Abstract
This study investigated the effect of aging on mitochondria in granulosa cells (GCs) collected from the antral follicles of young and aged cows (25–50 months and over 140 months in age, respectively). When GCs were cultured under 20% O2 for 4 days, mitochondrial DNA copy number (Mt-number), determined by real-time PCR, increased throughout the culture period, and the extent of increase was greater in the GCs of young cows than in those of old cows. In a second experiment, GCs were cultured under 20% O2 for 24 h. Protein levels of TOMM20 and TFAM in GCs were lower in aged cows than in young cows, and the amount of reactive oxygen species and the mitochondrial membrane potential were higher, whereas ATP content and proliferation activity were lower, respectively. Glucose consumption and lactate production were higher in the GCs of aged cows than in those of young cows. When GCs were cultured under 5% or 20% O2 for 24 h, low O2 decreased ATP content and increased glucose consumption in GCs of both age groups compared with high O2; however, low O2 decreased the Mt-number only in the GCs of young cows. In conclusion, we show that aging affects mitochondrial quantity, function, and response to differential O2 tensions in GCs.
Keywords: Aging, Cow, Granulosa cells, Mitochondria
Oocyte development occurs in follicles present in the cortex of the ovaries. In follicles, the oocytes are surrounded by granulosa cells (GCs), which support oocyte growth by providing energy substrates and other small molecules via gap junctions [1]. The quality of GCs is a decisive factor in proper oocyte development [2, 3]. During follicle growth, especially from the early antral follicle to the antral follicle stage, the number of GCs increases extensively. RNA-seq analysis of porcine GCs has revealed that GC metabolism changes from oxidative phosphorylation to glycolysis during follicle development from the early antral follicle to the antral follicle stage [4]. In addition, GCs collected from antral follicles depend mainly on glycolysis for their energy needs [5, 6]. The molecular mechanism for the activation of glycolysis is still unclear; however, it has been suggested that the clustering of cells activates hypoxia-inducible factor 1 (HIF1) and mitophagy [7] and that HIF1 activation in bovine GCs under lowered oxygen tension induces proliferation and activation of glycolysis, and reduces mitochondrial function and number compared with that observed under high oxygen tension (20%) [8]. Therefore, the metabolic switch is an important event in GCs for proper follicle development.
Aging is responsible for low fertility in females, and accumulating evidence shows that mitochondrial dysfunction in oocytes is associated with a low pregnancy rate in humans [9, 10]. In addition, a low number of GCs in aged antral follicles [11], low proliferation and shorter telomere length in GCs [12], and low quality of oocytes [13] have been reported in aged cows compared with their younger counterparts. However, only few studies have addressed age-associated events in the mitochondria of GCs [14]. Mitochondria play a crucial role in cellular energy production; their quantity and quality profoundly affect cellular metabolism, and mitochondrial dysfunction shifts cellular metabolism towards glycolysis [15]. Therefore, age-associated mitochondrial dysfunction could affect GC metabolism and metabolic changes in GCs during follicle development. In this study, we collected GCs from young and aged cows, cultured them under low and high oxygen tension, and examined their mitochondrial functions and quality.
Materials and Methods
Cows used for GC collection
Similar to humans, cows have a long reproductive life, and their reproductive ability gradually declines with age. Age-associated decline in the quality and quantity of oocytes and granulosa cells of Japanese black cows (Bos taurus) has been reported previously [12, 13, 16, 17]. In Japan, the age and breed of all cows at any slaughterhouse can be identified by their ear tags. Based on this information, young Japanese Black cows (25–50 months of age) and aged Japanese Black cows (over 140 months of age) were selected as donors.
Medium, chemicals, and oxygen concentrations of the culture
All reagents were purchased from Nacalai Tesque (Kyoto, Japan), unless otherwise stated. Medium-199 (Gibco, Paisley, UK), supplemented with 5% FCS and antibiotics, was used for culturing GCs. Although moderation of oxygen availability is key to obtaining follicle viability in vitro [18, 19], the kinetics of oxygen concentration in the inner follicle, along with follicle development from early antral follicles to pre-ovulatory large follicles, is still unclear. In general, 5% and 20% oxygen tensions are used as physiological and atmospheric conditions, respectively, for the study of embryo culture [20]. Therefore, in vitro culture of GCs was conducted under 5% CO2 in air (high oxygen condition) at 38.5°C, unless stated otherwise. The low oxygen tension used was 5% O2, 5% CO2, and 90% N2 at 38.5°C.
Ovary collection and GC preparation
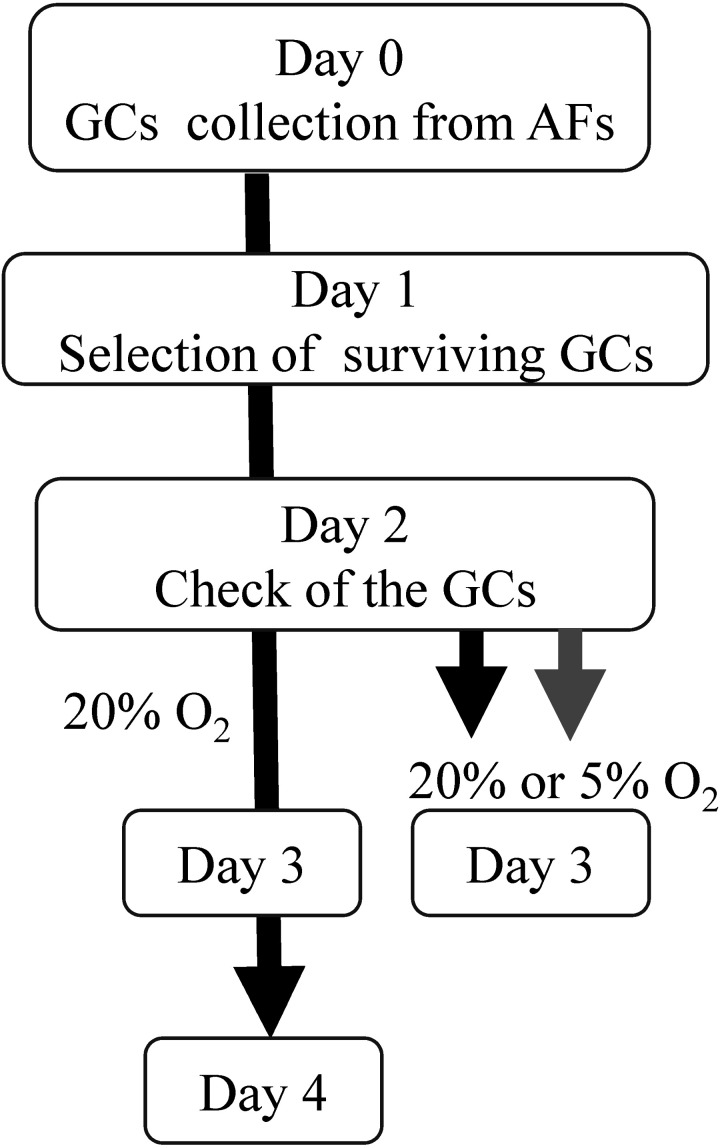
Ovaries were collected from a local slaughterhouse and transported to the laboratory within 4 h. The ovaries, being inedible, had been discarded, and their collection from the slaughterhouse for experimental use was allowed by the Committee for the Care and Welfare of Experimental Animals at Tokyo University of Agriculture. GCs (3–5 mm in diameter), collected from at least 7 young or aged cows, were aspirated from the antral follicles, pooled, and used for all experiments. As the GCs contained dead cells, surviving GCs were selected as follows: The GCs were washed in culture medium and cultured on plastic petri dishes (60 mm NUNC) overnight under high oxygen conditions. Twenty-four hours after collection, the surviving GCs attached to the culture plate were collected by Accumax (Innovative Cell Technologies, San Diego, CA, USA) treatment. The GCs were re-seeded in 96-well plates (Cat. No 353072; BD Biosciences, Franklin Lakes, NJ, USA) at a final concentration of 200,000 cells/ml and cultured under high oxygen conditions. The next day (2 days after collection), the morphology and density (70–80% confluent) of the GCs were examined under a microscope (Olympus, Tokyo, Japan), and the GCs were subsequently cultured under high or low oxygen conditions. The schematic design for GC preparation is described in Fig. 1.
Fig. 1.
Granulosa cells (GCs) were collected from the antral follicles (3–5 mm in diameter) and cultured for 1 day to separate surviving GCs attached to the culture dish. On day 1, the surviving GCs were retrieved from the share and subsequently cultured under 5% CO2 in air. On day 2, GCs with 70–80% confluence were subsequently incubated under atmospheric conditions of 5% CO2 in air for 2 days, or under 5% O2, 5% CO2, and 90% N2 for 24 h.
Collection of GCs and spent culture medium
At the end of the culture period (day 3 after collection), all media were collected from each culture well and subjected to centrifugation (7,000 × g, 10 min) to separate cellular debris, and the supernatant was used for subsequent experiments. The medium was used for glucose and lactate measurements or for DNA extraction.
DNA extraction from GCs and spent culture medium
Cell-free mitochondrial DNA (cf-mtDNA) in the medium was extracted by mixing an equal volume of medium with the DNA extraction buffer (final concentration, Tris-HCl, 20 mM; Nonidet-40, 0.9%; Tween 20, 0.9%; and proteinase K, 0.4 mg/ml) followed by heating at 55°C for 30 min and at 98°C for 10 min. DNA from GCs was extracted by incubating GCs with DNA extraction buffer at 55°C followed by heating at 98°C for 10 min.
ATP content in GC
To measure the ATP content, at the end of the culture period, GCs in each well were frozen and thawed three times with 100 μl water, and the water was collected from each well following vigorous pipetting. Half of the water (50 μl) was used to determine the ATP content by measuring the luminescence generated during an ATP-dependent luciferin-luciferase reaction (ATP assay kit; Toyo-Inc., Tokyo, Japan), as described previously [17]. The other half (50 μl) was mixed with DNA extraction buffer as described above to extract DNA. The extracted DNA was subjected to RT-PCR, targeting a single copy gene to determine the total cell number in the corresponding sample. Thereafter, the ATP contained in 20,000 GCs (number of GCs in a well) was calculated.
Measurement of mitochondrial genome copy number (Mt-number) in the medium and GCs
DNA in GCs or spent culture medium was extracted as described above. The DNA was subjected to RT-PCR using the CFX ConnectTM Real Time system (Bio-Rad, Hercules, CA, USA) and KAPA SYBR® FAST qPCR Kits (Roche, Indianapolis, IN, USA). The primers used were: 5-ACCCTTGTACCTTTGCAT-3 and 5-TCTGGTTTCGGGCTCGTTAG-3 (81 bp), targeting the mitochondrial genome (NC_006853.1), and 5-CCCTTACTGGTTGTGGCACT-3 and 5-TTCCACTCTGCACAGTAGCG-3 (83 bp), targeting a 1 copy gene (NC_037334.1). To determine the mitochondrial copy number per cell, the Mt-number in the sample was divided by the cell number in the sample, which was determined by real-time PCR targeting 1 copy gene. The PCR program used was as follows: 95°C for 3 min, followed by 40 cycles of 98 °C for 5 sec and 59 °C for 11 sec. The quality of the PCR products was checked using melt analysis, and the size of the PCR products was determined using electrophoresis. At every run of the PCR, a standard curve was generated using PCR products of the corresponding gene cloned into a vector using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen, Waltham, MA USA). The product was sequenced for confirmation prior to use. Using Avogadro’s constant and molar quantity of the standard vector, the copy number of the DNA was calculated. The PCR efficiency was > 1.98.
Measurement of glucose and lactate levels
At the end of the culture period, the spent culture medium was collected, and glucose in the medium was measured using a glucose lab assay kit (LabAssay™ Glucose; Fujifilm Osaka, Japan) according to the manufacturer’s protocol. To calculate glucose consumption of GCs, the glucose concentration in the spent culture medium was subtracted from that of the original medium and divided by the total cell number in the corresponding well, which was determined by real-time PCR targeting 1 copy gene. In addition, lactate in the medium was measured using the Lactate Assay Kit K604-100 (BioVision, Milpitas, CA, USA). The value was also divided by the total cell number in the corresponding well, and glucose consumption and lactate production per 20,000 cells were calculated.
Measurement of reactive oxygen species (ROS) and mitochondrial membrane potential
GCs were cultured in a glass bottom plate (Thermo Fisher, Rochester, NY, USA) and cultured under high oxygen tension for 24 h. GCs were then stained with a combination of MitoSOX Red mitochondrial superoxide indicator (Invitrogen) and MitoTracker Green, or MitoTracker Orange and MitoTracker Green. Fluorescent images of the same area, including approximately 200 cells, were captured under Leica Application Suite Advanced Fluorescence with a Leica DMI 6000B microscope (Leica, Wetzlar, Germany). Fluorescence intensity was analyzed using ImageJ software (NIH, Bethesda, MD, USA). To determine the mitochondrial membrane potential, the fluorescence intensity of MitoTracker Orange was divided by that of MitoTracker Green. To determine the level of mitochondrial ROS, the fluorescence intensity of MitoSOX was divided by that of MitoTracker Green.
Western blotting analysis
In total, 25 × 104 cells were treated with 50 µl of cell lysis buffer (Complete Lysis-M; Roche, Basel, Switzerland) containing protease inhibitors (Complete protease inhibitor cocktail; Roche) and phosphatase inhibitors (PhosSTOP; Roche) and frozen at –80°C until further use. Protein concentration was determined using a PierceTM BCA Protein Assay Kit (Thermo Fisher Rockford, IL, USA), and 5 µg of protein was co-incubated with Laemmli sample buffer containing 2-mercaptoethanol at 95°C for 5 min. Proteins were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Trans-Blot Turbo Mini Transfer Packs; Bio-Rad) using the Trans-Blot Turbo Transfer System (Bio-Rad). The primary antibodies used were rabbit polyclonal anti-TOMM20 (ab56783; Abcam, Cambridge, UK) or anti-TFAM (ARP31400_P050; Aviva Systems Biology, San Diego, CA, USA). Horseradish peroxidase-conjugated donkey anti-rabbit antibodies (Abcam) were used as secondary antibodies. The membranes were digitized using the ImageQuant LAS 4000 Biomolecular imager and ImageQuant software (GE Healthcare, Buckinghamshire, UK). The expression level of each protein was normalized to that of β-actin. Western blotting was repeated four times.
Survival rate and proliferation of cells
GCs were collected using Accummax (Innovative Cell Technologies) and stained with Hoechst 33342 and propidium iodide to determine the number of dead cells (Hoechst and propidium iodide positive cells). Proliferation activity of cells was determined by a cell proliferation ELISA, BrdU assay kit (Roche).
Statistical analysis
Data of more than three groups were analyzed by one-way ANOVA following Fisher’s LSD. In addition, the interaction between oxygen tension and age of GCs was analyzed using two-way ANOVA (IBM SPSS Statistics Ver. 21). In other experiments, statistical significance was determined using the Student’s t-test. A value of P < 0.05 was considered significant.
Results
Mt-numbers increased in GCs under high oxygen tension.
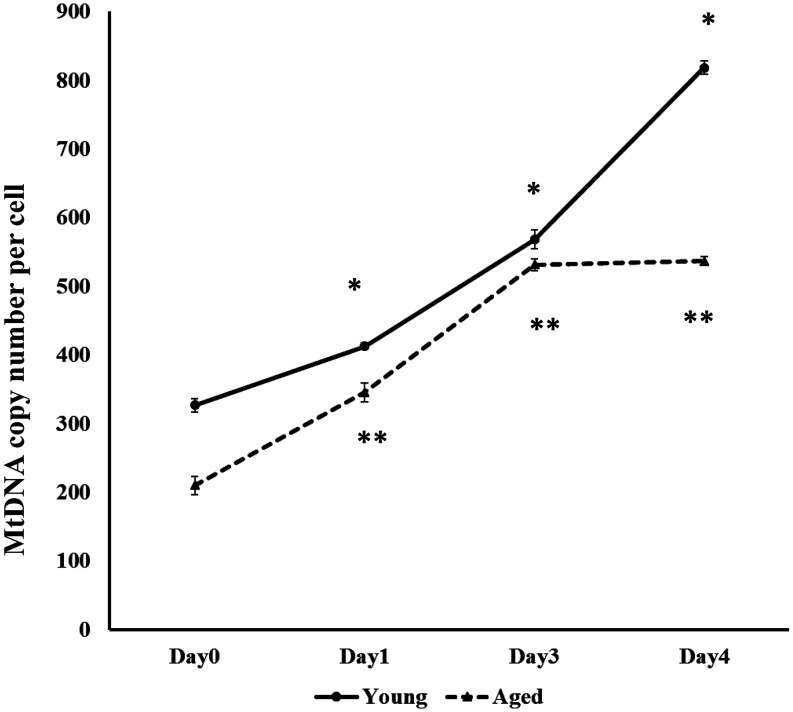
The Mt-number was greater in GCs of young cows than in those of aged cows throughout the culture period (days 0, 1, 3, and 4; Fig. 1), and the Mt-number in GCs of young cows increased from day 0 (GC collection) to day 4, but ceased to increase in GCs of aged cows at day 3 of incubation (Fig. 2). In addition to confirming the difference in Mt-number between young and aged cows, we conducted an additional experiment in which GCs were collected from antral follicles of 10 young and 10 aged cows, and the Mt-number per GC (Day 1) was examined using the same protocol. We found a significantly lower Mt-number in GCs of aged cows (449.0 ± 6.2, P < 0.05) than in those of young cows (507.7 ± 14.0).
Fig. 2.
Mitochondrial DNA copy number (Mt-number) per granulosa cell (GC) of young (solid line) and aged (dotted line) cows. GCs were collected from the antral follicles (day 0), and the surviving GCs were selected at day 1 and cultured under high oxygen tension for 2 days. GCs immediately after collection (day 0), surviving GCs separated on day 1, and GCs cultured for 2 days after re-seeding (days 3 and 4) were subjected to mitochondrial DNA copy measurement. * indicates significant (P < 0.05) differences between young and aged cows.
Comparison of mitochondria in GCs of young and aged cows
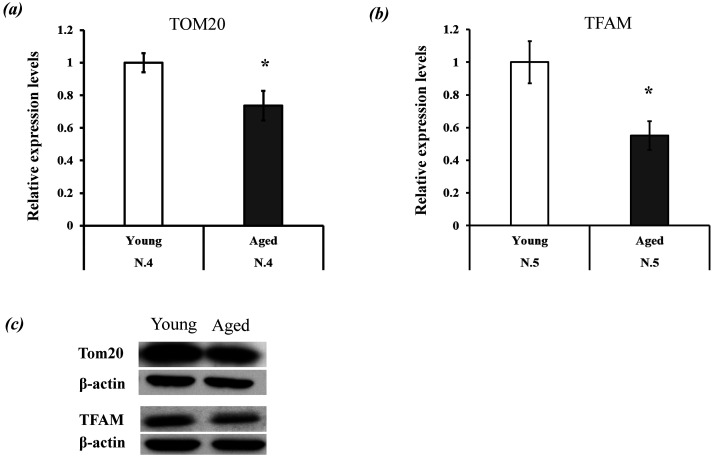
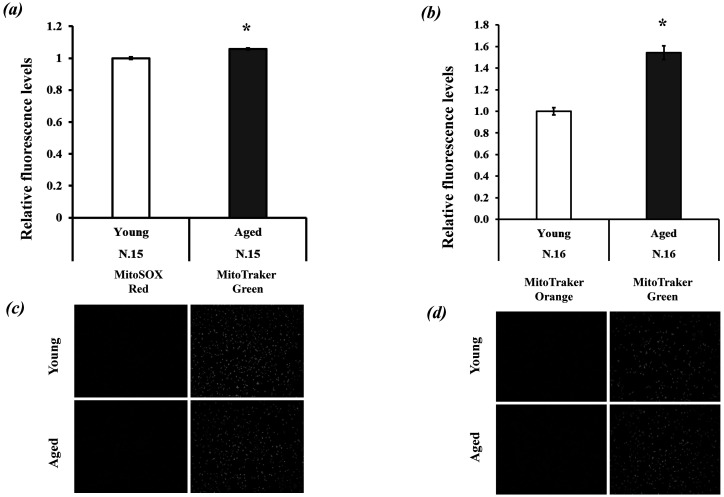
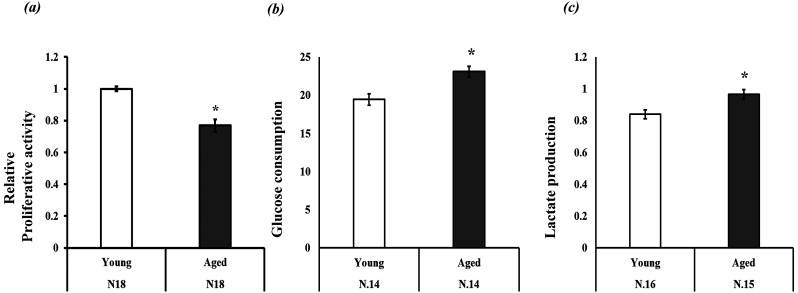
The first experiment demonstrated a significant difference in Mt-number (Mitochondrial DNA copy number) between the GCs of the two aged groups. Here, we examined the differences in mitochondrial function and quantity (mitochondrial protein expression levels) in GCs between young and aged cows. GCs were cultured under high oxygen tension for 24 h (from day 2 to day 3; Fig. 1), and then mitochondrial quantity and biogenesis were examined by evaluating TOMM20 (mitochondrial membrane protein) and TFAM protein expression levels. TFAM is a core mitochondrial transcription factor [21] used to examine mitochondrial number in cumulus and granulosa cells [22, 23]. We found significantly higher TOMM20 and TFAM protein expression levels in GCs of young cows than in GCs of aged cows (Fig. 3). Mitochondrial conditions were further examined by measuring mitochondrial ROS and mitochondrial membrane potential, which were both higher for aged GCs than for young GCs. (Fig. 4a and 4b). The proliferative activity of GCs was lower in aged cows than in young cows (Fig. 5a). Glucose and lactate content in the spent medium are markers of glycolytic activity. Aged GCs had a higher rate of consumption of glucose and concentration of lactate in the medium than young GCs (Fig. 5b and 5c).
Fig. 3.
Comparison of mitochondrial quantity and quality between the two age groups. (a–b) Expression levels of TOMM20 and TFAM. These expression levels were normalized to actin expression, and the expression levels of young granulosa cells (GCs) were defined as 1.0. (c) Band pattern of TOMM20, TFAM, and actin. * P < 0.05.
Fig. 4.
Relative fluorescence levels of (a) reactive oxygen species (ROS) and (b) mitochondrial membrane potential (MMP) and representative pictures of granulosa cells (GCs) stained with (c) MitoSOX and MitoTracker Green, and (d) MitoTracker Orange and MitoTracker Green. The values of young GCs were defined as 1.0. * P < 0.05.
Fig. 5.
Relative proliferative activity, glucose consumption, and lactate production of granulosa cells (GCs). (a) Proliferative activity of young GCs was defined as 1.0, (b) glucose consumption (μM/20,000 cells) and lactate production (μM/20,000 cells). * P < 0.05.
Changes in mitochondrial function and number in response to low oxygen tension
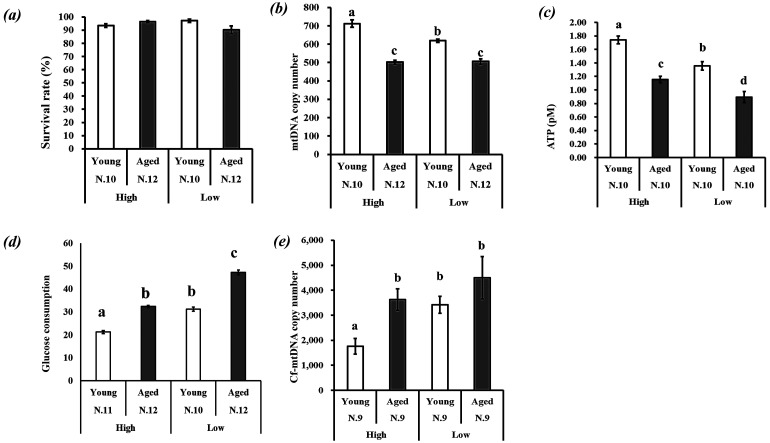
Two days after collection, GCs of young and aged cows were cultured for 24 h under two different oxygen tension conditions: high oxygen (20%) or low oxygen (5%) (Fig. 1). The survival rate of GCs under the two oxygen tension conditions did not differ among the four groups (Fig. 6a). In response to lower oxygen tension, Mt-number decreased in GCs of young cows, whereas it did not change in GCs of aged cows (Fig. 6b). There was a significant interaction (as determined by two-way ANOVA) between the effects of age and of oxygen tension on Mt-number of GCs (P < 0.05). ATP content in GCs was lower for aged cows, and low oxygen tension decreased the ATP content in GCs of both age groups (Fig. 6c). Glucose consumption was higher for GCs of aged cows under both oxygen tension conditions; in response to low oxygen tension, glucose consumption increased for GCs of both age groups (Fig. 6d). Cell-free mitochondrial DNA content in the medium reflects the dynamics of Mt-number in cells [24]. Higher cf-mtDNA content was found in the spent culture medium of GCs derived from aged cows compared with that of their younger counterparts. Low oxygen tension increased the amount of cf-mtDNA in the spent culture medium of GCs derived from young cows but not in the medium of those of aged cows (Fig. 6e).
Fig. 6.
Response of granulosa cells (GCs) to high and low oxygen tensions. On day 2, GCs of young and aged cows with 70–80% confluence were cultured under conditions of 5% (low) or 20% (high) O2 for 24 h, and then GCs or spent culture medium were used for the experiments. (a) Survival rate of GCs, (b) Mt-number in GCs, (c) ATP (pM) content in 20,000 GCs, (d) Glucose consumption (μM/20,000 GCs), and (e) mitochondrial DNA content in spent culture medium. The cell-free Mt-number (Cf-mtDNA copy number) was normalized to the GC number in a well: 20,000 GCs were cultured in 100 μl of medium. (a–d) Different superscripts indicate significant differences (P < 0.05).
Discussion
This study showed age-associated reduction in the mitochondrial function and quantity of GCs and their differential response to high and low oxygen tension.
Age-associated changes in the properties of bovine GCs have been reported previously. Goto et al. reported that GCs of aged cows had lower proliferation activity and shorter telomere lengths than GCs of young cows [12]. This study also confirmed the low proliferation of GCs derived from aged cows. As for the mitochondrial number in GCs, a clinical study showed that, for GCs aspirated from large antral follicles of women, the Mt-number positively correlated with maternal age [14]. In contrast, it has been reported that cumulus cells of women with diminished ovarian reserve had fewer Mt-numbers than those in women with normal ovarian reserve [25]. In addition, the Mt-number in cumulus cells is a marker of the developmental ability of oocytes [26]. In this study, we found that the Mt-number and mitochondrial protein levels in GCs derived from mid-sized antral follicles (3–5 mm in diameter) were lower in aged cows throughout the culture period. Therefore, we concluded that Mt-number in GCs decreased with age; the difference in the literature may be due to experimental conditions, including the size of the antral follicles aspirated and hormonal treatments.
When GCs were cultured under high oxygen tension, the Mt-number increased in the GCs of both age groups until day 3. Inner follicular oxygen conditions are believed to be hypoxic, and exposure to high oxygen tension induces oxidative stress. Mild oxidative stress induces increased mitochondrial biosynthesis through AMPK and peroxisome proliferator-activated receptor gamma co-activator 1a/b (PGC-1α) [27]; thus, once GCs were collected and cultured in vitro, high oxygen tension induced mitochondrial proliferation in GCs. At day 3 of incubation, we observed that the proliferation of mitochondria ceased in the GCs of aged cows, whereas it continued to increase in the GCs of young cows. Low mitochondrial biogenesis in the GCs of aged cows was confirmed by low protein levels of TOMM20, a mitochondrial membrane protein, and TFAM, a key regulator of mitochondrial synthesis. Regarding age-associated low mitochondrial generation, it has been reported that telomere shortening represses the expression of PGC-1a/b, the master regulators of mitochondrial biogenesis and function [28], and age-related shortening of telomere length in GCs of early antral and antral follicles of bovine cows has also been reported [29]. In addition, it has been reported that, under high oxidative stress, a decrease in mitochondrial generation could be a countermeasure to decrease the number of mitochondria rendered dysfunctional by ROS [30]. Consistent with this notion, we observed high ROS content in GCs of aged cows; therefore, age-associated low mitochondrial proliferation might be due to excessive ROS or shorter telomere length.
Mitochondrial membrane potential is a marker of mitochondrial function. The inner mitochondrial membrane has electron transport chain-protein complexes that create a high electric potential between the inner and outer membrane, and the potential provides energy for ATP production. We found that mitochondria in GCs of aged cows had higher mitochondrial membrane potential, whereas ATP generation was low. This result indicates that the mechanism of transmitting the membrane potential for ATP production may be dysfunctional in the mitochondria of aged GCs. Glucose consumption and lactate production are markers of glycolysis, and they are higher in GCs of aged cows than in those of young cows. These results suggest that, under high oxygen tension, GCs of aged cows depend more on glycolysis, likely due to the poor quality and quantity of mitochondria.
A previous report has shown that, on culturing bovine GCs under low oxygen tension, glycolytic activity and proliferation of GCs are activated, whereas mitochondrial quantity is decreased [8]. In addition, hypoxic conditions have been reported to reduce oxidative respiration and upregulate mitophagy [31]. In the present study, in response to low oxygen tension, ATP content and Mt-number decreased, whereas glucose consumption increased in GCs of young cows. Taken together, the results show that low oxygen tension functions as a metabolic switch that causes GCs to shift their metabolism from oxidative phosphorylation to glycolysis; however, this is not the case in the GCs of aged cows. In response to low oxygen tension, mitochondrial DNA content did not change in the GCs of aged cows. The causal factor of the low responsivity of Mt-number in the GCs of aged cows is unclear, but it is speculated that GCs of aged cows rely less on mitochondrial respiration or have lower mitochondrial clearance abilities than those of young GCs. In line with this hypothesis, age-related decline in autophagy in geriatric satellite cells has been reported [32].
In a previous study, cf-mtDNA in the medium was used to examine the dynamics of Mt-number in cells, and when GCs were treated with the mitochondrial uncoupler, the cf-mtDNA content in the medium significantly increased without any cellular death [24]. In addition, it has been reported that, when GCs are treated with an inhibitor of autophagy (bafilomycin), the amount of cf-mtDNA in the medium increases considerably. The authors suggested that autophagy plays a role in cytoplasmic mitochondrial DNA degradation; excessive cytoplasmic mitochondrial DNA cannot be managed by the intrinsic DNA degradation system, and it overflows into the culture medium [33]. In our study, the cf-mtDNA content in the medium of young GCs significantly increased in response to reducing atmospheric oxygen tension, whereas the Mt-number in the GCs decreased. These results suggest that high mitochondrial DNA content in the medium reflects a reduction in Mt-number in GCs. Interestingly, although the survival rate of the GCs did not differ for any combination of oxygen tension and GC origin (Fig. 6a), the cf-mtDNA content in the medium was higher for GCs of aged cows than for GCs of young cows under high oxygen tension, and low oxygen tension significantly increased the cf-mtDNA in the medium of GCs from young cows only. Based on these results, we hypothesize the following: 1) poor mitochondrial quality in GCs of aged cows increased the number of mitochondria to be degenerated, 2) due to low ability to respond to low oxygen tension, mitochondrial degeneration was not activated in GCs of aged cows, and 3) low cytoplasmic DNA processing capacity (autophagy and DNAse) in GCs of aged cows allowed for the secretion of cf-mtDNA into the medium.
In conclusion, we found that aging affects the quality and quantity of mitochondria in bovine GCs, which influences the metabolism and functions of GCs. It has been reported that oxygen and energy demand gradually increase with an increase in the growth of oocytes [18, 34], and oxygen consumption of the oocyte cumulus cell complexes is upregulated to a great extent during meiotic maturation of the oocytes [35]; thus, the metabolism of surrounding cells is adapted to control the flow of metabolism towards the oocytes [5]. Therefore, the age-associated changes in GC function and metabolism might affect this important energy homeostasis of oocytes. However, the question remains as to how age-associated mitochondrial deterioration in GC quality affects oocyte growth, which is a target for further research.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
References
- 1.Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol 2017; 79: 237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munakata Y, Ueda M, Kawahara-Miki R, Kansaku K, Itami N, Shirasuna K, Kuwayama T, Iwata H. Follicular factors determining granulosa cell number and developmental competence of porcine oocytes. J Assist Reprod Genet 2018; 35: 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munakata Y, Ichinose T, Ogawa K, Itami N, Tasaki H, Shirasuna K, Kuwayama T, Iwata H. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology 2016; 86: 1789–1798.e1. [DOI] [PubMed] [Google Scholar]
- 4.Munakata Y, Kawahara-Miki R, Shiratsuki S, Tasaki H, Itami N, Shirasuna K, Kuwayama T, Iwata H. Gene expression patterns in granulosa cells and oocytes at various stages of follicle development as well as in in vitro grown oocyte-and-granulosa cell complexes. J Reprod Dev 2016; 62: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetica P, Pintos L, Dalvit G, Beconi M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 2002; 124: 675–681. [PubMed] [Google Scholar]
- 6.Xie HL, Zhu S, Zhang J, Wen J, Yuan HJ, Pan LZ, Luo MJ, Tan JH. Glucose metabolism during in vitro maturation of mouse oocytes: An study using RNA interference. J Cell Physiol 2018; 233: 6952–6964. [DOI] [PubMed] [Google Scholar]
- 7.Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH. Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab 2019; 30: 720–734.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiratsuki S, Hara T, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol 2016; 437: 75–85. [DOI] [PubMed] [Google Scholar]
- 9.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, Kokocinski F, Cohen J, Munne S, Wells D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet 2015; 11: e1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Keilty D, Zhang ZF, Chian RC. Mitochondria in oocyte aging: current understanding. Facts Views Vis ObGyn 2017; 9: 29–38. [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata H. Age-associated changes in granulosa cells and follicular fluid in cows. J Reprod Dev 2017; 63: 339–345. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto H, Iwata H, Takeo S, Nisinosono K, Murakami S, Monji Y, Kuwayama T. Effect of bovine age on the proliferative activity, global DNA methylation, relative telomere length and telomerase activity of granulosa cells. Zygote 2013; 21: 256–264. [DOI] [PubMed] [Google Scholar]
- 13.Iwata H. Age-associated events in bovine oocytes and possible countermeasures. Reprod Med Biol 2016; 15: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, Guo Y, Wang S, Wang Y, Duan N, Xu B, Yin J, Yao Y. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod 2017; 32: 2465–2473. [DOI] [PubMed] [Google Scholar]
- 15.Halbrook CJ, Nwosu ZC, Lyssiotis CA. Fine-tuning mitochondrial dysfunction and reductive carboxylation. Trends Endocrinol Metab 2018; 29: 599–602. [DOI] [PubMed] [Google Scholar]
- 16.Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev 2011; 23: 424–432. [DOI] [PubMed] [Google Scholar]
- 17.Kansaku K, Takeo S, Itami N, Kin A, Shirasuna K, Kuwayama T, Iwata H. Maternal aging affects oocyte resilience to carbonyl cyanide-m-chlorophenylhydrazone-induced mitochondrial dysfunction in cows. PLoS One 2017; 12: e0188099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heise MK, Koepsel R, McGee EA, Russell AJ. Dynamic oxygen enhances oocyte maturation in long-term follicle culture. Tissue Eng Part C Methods 2009; 15: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talevi R, Sudhakaran S, Barbato V, Merolla A, Braun S, Di Nardo M, Costanzo V, Ferraro R, Iannantuoni N, Catapano G, Gualtieri R. Is oxygen availability a limiting factor for in vitro folliculogenesis? PLoS One 2018; 13: e0192501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belli M, Zhang L, Liu X, Donjacour A, Ruggeri E, Palmerini MG, Nottola SA, Macchiarelli G, Rinaudo P. Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum Reprod 2019; 34: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morozov YI, Parshin AV, Agaronyan K, Cheung ACM, Anikin M, Cramer P, Temiakov D. A model for transcription initiation in human mitochondria. Nucleic Acids Res 2015; 43: 3726–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan Y, Zhang S, Gong F, Lu C, Lin G, Hu L. The mitochondrial DNA copy number of cumulus granulosa cells may be related to the maturity of oocyte cytoplasm. Hum Reprod 2020; 35: 1120–1129. [DOI] [PubMed] [Google Scholar]
- 23.Hoque SAM, Kawai T, Zhu Z, Shimada M. Mitochondrial protein turnover is critical for granulosa cell proliferation and differentiation in antral follicles. J Endocr Soc 2018; 3: 324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kansaku K, Munakata Y, Itami N, Shirasuna K, Kuwayama T, Iwata H. Mitochondrial dysfunction in cumulus-oocyte complexes increases cell-free mitochondrial DNA. J Reprod Dev 2018; 64: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucret L, Chao de la Barca JM, Morinière C, Desquiret V, Ferré-L’Hôtellier V, Descamps P, Marcaillou C, Reynier P, Procaccio V, May-Panloup P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum Reprod 2015; 30: 1653–1664. [DOI] [PubMed] [Google Scholar]
- 26.Desquiret-Dumas V, Clément A, Seegers V, Boucret L, Ferré-L’Hotellier V, Bouet PE, Descamps P, Procaccio V, Reynier P, May-Panloup P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod 2017; 32: 607–614. [DOI] [PubMed] [Google Scholar]
- 27.Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 2009; 296: C116–C123. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Liu X, Ding X, Wang F, Geng X. Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2019; 20: 1–16. [DOI] [PubMed] [Google Scholar]
- 29.Kin A, Kansaku K, Sumiya M, Itami N, Sirasuna K, Kuwayama T, Iwata H. Effect of aging on telomere lengths in bovine oocytes and granulosa cells. J Mamm Ova Res 2017; 34: 37–43. [Google Scholar]
- 30.Yoboue ED, Devin A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol 2012; 2012: 403870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 2010; 1797: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 32.García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P. Autophagy maintains stemness by preventing senescence. Nature 2016; 529: 37–42. [DOI] [PubMed] [Google Scholar]
- 33.Kansaku K, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Mitochondrial cell-free DNA secreted from porcine granulosa cells. Zygote 2019; 27: 272–278. [DOI] [PubMed] [Google Scholar]
- 34.Wycherley G, Kane MT, Hynes AC. Oxidative phosphorylation and the tricarboxylic acid cycle are essential for normal development of mouse ovarian follicles. Hum Reprod 2005; 20: 2757–2763. [DOI] [PubMed] [Google Scholar]
- 35.Sutton ML, Cetica PD, Beconi MT, Kind KL, Gilchrist RB, Thompson JG. Influence of oocyte-secreted factors and culture duration on the metabolic activity of bovine cumulus cell complexes. Reproduction 2003; 126: 27–34. [DOI] [PubMed] [Google Scholar]