Abstract
Background
Several studies have shown the health effects of air pollutants, especially in China, North American and Western European countries. But longitudinal cohort studies focused on health effects of long-term air pollution exposure are still limited in Southeast Asian countries where sources of air pollution, weather conditions, and demographic characteristics are different. The present study examined the association between long-term exposure to air pollution and self-reported morbidities in participants of the Thai cohort study (TCS) in Bangkok metropolitan region (BMR), Thailand.
Methods
This longitudinal cohort study was conducted for 9 years from 2005 to 2013. Self-reported morbidities in this study included high blood pressure, high blood cholesterol, and diabetes. Air pollution data were obtained from the Thai government Pollution Control Department (PCD). Particles with diameters ≤10 μm (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and carbon monoxide (CO) exposures were estimated with ordinary kriging method using 22 background and 7 traffic monitoring stations in BMR during 2005–2013. Long-term exposure periods to air pollution for each subject was averaged as the same period of person-time. Cox proportional hazards models were used to examine the association between long-term air pollution exposure with self-reported high blood pressure, high blood cholesterol, diabetes. Results of self-reported morbidity were presented as hazard ratios (HRs) per interquartile range (IQR) increase in PM10, O3, NO2, SO2, and CO.
Results
After controlling for potential confounders, we found that an IQR increase in PM10 was significantly associated with self-reported high blood pressure (HR = 1.13, 95% CI: 1.04, 1.23) and high blood cholesterol (HR = 1.07, 95%CI: 1.02, 1.12), but not with diabetes (HR = 1.05, 95%CI: 0.91, 1.21). SO2 was also positively associated with self-reported high blood pressure (HR = 1.22, 95%CI: 1.08, 1.38), high blood cholesterol (HR = 1.20, 95%CI: 1.11, 1.30), and diabetes (HR = 1.21, 95%CI: 0.92, 1.60). Moreover, we observed a positive association between CO and self-reported high blood pressure (HR = 1.07, 95%CI: 1.00, 1.15), but not for other diseases. However, self-reported morbidities were not associated with O3 and NO2.
Conclusions
Long-term exposure to air pollution, especially for PM10 and SO2 was associated with self-reported high blood pressure, high blood cholesterol, and diabetes in subjects of TCS. Our study supports that exposure to air pollution increases cardiovascular disease risk factors for younger population.
Keywords: Long-term air pollution exposure, High blood pressure, High blood cholesterol, Diabetes, Cardiovascular disease risk factors
Highlights
-
•
Evidence on health effects of long-term air pollution exposure are still limited in Southeast Asia.
-
•
SO2 was associated with an increased risks of self-reported high blood pressure, high blood cholesterol, and diabetes.
-
•
Increase of PM10 was also associated with the incidences of high blood pressure and high blood cholesterol.
-
•
The effects were observed even at the level below the National Standard Limit.
-
•
This study suggests air pollution increases cardiovascular disease risk factors for younger population.
1. Introduction
Air pollution is one of the most serious environmental problems worldwide, especially in developing countries. Exposure to air pollution has detrimental effects on health and is one of the hardest environmental risks to avoid. Many epidemiological studies have found the effects of air pollution on mortality and morbidity for cardiovascular (Brook et al., 2010; Gold and Mittleman, 2013; Pope et al., 2004) and respiratory diseases (Analitis et al., 2006; Dockery and Pope, 1994; Hoek et al., 2013; Middleton et al., 2008). Additionally, long-term exposure to air pollution has been linked with hypertension (Bai et al., 2018; Coogan et al., 2012, 2017; Zhang et al., 2018), diabetes (Andersen et al., 2012; Brook et al., 2013; Eze et al., 2014; Hansen et al., 2016; Liang et al., 2019; Park et al., 2015; Qiu et al., 2018), dyslipidaemias (Bo et al., 2019a; Mao et al., 2020; Yang et al., 2018), liver cancer (Pan et al., 2016; Pedersen et al., 2017), and kidney disease (Bowe et al., 2017a, 2017b).
As with other developing countries, Thailand experiences air pollution, mostly from vehicular emissions in cities, biomass burning and transboundary haze in rural and border areas, and industrial discharges in concentrated industrialized zones (Vichit-Vadakan and Vajanapoom, 2011). These processes emit air pollution in the atmosphere and seriously affect human and environmental health. According to the Pollution Control Department (2019), the annual PM10 (a particulate matter less than 10 μm in aerodynamic diameter), PM2.5 (a particulate matter less than 2.5 μm in aerodynamic diameter), and ozone (O3) had still exceeded their national standards in many areas of Thailand, whereas other gaseous pollutants such as nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO) were well below that standard. However, the concentrations of NO2, SO2, and CO in some areas of Thailand, especially for Bangkok were periodically above the annual or 24-h mean values as determined by WHO air quality guidelines (WHO Regional Office for Europe, 2006).
Bangkok is the capital and most populous city of Thailand. Over 10 million people (15% of the Thai population) live in Bangkok. The climate in Bangkok is hot (normally above 30 °C) and humid (monthly average range of humidity is between 74% and 85%) throughout the year (Bangkok Climate, 2016). Bangkok has experienced serious urban air pollution problems because of rapid economic development and urbanization. Chuersuwan et al. (2008) has demonstrated that the major sources of PM10 at roadside location in Bangkok are vehicle emissions and biomass burning, which contributes roughly 33% each. Other gaseous pollutants, including NO2, SO2, and CO are also generated from vehicle exhaust. O3 in Bangkok could be formed due to vehicle emissions, where ozone precursors have been emitted (i.e. nitrogen oxide (NOx), CO, and volatile organic compounds (VOCs)), and sunlight is present. The favorable meteorological conditions, such as high temperature and strong solar radiation, could also enhance the O3 formation in Bangkok. Air pollution's contribution to mortality was greater in Bangkok, than in Hong Kong, Shanghai and Wuhan in China (Taneepanichskul et al., 2018; Wong et al., 2008). Short-term studies in Thailand also found associations between air pollution and respiratory and cardiovascular mortality (Ostro et al., 1999; Taneepanichskul et al., 2018) and morbidity (Buadong et al., 2009; Phosri et al., 2019; Trang and Tripathi, 2014).
Although health effects of air pollutants are well documented in many countries especially in China, North American and Western Europe, long-term studies are still limited in Southeast Asian countries, including Thailand where sources of air pollution, weather conditions, demographic characteristics (Phosri et al., 2019) and lifestyle (Strak et al., 2017) in each country are different. Moreover, no longitudinal cohort study has examined the association between long-term exposure to air pollution and health effects in Thailand. This study examined the association between long-term exposure to air pollution and self-reported morbidities in Bangkok metropolitan region (BMR), Thailand from 2005 to 2013.
2. Methods
2.1. Study design and participants
The population and recruitment of the Thai Cohort Study (TCS) was previously described in detail (Seubsman et al., 2011, 2012; Sleigh et al., 2008). Briefly, the Thai Health-Risk Transition Project began in 2004 with the aim of studying changes in the health status of the Thai population associated with rapid modernization and industrialization. Part of this study project has involved assembling a cohort of adult community dwelling Thais whose health status could be followed through time along with their risk behavior and socio-demographic and economic profiles. The target population was persons studying by correspondence via Sukhothai Thammathirat Open University (STOU). This group was chosen because STOU students lived throughout the country and display considerable variation in lifestyle, family structure, socio-economic status, domestic and occupational environment and personal behavior.
The cohort population is similar to the general Thai population in terms of median age, geographic residence and median income (Sleigh et al., 2008). They are however, overall, more highly educated than the Thai population. This means this study population is able to represent potential future health transitions in Thailand, as education levels in the general population increase. In 2005 a health questionnaire was mailed out to all students currently enrolled at STOU; 87,151 participants responded and formed the baseline cohort population. Two follow-ups were conducted in 2008/2009 (n = 60,569) and 2012/2013 (n = 42,785). Our study period was from 2005, the start of the cohort, until 2013 when the last survey was completed.
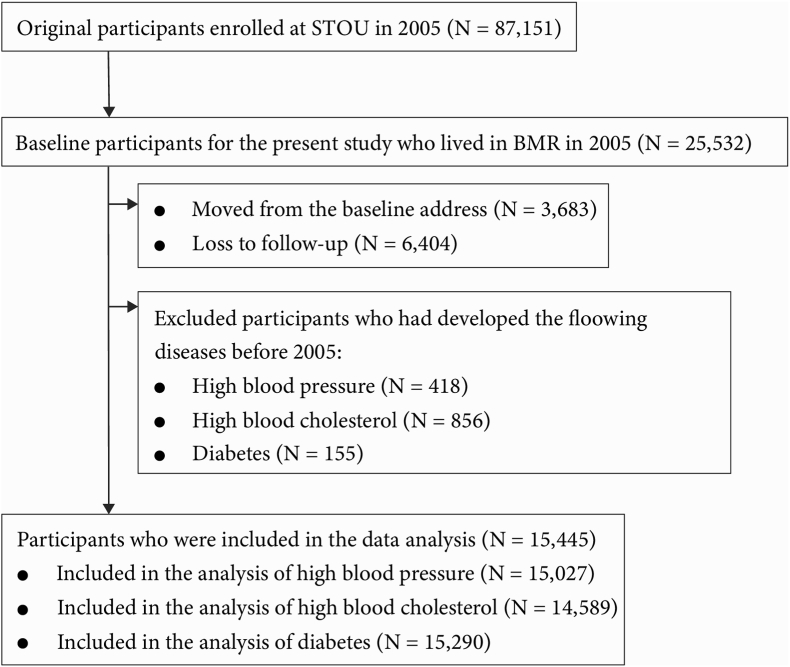
As shown in Fig. 1, we extracted the data of 25,532 subjects in TCS who lived in BMR, including Bangkok, Nonthaburi, Samutprakarn, Samut sakhon, Nakhon pathom, and Pathum thani in 2005. We excluded 3,683 subjects who moved from their baseline address and 6,404 subjects who were lost to follow-up. In addition, those who had developed diabetes (n = 155), high blood pressure (n = 418), and high cholesterol (n = 856) before 2005 were also excluded.
Fig. 1.
Flowchart of participant selection.
2.2. Data collection
Self-reported morbidities, including diabetes (Papier et al., 2017), high blood pressure (Rimpeekool et al., 2017; Thawornchaisit et al., 2014), high cholesterol (Lim et al., 2012; Rimpeekool et al., 2017), and information on other self-reported comorbidities, sex, age, and various subjects associated with health, including demography, social networking, work, health services, injury, environment, food and physical activity, smoking, alcohol and transport collected at 3 time points in 2005, 2009, and 2013 were obtained from TCS participants in the Thai Health Research Project (Seubsman et al., 2011, 2012; Sleigh et al., 2008).
2.3. Exposure assessment
Hourly concentrations of PM10 and other gaseous pollutants, including SO2, NO2, CO, and O3 were obtained from the Pollution Control Department (PCD) in Thailand. The chemiluminescence method was applied for measuring O3 and NO2; UV-fluorescence for SO2; Tapered Element Oscillation Microbalance (TEOM) for PM10; and Non-Dispersive Infrared Detection for CO. Same period daily average temperature (in degrees Celsius; °C) and relative humidity (in percent; %) were obtained from the Thai Meteorological Department. Meteorological data was used from only one meteorological station which is located in Bangkok and had complete data of temperature and relative humidity from 2004 until 2013. Additionally, almost all of study subjects lived in Bangkok. Therefore, we selected the main meteorological station in Bangkok to represent environmental conditions for all participants.
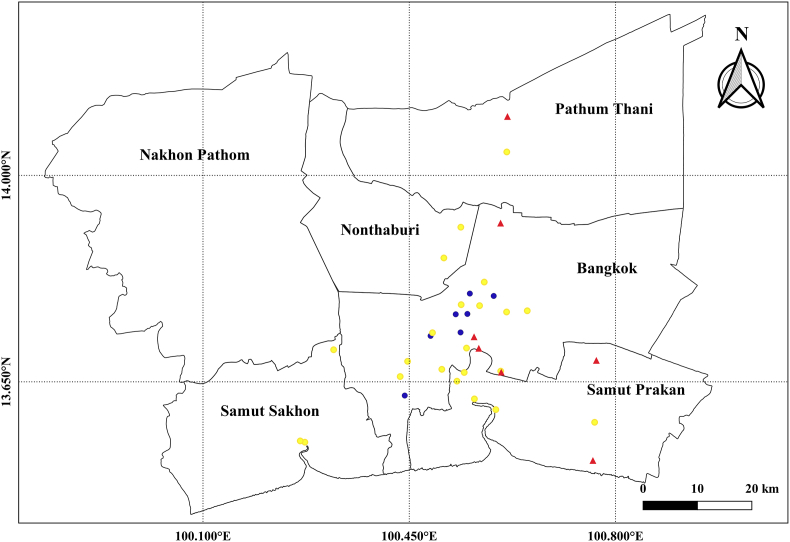
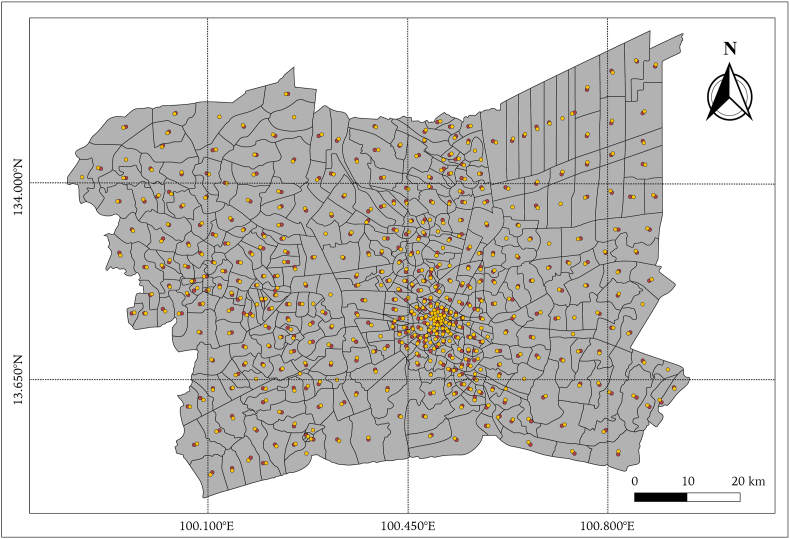
Air pollution exposure, including daily average of PM10, SO2, NO2, CO, and daily maximum 8-hr O3 average (Lim et al., 2018) were estimated with ordinary kriging method (Geniaux et al., 2017; Leem et al., 2006; Liu and Rossini, 1996) which is a linear prediction model to estimate a value at a point of an unobserved location for which a variogram is known, based on the weighted average of surrounding monitoring stations (Wackernagel, 1995). We included hourly mean air pollution concentrations as a dependent variable and geographical data, and latitudinal and longitudinal coordinates, as potential predictors (independent variables). We conducted measurements of concentrations of air pollutants from 22 background and 7 traffic monitoring sites in BMR during 2005–2013 (Fig. 2) in order to generate a long-term annual average from all discontinuous site-specific measurements. For the prediction, we generated the grids of 100x100 m. Then the average concentration for each air pollutant at the district levels were estimated by the concentrations from the grids closest to the centroid of each district (Fig. 3). We developed and validated a separate model for each air pollutant to estimate grid-specific air pollution concentrations. Good model performance from 2005 to 2013 was achieved, with leave-one-out cross validation R2 value of 0.99 for PM10, O3, NO2, SO2, and 0.98 for CO. Model predictions had little bias, with cross-validated slopes (predicted vs observed) of 0.99 for PM10, O3, NO2, SO2, and 0.98 for CO.
Fig. 2.
Map of the study area (BMR). Ambient monitoring stations are denoted by yellow circles; traffic monitoring stations are denoted by blue circles; meteorological monitoring stations are denoted by triangles.
Fig. 3.
The grid of nearest centroid point of each district in the study area (BMR).
Air pollution exposure for each subject was averaged from the start of cohort (year 2005) to the year of disease occurrence; the exposure until the end of cohort (year 2013) was considered if the subject did not develop any disease during the cohort period. Moreover, we averaged the exposure until the first follow-up of cohort (year 2009) if the subject without disease in 2009 was lost to follow-up or changed the address in 2013.
2.4. Covariates
Information on a wide range of potential covariates was collected using a standard self-administered questionnaire. We used data in 2005 on age (years), sex (male/female), BMI (kg/m2), smoking status (never-smokers/former-smokers/current-smokers), smoking intensity (number per day), alcohol drinking (non-users/former-users/occasional-users/current-users), daily alcohol intake (less than 2 glasses/2–3 glasses/4–5 glasses/6 glasses or more), strenuous and moderate exercise (times per week), education level (junior high school or equivalent/completed high school or equivalent/post-high school diploma or certificate/bachelor or higher university degree), average monthly income (≤3,000 Thai baht/3,001–7,000 Thai baht/7,001–10,000 Thai baht/10,001–20,000 Thai baht/20,001–30,000 Thai baht/≥30,000 Thai baht), marital status (single/living with partner or married/divorced or separated/widowed), high fat, sodium and sugar consumption and sugar-sweetened soft drink consumption (never or less than once a month/1–3 times per month/1–2 times per week/3–6 times per week/once a day or more), vegetables and fruit consumption (serves/day), and other self-reported comorbidities (i.e. cancer, chronic bronchitis, asthma, stroke, coronary heart disease).
2.5. Statistical analysis
We used a time-varying Cox proportional hazards model to investigate associations between long-term air pollution exposure (PM10, O3, NO2, SO2 and CO) and the development of diabetes, high blood pressure, and high cholesterol during 2005–2013. The concentrations of air pollutants were included as time-dependent variables in the Cox regression model. The time-scale used in the Cox regression model is time-in-study (i.e. follow-up time). Person-time was calculated from the enrollment of cohort in 2005 until the year of the occurrence of diabetes or high blood pressure or high cholesterol, loss to follow-up, moving from the study area, death, or end of follow-up, whichever occurred first (Coogan et al., 2012; Liang et al., 2019; Lim et al., 2018). Study subjects were censored at the time of disease occurrence, at the end of the study period (year 2013), or when they were lost to follow-up or move outside the study area.
A wide range of covariates were selected based on the previous literature (Bai et al., 2018; Bo et al., 2019a, 2019b; Lao et al., 2019; Liang et al., 2019; Qiu et al., 2018; Renzi et al., 2018; Zhang et al., 2018). Five models were developed by gradually including these covariates: a crude model (Model I), and a model adjusted for age and sex only (Model II). The 3rd model (Model III) was adjusted for age, sex, body mass index (BMI), smoking status, alcohol intake, physical activity, high fat, sodium and sugar consumption, intake of fruit and vegetables, sugar-sweetened soft drink consumption, marital status, education level, and income. The 4th model (Model IV) was adjusted for covariates in the third model plus 2-year average of temperature and relative humidity during the year of the disease occurrence or the last follow-up and the preceding year were conducted for each air pollutant. Moreover, an additional model (Model V) adjusting for other self-reported comorbidities (i.e. cancer, chronic bronchitis, asthma, stroke, coronary heart disease) and city of residence. Self-reported comorbidities were considered until the start of cohort in 2005.
Two-pollutant models were used to examine the robustness of the effect estimate. The results were presented as hazard ratios (HRs) per interquartile range (IQR) increase in PM10, O3, NO2, SO2, and CO, with 95% confidence intervals (CIs). All statistical analyses were conducted using R statistical project (version 3.6.1). P < 0.05 was considered statistically significant.
3. Results
As shown in Table 1, we included 15,027, 14,589, and 15,290 subjects for study of high blood pressure, high blood cholesterol, and diabetes, respectively. Around 60% of TCS's subjects were female and their age range was from 17 to 87 years old in 2005. More than 60% of them were younger than 35 years old. More than a half of study subjects lived in Bangkok. Over 80% of study subjects were low to middle-income earners (<20,000 baht/month). In 2005, around 70% of study subjects had a highest attained education level lower than bachelor degree. During this study period (2005–2013), the proportion of study subjects who developed high blood pressure, high cholesterol, and diabetes were around 7.0%, 17.7%, and 2.0%, respectively.
Table 1.
Basic characteristics of study subjects at the baseline in 2005.
| Variables | Entire Population (N = 25,532) | High blood pressure (N = 15,027) | High cholesterol (N = 14,589) | Diabetes (N = 15,290) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 9946 (39.0) | 5,671 (37.7) | 5,483 (37.6) | 5,855 (38.3) |
| Female | 15586 (61.0) | 9,356 (62.3) | 9,106 (62.4) | 9,435 (61.7) |
| Age, years | ||||
| Mean ± SD | 30.9 ± 8.4 | 32.1 ± 8.3 | 32.0 ± 8.3 | 32.3 ± 8.5 |
| Range | [16.0, 87.0] | [17.0, 87.0] | [17.0, 87.0] | [17.0, 87.0] |
| Age groups | ||||
| < 35 years old | 17950 (70.3) | 9,684 (64.4) | 9,544 (65.4) | 9,743 (63.7) |
| ≥ 35 years old | 7581 (29.7) | 5,343 (35.6) | 5,045 (34.6) | 5,547 (35.3) |
| Body mass index, kg/m2 | ||||
| Mean ± SD | 21.8 ± 3.6 | 21.9 ± 3.5 | 21.9 ± 3.6 | 22.0 ± 3.6 |
| Smoking status, n (%) | ||||
| Never smokers | 18446 (72.2) | 11,069 (73.7) | 10,746 (73.7) | 11,215 (73.3) |
| Former smokers | 4809 (18.8) | 2,764 (18.4) | 2,687 (18.4) | 2,854 (18.7) |
| Current smokers | 2277 (8.9) | 1,196 (8.0) | 1,158 (7.9) | 1,223 (8.0) |
| Alcohol drinking, n (%) | ||||
| Non-users | 14828 (58.1) | 4,366 (29.1) | 4,252 (29.1) | 4,438 (29.0) |
| Former-users | 2247 (8.8) | 1,301 (8.7) | 1,247 (8.5) | 1,333 (8.7) |
| Occasional-users | 7015 (27.5) | 8,539 (56.8) | 8,282 (56.8) | 8,660 (56.6) |
| Current-users | 1086 (4.3) | 621 (4.1) | 604 (4.1) | 656 (4.3) |
| Moderate Exercise, n (%) | ||||
| 0 session/ week | 13089 (51.3) | 7,877 (52.4) | 7,625 (52.3) | 7,995 (52.3) |
| 1-2 sessions/ week | 5850 (22.9) | 3,409 (22.7) | 3,297 (22.6) | 3,469 (22.7) |
| At least three times a week | 5461 (21.4) | 3,128 (20.8) | 3,063 (21.0) | 3,193 (20.9) |
| Education levels, n (%) | ||||
| Junior high school or equivalent | 1226 (4.8) | 705 (4.7) | 698 (4.8) | 729 (4.8) |
| Completed high school or equivalent | 11730 (45.9) | 6,713 (44.7) | 6,613 (45.3) | 6,821 (44.6) |
| Post-high school diploma or certificate | 6230 (24.4) | 3,503 (23.3) | 3,434 (23.5) | 3,545 (23.2) |
| Bachelor or higher university degree | 6263 (24.5) | 4,063 (27.0) | 3,805 (26.1) | 4,150 (27.1) |
| Income (monthly), n (%) | ||||
| ≤ 3,000 Baht | 1496 (5.9) | 792 (5.3) | 789 (5.4) | 800 (5.2) |
| 3,001 – 7,000 Baht | 6617 (25.9) | 3,475 (23.1) | 3,473 (23.8) | 3,512 (23.0) |
| 7,001 – 10,000 Baht | 6457 (25.3) | 3,722 (24.8) | 3,664 (25.1) | 3,749 (24.5) |
| 10,001 – 20,000 Baht | 6543 (25.6) | 4,161 (27.7) | 4,006 (27.5) | 4,232 (27.7) |
| 20,001 – 30,000 Baht | 2046 (8.0) | 1,401 (9.3) | 1,294 (8.9) | 1,443 (9.4) |
| ≥ 30,000 Baht | 1877 (7.4) | 1,225 (8.2) | 1,106 (7.6) | 1,294 (8.5) |
| Incidence of self-reported morbidities (2005-2013), n (%) | ||||
| Hypertension | 1,055 (7.0) | |||
| Hyper cholesterol | 2,589 (17.7) | |||
| Diabetes | 304 (2.0) | |||
| Prevalence of self-reported comorbidities (until 2005), n (%) | ||||
| Cancer | 221 (0.9) | 102 (0.7) | 94 (0.6) | 102 (0.7) |
| Stroke | 57 (0.2) | 32 (0.2) | 30 (0.2) | 39 (0.3) |
| Coronary heart disease | 106 (0.4) | 54 (0.4) | 51 (0.3) | 60 (0.4) |
| Asthma | 1013 (4.0) | 568 (3.8) | 551 (3.8) | 576 (3.8) |
| Chronic bronchitis | 550 (2.2) | 327 (2.2) | 317 (2.2) | 329 (2.2) |
| High blood pressure | 1324 (5.2) | - | 740 (5.1) | 855 (5.6) |
| Diabetes | 325 (1.3) | 180 (1.2) | 170 (1.1) | - |
| High cholesterol | 2922 (11.4) | 1881 (12.5) | - | 1993 (13.0) |
| Marital status | ||||
| Single | 15875 (62.2) | 7,853 (52.3) | 7,686 (52.7) | 7,930 (51.9) |
| Living with partner or married | 8511 (33.3) | 6,500 (43.2) | 6,250 (42.8) | 6,672 (43.6) |
| Divorced or separated | 979 (3.8) | 567 (3.8) | 545 (3.7) | 582 (3.8) |
| Widowed | 167 (0.7) | 117 (0.8) | 114 (0.8) | 119 (0.8) |
| High fat consumption (Deep fried food) | ||||
| Never or less than once a month | 707 (2.8) | 433 (2.9) | 420 (2.9) | 432 (3.0) |
| 1-3 times per month | 3583 (14.0) | 2,108 (14.0) | 2,039 (14.0) | 2,153 (14.8) |
| 1-2 times per week | 7568 (29.6) | 4,481 (29.8) | 4,366 (29.9) | 4,586 (31.4) |
| 3-6 times per week | 9278 (36.3) | 5,409 (36.0) | 5,227 (35.8) | 5,507 (37.7) |
| Once a day or more | 4158 (16.3) | 2,458 (16.4) | 2,401 (16.5) | 2,486 (17.0) |
| High sodium consumption (Canned food) | ||||
| Never or less than once a month | 9063 (35.5) | 5,510 (36.7) | 5,336 (36.6) | 5,624 (36.8) |
| 1-3 times per month | 10910 (42.7) | 6,446 (42.9) | 6,257 (42.9) | 6,566 (42.9) |
| 1-2 times per week | 3894 (15.3) | 2,183 (14.5) | 2,130 (14.6) | 2,204 (14.4) |
| 3-6 times per week | 1184 (4.6) | 644 (4.3) | 627 (4.3) | 648 (4.2) |
| Once a day or more | 237 (0.9) | 105 (0.7) | 103 (0.7) | 104 (0.7) |
| High sugar consumption (Food/dessert with coconut milk) | ||||
| Never or less than once a month | 3805 (14.9) | 2,224 (14.8) | 2,164 (14.8) | 2,271 (14.9) |
| 1-3 times per month | 8495 (33.3) | 5,045 (33.6) | 4,917 (33.7) | 5,143 (33.6) |
| 1-2 times per week | 7504 (29.4) | 4,449 (29.6) | 4,305 (29.5) | 4,518 (29.5) |
| 3-6 times per week | 4221 (16.5) | 2,458 (16.4) | 2,363 (16.2) | 2,492 (16.3) |
| Once a day or more | 1312 (5.1) | 742 (4.9) | 729 (5.0) | 754 (4.9) |
| Sugar-sweetened soft drink consumption | ||||
| Never or less than once a month | 5979 (23.4) | 3,761 (25.0) | 3,632 (24.9) | 3,810 (24.9) |
| 1-3 times per month | 6332 (24.8) | 3,816 (25.4) | 3,693 (25.3) | 3,916 (25.6) |
| 1-2 times per week | 5598 (21.9) | 3,314 (22.1) | 3,205 (22.0) | 3,356 (21.9) |
| 3-6 times per week | 4718 (18.5) | 2,587 (17.2) | 2,534 (17.4) | 2,628 (17.2) |
| Once a day or more | 2668 (10.4) | 1,418 (9.4) | 1,393 (9.5) | 1,443 (9.4) |
| Vegetables consumption (serves/day) | ||||
| 0 serve/day | 409 (1.6) | 238 (1.6) | 234 (1.6) | 241 (1.6) |
| 1 serve/day | 12574 (49.2) | 7,475 (49.7) | 7,214 (49.4) | 7,608 (49.8) |
| 2 serves/day | 6913 (27.1) | 4,142 (27.6) | 4,041 (27.7) | 4,223 (27.6) |
| 3 serves/day | 2738 (10.7) | 1,587 (10.6) | 1,543 (10.6) | 1,609 (10.5) |
| More than 3 serves/day | 2200 (8.6) | 1,223 (8.1) | 1,198 (8.2) | 1,242 (8.1) |
| Fruit consumption (serves/day) | ||||
| 0 serve/day | 758 (3.0) | 437 (2.9) | 433 (3.0) | 449 (2.9) |
| 1 serve/day | 8637 (33.8) | 5,143 (34.2) | 4,954 (34.0) | 5,243 (34.3) |
| 2 serves/day | 5946 (23.3) | 3,548 (23.6) | 3,445 (23.6) | 3,615 (23.6) |
| 3 serves/day | 3770 (14.8) | 2,265 (15.1) | 2,216 (15.2) | 2,307 (15.1) |
| More than 3 serves/day | 5725 (22.4) | 3,270 (21.8) | 3,179 (21.8) | 3,305 (21.6) |
| City of residence, n (%) | ||||
| Bangkok | 14854 (58.2) | 8,719 (58.0) | 8,452 (57.9) | 8,912 (58.3) |
| Nonthaburi | 3012 (11.8) | 1,751 (11.7) | 1,668 (11.4) | 1,773 (11.6) |
| Samut Prakarn | 2816 (11.0) | 1,688 (11.2) | 1,649 (11.3) | 1,717 (11.2) |
| Phathum Thani | 2246 (8.8) | 1,246 (8.3) | 1,209 (8.3) | 1,252 (8.2) |
| Nakhon pathom | 1486 (5.8) | 937 (6.2) | 922 (6.3) | 942 (6.2) |
| Samutsakorn | 1118 (4.4) | 686 (4.6) | 689 (4.7) | 694 (4.5) |
Annual summary values of the environmental variables were presented with their mean, standard deviation (SD), range, and IQR in Table 2. Average concentration of PM10 during study period nearly exceeded annual PM10 standard limit in Thailand (PM10 < 50 μg/m3). Other gaseous pollutants in this study were well below their national standards, but there is still no annual standard limit of O3 and CO in Thailand. Annual average temperatures ranged from 28.4 °C to 29.3 °C, while annual mean relative humidity ranged from 69.5% to 75.7% (Table 2). As shown in Table 3, air pollutants were positively correlated with each other (P < 0.05), while they were negatively correlated with temperature and relative humidity (P < 0.05).
Table 2.
Annual average concentration of environmental variables during the study period.
| Environmental Variable | Mean ± SD | Range | IQR |
|---|---|---|---|
| PM10 (μg/m3) | 44.4 ± 14.4 | 20.5–125.3 | 15.3 |
| O3 (ppb) | 30.2 ± 5.1 | 14.1–41.2 | 7.0 |
| NO2 (ppb) | 19.4 ± 4.1 | 12.3–39.2 | 4.4 |
| SO2 (ppb) | 5.7 ± 3.2 | 1.2–15.8 | 4.2 |
| CO (ppm) | 0.72 ± 0.2 | 0.24–1.7 | 0.2 |
| Temperature () | 29.0 ± 0.3 | 28.4–29.3 | 0.4 |
| Relative humidity (%) | 72.7 ± 2.0 | 69.5–75.7 | 3.1 |
Table 3.
Correlation coefficient between air pollution and weather variables during the study period.
| PM10 | O3 | NO2 | SO2 | CO | |
|---|---|---|---|---|---|
| O3 | 0.29 | ||||
| NO2 | 0.45 | 0.34 | |||
| SO2 | 0.16 | 0.04 | 0.09 | ||
| CO | 0.3 | 0.17 | 0.54 | 0.13 | |
| Temperature | −0.13 | −0.06 | −0.27 | −0.08 | −0.24 |
| Relative humidity | −0.34 | −0.45 | −0.21 | −0.18 | −0.08 |
Table 4 presents the estimated HRs in self-morbidities for an increment of IQR in PM10, O3, NO2, SO2 and CO in Model I to Model V. Regardless of the model used, PM10 and SO2 were significantly associated with incidences of high blood pressure and high cholesterol. Furthermore, we found the positive association between CO exposure and incidence of high blood pressure, but not for high cholesterol and diabetes. In contrast, O3 and NO2 generally showed negative association with incidences of high blood pressure, high cholesterol, and diabetes in model.
Table 4.
Estimated Hazard Ratios (95% CI) for self-reported morbidities in 2005–2013 for an increment of IQR increase in pollutant concentrations.
| PM10 (μg/m3) | O3 (ppb) | NO2 (ppb) | SO2 (ppb) | CO (ppm) | |
|---|---|---|---|---|---|
| High blood pressure | |||||
| Model I | 1.52 (1.40, 1.64)* | 0.82 (0.74, 0.91)* | 0.95 (0.88, 1.03) | 1.93 (1.73, 2.14)* | 1.15 (1.08, 1.24)* |
| Model II | 1.55 (1.43, 1.67)* | 0.77 (0.70, 0.86)* | 0.94 (0.86, 1.01) | 2.28 (2.05, 2.54)* | 1.16 (1.08, 1.24)* |
| Model III | 1.54 (1.42, 1.67)* | 0.77 (0.69, 0.86)* | 0.93 (0.86, 1.02) | 2.32 (2.07, 2.60)* | 1.15 (1.07, 1.24)* |
| Model IV | 1.13 (1.04, 1.23)* | 0.89 (0.80, 0.99)* | 1.01 (0.94, 1.09) | 1.22 (1.08, 1.38)* | 1.07 (1.00, 1.15) |
| Model V | 1.18 (1.07, 1.30)* | 0.90 (0.80, 1.02) | 1.03 (0.94, 1.13) | 1.66 (1.34, 2.07)* | 1.09 (1.00, 1.18)* |
| High cholesterol | |||||
| Model I | 1.62 (1.54, 1.69)* | 0.83 (0.78, 0.89)* | 0.89 (0.85, 0.94)* | 1.82 (1.71, 1.95)* | 1.03 (0.99, 1.08) |
| Model II | 1.62 (1.55, 1.70)* | 0.80 (0.75, 0.86)* | 0.87 (0.82, 0.92)* | 2.06 (1.92, 2.20)* | 1.02 (0.97, 1.07) |
| Model III | 1.63 (1.55, 1.70)* | 0.80 (0.75, 0.86)* | 0.85 (0.81, 0.90)* | 2.11 (1.96, 2.26)* | 1.02 (0.97, 1.07) |
| Model IV | 1.07 (1.02, 1.12)* | 1.00 (0.93, 1.07) | 0.97 (0.92, 1.02) | 1.20 (1.11, 1.30)* | 1.02 (0.97, 1.07) |
| Model V | 1.10 (1.04, 1.16)* | 1.03 (0.95, 1.11) | 0.93 (0.87, 0.99)* | 1.68 (1.48, 1.91)* | 1.02 (0.96, 1.07) |
| Diabetes | |||||
| Model I | 1.71 (1.48, 1.96)* | 0.83 (0.68, 1.01) | 0.95 (0.83, 1.10) | 1.71 (1.40, 2.10)* | 1.07 (0.93, 1.22) |
| Model II | 1.76 (1.53, 2.02)* | 0.78 (0.64, 0.95)* | 0.92 (0.79, 1.07) | 2.15 (1.74, 2.65)* | 1.05 (0.92, 1.21) |
| Model III | 1.81 (1.56, 2.09)* | 0.81 (0.66, 1.00)* | 0.89 (0.75, 1.05) | 2.19 (1.75, 2.75)* | 1.04 (0.89, 1.20) |
| Model IV | 1.05 (0.91, 1.21) | 0.97 (0.78, 1.21) | 1.04 (0.89, 1.22) | 1.21 (0.92, 1.60) | 1.06 (0.92, 1.22) |
| Model V | 1.09 (0.91, 1.30) | 1.01 (0.79, 1.30) | 0.97 (0.81, 1.17) | 1.88 (1.24, 2.85)* | 1.00 (0.85, 1.18) |
Significance indicated by *P < 0.05. Model I: no adjustment; Model II: adjusted for age and sex; Model III: further adjusted for BMI, smoking status, alcohol intake, physical activity, food consumption, intake of fruit and vegetables, marital status, education level, and income. Specifically, food consumption in model III: high blood pressure models adjusted for high sodium consumption; high cholesterol models adjusted for high fat consumption; diabetes models adjusted for high sugar consumption and sugar-sweetened soft drink consumption. Model IV (Main model): further adjusted for temperature and relative humidity; Model V: further adjusted for other self-reported comorbidities and city of residence.
Although the HRs of high blood pressure and cholesterol exceeded 1.5 per IQR increase in PM10 and SO2 in Model I, II and III, the associations became weaker in Model IV (main model) which adjusted for 2-year average of temperature and relative humidity. In Model IV, the HRs of PM10 were 1.13 (95%CI: 1.04, 1.23) for high blood pressure and 1.07 (95%CI: 1.02, 1.12) for high cholesterol. The HRs of SO2 were 1.22 (95%CI: 1.08, 1.38) for high blood pressure and 1.20 (95%CI: 1.11, 1.30) for high cholesterol, which were slightly higher than those of PM10. Although the association of SO2 with diabetes was not significant in the main model (HR = 1.21, 95%CI: 0.92, 1.6), the association became significant (HR = 1.88, 95%CI: 1.24, 2.85) after adjusted for other self-reported co-morbidities and city of residence (Model V). We found no clear association between PM10 with incidences of diabetes (HR = 1.05, 95%CI: 0.91, 1.21), and all self-reported morbidities with O3 and NO2 in the main model and our sensitivity analysis in Model V. In two-pollutants model (Table S1), the associations of PM10 and SO2 with incidences of self-morbidities were not essentially changed after adjusted by co-pollutants.
4. Discussions
In this study, we examined the association of long-term air pollution with self-reported morbidity of high blood pressure, high cholesterol, and diabetes in subjects of TCS in BMR, Thailand. Long-term exposure to PM10 was positively associated with incidences of high blood pressure and high blood cholesterol, but not for diabetes in the main model. Additionally, we also found the positive association of SO2 with all self-reported morbidities, and CO with incidence of high blood pressure. However, we did not observe the clear association of O3 and NO2 with self-reported morbidities.
Recent longitudinal studies have consistently reported that an increase of air pollutant concentrations (e.g. PM2.5, NO2, NOx) were associated with hypertension incidence (Bai et al., 2018; Bo et al., 2019b; Coogan et al., 2012), diabetes morbidity (Andersen et al., 2012; Bai et al., 2018; Coogan et al., 2012; Lao et al., 2019; Liang et al., 2019; Qiu et al., 2018) and mortality (Brook et al., 2013; Lim et al., 2018). Nonetheless, some previous studies did not find the association of long-term exposure to air pollution with hypertension and diabetes (Adar et al., 2018; Chen et al., 2015; Lazarevic et al., 2015). In this study, we observed significant positive associations of hypertension with PM10 and CO, but not with O3 and NO2. Long-term SO2 exposure was also positively associated with hypertension and diabetes. However, we did not find the clear association between diabetes with other pollutants in the main model.
Several biological mechanisms linked particulate air pollution to the development of hypertension and diabetes, include the elicitation of local and systemic inflammation and oxidative stress, endothelial dysfunction, and the triggering of autonomic nervous system imbalance (Brook et al., 2004, 2009, 2010; Brook and Rajagopalan, 2009; Coogan et al., 2012). Other proposed mechanisms connecting air pollution exposure and promotion of insulin resistance have also been suggested by animal and human studies (Evans et al., 2002; Kelly, 2003; Liu et al., 2014; Rajagopalan and Brook, 2012; Sun et al., 2009). In addition, the trigger of autonomic nervous system imbalance by particulate matter which can promote to vasoconstriction (Brook, 2008; Brook et al., 2002), contributes to hypertension and impaired insulin sensitivity (Carnethon et al., 2003; Sun et al., 2009).
Previous studies observed the associations between long-term PM2.5 exposure with dyslipidemias incidence (Bo et al., 2019a) and prevalence (Mao et al., 2020). Similarly, we also found the positive association between PM10 and SO2 with incident high blood cholesterol, but not for other pollutants. The biological mechanisms underlying the relationship between long-term exposure to air pollution and changed blood lipids is still unclear. Some evidences suggested that air pollution inhalation could induce inflammation and oxidative stress, interfering with lipids metabolism and oxidation, and contributing to altered blood lipid levels (Araujo and Nel, 2009; Mao et al., 2020; Poursafa et al., 2014; Xu et al., 2011). Besides, intervention (Chen et al., 2016) and experimental (Mendez et al., 2013) studies suggested that decreases in DNA methylation (Bind et al., 2014), especially on genes elicited by inhaled air pollution also related to lipid metabolism and inflammation pathways.
In this study, Cox regression model was selected in order to compare our results of HRs to previous studies which also used the same method to examine the associations between long-term exposure to air pollutants, especially for PM10 and PM2.5 and the incidences of dyslipidemia (Bo et al., 2019a), diabetes and hypertension (Andersen et al., 2012; Bai et al., 2018; Coogan et al., 2012; Lao et al., 2019; Liang et al., 2019; Qiu et al., 2018). The magnitude of the effects for PM10 in our main model (model IV), which is represented by HRs, is not much different to most of the previous studies. For example, Yang et al. (2020) showed meta-analyses of diabetes incidence with PM2.5 (11 studies; HR = 1.10, 95% CI = 1.04–1.17 per 10 μg/m3 increment) and PM10 (6 studies; HR = 1.11; 95% CI = 1.00–1.22 per 10 μg/m3 increment). However, few previous studies observed the magnitude of HRs between PM2.5 and the incidences of hypertension and diabetes (Coogan et al., 2012; Lao et al., 2019) larger than our study. The evidences of long-term effects of SO2 on morbidities, especially for the incidences of diabetes and high blood cholesterol are still limited. Although the sample size of our study was smaller than most of previous studies, data richness in terms of detailed availability of potential confounding allows a thorough adjustment in the model resulting to a more robust estimation.
Many previous cross-sectional studies might not be able to detect the association between air pollution and hypertension, high cholesterol, and diabetes because these diseases are a chronic process (Chen et al., 2015; Lazarevic et al., 2015). Furthermore, air pollution exposure in some studies (Chuang et al., 2011; Shanley et al., 2016) were estimated based on the proximity of residences to fixed monitoring stations, assigning the same exposure level to entire communities (districts, counties or cities) which may introduce misclassification and inconsistent results. In this longitudinal study, we are able to detect developments or changes in the characteristics of the target population at both the group and the individual level because several observations of the same subjects were conducted over a period of time. Additionally, we also used ordinary kriging method to evaluate spatial representativeness of monitoring stations which can improve the accuracy of air pollution exposure estimates.
Previous studies from TCS suggested the characteristic factors which have been reported to correlate with morbidities of high blood pressure, high blood lipids, and diabetes (Papier et al., 2016, 2017; Rimpeekool et al., 2017; Seubsman et al., 2010). Rimpeekool et al. (2017) reported increased risk of high blood pressure, high blood lipids, and obesity among Thai adults who have low physical activity, unhealthy eating (high levels of sugar, fat and sodium, and little fibre), and seldom/rarely use nutritional labelling. In men and women, type 2 diabetes mellitus (T2DM) was positively associated with age, BMI, smoking, and alcohol intake (Papier et al., 2016). The sociodemographic (i.e. education, income, household assets, and housing type) and lifestyle changes that have been accompanied with Thailand's economic development were also associated strongly with obesity and T2DM risk in a large cohort of Thai adults (Papier et al., 2016; Seubsman et al., 2010). Seubsman et al. (2010) reported that obesity increased with age and was more prevalent among males than females. In addition, Thais who lived in urban residence associated with unhealthy diets for both sexes (Papier et al., 2017).
Our study has several potential limitations. First, we can include only subjects who lived in BMR from 2005 until 2013 due to the availability of air pollution data. Moreover, the study period was also based on the TCS which started in 2005 and ended in 2013, where this 9-year period is long enough, to some extent, to observe long-term health effects of air pollution in the study area. Second, we could not include traffic variables and land use data into the ordinary kriging due to the limitations of the data in Thailand. However, we used air pollution data from traffic monitoring stations for exposure assessment to generate ordinary kriging for traffic-related air pollution such as NO2 and CO. Third, self-report of disease diagnosis was also a limitation. Although the diseases that each subject got in this study were diagnosed and confirmed by the doctor, we knew only the year that each subject got disease but we did not know the date. Hence, we can only match the health outcomes data with annual air pollution exposure.
Notwithstanding, most of the cohort members were relatively young (aged 20–39 years old) and had not developed cardiovascular disease yet, we may find the association between air pollution and cardiovascular disease risk factors for younger population from this study. Thus, this study may clarify the process from exposure to air pollutants to development of cardiovascular diseases, by affecting risk factors. A lot of data was also recorded in this study such as BMI, education level, income, marital status, regular exercise, alcohol consumption, smoking status, food and drink consumption, intake of vegetables and fruit. Therefore, we can control these variables in our model. Furthermore, we also applied ordinary kriging method which can evaluate spatial representativeness of monitoring stations and improve the accuracy of air pollution exposure estimates.
5. Conclusions
Long-term exposure to air pollution, particularly for PM10 and SO2 was associated with self-reported high blood pressure, high blood cholesterol, and diabetes in subjects of TCS. Our findings could be a benefit and helpful for understanding long-term effects of air pollution on risk factors for cardiovascular diseases, as well as their mechanisms under current situation in Thailand. Moreover, this study may contribute to the establishment and improvement of long-term air pollution control strategies in Thailand for preventing public health issues. Further epidemiological studies are needed to understand and identify plausible mechanisms underlying the association, as well as longitudinal studies to confirm the causal relationship between long-term air pollution exposure with diabetes and high blood cholesterol. In the advent of more granular exposure data, further studies can focus on the sources of air pollution and develop new or advanced method for exposure assessment, as well as long-term effects of weather on the morbidities.
CRediT author statement
Kanawat Paoin: Conceptualization, Formal analysis, Writing - original draft. Kayo Ueda: Conceptualization, Supervision, Writing - review & editing. Thammasin Ingviya: Methodology, Writing - review & editing. Suhaimee Buya: Methodology. Arthit Phosri: Methodology, Writing - review & editing. Xerxes Tesoro Seposo: Methodology, Writing - review & editing. Sam-ang Seubsman: Data collection, Data curation, Funding acquisition. Matthew Kelly: Supervision, Writing - review & editing. Adrian Sleigh: Supervision, Writing - review & editing, Funding acquisition. Akiko Honda: Supervision. Hirohisa Takano: Supervision.
Ethics and consent
Approval for the study was obtained from Sukhothai Thammathirat Open University Research and Development Institute (protocol number 0522/10) and the Australian National University Human Research Ethics Committee (protocol numbers 2004/344 and 2009/570) and the Graduate School of Engineering, Kyoto University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the International Collaborative Research Grants Scheme with joint grants from the Wellcome Trust UK (grant number GR071587MA) and the Australian National Health and Medical Research Council (NHMRC) (grant number 268055). It was also supported by a global health grant from the NHMRC (grant number 585426). The authors would like to express our sincere gratitude to the staff at Sukhothai Thammathirat Open University (STOU), who assisted with student contact, and the STOU students who are participating in the cohort study, the Pollution Control Department of the Ministry of Natural Resources and Environment, and Thai Meteorological Department for providing the useful data for analyses in this study. We thank Dr. Vasoontara Yiengprugsawan and Dr. Benjawan Tawatsupa for guiding us through the complex data processing, and we also thank Dr. Suphanat Wongsanuphat, Mr. Thatkiat Meema, Dr. Vera Ling Hui Phung, and Dr. Kraiwuth Kallawicha for their guidance and encouragements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2020.110330.
Contributor Information
Kayo Ueda, Email: uedak@health.env.kyoto-u.ac.jp.
Thai Cohort Study Team Thailand:
Jaruwan Chokhanapitak, Chaiyun Churewong, Suttanit Hounthasarn, Suwanee Khamman, Daoruang Pandee, Suttinan Pangsap, Tippawan Prapamontol, Janya Puengson, Wimalin Rimpeekool, Yodyiam Sangrattanakul, Sam-ang Seubsman, Boonchai Somboonsook, Nintita Sripaiboonkij, Pathumvadee Somsamai, Benjawan Tawatsupa, Arunrat Tangmunkongvorakul, Duangkae Vilainerun, and Wanee Wimonwattanaphan
Thai Cohort Study Team Australia:
Chris Bain, Emily Banks, Cathy Banwell, Janneke Berecki-Gisolf, Bruce Caldwell, Gordon Carmichael, Tarie Dellora, Jane Dixon, Sharon Friel, David Harley, Susan Jordan, Matthew Kelly, Tord Kjellstrom, Lynette Lim, Roderick McClure, Anthony McMichael, Tanya Mark, Adrian Sleigh, Lyndall Strazdins, Tam Tran, Vasoontara Yiengprugsawan, and Jiaying Zhao
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adar S.D., Chen Y.H., D’souza J.C., O’neill M.S., Szpiro A.A., Auchincloss A.H., Kaufman J.D. Longitudinal analysis of long-term air pollution levels and blood pressure: a cautionary tale from the multi-ethnic study of atherosclerosis. Environ. Health Perspect. 2018;126(10):1–11. doi: 10.1289/EHP2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Analitis A., Katsouyanni K., Dimakopoulou K., Samoli E., Nikoloulopoulos A.K., Petasakis Y., Pekkanen J. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17(2):230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- Andersen Z.J., Raaschou-Nielsen O., Ketzel M., Jensen S.S., Hvidberg M., Loft S., Sørensen M. Diabetes incidence and long-term exposure to air pollution. Diabetes Care. 2012;35:92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo J.A., Nel A.E. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 2009;6(24):1–19. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Chen H., Hatzopoulou M., Jerrett M., Kwong J.C., Burnett R.T., Weichenthal S. Exposure to ambient ultrafine particles and nitrogen dioxide and incident hypertension and diabetes. Epidemiology. 2018;29(3):323–332. doi: 10.1097/EDE.0000000000000798. [DOI] [PubMed] [Google Scholar]
- Bangkok Climate Relative humidity in Bangkok, Thailand. 2016. http://www.bangkok.climatemps.com/humidity.php
- Bind M., Lepeule J., Zanobetti A., Gasparrini A., Baccarelli A.A., Coull B.A., Schwartz J. Air pollution and gene-specific methylation in the normative aging study. Epigenetics. 2014;9(3):448–458. doi: 10.4161/epi.27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo Y., Chang L.Y., Guo C., Zhang Z., Lin C., Chuang Y.C., Yeoh E.K. Association of long-term exposure to fine particulate matter and incident dyslipidaemia: a longitudinal cohort study. Environ. Res. 2019;173:359–365. doi: 10.1016/j.envres.2019.03.034. [DOI] [PubMed] [Google Scholar]
- Bo Y., Guo C., Lin C., Chang L.Y., Chan T.C., Huang B., Yeoh E.K. Dynamic changes in long-term exposure to ambient particulate matter and incidence of hypertension in adults: a natural experiment. Hypertension. 2019;74(3):669–677. doi: 10.1161/HYPERTENSIONAHA.119.13212. [DOI] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Li T., Yan Y., Xian H., Al-Aly Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. The Lancet Planetary Health. 2017;1 doi: 10.1016/S2542-5196(17)30117-1. [DOI] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Li T., Yan Y., Xian H., Al-Aly Z. Journal of the American Society of Nephrology; 2017. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008;115(6):175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Brook J.R., Urch B., Vincent R., Rajagopalan S., Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.CIR.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Cakmak S., Turner M.C., Brook J.R., Crouse D.L., Peters P.A., Burnett R.T. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36(10):3313–3320. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R.D., Franklin B., Cascio W., Hong Y., Howard G., Lipsett M., Tager I. Air pollution and cardiovascular disease A statement for healthcare professionals from the expert panel on population and prevention science of the American heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Rajagopalan S. Particulate matter, air pollution, and blood pressure. Journal of the American Society of Hypertension. 2009;3(5):332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Rajagopalan S., Pope C.A., Brook J.R., Bhatnagar A., Diez-Roux A.V., Kaufman J.D. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the american heart association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook R.D., Urch B., Dvonch J.T., Bard R.L., Speck M., Keeler G., Brook J.R. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buadong D., Jinsart W., Funatagawa I., Karita K., Yano E. Association between PM10 and O3 levels and hospital visits for cardiovascular diseases in Bangkok, Thailand. J. Epidemiol. 2009;19(4):182–188. doi: 10.2188/jea.JE20080047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon M.R., Golden S.H., Folsom A.R., Haskell W., Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes the atherosclerosis risk in communities study, 1987–1998. Circulation. 2003;107:2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- Chen R., Meng X., Zhao A., Wang C., Yang C., Li H., Kan H. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: a randomized crossover trial. Environ. Int. 2016;94:614–619. doi: 10.1016/j.envint.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Chen S., Wu C., Lee J., Hoffmann B., Peters A., Brunekreef B. Associations between long-term air pollutant exposures and blood pressure in elderly residents of taipei city: a cross-sectional study. Environ. Health Perspect. 2015;123(8):779–784. doi: 10.1289/ehp.1408771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K.J., Yan Y.H., Chiu S.Y., Cheng T.J. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup. Environ. Med. 2011;68(1):64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Chuersuwan N., Nimrat S., Lekphet S., Kerdkumrai T. Levels and major sources of PM2.5 and PM10 in Bangkok metropolitan region. Environ. Int. 2008;34(5):671–677. doi: 10.1016/j.envint.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Coogan P.F., White L.F., Jerrett M., Brook R.D., Su J.G., Seto E., Rosenberg L. Air pollution and incidence of hypertension and diabetes in african American women living in Los Angeles. Circulation. 2012;125(6):767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan P.F., White L.F., Yu J., Brook R.D., Burnett R.T., Marshall J.D., Jerrett M. Long-term exposure to NO2 and ozone and hypertension incidence in the black women's health study. Am. J. Hypertens. 2017;30(4):367–372. doi: 10.1093/ajh/hpw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery D., Pope A. Acute respiratory effects of particulate air pollution. Annu. Rev. Publ. Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Evans J.L., Goldfine I.R.A.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Eze I.C., Schaffner E., Fischer E., Schikowski T., Adam M., Imboden M., Probst-Hensch N. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ. Int. 2014;70:95–105. doi: 10.1016/j.envint.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Geniaux G., Martinetti D., Gabriel E., Parent E., Desassis N., Allard D., Romary T. Analyzing spatio-temporal data with R: everything you always wanted to know-but were afraid to ask. J. Soc. Fr. Stat. 2017;158(3):124–158. [Google Scholar]
- Gold D.R., Mittleman M.A. New insights into pollution and the cardiovascular system: 2010 to 2012. Circulation. 2013;127(18):1903–1913. doi: 10.1161/CIRCULATIONAHA.111.064337. [DOI] [PubMed] [Google Scholar]
- Hansen A.B., Ravnskjær L., Loft S., Andersen K.K., Bräuner E.V., Baastrup R., Andersen Z.J. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ. Int. 2016;91:243–250. doi: 10.1016/j.envint.2016.02.036. [DOI] [PubMed] [Google Scholar]
- Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Brunekreef B., Kaufman J.D. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Health. 2013;12(43):1–15. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F.J. Oxidative stress: its role in air pollution and adverse health effects. Occup. Environ. Med. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao X.Q., Guo C., Chang L., yun Bo Y., Zhang Z., Chuang Y.C., Chan T.C. Long-term exposure to ambient fine particulate matter (PM 2.5) and incident type 2 diabetes: a longitudinal cohort study. Diabetologia. 2019;62:759–769. doi: 10.1007/s00125-019-4825-1. [DOI] [PubMed] [Google Scholar]
- Lazarevic N., Dobson A.J., Barnett A.G., Knibbs L.D. Long-term ambient air pollution exposure and self-reported morbidity in the Australian Longitudinal Study on Women's Health: a cross-sectional study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem J.H., Kaplan B.M. Exposures to air pollutants during pregnancy and preterm delivery. Environ. Health Perspect. 2006;114(6):905–910. doi: 10.1289/ehp.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Yang X., Liu F., Li J., Xiao Q., Chen J., Gu D. Long-term exposure to ambient fine particulate matter and incidence of diabetes in China: a cohort study. Environ. Int. 2019;126:568–575. doi: 10.1016/j.envint.2019.02.069. [DOI] [PubMed] [Google Scholar]
- Lim C.C., Hayes R.B., Ahn J., Shao Y., Debra T., Jones R.R., Thurston G.D. Association between long-term exposure to ambient air pollution and diabetes mortality in the US. Environ. Res. 2018;165:330–336. doi: 10.1016/j.envres.2018.04.011.Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L.L., Seubsman S., Sleigh A., Bain C. Validity of self-reported abdominal obesity in Thai adults: a comparison of waist circumference, waist-to-hip ratio and waist-to-stature ratio. Nutr. Metabol. Cardiovasc. Dis. 2012;22:42–49. doi: 10.1016/j.numecd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Liu L.J.S., Rossini A. Use of kriging models to predict 12-hour mean ozone concentrations in metropolitan Toronto—a pilot study. Environ. Int. 1996;22:677–692. [Google Scholar]
- Liu Cuiqing, Ying Z., Harkema J., Sun Q., Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2014;41(2):361–373. doi: 10.1177/0192623312464531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S., Chen G., Liu F., Li N., Wang C., Liu Y., Li S. Long-term effects of ambient air pollutants to blood lipids and dyslipidemias in a Chinese rural population. Environ. Pollut. 2020;256:113403. doi: 10.1016/j.envpol.2019.113403. [DOI] [PubMed] [Google Scholar]
- Mendez R., Zheng Z., Fan Z., Rajagopalan S., Sun Q., Zhang K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am. J. Tourism Res. 2013;5(2):224–234. doi: 10.1088/1361-648X/aa68eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton N., Yiallouros P., Kleanthous S., Kolokotroni O., Schwartz J., Dockery D.W., Koutrakis P. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: the effect of short-term changes in air pollution and dust storms. Environ. Health. 2008;7(39):1–16. doi: 10.1186/1476-069X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B., Chestnut L., Vichit-Vadakan N., Laixuthai A. The impact of particulate matter on daily mortality in Bangkok, Thailand. J. Air Waste Manag. Assoc. 1999;49(9):100–107. doi: 10.1080/10473289.1999.10463875. [DOI] [PubMed] [Google Scholar]
- Pan W.C., Wu C. Da, Chen M.J., Huang Y.T., Chen C.J., Su H.J., Yang H.I. Fine particle pollution, alanine transaminase, and liver cancer: a Taiwanese prospective cohort study (REVEAL-HBV) J. Natl. Cancer Inst. 2016;108(3):1–7. doi: 10.1093/jnci/djv341. [DOI] [PubMed] [Google Scholar]
- Papier K., Jordan S., D'Este C., Bain C., Peungson J., Banwell C., Sleigh A. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: results from the Thai cohort study. BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papier K., Jordan S., Bain C., D’este C., Thawornchaisit P., Seubsman S., Sleigh A. Validity of self-reported diabetes in a cohort of Thai adults. Global J. Health Sci. 2017;9(7) doi: 10.5539/gjhs.v9n7p1. [DOI] [Google Scholar]
- Park S.K., Adar S.D., O'Neill M.S., Auchincloss A.H., Szpiro A., Bertoni A.G., Diez-Roux A.V. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am. J. Epidemiol. 2015;181(5):327–336. doi: 10.1093/aje/kwu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M., Andersen Z.J., Stafoggia M., Weinmayr G., Galassi C., Sørensen M., Raaschou-Nielsen O. Ambient air pollution and primary liver cancer incidence in four European cohorts within the ESCAPE project. Environ. Res. 2017;154:226–233. doi: 10.1016/j.envres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Phosri A., Ueda K., Phung V.L.H., Tawatsupa B., Honda A., Takano H. Effects of ambient air pollution on daily hospital admissions for respiratory and cardiovascular diseases in Bangkok, Thailand. Sci. Total Environ. 2019;651(September):1144–1153. doi: 10.1016/j.scitotenv.2018.09.183. [DOI] [PubMed] [Google Scholar]
- Pollution Control Department Booklet on Thailand state of pollution 2018. 2019. http://www.pcd.go.th/file/Booklet on Thailand State of Pollution 2018.pdf Retrieved from.
- Pope C.A., Burnett R.T., Thurston G.D., Thun M.J., Calle E.E., Krewski D., Godleski J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Poursafa P., Mansourian M., Motlagh M., Ardalan G., Kelishadi R. Is air quality index associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Environ. Res. 2014;134:105–109. doi: 10.1016/j.envres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Qiu H., Schooling C.M., Sun S., Tsang H., Yang Y., Lee R. S. yin, Tian L. Long-term exposure to fine particulate matter air pollution and type 2 diabetes mellitus in elderly: a cohort study in Hong Kong. Environ. Int. 2018;113:350–356. doi: 10.1016/j.envint.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Brook R.D. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61(12):3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi M., Cerza F., Gariazzo C., Agabiti N., Cascini S., Di Domenicantonio R., Cesaroni G. Air pollution and occurrence of type 2 diabetes in a large cohort study. Environ. Int. 2018;112:68–76. doi: 10.1016/j.envint.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Rimpeekool W., Yiengprugsawan V., Kirk M., Banwell C., Seubsman S., Sleigh A. Nutrition label experience, obesity, high blood pressure, and high blood lipids in a cohort of 42,750 Thai adults. PloS One. 2017;12(12):1–12. doi: 10.1371/journal.pone.0189574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubsman S.A., Kelly M., Sleigh A., Peungson J., Chokkanapitak J., Vilainerun D. Methods used for successful follow-up in a large scale national cohort study in Thailand. BMC Res. Notes. 2011;4(166) doi: 10.1186/1756-0500-4-166. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21615963 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubsman S.A., Lim L.L., Banwell C., Sripaiboonkit N., Kelly M., Bain C., Sleigh A.C. Socioeconomic status, sex, and obesity in a large national cohort of 15-87-year-old open university students in Thailand. J. Epidemiol. 2010;20(1):13–20. doi: 10.2188/jea.JE20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubsman S.A., Yiengprugsawan V., Sleigh A.C., Team the T.C.S. A large national Thai cohort study of the health-risk transition based on Sukhothai Thammathirat open university students. ASEAN Journal of Open and Distance Learning. 2012;4(1) http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=23750340 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Shanley R.P., Hayes R.B., Cromar K.R., Ito K., Gordon T., Ahn J. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology. 2016;27(2):291–298. doi: 10.1097/EDE.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh A.C., Seubsman S.A., Bain C., Vilainerun D., Khamman S., Somboonsook B., Dellora T. Cohort profile: the Thai cohort of 87 134 Open University students. Int. J. Epidemiol. 2008;37:266–272. doi: 10.1093/ije/dym161. [DOI] [PubMed] [Google Scholar]
- Strak M., Janssen N., Beelen R., Schmitz O., Karssenberg D., Houthuijs D., Hoek G. Associations between lifestyle and air pollution exposure: potential for confounding in large administrative data cohorts. Environ. Res. 2017;156:364–373. doi: 10.1016/j.envres.2017.03.050. [DOI] [PubMed] [Google Scholar]
- Sun Q., Yue P., Deiuliis J.A., Lumeng C.N., Kampfrath T., Mikolaj M.B., Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneepanichskul N., Gelaye B., Grigsby-Toussaint D.S., Lohsoonthorn V., Jimba M., Williams M.A. Short-term effects of particulate matter exposure on daily mortality in Thailand: a case-crossover study. Air Quality, Atmosphere and Health. 2018;11(6):639–647. doi: 10.1007/s11869-018-0571-7. [DOI] [Google Scholar]
- Thawornchaisit P., De Looze F., Reid C.M., Seubsman S., Sleigh A., Thai Cohort Study Team T. Validity of self-reported hypertension: findings from the Thai cohort study compared to physician telephone interview. Global J. Health Sci. 2014;6(2):1–11. doi: 10.5539/gjhs.v6n2p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang N.H., Tripathi N.K. Spatial correlation analysis between particulate matter 10 (PM10) hazard and respiratory diseases in chiang mai province, Thailand. International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences - ISPRS Archives. 2014;XL-8(1):185–191. doi: 10.5194/isprsarchives-XL-8-185-2014. [DOI] [Google Scholar]
- Vichit-Vadakan N., Vajanapoom N. Health impact from air pollution in Thailand: current and future challenges. Environ. Health Perspect. 2011;119(5):A197–A198. doi: 10.1289/ehp.1103645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernagel H. Multivariate Geostatistics. Springer; Berlin, Heidelberg: 1995. Ordinary kriging. [DOI] [Google Scholar]
- WHO Regional Office for Europe . 2006. Air Quality Guidelines: Global Update. [DOI] [Google Scholar]
- Wong C.M., Vichit-Vadakan N., Kan H., Qian Z. Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ. Health Perspect. 2008;116(9):1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Xu X., Zhong M., Hotchkiss I.P., Lewandowski R.P., Wagner J.G., Sun Q. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 2011;8(20) doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.Y., Bloom M.S., Markevych I., Qian Z. (Min), Vaughn M.G., Cummings-Vaughn L.A., Dong G.H. Exposure to ambient air pollution and blood lipids in adults: the 33 Communities Chinese Health Study. Environ. Int. 2018;119:485–492. doi: 10.1016/j.envint.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Yang B.Y., Fan S., Thiering E., Seissler J., Nowak D., Dong G.H., Heinrich J. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ. Res. 2020;180:108817. doi: 10.1016/j.envres.2019.108817. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Guo C., Lau A.K.H., Chan T.C., Chuang Y.C., Lin C., Lao X.Q. Long-term exposure to fine particulate matter, blood pressure, and incident hypertension in taiwanese adults. Environ. Health Perspect. 2018;126(1):1–8. doi: 10.1289/EHP2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.