Abstract
Kisspeptin has an indispensable role in gonadotropin-releasing hormone/gonadotropin secretion in mammals. In rodents, kisspeptin neurons are located in distinct brain regions, namely the anteroventral periventricular nucleus-periventricular nucleus continuum (AVPV/PeN), arcuate nucleus (ARC), and medial amygdala (MeA). Among them, the physiological role of AVPV/PeN kisspeptin neurons in males has not been clarified yet. The present study aims to investigate the acute effects of the olfactory and/or mating stimulus with a female rat on hypothalamic and MeA Kiss1 mRNA expression, plasma luteinizing hormone (LH) and testosterone levels in male rats. Intact male rats were exposed to the following stimuli: exposure to clean bedding; exposure to female-soiled bedding as a female-olfactory stimulus; exposure to female-soiled bedding and mating stimulus with a female rat. The mating stimulus significantly increased the number of the AVPV/PeN Kiss1 mRNA-expressing cells in males within 5 minutes after the exposure, and significantly increased LH and testosterone levels, followed by an increase in male sexual behavior. Whereas, the males exposed to female-soiled bedding showed a moderate increase in LH levels and no significant change in testosterone levels and the number of the AVPV/PeN Kiss1 mRNA-expressing cells. Importantly, none of the stimuli affected the number of Kiss1 mRNA-expressing cells in the ARC and MeA. These results suggest that the mating-induced increase in AVPV/PeN Kiss1 mRNA expression may be, at least partly, involved in stimulating LH and testosterone release, and might consequently ensure male mating behavior. This study would be the first report suggesting that the AVPV/PeN kisspeptin neurons in males may play a physiological role in ensuring male reproductive performance.
Keywords: Kisspeptin, Gonadotropin-releasing hormone, Luteinizing hormone, Male sexual behavior, Testosterone
Accumulating evidence suggests that kisspeptin neurons play an essential role in mammalian reproductive function [1, 2]. Kisspeptin neurons are indispensable for gonadotropin-releasing hormone (GnRH) and consequent luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release in mammals including rodents [3,4,5], ruminants [6,7,8] and primates [9, 10]. Indeed, plasma LH and FSH levels are undetectable in male and female Kiss1 (kisspeptin gene) knockout (KO) rats [3]. Cell bodies of kisspeptin neurons are located in distinct brain regions, such as the anteroventral periventricular nucleus (AVPV)/periventricular nucleus (PeN)/preoptic area (POA), arcuate nucleus (ARC) and medial part of the amygdala (MeA) [4, 11,12,13] in the brain of mammalian species including rodents [14,15,16,17], ruminants [7, 18,19,20], primates [21,22,23]. Since the discovery of kisspeptin neurons, the role of kisspeptin neurons in reproduction has been intensively studied mainly in females.
In male mammals, it is postulated that the ARC kisspeptin neurons play a role in the regulation of tonic GnRH/gonadotropin release [3, 24,25,26] similar to females. The ARC kisspeptin neurons are suggested to serve as a GnRH pulse generator, which governs pulsatile gonadotropin release to control various reproductive events, such as puberty onset, spermatogenesis/folliculogenesis and sex steroid synthesis in both sexes [3, 25, 27]. On the other hand, a number of evidence suggests that the AVPV/PeN/POA kisspeptin neurons mediate the estrogen-positive feedback to induce GnRH/LH surge that leads to ovulation in female mice [28], rats [29], sheep [30], goats [31], cattle [32], musk shrews [33], pigs [34] and monkeys [35]. Moreover, our previous study suggests that the AVPV/PeN kisspeptin neurons integrate the external information, such as a male-olfactory stimulus, to enhance GnRH/LH surge in female rats [36]. Whereas, the number of AVPV/PeN/POA kisspeptin neurons in males is lower than in females in rodents [37] and monkeys [35]. Thus, the physiological importance of the AVPV/PeN/POA kisspeptin neurons in males has not been clarified yet. The AVPV/PeN Kiss1 mRNA expression in male mice is augmented by exogenous androgen [38] and the POA kisspeptin neurons in male monkeys [35, 39] and goats [31] are activated by exogenous estrogen followed by a surge-like increase in plasma LH levels as shown in females. These studies suggest that the AVPV/PeN/POA kisspeptin neurons may also function to control reproduction in males.
The sex-related olfactory stimulus and/or mating stimulus are suggested to be involved in a rapid induction of GnRH release, and the GnRH may directly or indirectly (via LH and then testosterone increase) enhance mating behavior in male animals [40,41,42]: Previous studies reported that plasma LH levels increase after female urine exposure within 15 min in male mice [43]; plasma LH and testosterone levels increase after copulation within 10 min and 60 min, respectively, in male rats [44]; rapid LH or testosterone increase after copulation has been reported in other mammals including rabbits (testosterone: within 45 min) [45], cows (LH: within 60 min) [46] and pigs (LH: within 20 min) [47]. Further, a central administration of GnRH facilitated male-sexual behavior in gonad-intact male rats within 15 min [40] and testosterone-implanted castrated male rats within 30 min [41], suggesting that the GnRH may rapidly and positively affect the brain circuit involved in the male sexual behavior. These results suggest that the olfactory stimulus and/or mating stimulus may rapidly stimulate kisspeptin neurons, consequently enhances GnRH release and then LH and testosterone release in circulation in male mammals. Further, the MeA kisspeptin neurons are suggested to mediate pheromonal cues to increase LH release in male mice [43, 48]. Thus, we hypothesized that a rapid increase in Kiss1 mRNA expression in the brain of male rats may mediate the signals originated from the olfactory and/or mating stimuli from/with female rats to enhance GnRH/LH and then testosterone release; eventually, the information(s) augment male sexual behavior.
The present study, thus, aims to investigate the acute effects of the olfactory stimulus derived from female rats and/or mating stimulus with a female rat on Kiss1 mRNA expression in the brain, LH and testosterone release in male rats. To address this issue, we examined the effects of three-stimulus, such as exposure to clean bedding, exposure to female-soiled bedding as a female-olfactory stimulus, and exposure to female-soiled bedding and mating stimulus with a female rat on the number of the Kiss1 mRNA-expressing cells in the AVPV/PeN, ARC, and MeA, and plasma LH and testosterone levels in intact male rats. Kiss1 mRNA expression in the nuclei was investigated within 5 min after the stimuli, because plasma LH levels increased within 12 min after the stimuli in the present study. Sexual behavior was also analyzed in the males mated with a female rat.
Materials and Methods
Animals
Adult males (ages 10–13 wk; 300–400 g body weight) and females (ages 10–12 wk; 250–300 g body weight) Wistar-Imamichi strain rats were maintained under a controlled environment (14 h light and 10 h darkness, lights on at 0500 h; 23 ± 3°C) and allowed free access to standard laboratory rat chow (CE2; Clea, Tokyo, Japan) and water. All male and female rats were sexually experienced before the experiments. Sixteen male rats were subjected to the analysis for plasma LH and testosterone levels, and the other fifteen males were subjected to the histological analysis for Kiss1 mRNA expression in the brain after exposure to clean or female-soiled bedding, or exposure to female-soiled bedding and mating with a female rat. The surgical procedures for all animals were performed under ketamine (27.0 mg kg-1)/xylazine (5.3 mg kg-1) mixture and inhalant isoflurane (1–3%) anesthesia, if not otherwise specified. Seven days before blood or brain tissue sampling from the males, the females for bedding/mating stimulus were bilaterally ovariectomized (OVX) and subcutaneously implanted with Silastic tubing (1.0-mm inner diameter; 1.5-mm outer diameter; 20 mm in length; Dow Corning, Midland, MI, USA) containing crystalline estradiol-17β (E2) (Sigma, St Louis, MO, USA) to mimic a proestrous level of plasma E2 to ensure the olfactory stimulus from the female and acceptance of copulation by a male rat: The OVX + E2 females were confirmed to show robust lordosis behavior [49], and the plasma E2 levels in OVX rats with the same E2 treatment were 1.86 ± 0.34 nmol/l as determined in our previous study [50]. All animal experiments were conducted in accordance with the Guidelines of the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University (Accession number 2018031358).
Olfactory or mating stimulation for male rats
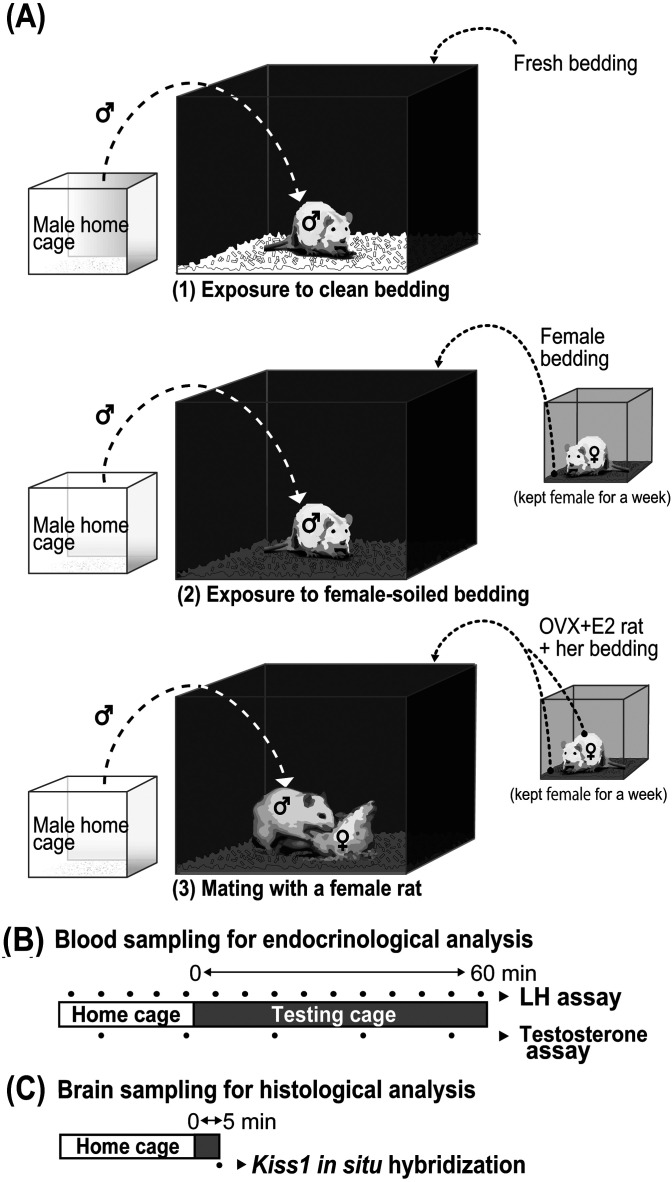
Intact male rats were divided into three groups: exposure to clean bedding, exposure to female-soiled bedding, and placed with a female for mating with female-soiled bedding (Fig. 1A). The female-soiled bedding was prepared by housing an OVX + E2 female rat in a clean acrylic cage covered with bedding for seven days. On the day of blood or brain sampling, the testing cages (60 cm long × 45 cm wide × 45 cm high, the front side was transparent, and the other sides were opaque) were covered with clean (fresh) or female-soiled bedding 30 min before introducing a male. The OVX + E2 female rat was placed in the testing cage covered with their own female-soiled bedding at the same time. A male rat was then transferred from his home cage to the testing cage at 1730 h and kept there for 60 min for blood sampling (Fig. 1B) or 5 min for brain sampling (Fig. 1C).
Fig. 1.
Experimental scheme of olfactory or mating stimulation. Intact male rats were divided into three groups: exposure to clean bedding, exposure to female-soiled bedding, and placed with a female for mating with female-soiled bedding. The males were kept in their home cages and then introduced to each testing cage containing fresh bedding or female-soiled bedding without/with an ovariectomized (OVX) + estradiol-17β (E2) female rat. The fresh bedding or the female-soiled bedding taken from the cage, where an OVX + E2 female rat had been kept for a week, was placed into a testing cage without/with an OVX + E2 rat 30 min before introducing a male rat into the testing cage (A). Male rats were subjected to the blood sampling in their home cage (for the first 24 min) and then in the testing cage (for the last 60 min) at 6 min intervals, and the plasma samples were used for luteinizing hormone (LH) and testosterone assays (B). Five minutes after the onset of the exposure to each stimulus in the testing cage, the male brain samples were taken for histological analysis for Kiss1 mRNA expression (C).
Blood sampling for analyzing plasma LH and testosterone levels and monitoring for male sexual behavior
A silicon cannula (inner diameter 0.5 mm; outer diameter 1.0 mm; Shin-Etsu Polymer, Tokyo, Japan) was inserted into the right atrium through the jugular vein on a day before the blood sampling in male rats (n = 4–6 in each group). To determine the effect of olfactory and/or mating stimuli on the plasma LH and testosterone levels, blood samples (150 μl) were obtained from free-moving conscious intact male rats (n = 4–6 in each group) for 84 min at 6 min intervals: Male rats were subjected to the blood sampling in their home cage (for the first 24 min) and then in the testing cage (for the last 60 min). Plasma samples were obtained by immediate centrifugation and stored at –20°C until assayed for LH and testosterone. The sexual behavior of the male rats placed in the testing cage with a female rat was recorded on a video camera and the total number of mounting, intromission, and ejaculation of the males was counted every 6 minutes according to previous studies [51, 52].
LH and testosterone assays
Plasma LH concentrations in 50-μl plasma samples were determined by a double-antibody radioimmunoassay (RIA) with a rat LH RIA kit provided by the National Hormone and Peptide Program (Baltimore, MD, USA), and were expressed in terms of the NIDDK rat LH RP-3. The least detectable level of LH assay was 156 pg/ml, and the intra- and inter-assay coefficients of variation were 6.75 and 2.65% at 0.98 ng/ml, respectively.
Plasma testosterone levels were determined by an enzyme-linked sorbent immunoassay using a testosterone ELISA kit (Cat No. 582701, Cayman Chemicals, Ann Arbor, MI, USA, RRID: AB_328059) according to the manufacturer instructions. Testosterone extracted from 40-μl plasma samples with a mixture of hexane and ether (3:2) was dissolved in 0.1% gelatin-0.05 M phosphate solution and then quantified. The least detectable level of testosterone was 6 pg/ml, and the intra- and inter-assay of coefficients of variation were 2.61 and 11.06% at 1.40 ng/ml, respectively.
Brain sampling
Five minutes after the transfer to the testing cages, male rats (n = 5 in each group) were deeply anesthetized with sodium pentobarbital and perfused with 0.05 M PBS followed by 4% paraformaldehyde in 0.05 M PB to obtain the brain sample for analysis of Kiss1 mRNA expression. The timing of the brain sampling for Kiss1 mRNA analysis was chosen, because the plasma LH levels in male rats were significantly increased 12 minutes after mating stimulation and thereafter with kisspeptin being a dominant stimulator for GnRH/LH release in male rats [3]. Note that all male rats in the group for mating stimulus immediately showed mating behavior. Brains were immediately removed from the skull, postfixed with the same fixative at 4°C overnight, and then immersed in 30% sucrose in 0.05 M PB at 4°C for 2–3 days until they sank. Serial 50-μm coronal sections containing the AVPV/PeN, ARC, and MeA were obtained.
In situ hybridization of Kiss1 mRNA
In situ hybridization for Kiss1 was performed in the brain sections taken from male rats in each group as previously described [36, 37]. The brain sections were hybridized with 1 μg/ml DIG-labeled anti-sense Kiss1 cRNA probe (position 33-348; GenBank accession no. AY196983) overnight at 60°C. The sections were washed with 2 × SSC containing 50% formamide for 15 min at 60°C twice, then treated with 20 μg/ml RNase A for 30 min at 37°C and alkaline phosphatase-conjugated anti-DIG antibody (sheep IgG, dilution 1:1000; Roche Diagnostics, Indianapolis, IN, USA, RRID: AB_514497) 2 h at 37°C, and then treated with a chromogen solution (337 μg/ml 4-nitroblue tetrazolium chloride, 175 μg/ml 5-Bromo-4-Chloro-3-indoyl-phosphate) for 1.5 h. The number of Kiss1 mRNA-expressing cells on each brain section was bilaterally counted twice by a blind investigator under a microscope, and the average per section was calculated in each group. The number of Kiss1 mRNA-expressing cells was counted every second section through the AVPV/PeN (from 0.48 mm anterior to 0.48 mm posterior to the bregma; eleven sections in total) or every fourth section through the ARC (rostral division, from 1.80 to 2.60 mm; middle division, from 2.60 to 3.40 mm; caudal division, from 3.40 to 4.20 mm posterior to the bregma; three sections for each division of the ARC and nine sections in total) and MeA (from 2.40 to 3.60 mm posterior to the bregma; four sections in total) according to the rat brain atlas [53]. No positive signal for Kiss1 mRNA was detected in the brain sections hybridized with the corresponding sense probe as described previously [36].
Data and statistical analysis
Statistical differences in the plasma LH or testosterone concentrations were determined by two-way (stimulus and time as main effects) ANOVA, followed by the Bonferroni test using js-STAR software (http://www.kisnet.or.jp/nappa/software/star/). Statistical differences in the number of mounts, intromission or ejaculation in male rats were determined by one-way repeated measures ANOVA, followed by the Bonferroni test using js-STAR software. Statistical differences in the number of Kiss1 mRNA-expressing cells per section in the AVPV/PeN, ARC (rostral, middle, caudal divisions) or MeA were determined by one-way factorial ANOVA, followed by the Bonferroni test using js-STAR software.
Results
The mating stimulus with a female rat increased plasma LH and testosterone levels in intact male rats
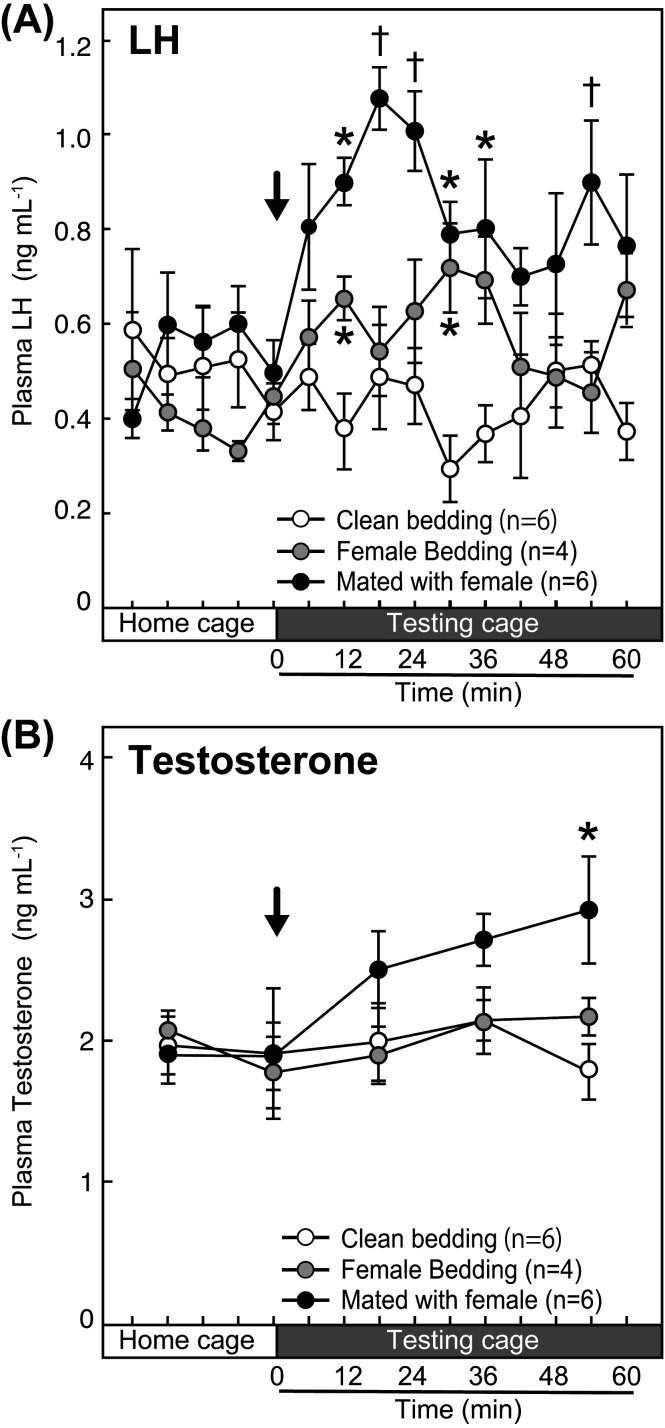
The profiles of changes in mean plasma LH concentrations in intact male rats in each group were shown in Fig. 2A. The LH level showed an immediate increase in intact male rats after the mating with a female rat, while the LH level gradually increased after the exposure to female-soiled bedding. The LH level was stable when the male rats were exposed to the clean bedding. Two-way ANOVA analysis (main effects, stimuli and time) revealed significant effects of stimuli (F2,13 = 6.37, P < 0.05), time (F14,182 = 2.72, P < 0.05) and interaction between stimuli and time (F28,182 = 2.57, P < 0.05) on the plasma LH levels (Fig. 2A). Specifically, the plasma LH levels in the male rat at 18, 24, and 54 minutes after the mating with a female rat were significantly higher (†, P < 0.05, analyzed by the Bonferroni test) than those in clean-bedding- and female-soiled-bedding-exposed male rats (Fig. 2A). The plasma LH levels in the male rat at 12, 30, and 36 minutes after the mating were significantly higher (*, P < 0.05, analyzed by the Bonferroni test) than those in clean-bedding-exposed male rats (Fig. 2A). The plasma LH levels in the male rat at 12 and 30 min after the exposure to female bedding were significantly higher (*, P < 0.05, analyzed by the Bonferroni test) than those in clean-bedding-exposed male rats (Fig. 2A).
Fig. 2.
Effects of olfactory and/or mating stimulation on plasma luteinizing hormone (LH) and testosterone levels in intact male rats. The changes in the mean plasma LH levels of intact male rats in each group that were exposed to the clean bedding, the female-soiled bedding, or female-soiled bedding and mated with an ovariectomized (OVX) + estradiol-17β (E2) rat (A). The changes in the mean plasma testosterone levels in each group (B). Arrows indicate the onset of each bedding exposure and/or mating with a female. †, values indicating significant difference compared with the value in the males exposed to clean bedding and female-soiled bedding (P < 0.05, two-way ANOVA). *, values indicating significant difference compared with the value in the males exposed to clean bedding (P < 0.05, two-way ANOVA).
The mean plasma testosterone concentrations in the male rats mated with a female gradually increased, while testosterone levels were stable in the males exposed to the clean or female-soiled bedding during the sampling period (Fig. 2B). Two-way ANOVA analysis (main effects, stimuli and time) revealed significant effects of time (F4,52 = 4.94, P < 0.05) and interaction between stimuli and time (F8,52 = 2.99, P < 0.05), but no significant effects of stimuli (F2,13 = 0.98, P > 0.05), on the plasma testosterone levels (Fig. 2B). Specifically, the plasma testosterone levels in the male rat at 54 minutes after the mating were significantly higher (P < 0.05, analyzed by the Bonferroni test) than those of male rats exposed to clean bedding (Fig. 2B).
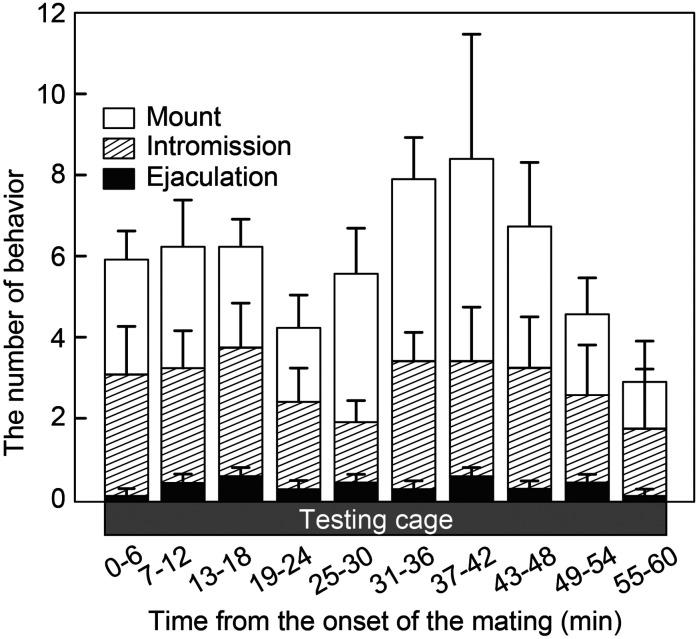
Temporal analysis of the number of male-type sexual behavior
Temporal changes in the number of mounting, intromission, and ejaculation, and the total number of these sexual behavior in male rats for 60 min after the cohabitation with a female rat are shown in Fig. 3. All male rats showed sexual behaviors during the 60 min blood sampling/behavior test period. The mounting and intromission behavior seemed to show a biphasic increase during the period, but no significant difference was found in the number of mounting (F9,45 = 0.78, P > 0.05), intromission (F9,45 = 0.33, P > 0.05), ejaculation (F9,45 = 0.72, P > 0.05) or total (F9,45 = 0.56, P > 0.05) in male rats (Fig. 3).
Fig. 3.
Temporal changes in the number of male-type sexual behaviors in intact male rats after the introduction to the female-soiled bedding and a female rat. The number of mounting, intromission, and ejaculation were measured every 6 min for 60 min in intact male rats exposed to female-soiled bedding and mated with an ovariectomized (OVX) + estradiol-17β (E2) rats. Values are the mean ± SEM. There was no significant difference between each time point (one-way ANOVA).
The mating stimulus with a female rat increased Kiss1 mRNA expression in the AVPV/PeN, but not in the ARC and MeA in intact male rats
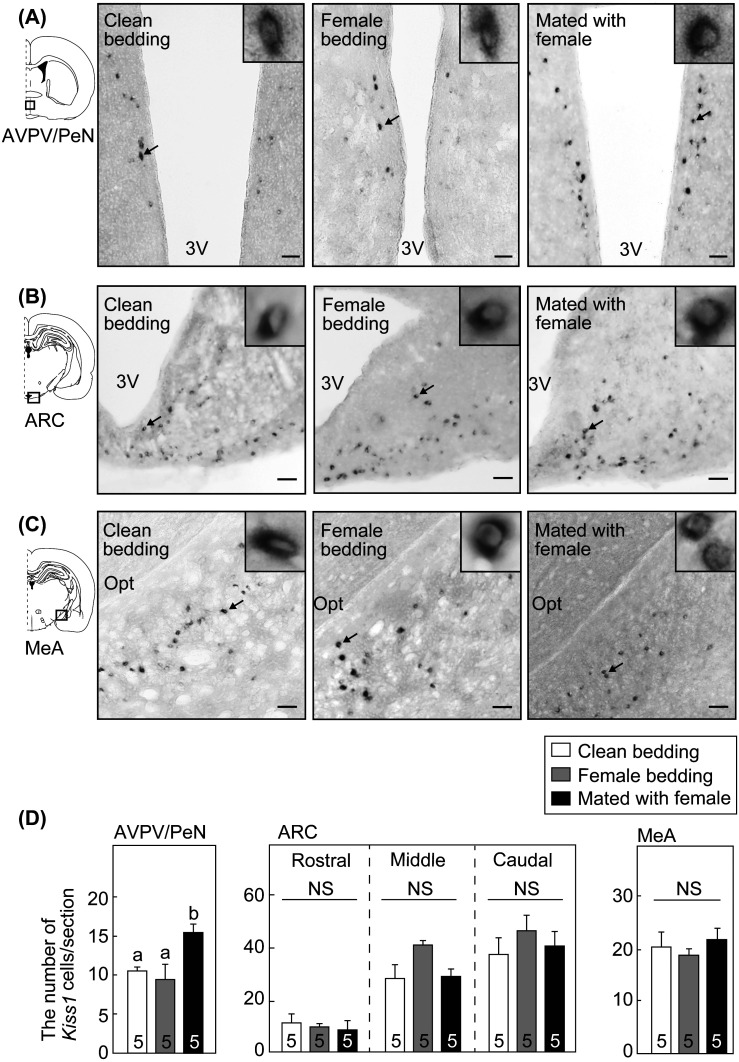
Kiss1 mRNA-expressing cells in the AVPV/PeN, ARC and MeA in representative intact male rats are shown in Fig. 4. Kiss1 mRNA-expressing cells were found in the AVPV/PeN (Fig. 4A), ARC (Fig. 4B) and MeA (Fig. 4C) of all male rats exposed to clean or female-soiled bedding, and mated with a female rat.
Fig. 4.
Effects of olfactory and/or mating stimulation on Kiss1 mRNA expression in the anteroventral periventricular nucleus-periventricular nucleus continuum (AVPV/PeN), arcuate nucleus (ARC) and medial amygdala (MeA) in intact male rats. Kiss1 mRNA expression determined by in situ hybridization in the AVPV/PeN (A), ARC (B) and MeA (C) in representative intact male rats exposed to clean bedding, female-soiled bedding, or female-soiled bedding with a female rat. The boxed area in the schematic illustration of the coronal section of the rat brain shows the location of the AVPV/PeN, ARC, or MeA examined. Insets show Kiss1 mRNA-expressing cells at higher-magnification in each brain area indicated by arrows in the photomicrographs. Scale bars, 100 μm; 3 V, the third ventricle; Opt, optic tract. The number of Kiss1 mRNA-expressing cells per section in the AVPV/PeN, ARC (the rostral, middle, and caudal divisions), and MeA of intact male rats exposed to clean bedding, female-soiled bedding, or female-soiled bedding with a female rat (D). The value with different characters shows a significant difference within the three groups (P < 0.05, one-way ANOVA followed by Bonferroni test). There was no significant difference (NS) between the groups (one-way ANOVA) in the number of the Kiss1 mRNA-expressing cells per section in the ARC (the rostral, middle, and caudal divisions) and MeA. Values are the means ± SEM. The number in each column indicates the number of animals used.
The mating stimulus significantly increased the number of Kiss1 mRNA-expressing cells in the AVPV/PeN, but not in the ARC and MeA of intact male rats (Fig. 4D). Specifically, one-way ANOVA analysis showed a significant effect of the stimulation on the number of Kiss1 mRNA-expressing cells in the AVPV/PeN in the males (F2,12 = 7.23, P < 0.05) (Fig. 4D), and the number in the AVPV/PeN in male rats mated with a female rat was significantly higher than the rats exposed to clean bedding or female-soiled bedding (P < 0.05, analyzed by the Bonferroni test) (Fig. 4D). No significant difference was found in the number of Kiss1 mRNA-expressing cells in the rostral ARC (F2,12 = 0.18, P > 0.05, Fig. 4D), middle ARC (F2,12 = 4.74, P > 0.05, Fig. 4D), caudal ARC (F2,12 = 0.57, P > 0.05, Fig. 4D) or MeA (F2,12 = 0.54, P > 0.05, Fig. 4D) in male rats between groups.
Discussion
The present study demonstrated that the AVPV/PeN kisspeptin neurons are, at least partly, involved in ensuring the mating behavior in male rodents, because the mating stimulus with a female rat rapidly (within 5 min) increased the number of AVPV/PeN Kiss1 mRNA-expressing cells and then LH release followed by testosterone release in male rats. Further, our previous study showed that kisspeptin neurons are indispensable for male-type behavior: Kiss1 KO male rats failed to show the ejaculation, even if the KO rats were supplemented with exogenous testosterone [52]. The central GnRH has been suggested to be involved in enhancement of male sexual behavior in a previous study [41]: The central GnRH administration restored mounting behavior in hyperprolactinemic male rats showing deficits of the behavior. Taken together, the present findings suggest that the mating stimulus induces Kiss1 mRNA expression in the AVPV/PeN to enhance GnRH/LH and then testosterone release, consequently strengthening male sexual behavior. To our knowledge, this is the first report suggesting a physiological role of AVPV/PeN kisspeptin neurons in males.
Interestingly, solely female-olfactory stimulus failed to increase AVPV/PeN Kiss1 mRNA expression and plasma testosterone levels in male rats, suggesting that physical stimulus caused by mating with a female but not female-derived olfactory stimulus is mainly involved in the induction of the AVPV/PeN Kiss1 mRNA expression to activate the hypothalamic-pituitary-gonadal (HPG) axis. We have previously demonstrated that the male-derived olfactory stimulus increased c-Fos expression in the AVPV/PeN kisspeptin neurons and LH release in estrogen-primed-female rats [36], suggesting that the male derived-olfactory stimulus activated AVPV/PeN kisspeptin neurons in females and that the olfactory stimulus derived from the mates is less important in male rats than females for the activation of AVPV/PeN kisspeptin neurons. The notion is consistent with a previous study, showing that exposure to a mate’s bedding increased the number of AVPV/PeN kisspeptin neurons in female mice, but not in males [54]. Thus, these results suggest a sexual differentiation of neural responses to sex-related olfactory cues to affect kisspeptin neurons and then GnRH and gonadotropin release.
The present results concerning the number of Kiss1 mRNA-expressing cells suggest that the ARC and MeA kisspeptin neurons in male rats would be less important than AVPV/PeN kisspeptin neurons for the induction of the LH release after copulation. On the other hand, a recent study showed that exposure to female urine (including pheromonal signals) for 30 min increased the number of kisspeptin neurons co-expressing c-Fos in the MeA in intact male mice, suggesting that activation of the MeA kisspeptin neurons may be involved in an increase in LH release in intact male mice [43]. Moreover, it is suggested that the MeA kisspeptin neurons were positively regulated by sex steroids in male mice [55]. These studies suggest that there might be species difference in the central mechanism regulating kisspeptin neurons and sexual behavior in males. Further studies are required to clarify whether the MeA and/or ARC kisspeptin neurons are also involved in the enhancement of LH and testosterone release by the olfactory/mating stimulus in the male rodents and other mammalian species.
Interestingly, the number of Kiss1 mRNA-expressing cells in the AVPV/PeN increased rapidly after the mating stimulus. This rapid increase in Kiss1 mRNA expression is consistent with previous studies, suggesting that mRNA expression of some peptides increased within 15 min after the stimuli: corticotropin-releasing hormone mRNA expression in the hypothalamic paraventricular nucleus was induced by isotonic saline injection as a mild stressor [56]; brain natriuretic peptide mRNA expression in the cultured ventricular cardiocytes was induced by endothelin-1 stimulation [57]. These results suggest that some peptide mRNA could be rapidly expressed after the stimuli as immediate early genes do. Generally, the amount of mRNA in the cell depends on both the rates of mRNA transcription in the nucleus and mRNA degradation in the cytoplasm [58]. Thus, the rapid increase in the AVPV/PeN Kiss1 mRNA expression in the current study would be the results of an increase in transcription and/or decrease in degradation of the Kiss1 mRNA. Future studies are required to clarify if the rapid increase in the AVPV/PeN Kiss1 mRNA expression would be associated with an activation of the AVPV/PeN kisspeptin neurons.
In conclusion, the present results suggest that the increase in the number of Kiss1 mRNA-expressing cells in the male AVPV/PeN induced by the mating stimulus may be involved in stimulating the HPG axis, and might consequently assure the mating behavior in male rats. The current study suggests that the AVPV/PeN kisspeptin neurons in males may play a physiological role in ensuring male reproductive performance.
Conflict of Interest
The authors have nothing to disclose.
Acknowledgments
This work was supported in part by the Naito Foundation to NI; JSPS KAKENHI Grant Numbers 14J04328, 16H06742, 19K15966 to YW, 18K06860 to HO, 18H03973 to HT and 16K07987, 19H03103 to NI. We are grateful to the National Hormone and Peptide Program for the rat LH assay kit. The radioimmunoassay was performed at the Nagoya University Radioisotope Research Center. We thank Dr Nicola Skoulding for editorial assistance.
References
- 1.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 2007; 104: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 3.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 4.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077. [DOI] [PubMed] [Google Scholar]
- 5.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004; 80: 264–272. [DOI] [PubMed] [Google Scholar]
- 6.Smith JT, Saleh SN, Clarke IJ. Seasonal and cyclical change in the luteinizing hormone response to kisspeptin in the ewe. Neuroendocrinology 2009; 90: 283–291. [DOI] [PubMed] [Google Scholar]
- 7.Hashizume T, Saito H, Sawada T, Yaegashi T, Ezzat AA, Sawai K, Yamashita T. Characteristics of stimulation of gonadotropin secretion by kisspeptin-10 in female goats. Anim Reprod Sci 2010; 118: 37–41. [DOI] [PubMed] [Google Scholar]
- 8.Goto Y, Endo N, Nagai K, Ohkura S, Wakabayashi Y, Tanaka A, Matsui H, Kusaka M, Okamura H, Tanaka T. Ovarian and hormonal responses to follicular phase administration of investigational metastin/kisspeptin analog, TAK-683, in goats. Reprod Domest Anim 2014; 49: 338–342. [DOI] [PubMed] [Google Scholar]
- 9.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 2005; 90: 6609–6615. [DOI] [PubMed] [Google Scholar]
- 10.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 2005; 102: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 2009; 21: 673–682. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 2011; 152: 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 2009; 30: 26–33. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Bosch MA, Qiu J, Rønnekleiv OK, Kelly MJ. 17β-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol 2015; 29: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 2011; 152: 2387–2399. [DOI] [PubMed] [Google Scholar]
- 16.Yeo SH, Clarkson J, Herbison AE. Kisspeptin-Gpr54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice. Neuroendocrinology 2014; 100: 191–197. [DOI] [PubMed] [Google Scholar]
- 17.Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, Breen KM. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology 2017; 158: 3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polkowska J, Wójcik-G Adysz A, Chmielewska N, Wa Kowska M. Expression of kisspeptin protein in hypothalamus and LH profile of growing female lambs. Reprod Fertil Dev 2018; 30: 609–618. [DOI] [PubMed] [Google Scholar]
- 19.Polkowska J, Cieślak M, Wańkowska M, Wójcik-Gładysz A. The effect of short fasting on the hypothalamic neuronal system of kisspeptin in peripubertal female lambs. Anim Reprod Sci 2015; 159: 184–190. [DOI] [PubMed] [Google Scholar]
- 20.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 2012; 153: 2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terasawa E, Garcia JP, Seminara SB, Keen KL. Role of kisspeptin and neurokinin B in puberty in female non-human primates. Front Endocrinol (Lausanne) 2018; 9: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 2009; 30: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenealy BP, Keen KL, Garcia JP, Richter DJ, Terasawa E. Prolonged infusion of estradiol benzoate into the stalk median eminence stimulates release of GnRH and kisspeptin in ovariectomized female rhesus macaques. Endocrinology 2015; 156: 1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia JP, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Role of kisspeptin and neurokinin B signaling in male rhesus monkey puberty. Endocrinology 2018; 159: 3048–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology 2019; 160: 557–567. [DOI] [PubMed] [Google Scholar]
- 26.Minabe S, Sato M, Inoue N, Watanabe Y, Magata F, Matsuda F, Uenoyama Y, Ozawa H, Tsukamura H. Neonatal estrogen causes irreversible male infertility via specific suppressive action on hypothalamic Kiss1 neurons. Endocrinology 2019; 160: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 29.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 30.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 2006; 401: 225–230. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, Wakabayashi Y, Okamura H, Maeda KI, Uenoyama Y, Tsukamura H, Ohkura S. The luteinising hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol 2015; 27: 57–65. [DOI] [PubMed] [Google Scholar]
- 32.Hassaneen A, Naniwa Y, Suetomi Y, Matsuyama S, Kimura K, Ieda N, Inoue N, Uenoyama Y, Tsukamura H, Maeda KI, Matsuda F, Ohkura S. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev 2016; 62: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue N, Sasagawa K, Ikai K, Sasaki Y, Tomikawa J, Oishi S, Fujii N, Uenoyama Y, Ohmori Y, Yamamoto N, Hondo E, Maeda K, Tsukamura H. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci USA 2011; 108: 17527–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomikawa J, Homma T, Tajima S, Shibata T, Inamoto Y, Takase K, Inoue N, Ohkura S, Uenoyama Y, Maeda K, Tsukamura H. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod 2010; 82: 313–319. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol 2014; 26: 909–917. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y, Ikegami K, Ishigaki R, Ieda N, Uenoyama Y, Maeda KI, Tsukamura H, Inoue N. Enhancement of the luteinising hormone surge by male olfactory signals is associated with anteroventral periventricular Kiss1 cell activation in female rats. J Neuroendocrinol 2017; 29: e12505. [DOI] [PubMed] [Google Scholar]
- 37.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 38.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146: 2976–2984. [DOI] [PubMed] [Google Scholar]
- 39.Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female Rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology 2017; 105: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorsa DM, Smith ER. Facilitation of mounting behavior in male rats by intracranial injections of luteinizing hormone-releasing hormone. Regul Pept 1980; 1: 147–155. [DOI] [PubMed] [Google Scholar]
- 41.Dennison E, Bain PA, Bartke A, Meliska CJ. Systemically administered gonadotrophin-releasing hormone enhances copulatory behaviour in castrated, testosterone-treated hyperprolactinaemic male rats. Int J Androl 1996; 19: 253–259. [DOI] [PubMed] [Google Scholar]
- 42.Hawken PA, Martin GB. Sociosexual stimuli and gonadotropin-releasing hormone/luteinizing hormone secretion in sheep and goats. Domest Anim Endocrinol 2012; 43: 85–94. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S, Tang C, Sing K, Kim HW, Millar RP, Tello JA. Medial amygdala kiss1 neurons mediate female pheromone stimulation of luteinizing hormone in male mice. Neuroendocrinology 2019; 108: 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamel F, Wright WW, Mock EJ, Frankel AI. The influence of mating and related stimuli on plasma levels of luteinizing hormone, follicle stimulating hormone, prolactin, and testosterone in the male rat. Endocrinology 1977; 101: 421–429. [DOI] [PubMed] [Google Scholar]
- 45.Haltmeyer GC, Eik-Nes KB. Plasma levels of testosterone in male rabbits following copulation. J Reprod Fertil 1969; 19: 273–277. [DOI] [PubMed] [Google Scholar]
- 46.Katongole CB, Naftolin F, Short RV. Relationship between blood levels of luteinizing hormone and testosterone in bulls, and the effects of sexual stimulation. J Endocrinol 1971; 50: 457–466. [DOI] [PubMed] [Google Scholar]
- 47.Ellendorff F, Parvizi N, Pomerantz DK, Hartjen A, König A, Smidt D, Elsaesser F. Plasma luteinizing hormone and testosterone in the adult male pig: 24 hour fluctuations and the effect of copulation. J Endocrinol 1975; 67: 403–410. [DOI] [PubMed] [Google Scholar]
- 48.Stephens SBZ, Kauffman AS. Regulation and possible functions of kisspeptin in the medial amygdala. Front Endocrinol (Lausanne) 2017; 8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakakibara M, Deura C, Minabe S, Iwata Y, Uenoyama Y, Maeda KI, Tsukamura H. Different critical perinatal periods and hypothalamic sites of oestradiol action in the defeminisation of luteinising hormone surge and lordosis capacity in the rat. J Neuroendocrinol 2013; 25: 251–259. [DOI] [PubMed] [Google Scholar]
- 50.Tsukamura H, Maeda KI, Yokoyama A. Effect of the suckling stimulus on daily LH surges induced by chronic oestrogen treatment in ovariectomized lactating rats. J Endocrinol 1988; 118: 311–316. [DOI] [PubMed] [Google Scholar]
- 51.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 2007; 51: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura S, Uenoyama Y, Ikegami K, Dai M, Watanabe Y, Takahashi C, Hirabayashi M, Tsukamura H, Maeda KI. Neonatal kisspeptin is steroid-independently required for defeminisation and peripubertal kisspeptin-induced testosterone is required for masculinisation of the brain: A behavioural study using Kiss1 knockout rats. J Neuroendocrinol 2016; 28: 10. [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2006: Elsevier. [DOI] [PubMed] [Google Scholar]
- 54.Taziaux M, Bakker J. Absence of female-typical pheromone-induced hypothalamic neural responses and kisspeptin neuronal activity in alpha-fetoprotein knockout female mice. Endocrinology 2015; 156: 2595–2607. [DOI] [PubMed] [Google Scholar]
- 55.Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-alpha but not estrogen receptor-beta. Endocrinology 2016; 157: 4021–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dent GW, Smith MA, Levine S. Rapid induction of corticotropin-releasing hormone gene transcription in the paraventricular nucleus of the developing rat. Endocrinology 2000; 141: 1593–1598. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, Nishino K, Yoshimasa T, Nakao K. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 1995; 96: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada T, Nagahama M, Akimitsu N. Interplay between Transcription and RNA Degradation. In: Gene Expression and Regulation in Mammalian Cells—Transcription From General Aspects.2018; Chapter 6: 97–114. (https://www.intechopen.com/books/gene-expression-and-regulation-in-mammalian-cells-transcription-from-general-aspects/interplay-between-transcription-and-rna-degradation) [Google Scholar]