 Apr;420:97–108. doi: 10.1016/j.canlet.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017 May;9((391)):eaal4682. doi: 10.1126/scitranslmed.aal4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss A, Park-Simon TW, Albanell J, Lassen U, Cortés J, Dieras V, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs. 2018 Oct;36((5)):848–59. doi: 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foreman PK, Gore M, Kobel PA, Xu L, Yee H, Hannum C, et al. ErbB3 inhibitory surrobodies inhibit tumor cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012 Jul;11((7)):1411–20. doi: 10.1158/1535-7163.MCT-12-0068. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz LJ, Hutchinson KE, Rexer BN, Estrada MV, Gonzalez Ericsson PI, Sanders ME, et al. An ERBB1-3 Neutralizing Antibody Mixture With High Activity Against Drug-Resistant HER2+ Breast Cancers With ERBB Ligand Overexpression. J Natl Cancer Inst. 2017 Nov;109((11)):djx065. doi: 10.1093/jnci/djx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osada T, Hartman ZC, Wei J, Lei G, Hobeika AC, Gwin WR, et al. Polyfunctional anti-human epidermal growth factor receptor 3 (anti-HER3) antibodies induced by HER3 vaccines have multiple mechanisms of antitumor activity against therapy resistant and triple negative breast cancers. Breast Cancer Res. 2018 Aug;20((1)):90. doi: 10.1186/s13058-018-1023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veikkolainen V, Vaparanta K, Halkilahti K, Iljin K, Sundvall M, Elenius K. Function of ERBB4 is determined by alternative splicing. Cell Cycle. 2011 Aug;10((16)):2647–57. doi: 10.4161/cc.10.16.17194. [DOI] [PubMed] [Google Scholar]
- 41.Wege AK, Chittka D, Buchholz S, Klinkhammer-Schalke M, Diermeier-Daucher S, Zeman F, et al. HER4 expression in estrogen receptor-positive breast cancer is associated with decreased sensitivity to tamoxifen treatment and reduced overall survival of postmenopausal women. Breast Cancer Res. 2018 Nov;20((1)):139. doi: 10.1186/s13058-018-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canfield K, Li J, Wilkins OM, Morrison MM, Ung M, Wells W, et al. Receptor tyrosine kinase ERBB4 mediates acquired resistance to ERBB2 inhibitors in breast cancer cells. Cell Cycle. 2015;14((4)):648–55. doi: 10.4161/15384101.2014.994966. [DOI] [PMC free article] [PubMed] [Google Scholar]