Abstract
The current global outbreak of COVID-19 due to SARS-CoV-2 is an unprecedented humanitarian crisis. Considering the gravity of its impact there is an immediate need to develop a detection technique that is sensitive, specific, fast, and affordable for the clinical diagnosis of the disease. Real time Polymerase Chain Reaction (RT-PCR)-based detection platforms are contemplated to be the gold standard to detect viral RNA. However, that may be susceptible to errors, and there is a risk of obtaining false results, which ultimately compromises the strategy of efficient disease management. Several modern techniques exhibiting assured results with enhanced sensitivity and specificity against the SARS-CoV-2 associated viral components or immune response against it have been developed and may be implemented. The review deals with the conventional RT-PCR detection techniques and compares them to other detection platforms viz., biosensor based detection of antigens, fluorescent or colorimetric detection systems including CRISPR-Cas 13 based SHERLOCK kit, CRISPR Cas-9 based FELUDA test kit, CRISPR DETECTR kit, Next Generation Sequencing or microarray-based kits. These modern techniques are great as a point of care detection methods but should be followed by RT PCR based detection for the confirmation of COVID-19 status.
Keywords: SARS-CoV-2, RT-PCR, Biosensors, Nucleic-acid amplification, CRISPR-Cas, Next-generation sequencing, Microarray, Immunosensor, Serological test
Abbreviations: ACE2, Angiotensin- Converting Enzyme 2; ASEA, advanced strand exchange amplification; BALF, broncho-alveolar lavage fluid; BTO, Billion To One; CARs, chimeric antigen receptors; CDC, Centers for Disease Control and Prevention; Cell-SELEX, Systematic Evolution of Ligands by exponential enrichment; CPEs, Cytopathic effects; CRISPR, clusters of regularly interspaced short palindromic repeats; E-gene, envelope protein; EUA, Emergency Use Authorization; Feluda, FnCas9 Editor Linked Uniform Detection Assay; FET, Field-effect transistor; Gr-FET, graphene field-effect transistor; Ig, Immunoglobulin; LAMP, Loop-mediated isothermal amplification; LSPR, localized surface plasmon resonance; N-gene, nucleocapsid protein; NGS, Next Generation Sequencing; NMPA, National Medical Products Administration; POC, point of care; PNAs, peptide nucleic acids; PPT, plasmonic photothermal; RCA, rolling circle amplification; RdRp, RNA dependent RNA polymerase; RT-PCR, Reverse Transcription Polymerase Chain Reaction; RUO, Research Use Only
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an enveloped virus having a positive-sense single-stranded RNA, which belongs to the Coronaviridae family [1]. They were reported in China to cause a severe pneumonic respiratory disease termed Coronavirus disease-2019 or in short COVID-19 [2]. To date, millions of people have been infected, and more than 300,000 fatalities have been reported [3]. The numbers are still on the rise. The accurate, cheap, and fast detection of the SARS-CoV-2 has become a matter of immense importance. This could be advantageous in controlling the infection sources and preventing disease progression in patients as well as healthcare professionals who come in close contact.
The nucleic acid-based detection methods experienced rapid development and have become extensive and comprehensive technology. The real-time reverse transcriptase Polymerase Chain Reaction- methods (RT-PCR) are known for their high specificity and sensitivity and are therefore considered to be the “gold standard” for the detection of viral RNA [4]. Subsequently, other PCR based methods were also developed with slight modifications. Most of the kits in the market which were available immediately after the pandemic outbreak was based on RT-PCR based assays which relied on the genetic similarities of other coronaviruses. Once the genomic constitution of SARS CoV-2 was deciphered on January 9th, 2020 [5], more robust kits were possible to develop.
The real-time RT-PCR based molecular diagnosis essentially includes viral RNA extraction from patient body fluids, synthesis of complementary first strand DNA followed by real-time amplification. Each step is very crucial and may directly affect the precision of diagnosis. Therefore, for the fast and authentic detection of SARS-CoV-2, sensitive immunological diagnostic tools were developed. These immunoassays either can detect the viral antibody (IgA/ IgM) and IgG or can directly detect the viral antigens in the clinical samples without needing sample pre-processing. Automated fluorescent immunoassays can also quantitatively detect the target biomolecules i.e., either IgM/ IgG or viral antigen.
Among other non-PCR-based RNA detection techniques, Field-effect transistor (FET) based biosensing devices are significantly useful in the rapid detection of SARS-CoV-2 [6]. Due to high carrier mobility, electronic conductivity, and the large specific area, these biosensors have been reported to be of great use for several sensing and testing platforms [7]. The graphene-based FETs can detect nearby changes on its surface and offers an optimum sensing environment for low-noise and ultra-sensitive detection [8].
In addition to these, various other detection methods which can be applied for reducing the time and are simpler includes Loop-mediated isothermal amplification (LAMP) [9], rolling circle amplification (RCA) [10]; ultrafast advanced strand exchange amplification (ASEA) [11] technique, nanosensor based platforms [12], Microarray [13] and next-generation sequencing (NGS) -based CoVID-19 kits [14] are also under development. Some of the non-PCR based detection kits are mentioned in Table 1 .
Table 1.
Some non-PCR based detection tests.
| Name of Test | Organization/Manufacturer | Type/Technique of Test | Reference |
|---|---|---|---|
| ID NOW COVID-19 |
Abbott Diagnostics Scarborough, Inc. D2 |
Isothermal nucleic acid amplification technique | [61,62] |
| Nucleic Acid reagent test kit for novel coronavirus |
Anbio (Xiamen) Biotechnology Co., Ltd. | hybridization capture immunofluorescence | [66] |
| Nucleic Acid reagent test kit for novel coronavirus 2019 -nCoV (RNA sothermal amplification lateral flow assay) | Wuhan Zhongzhi Biotechnologies Inc. | RNA isothermal amplification lateral flow assay | [66] |
| RapCov™ Rapid COVID-19 Test | Advaite | lateral flow immunoassay | [66] |
| SARS-CoV-2 (2019-nCoV) Spike Detection ELISA Kit (RUO) | Sino Biological | ELISA | [15] |
| Wantai SARS-CoV-2 Ab Rapid Test kit | Beijing Wantai Biological Pharmacy Enterprise Co. Ltd. (China) | ELISA | [66] |
| IgG Antibody test kit for novel coronavirus 2019-nCoV | Bioscience (Chongqing) Diagnostic Technology Co., Ltd | magnetic particle-based chemiluminescence immunoassay | [66] |
| SARS -CoV -2 IgG/IgM kit | Biotime | colloidal gold lateral flow immunoassay | [66] |
| CORONAVIR US IgG/IgM (COVID -19) | Ebram Productos Laboratoriais | chromatograph is rapid qualitative immunoassay | [66] |
| COVID-19 Ag ECO Teste | Eco DiagnosticaLtda | immunochromatographic assay enhanced with colloidal gold. | [66] |
| Standard Q COVID-19 Ag | SD Biosensor | Chromatographic immunoassay | [15] |
Although so many testing kits have been launched commercially, a huge difference of opinions about the right and effective detection method of the virus and the most appropriate policies for testing asymptomatic carriers are yet to be sorted. Several point-of-care (POC) assay methods are under development and are awaiting the approval of regulatory agencies. Some of them have received Emergency Use Authorization (EUA) whereas other products are approved for research use only (RUO) [15]. There are several misconceptions and myths among general nontechnical persons about PCR based methods and immunoassays [16]. Here, in this review, we have critically highlighted the advantages and limitations of the RT-PCR detection technique and discussed other Non-PCR-based techniques for the detection of SARS-CoV-2 to come up with a probable solution.
2. PCR-based detection: the conventional way
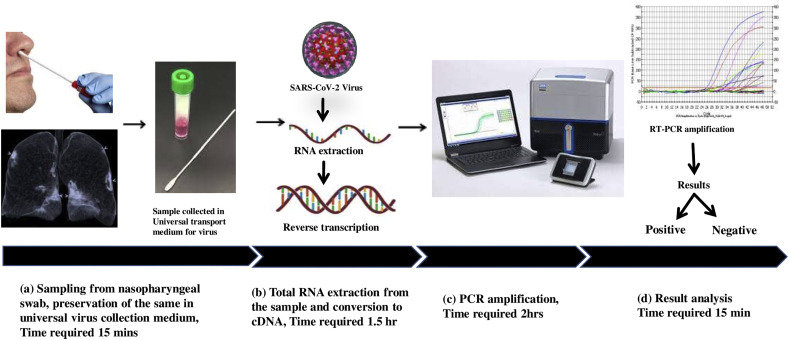
The real-time RT-PCR technique is a principal method that is implemented for the detection of all types of coronaviruses, including the novel SARS-CoV-2 virus [17]. This is usually executed as per the manufacturer's protocol to convert the viral RNA into cDNA followed by RT-PCR amplification. As per the guidelines of the Centers for Disease Control and Prevention (CDC), USA, nasopharyngeal, oropharyngeal, nasal swab from the upper respiratory tract, and bronchoalveolar lavage fluid (BALF), tracheal aspirate, pleural fluid, and lung biopsy are considered as the sources of specimen collection. These are immediately transferred to sterile transport tubes containing universal viral transport medium for further analysis as per protocol (Fig. 1 ). Most of the test kits target three different viral genes, so that if one gene mutates and the RT-PCR gives a negative result, then other assays might be positive. These targets are the N-gene for viral nucleocapsid protein, RdRp gene coding for RNA dependent RNA polymerase, Orf1 gene for human RNA polymerase protein, and the E-gene coding for envelope protein. There are some limited numbers of kits that target the spike protein or the S gene.
Fig. 1.
Approximate Timeline for Detection of SARS-CoV-2 by RT-PCR.
2.1. Problems associated with PCR-based detection at each sequential step
The RT-PCR method involves sample handling and post-PCR analysis, which consumes a lot of time. The RT-PCR is also susceptible to contamination, and therefore researchers are focussing on the improvement of the efficacy of the real-time RT-PCR [18]. The limitations of the detection in each step are discussed in the following paragraphs.
2.1.1. Source of sample
The detection will give an accurate result if the sample collected has an adequate amount of viral RNA. The extraction depends on the efficiency of the health worker collecting the swab. The virus residing in the respiratory tract may be found either in the nasopharyngeal swab or sputum and might not be present in both [19]. Therefore the next hunt was to search for specimens that will maximize the efficacy of the detection. The bronchoalveolar lavage fluid (BALF) can be used for assessment only if collected by an expert operator with a suction instrument. After collection, there is a chance of contamination of the sample which should be considered with the utmost care.
2.1.2. Possible error of RNA extraction process
Then viral RNA is extracted by solid-phase extraction method where stationary phase consists of a silica gel membrane on which the RNA binds and later eluted. If the sample is either contaminated or contain viral RNA inadequately, the PCR may give false results. It is difficult to extract RNA if the viral load is very low in the sample and carrying the sample to the laboratory may cause denaturation of the RNA [19]. So, skilled personnel is required for the collection, handling, and RNA extraction.
2.1.3. Choice of enzyme
At least two important enzymes with high efficacy are required for the protocol of RT-PCR viz., reverse transcriptase and DNA polymerase. Several manufacturers offer improved quality of enzymes, the right choice of the enzyme is very crucial for nucleotide amplification.
2.1.4. The PCR protocol
During early infection, a swab may contain very little amount of viral RNA. The minor mistake in the collection and extraction of the sample can create a major difference in the result of test analysis since DNA is amplified exponentially in each step of PCR. SARS-CoV-2 can mutate rapidly [20]. Therefore, the mutations in the probe and the primer targeted regions in the genome of SARS-CoV-2 may lead to false-negative results.
2.1.5. Choice of target genes
Even though the design of real-time RT-PCR assay was based on the conserved regions of the SARS-CoV-2 genome and was considered to be precise, mismatches within the probes, the primers, and the target sequences caused by the variability can lead to false-negative results and thus affecting the performance of the assay. This problem could be avoided by targeting multiple genes. To meet up the huge demand for detection kits, initially, several real-time RT-PCR kits for detection of SARS-CoV-2 have been rapidly approved, but their quality varies. Some of the RT-PCR based detection kits targeting genes are highlighted in Table 2 .
Table 2.
Commercial kit based on RT PCR principle targeting different genes.
2.1.6. Other general issues
The RT-PCR technique, however, has some flaws and constraints. Viral preservation solutions vary by different manufacturers so the sensitivity and quality of the detection kits, all may lead to the risk of obtaining false-negative results and thus compromising the accuracy of detection [16]. More importantly, the viral load may be undetectable in patients with persistent and frequent antiviral medications like anti-HIV drugs [21]. Therefore, some novel virus detection techniques with more precision than real-time RT-PCR are needed to be developed and implemented for accurate detections.
3. Isothermal nucleic acid amplification-based detection techniques
3.1. LAMP-based assay
Loop-mediated isothermal amplification or LAMP is a new isothermal nucleic acid amplification technique having a high efficacy. The LAMP-based detection technique shows enhanced sensitivity and specificity as it possesses the feature to amplify exponentially and six target sequences, which are detected by the four different primers concomitantly, and therefore DNAs and RNAs are usually amplified using this technique [9]. Expensive instrumentations or reagents are not required in the rapid LAMP-assay; thus implementation of the LAMP-based technique may help in the reduction of expenses during coronavirus detection [22]. The analysis of the amplified products is usually done via gel electrophoresis. The attainability of LAMP-based assay for SARS-virus detection was exhibited in a study where the ORF1b region was selected to detect the virus SARS-CoV-2 which was then amplified by LAMP technique using 6 primers, and the result so obtained after amplification has undergone gel electrophoresis [23]. It was found that the sensitivity and the rate of detection were the same as the conventional PCR-based detection technique. In another study, the LAMP technique was successfully implemented by executing agarose gel electrophoresis for diagnosing HCoV-NL63, with desired specificity and sensitivity in clinical specimens and cell cultures. The detection limit came around a copy of the RNA template per reaction [24]. The amplification is usually noticed by the fluorescence dye or magnesium pyrophosphate which allowed real-time analysis of the fluorescence or turbidity of pyrophosphate which has efficiently surpassed the endpoint detection limitations [25].
The RT-lamp technique was refined with the help of a quenching probe (QProbe), which was used to detect signs and have similar efficiency to real-time RT-PCR [26]. To make the LAMP-detection technique more reliable researchers replaced the intercalating dye and were implemented for sequence-specific validation of LAMP amplicons on a real-time basis [27]. However, the optimum temperature for LAMP-assay's efficient performance is around 65 °C, thus limiting its utilization. Another type of LAMP-based technique involving the use of phosphorothioated primers, known as PS-LAMP, was developed which allowed the more effective formation of the hairpin and growing concatamers' termini extension, thus enabling the technique to function at relatively lower temperatures, and specific and sensitive detection of amplicons was possible at around 40 °C [28]. It does not have sufficient supportive literature like RT-PCR for detecting SARS-CoV-2 and its clinical setting is being assessed [21].
3.2. RCA-based techniques
The rolling circle amplification (RCA) can amplify the signal of each circle up to 109-fold within 90 min, and therefore, RCA has gathered remarkable attention in the determination of nucleic acid. To detect SARS-CoV using RCA, an effective assay was set up in solid as well as liquid phases, and on a fewer number of respiratory specimens, the initial results were put up. The RCA can be carried out under isothermal conditions without obtaining any false-positive results and requires minimum reagents, thus making it a better alternative for detection techniques based on PCR [10]. However, this technique is not deployed for the detection of the SARS-CoV-2 virus and should be worked on for its further clinical trial.
4. Biosensor-based virus detection techniques
Due to the affordable instrumentation, easy operation, fast analysis proficiency, and minimum or no sample pre-treatment, the biosensors can serve to be a potential method to rationalize diagnostic protocols for viruses [12]. Therefore, biosensing techniques have been developed into the detection tools for specific, rapid detection of pathogens.
The virus biosensors based on affinity interactions are categorized into four groups [29] based on the viral targets and affinity reagents. They are immune/ antibody-based biosensors, DNA-based biosensors, cell-based biosensors, and antigen-based biosensors. Immunosensor is a kind of biosensor which mainly depends on the antigen-antibody specific interactions resulting in generations of responses that can be quantified and assessed. Aptamers also can recognize the viral antigens of interest with high affinity and precision. These aptamers either include peptide molecules comprised of approximately 40 to 60 bases or single-stranded DNA/RNA or peptides. The Cell-SELEX (Systematic Evolution of Ligands by exponential enrichment) method is used to select aptamers from the mixture of random oligonucleic acid [30]. The peptide aptamers are manipulated proteins comprised of specific target binding sites situated at peptide loops or surfaces, and thus, these peptide aptamers mimic the function of antibodies [31]. By acquiring a three-dimensionally preferred orientation that distinguishes targets based on fine structural variations, the virus targets are identified by the DNA aptamers [32]. RNA aptamer-based detection of C-terminal region of N protein of SARS virus was developed by Ahn et al., which could detect the antigen up to 2 pg/ml concentration and with a dissociation constant of 1.65 nm [33].
The diagnostic applications of antigenic probe-based biosensors mostly rely on the detection of surface antigens such as nucleocapsid and envelope proteins or the whole virus particles using antigen-specific antibodies originated from patient sera [34,35]. The reliability of these antigen probe-based biosensors is constrained by the number of antibodies produced during various stages of infection, similar to the conventional enzyme-linked assays and serological evaluations.
Nucleic acid hybridization is the principle on which the DNA-based biosensors function. Short single-stranded DNAs consisting of around 20–40 base pairs are immobilized on the surface of the sensor retaining their reactivity, accessibility, and stability towards the target strands of DNA. Later peptide nucleic acids (PNAs) and their use were emphasized in biosensing techniques. PNAs are remarkably stable and can hybridize rapidly, strongly, and specifically, which makes them an essential and propitious tool in the detection of DNA [36].
Cytopathic effects (CPEs) like membrane degradation, degenerative morphological changes, detachment, and eventual cell death in cell cultures occur as a result of an infection [37]. The cell-attached biosensors quantitatively detect the signals induced by these CPEs in the form of optical signals, electrochemical resistance [38], and conductance, which enables the detection/diagnosis, studying, and monitoring of infections caused by viruses. Cell-based biosensors have a very high capability to detect infection causing virus particles.
4.1. Plasmonic biosensors
Pathogens like viruses and bacteria and the components associated with them, which are airborne and cannot be seen with naked eyes; pose a significant threat to health even when present in small amounts. To quantify the total bioaerosol and accurately detect the presence of SARS-CoV-2, plasmonic biosensors have been developed [39]. A localized surface plasmon resonance biosensor (LSPR) relying on succinimidyl-ester-functionalized gold nanoislands (SEF-AuNIs) was developed to quickly analyze the total concentration of bioaerosol [40].
The combination of LSPR sensing transduction and plasmonic photothermal (PPT) effect along with the plasmonic biosensor, possessing a dual functionality, gives an assured solution to the issue of SARS-CoV-2 detection. The AuNIs can carry out the detection of selected sequences from SARS-CoV-2 sensitively via the nucleic acid hybridization when functionalized with cDNA receptors. The AuNIs chip generates thermo-plasmonic heat when they are illuminated at their plasmonic resonance frequency, to enhance the sensing performance. The escalation in the in situ hybridization temperature is regulated by the localized PPT heat, thus facilitating the precise differentiation of two gene sequences that are alike. High sensitivity with a lower detection limit as low as 0.22 pM is exhibited by the LSPR biosensor, thus enabling accurate detection of the specified target in a mixture of multiple genes. As this virus sensor can provide fast detection and continuous monitoring of the ambient viruses, definitely function as an alternative to the gold standard, i.e., the PCR-based Covid-19 detection platforms [40]. For the application of this technique, certain drawbacks of SPR like the non-specific binding on the SPR disc, steric hindrance due to immobilization of receptors, difficulty to detect small molecules can be overcome by effective modification of sensor chip [41].
4.2. Bioelectric biosensor
A novel biosensor for the detection of the S1-spike protein of SARS-CoV-2 was developed for the steady monitoring of affected patients, especially the asymptomatic ones. The changes in the bioelectric responses of membrane-engineered mammalian Vero cells consisting of the human chimeric spike S1 antibody is measured by the biosensor according to the bioelectric recognition assay principle [42] and the membrane engineering-based molecular identification technology [43]. It was demonstrated that a substantial and marked change in the cellular bioelectric properties occurred due to the adherence of spike protein to the membrane-bound antibodies. Additionally, any cross-reactivity against the SARS-CoV-2 nucleocapsid protein was not reported. The detection with high specificity (pg/ng level) and selectivity of viral antigen as possible within 3 min using this biosensor, and did not require any prior processing of sample [44]. The reduced cell viability is a great concern for cell-based biosensors.
4.3. Graphene FET-based antigen/antibody biosensors
Graphene is a chemically stable two-dimensional material exhibiting extremely well electronic properties. The Gr-FET (graphene field-effect transistor), which has an unmatched sensitivity, was combined with highly specific antigen/antibody interaction for the development of immunosensors, which can rapidly detect SARS-CoV-2 with ease. GrFET involves the use of FET structure with graphene acting as the channel material and the body fluid environment acting as the liquid gate. The graphene surface is non-covalently crosslinked with either the human's ACE2 receptor or the S1 subunit antibody (CSAb) of spike glycoprotein [45]. The SARS-CoV-2's spike glycoprotein S1 antigen attaches to the CSAb and ACE2 on the graphene surface during the test [46], which results in GrFET's source-drain conductance change through field effect.
Presently, at the laboratory stage, the GrFET can detect concentrations as low as 0.1 nM and 0.2 pM of ACE2 and CSAb, respectively. If a linear sensing response is assumed, a LOD (limit of detection) as low as around 10 fM can be deduced at a signal-to-noise ratio of 1. Hence, these GrFET based antibody/antigen biosensors may serve as a potential alternative in achieving early diagnosis and help in better management of the crisis [47]. However, the set-up deals with great cost, high concentration of antibodies (250 μg/ml), and low throughput instrumentations.
5. Microarray-based detection techniques
Microarray detection is a high throughput technique with efficient performance in pathogen detection and quick results. Production of cDNA labeled with specified probes via reverse transcription from the coronavirus RNA is a pre-requisite for this method. These labeled cDNAs are then loaded into wells and are hybridized with fixed oligonucleotides in solid-phase on the microarray. This step is then followed by sequential steps of washing to omit the free DNAs. The detection of RNA from CoV is followed by using specified probes. The rapidity, specificity, and accuracy of microarray-based detection make it a superior choice for CoV-detection [13]. This test requires a high cost therefore, low-density non-fluorescent oligonucleotides are developed which can minimize the expense.
Following the sequence of TOR2, a 60-mer oligonucleotide microarray was successfully designed and implemented for the detection of SARS-CoV in the patient samples [48]. Due to the quick and unexpected mutations in the SARS-CoV, a new microarray technique to detect 24 SNP mutations amidst the gene encoding the spike (S) protein of SARS-CoV was developed, having 100% accuracy in detection of the sample. The foremost care should be taken that the diagnostic methods are efficient enough to for the broad-spectrum detection of coronavirus and can be easily deployed near/at POC, as there might be a sudden CoV outburst. The efficiency and sensitivity of this array were somewhat similar to that of the real-time RT-PCR [49]. The evaluation of the MAP (mobile analysis platform) which is based on the microarray chip, is a new, near-POC, and compact diagnostic platform. Its efficiency in the detection of viruses was remarkable [50]. However sufficient literature is lacking to implement this method in detecting SARS-CoV-2.
6. Serological tests for COVID-19
Blood-based tests are a potential alternative for detecting COVID-19 as they require a relatively short time to diagnose, and the active immune response against the virus can be very efficiently tested. The probable strategy for COVID-19 detection may include a test like western blotting, which identifies the viral proteins, and an enzyme-linked test like ELISA which identifies the patient's antibodies against the virus, thus enabling quick detection of antibodies against the viral proteins or the viral protein itself, with the help of only the serum. Out of the four major structural proteins of the SARS-CoV-2, have structural similarity to SARS-CoV [51], i.e., membrane (M), spike (S), envelope (E) which provides viroporin activity to the virus [52], and nucleocapsid (N), the spike protein has been primarily reported to have interactions with the host cells. The receptor-binding domain of spike protein can be used for lateral flow immunochemical devices [53]. However, it is still not confirmed whether most of the antibodies that are raised on SARS-CoV-2 infection are against S protein or for other components of the virus. As soon as the suspected antigenic protein is identified, antibodies against the antigen can be produced in the animals, which can lead to the production of antibodies that would probably react with any of the viral proteins during the western blotting and may even provide ELISA's protein anchor. On the ELISA, the proteins must be presented in the same way they are presented in the body, or else there might be a chance of obtaining a false-negative result due to the failure of antibody attachment. After the anchor is prepared, a secondary antibody could be developed, which interacts with the human antibodies attached to the anchor, giving a fluorimetric or colorimetric outcome that can be analyzed by quantification. It was suggested in a recent study that the monoclonal antibodies, i.e., CR3022, efficiently identified the S protein of SARS-CoV-2 [44]. Another recent study suggested that the N-protein and S-protein based ELISA, being highly sensitive, can play a crucial role during the screening of COVID-19. ELISA can be useful in eliminating the other febrile patients from the group of COVID-19 infected people and hence can help in better management of the disease [54]. The serological tests are based on the production of antibodies, where the immediate infection fails to give results. In the case of SARS-CoV-2 infection, antibodies are developed after three days of onset of symptoms or 1 week post-infection. Therefore it fails to give output for initial infection and mild infection in patients who fail to develop the antibodies [21].
7. NGS based platforms: a hope for rapid SARS-CoV-2 detection
Next-Generation Sequencing (NGS) can play a crucial role in the detection and characterization of viruses posing a threat to humanity. The NGS platforms, which were used for worldwide research on the Human Genome Project, are now going to be implemented for SARS-CoV-2 detection. One cancer molecular diagnostic company, “Billion To One (BTO)” [55] has designed a technique called the qSanger-COVID-19 test, which can run the detection as fast as 30 times the standard qPCR techniques. BTO has applied for EUA approval by the FDA.
Several other NGS-based COVID-19 kits are being employed for the observation of mutations in the viral population. Youseq, a company from the United Kingdom, has developed an exceptionally sensitive SARS-CoV-2 Coronavirus NGS Library Prep Kit which functions well in low viral titers. This kit is sufficient and does not require any additional reagents [56]. A Chinese company, BGI Biotechnology, introduced a metagenomic sequencing detection kit, which can diagnose and detect the novel coronavirus as well as other coronaviruses and respiratory disorders, and thus can rapid detection of viral sequences can be attained. The NMPA (National Medical Products Administration) of China sanctioned the testing kits called NGS COVID-19 kit, designed by the company BGI Biotechnology [14].
8. CRISPR-Cas-based SARS-CoV-2 detection
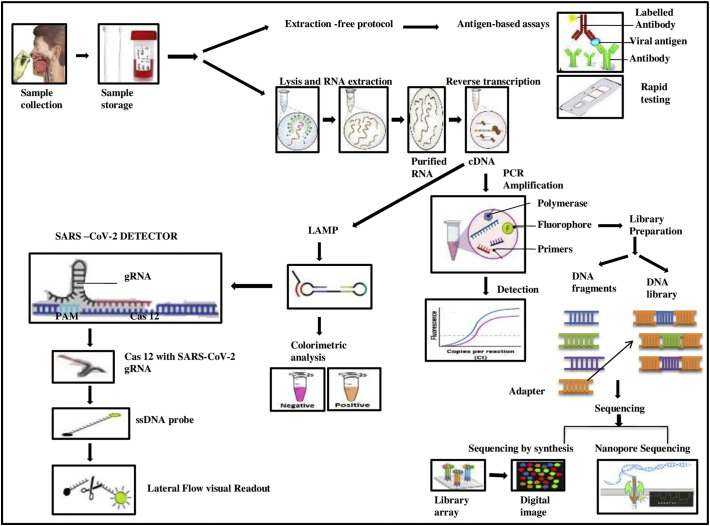
“Clusters of regularly interspaced short palindromic repeats” abbreviated as “CRISPR” have the repeated sequences of nucleotides and small bits of spacer sequences and CAS stands for CRISPR-associated proteins which function as nuclease enzyme. These are found as bacterial defense systems protecting them foreign invaders and are now widely employed for RNA editing, gene therapy, and even in the detection of viral genome detection. In recent years, CRISPR is being widely employed in the in vitro diagnostic field because of its allele specificity, which is critical for its successful application in the development of high-precision treatment and diagnosis. CRISPR-based DETECTR assay targets the E and the N2 gene of SARS-CoV-2 based on the CRISPR-Cas 12 system [57]. A lateral flow strip was developed where the probes were tagged with the streptavidin-biotin complex. This can detect as low as 10 copies per microlitre of viral RNA at about 40 min.
CRISPR-Cas13 is another such system used for rapid nucleic acid-sensing [58]. It was demonstrated in a recent study that Cas13 could affect and knockdown the genomes of many single-stranded RNA viruses that reside in mammals. The enzyme can be programmed to target these viruses, followed by their inhibition [59]. CRISPR-Cas13 system briefly includes the following three steps [60]: (1) recombinase polymerase amplification (RPA) for 25 min by isothermal incubation; (2) CRISPR-Cas13 based detection of the amplified viral RNA after incubation for 30 min; (3) a paper-dipstick based detection for displaying the result after 2 min incubation. Nucleic acid extraction to dipstick display employed for this method takes about an hour. Beginning from nucleic acid extraction, which is commonly employed for qRT-PCR tests, it is supposed to be completed in 1 h. Very similar to SHERLOCK diagnostic TEST another All-in-one dual CRISPR- Cas 12a (AIOD-CRISPR) assay was developed which is a very rapid and ultrasensitive visual detection method. The limit of detection is up to 5-11copies of viral RNA molecule per microlitre in about 90 min [61]. The scientists from the Council of Scientific & Industrial Research-Institute of Genomics and Integrative Biology (CSIR-IGIB) in New Delhi developed a kit named FnCas9 Editor Linked Uniform Detection Assay (FELUDA). This test kit uses CRISPR-Cas9 gene-editing protocol to target the genetic material of Sars-CoV-2 and can detect it within an hour [62].
9. Digital methods of SARS-CoV-2 detection mostly in asymptomatic patients
A hypothesis is being made regarding detection of COVID-19 asymptomatic subjects, using forced-cough cell phone recording harvesting Artificial Intelligence [63]. A survey was done through the MIT website (opensigma.mit.edu) by collecting COVID-19 cough recordings of 5320 people. A framework of AI speech processing has been developed which utilizes acoustic biomarker extractors and convert the recordings to Convolutional Neural Network (CNN) based architecture to pre-screen patients from cough recordings. It further develops a saliency map to monitor patients on a real-time basis, serves as a non-invasive method of detection with zero variable cost. This CNN-based method had been trained in 4256 samples and validated on 1064 samples.
A speech modeling and signal-processing framework have been proposed by Quatieri et al. from MIT to detect asymptomatic and symptomatic states of COVID-19 [64]. This framework based on complex coordination pattern among neuromotor of speech subsystems indulged in articulation, respiration, and phonation which is motivated by the specific feature of COVID-19 which involves lower (bronchial, diaphragm, lower tracheal) concerning the upper (laryngeal, pharyngeal, oral and nasal) respiratory tract inflammation, also by the increased prove of the virus' neurological display. The outcome was reduced complexity coordination patterns of subsystem among COVID 19 patients. This provides a direction of scalable, longitudinal analysis to study human behavior dynamics in naturalistic environments to track COVID-19 patients. A team led by Kylie Foy at MIT processed speech recordings of COVID-19 asymptomatic patients [65]. They found proof of vocal biomarkers as indicators, to predict the disease. These biomarkers are consequences of infection produced due to disruptions of muscle movement across the respiratory, laryngeal, and articulatory systems.
10. Conclusion
Presently, the COVID-19 diagnosis primarily relies on coronavirus RNA detection. The RT-PCR-based platforms are being widely practiced for the detection of SARS-CoV-2. Real-time RT-PCR would show the highest sensitivity and specificity among all available methods. RT PCR technique has some flaws which have been discussed in our review. RT-PCR may cause false-negative results when clinical specimens contained only low copy numbers of viral RNAs or they were subjected to inadequate procedures during the assays. Simple methods with small numbers of assay steps may reduce the risk of human errors, but in many cases, these types of kits/methods show lower sensitivity than real-time RT-PCR. The reduced sensitivity significantly elevates false-positive rates which are the weak-point of real-time RT-PCR, but they are also the case for most other methods. The various advantages and limitations of detection techniques are discussed in Table 3 . Nowadays multiplexed RT-PCR kits manufactured by several Healthcare units can detect two target sequences simultaneously, reverse transcription and PCR amplification are done in a single step with no cross-reactivity with other human coronaviruses, and thus required time also is reduced.
Table 3.
A comparison of different SARS-CoV-2 detection methods.
| Methods | Principle/ target of detection | Limit of detection | Time (h) | Merits |
|
Ref |
|---|---|---|---|---|---|---|
| RT-PCR | RNA template converted into cDNA which is amplified. Specific primer-probe based detection of viral RNA | 95%; 100 copies of RNA per ml of transport media (approx.) | 3–4 h |
|
|
[16,21] |
| RT-LAMP | Based on autocycling strand displacement DNA synthesis. Uses more than two sets of specific primer for detection. | a copy of the RNA template per reaction | 60–90 min |
|
|
[22] |
| NP antigen detection test | Viral antigen (nucleocapsid protein) detection. Point-of-care (PoC) test. |
0.58 copies per μl | 15–30 min |
|
|
[66] |
| Plasmonic biosensor | Use label free probe of biological analytes for detecting molecules (viral particles) at much lower concentration | 0.22 pM of viral particles | Few minutes |
|
|
[41] |
| Bioelectric biosensor | Uses biorecognition element that reacts with target and produce signal proportional to the concentration of the target. | 1 fg/ml | 3 min |
|
|
[44] |
| Microarray based techniques | The target DNA fragments with fluorescent probes bind with probes of DNA chip due to complementarity which is measured using fluorescence emission. | 100% | 10 min |
|
|
[13,50] |
| ELISA | Antibody detection using a specific antigen (enzyme substrate reaction). | 97.8% IgG | 4–6 h |
|
|
[21,54] |
| NGS based platform | Whole genome sequencing | 100% | 1–2 day |
|
|
[55,56] |
| CRISPR-Cas-based detection | Finds a specific bit of DNA inside a cell. | 10 copies per microlitre of viral RNA | 40–60 min | detection within minutes, low cost |
|
[59,61,62] |
| Digital platforms for detection/ tracking | By recording Cough or speech pattern or other physiological manifestations and applying AI | Can be detected symptomatic/ asymptomatic patients accurately | Few minutes | Yet not implemented in larger set up |
|
[[63], [64], [65]] |
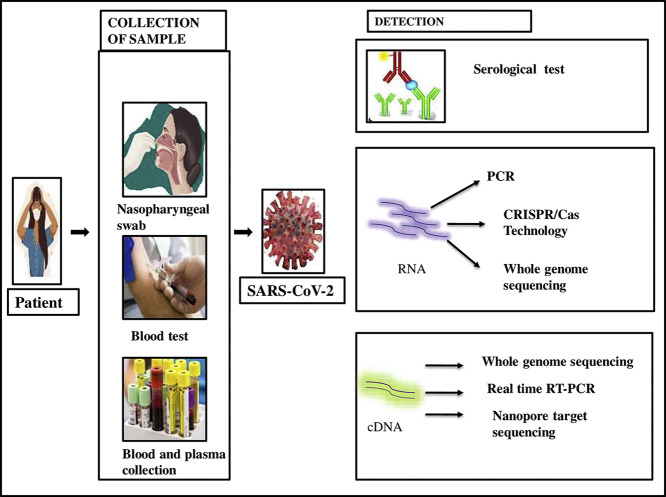
It is essential to choose the detection methods which are appropriate, accurate, sensitive, rapid, and specific. The collection of samples and various detection techniques are represented in Fig. 2 . Techniques like LAMP and Microarray-based detection have limitations in their implementation, e.g., LAMP requires a high temperature for its functioning, whereas microarray-based platforms are quite expensive. Thus, the development of more practical techniques with enhanced efficiency is the need of the hour. Significant attempts have been and are still being made to improve the diagnosis of COVID-19. The RT-PCR based methods are still the most reliable and can be a gold standard, and at least at present none of the new methods based on the sophisticated technology can be ‘alternative’ to real-time RT-PCR based methods. Modern sophisticated techniques definitely can be additional and supportive tools for diagnosis of COVID-19 and they can be the best for certain purposes, but would not be ‘alternative’ to real-time RT-PCR based methods for the first line of diagnosis of COVID-19 (detection of SARS-CoV-2). Some of the methods may be potentially applicable to asymptomatic or mild COVID-19 cases clinically, but may not be considered as the gold standard, or alternative to real-time RT-PCR. The RT may give a negative report when the test is performed at an early stage of infection due to lack of viral particle in the swab, secondly, improper collection of the swab. The antibody kits are based on predominantly IgG, or both for IgM and IgG. But when we are looking at picking up patients with active disease, testing for an IgG does not make much sense. This antibody test is not beneficial to test the infected cases but to record the percentage of the infected population. While doing a rapid antigen test, a positive result confirms the viral infection but the negative result is not reliable. One needs to follow up with RT-PCR to be sure about the infection status. The finest and the most economical alternative can be achieved by maintaining equilibrium between the disadvantages and advantages of several detection strategies according to specific purposes. Present CRISPR- Cas-based detection methods and immunosensors can directly measure the SARS-CoV-2 in a very short time and the limit of detection is very low, thus may be promising for the detection of samples having a very low viral load. These techniques may be very helpful for rapid diagnostic assays but yet to be implemented in a clinical setup. Therefore RT-PCR technique remains to be the most reliable method for COVID-19 diagnosis so far. However, if we consider the most up and coming technology in the field of medical diagnostics then we must take into consideration of brilliant prospects of CRISPR-Cas-based detection methods and their multifaceted biomedical applications.
Fig. 2.
Collection of sample and various detection techniques of SARS-CoV-2
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no competing interest.
Data availability
No data was used for the research described in the article.
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J.D., Drosten C., Gulyaeva A.A., Gulyaeva B.L., Penzar D. Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 2.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Bioscience trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 3.S.J. Fong, N. Dey and J. Chaki, An introduction to COVID-19, artificial intelligence for coronavirus outbreak. (2021) 1-22. Springer briefs in applied sciences and technology. Springer, Singapore.doi: 10.1007/978-981-15-5936-5_1. [DOI]
- 4.Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor symposia on quant. boil. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H.…Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. ISSN 0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik A.K., Dhau J.S., Gohel H., Mishra Y.K., Kateb B., Kim N.Y., Goswami D.Y. 2020. Electrochemical SARS-CoV-2 Sensing at Point-of-Care and Artificial Intelligence for Intelligent COVID-19 Management, ACS Applied Bio Materials. [DOI] [PubMed] [Google Scholar]
- 7.Cooper D.R., D’Anjou B., Ghattamaneni N., Harack B., Hilke M., Horth A.…Yu V. 2012. Experimental Review of Graphene, ISRN Condensed Matter Physics. [DOI] [Google Scholar]
- 8.Lei Y.M., Xiao M.M., Li Y.T., Xu L., Zhang H., Zhang Z.Y., Zhang G.J. Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens. Bioelectron. 2017;91:1–7. doi: 10.1016/j.bios.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12) doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.W.K. Wang, C.T. Fang, H.L. Chen, C.F. Yang, Y.C. Chen, M.L. Chen, S.Y. Chen, J.Y. Yang, J.H. Lin, P.C. Yang, S.C. Chang and Members of the SARS Research Group of National Taiwan University College of Medicine-National Taiwan University Hospital, Detection of severe acute respiratory syndrome coronavirus RNA in plasma during the course of infection, Journal of clinical microbiology, 43(2) (2005) 962–965. doi: 10.1128/JCM.43.2.962-965.2005. [DOI] [PMC free article] [PubMed]
- 11.Ma C., Jing H., Zhang P., Han L., Zhang M., Wang F., Niu S., Shi C. An ultrafast one-step assay for the visual detection of RNA virus. Chemical communications Cambridge, England. 2018;54(25):3118–3121. doi: 10.1039/c8cc00150b. [DOI] [PubMed] [Google Scholar]
- 12.Shinde S.B., Fernandes C.B., Patravale V.B. Recent trends in in-vitro nanodiagnostics for detection of pathogens. J. Control. Release. 2012;159(2):164–180. doi: 10.1016/j.jconrel.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Li J., Deng Z., Xiong W., Wang Q., Hu Y.Q. Comprehensive detection and identification of seven animal coronaviruses and human respiratory coronavirus 229E with a microarray hybridization assay. Inter virology. 2010;53(2):95–104. doi: 10.1159/000264199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R., Nagpal S., Kaushik S., Mendiratta S. COVID-19 diagnostic approaches: different roads to the same destination. Virus disease. 2020;31(2):97–105. doi: 10.1007/s13337-020-00599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R., Jervey S.R., Liu C. Assay techniques and test development for COVID-19 diagnosis. ACS central science. 2020;6(5):591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustin S.A., Nolan T. RT-qPCR testing of SARS-CoV-2: a primer. International Jl Molecular Sc. 2020;21(8):3004. doi: 10.3390/ijms21083004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Jl of molecular endocrinology. 2000;25(2):169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 18.van Elden L.J., van Loon A.M., van Alphen F., Hendriksen K.A., Hoepelman M.G. Van Kraaij, Oosterheert J.J., Schipper P., Schuurman R., Nijhuis M. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. The Journal of infectious diseases. 2004;189(4):652–657. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng W., Newbigging A.M., Le C., Pang B., Peng H., Cao Y., Wu J., Abbas G., Song J., Wang D.B., Cui M., Tao J., Tyrrell D.L., Zhang X.E., Zhang H., Le X.C. Molecular diagnosis of COVID-19: challenges and research needs. Anal. Chem. 2020;92(15):10196–10209. doi: 10.1021/acs.analchem.0c02060. [DOI] [PubMed] [Google Scholar]
- 20.Phan T. Genetic diversity and evolution of SARS-CoV-2, Infection, genetics and evolution. Journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younes N., Al-Sadeq D.W., Al-Jighefee H., Younes S., Al-Jamal O., Daas H.I., Yassine H.M., Nasrallah G.K. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12(6):582. doi: 10.3390/v12060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enosawa M., Kageyama S., Sawai K., Watanabe K., Notomi T., Onoe S., Mori Y., Yokomizo Y. Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. Jl of clinical microbiology. 2003;41(9):4359–4365. doi: 10.1128/jcm.41.9.4359-4365.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon L.L., Leung C.S., Tashiro M., Chan K.H., Wong B.W., Yuen K.Y., Guan Y., Peiris J.S. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop-mediated isothermal amplification assay. Clin. Chem. 2004;50(6):1050–1052. doi: 10.1373/clinchem.2004.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyrc K., Milewska A., Potempa J. Development of loop-mediated isothermal amplification assay for detection of human coronavirus-NL63. J. Virol. Methods. 2011;175(1):133–136. doi: 10.1016/j.jviromet.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori Y., Nagamine K., Tomita N., Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001;289(1):150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- 26.Shirato K., Semba S., El-Kafrawy S.A., Hassan A.M., Tolah A.M., Takayama I., Kageyama T., Notomi T., Kamitani W., Matsuyama S., Azhar E.I. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J. Virol. Methods. 2018;258:41–48. doi: 10.1016/j.jviromet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y.S., Stacy A., Whiteley M., Ellington A.D., Bhadra S. Amplicon competition enables end-point quantitation of nucleic acids following isothermal amplification. Chembiochem : a European journal of chemical biology. 2017;18(17):1692–1695. doi: 10.1002/cbic.201700317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai S., Jung C., Bhadra S., Ellington A.D. Phosphorothioated primers Lead to loop-mediated isothermal amplification at low temperatures. Anal. Chem. 2018;90(14):8290–8294. doi: 10.1021/acs.analchem.8b02062. [DOI] [PubMed] [Google Scholar]
- 29.Ozer T., Geiss B.J., Henry C.S. Review-chemical and biological sensors for viral detection. J. Electrochem. Soc. 2020;167(3) doi: 10.1149/2.0232003JES. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 31.Maity D., Manoharan M., Rajendra Kumar R.T. Development of the PANI/MWCNT nanocomposite-based fluorescent sensor for selective detection of aqueous Ammonia. ACS Omega. 2020;5(15):8414–8422. doi: 10.1021/acsomega.9b02885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaya S.I., Karadurmus L., Ozcelikay G., Bakirhan N.K., Ozkan S.A. Electrochemical virus detections with nanobiosensors. Nanosensors for Smart Cities. 2020:303–326. doi: 10.1016/B978-0-12-819870-4.00017-7. [DOI] [Google Scholar]
- 33.Ahn D.G., Jeon I.J., Kim J.D., Song M.S., Han S.R., Lee S.W., Jung H., Oh J.W. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst. 2009;134(9):1896–1901. doi: 10.1039/b906788d. [DOI] [PubMed] [Google Scholar]
- 34.de la Escosura-Muñiz A., Maltez-da Costa M., Sánchez-Espinel C., Díaz-Freitas B., Fernández-Suarez J., González-Fernández A., Merkoçi A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosensors & bioelectronics. 2010;26(4):1710–1714. doi: 10.1016/j.bios.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S., Kim D.K., Park T.J., Lee S.J., Lee S.Y. Label-free optical diagnosis of hepatitis B virus with genetically engineered fusion proteins. Talanta. 2010;82(2):803–809. doi: 10.1016/j.talanta.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 36.Pournaghi-Azar M.H., Ahour F., Hejazi M.S. Direct detection and discrimination of double-stranded oligonucleotide corresponding to hepatitis C virus genotype 3a using an electrochemical DNA biosensor based on peptide nucleic acid and double-stranded DNA hybridization. Anal. Bioanal. Chem. 2010;397(8):3581–3587. doi: 10.1007/s00216-010-3875-5. [DOI] [PubMed] [Google Scholar]
- 37.Campbell C.E., Laane M.M., Haugarvoll E., Giaever I. Monitoring viral-induced cell death using electric cell-substrate impedance sensing. Biosens. Bioelectron. 2007;23(4):536–542. doi: 10.1016/j.bios.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Kiilerich-Pedersen K., Poulsen C.R., Jain T., Rozlosnik N. Polymer based biosensor for rapid electrochemical detection of virus infection of human cells. Biosens. Bioelectron. 2011;28(1):386–392. doi: 10.1016/j.bios.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 39.Qiu G., Yue Y., Tang J., Zhao Y.B., Wang J. Total bioaerosol detection by a Succinimidyl-Ester-functionalized Plasmonic biosensor to reveal different characteristics at three locations in Switzerland. Environmental science & technology. 2020;54(3):1353–1362. doi: 10.1021/acs.est.9b05184. [DOI] [PubMed] [Google Scholar]
- 40.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional Plasmonic Photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 41.Antiochia R., Bollella P., Favero G., Mazzei F. Nanotechnology-based surface Plasmon resonance affinity biosensors for in vitro diagnostics. International journal of analytical chemistry. 2016;2981931 doi: 10.1155/2016/2981931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kintzios S., Pistola E., Panagiotopoulos P., Bomsel M., Alexandropoulos N., Bem F., Ekonomou G., Biselis J., Levin R. Bioelectric recognition assay (BERA) Biosens. Bioelectron. 2001;16(4–5):325–336. doi: 10.1016/s0956-5663(01)00127-0. [DOI] [PubMed] [Google Scholar]
- 43.Kokla A., Blouchos P., Livaniou E., Zikos C., Kakabakos S.E., Petrou P.S., Kintzios S. Visualization of the membrane engineering concept: evidence for the specific orientation of electro inserted antibodies and selective binding of target analytes. Journal of molecular recognition: JMR. 2013;26(12):627–632. doi: 10.1002/jmr.2304. [DOI] [PubMed] [Google Scholar]
- 44.Mavrikou S., Moschopoulou G., Tsekouras V., Kintzios S. Development of a Portable, Ultra-Rapid and Ultra-Sensitive Cell-Based Biosensor for the Direct Detection of the SARS-CoV-2 S1 Spike Protein Antigen. Sensors (Basel, Switzerland) 2020;20(11):E3121. doi: 10.3390/s20113121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu W., Nef C., Tarasov A., Wipf M., Stoop R., Knopfmacher O., Weiss M., Calame M., Schönenberger C. High mobility graphene ion-sensitive field-effect transistors by noncovalent functionalization. Nanoscale. 2013;5(24):12104–12110. doi: 10.1039/c3nr03940d. [DOI] [PubMed] [Google Scholar]
- 46.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging microbes & infections. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Qi Q., Jing Q., Ao S., Zhang Z., Ding M., Wu M., Liu K., Wang W., Ling Y., Zhang Z. Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor. arXiv preprint. 2020 https://arxiv.org/abs/2003.12529 arXiv:2003.12529. [Google Scholar]
- 48.Shi R., Ma W., Wu Q., Zhang B., Song Y., Guo Q., Xiao W., Wang Y., Zheng W. Design and application of 60mer oligonucleotide microarray in SARS coronavirus detection. Chinese science bulletin Kexue tongbao. 2003;48(12):1165–1169. doi: 10.1007/BF03183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza Luna L.K., Heiser V., Regamey N., Panning M., Drexler J.F., Mulangu S., Poon L., Baumgarte S., Haijema B.J., Kaiser L., Drosten C. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. J. Clin. Microbiol. 2007;45(3):1049–1052. doi: 10.1128/JCM.02426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.J. Hardick, D. Metzgar, L. Risen, C. Myers, M. Balansay, T. Malcom, T, ... and C. Gaydos, Initial performance evaluation of a spotted array Mobile Analysis Platform (MAP) for the detection of influenza A/B, RSV, and MERS coronavirus, Diagnostic microbiology and infectious disease. 91(3) (2018) 245–247. doi: 10.1016/j.diagmicrobio.2018.02.011. [DOI] [PMC free article] [PubMed]
- 51.Luo H., Ye F., Sun T., Yue L., Peng S., Chen J., Li G., Du Y., Xie Y., Yang Y., Shen J., Wang Y., Shen X., Jiang H. In vitro biochemical and thermodynamic characterization of nucleocapsid protein of SARS. Biophys. Chem. 2004;112(1):15–25. doi: 10.1016/j.bpc.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee S., Bhattacharyya D., Bhunia A. Host-membrane interacting interface of the SARS coronavirus envelope protein: immense functional potential of C-terminal domain. Biophys. Chem. 2020;266:106452. doi: 10.1016/j.bpc.2020.106452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerofolini L., Fragai M., Luchinat C., Ravera E. Orientation of immobilized antigens on common surfaces by a simple computational model: exposition of SARS-CoV-2 spike protein RBD epitopes. Biophys. Chem. 2020;265:106441. doi: 10.1016/j.bpc.2020.106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S., Xiong Z., Zheng S. Evaluation of Nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00461-20. e00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapid Microbiology. Next-Generation Sequencing Delivers 30x Faster COVID-19 Testing than qPCR. https://www.rapidmicrobiology.com/news/next-generation-sequencing-delivers-30x-faster-covid-19-testing-than-qpcr Accessed April 22, 2020.
- 56.Youseq SARS-COV-2 coronavirus NGS library prep kit. https://youseq.com/product/sars-cov-2-coronavirus-ngs-library-prep-kit/15
- 57.J.P. Broughton, X. Deng, G. Yu, C.L. Fasching, V. Servellita, J. Singh, …. C.Y. Chi, CRISPR–Cas12- based detection of SARS-CoV-2, Nat Biotechnol. (2020). https:// 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed]
- 58.Gootenberg J.S., Abudayyeh O.O., Lee J.W., Essletzbichler P., Dy A.J., Joung J., Verdine V., Donghia N., Daringer N.M., Freije C.A., Myhrvold C., Bhattacharyya R.P., Livny J., Regev A., Koonin E.V., Hung D.T., Sabeti P.C., Collins J.J., Zhang F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science (New York, N.Y) 2017;356(6336):438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freije C.A., Myhrvold C., Boehm C.K., Lin A.E., Welch N.L., Carter A., Metsky H., Luo C.Y., Abudayyeh O.O., Gootenberg J.S., Yozwiak N.L., Zhang F., Sabeti P.C. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Molecular cell. 2019;76(5) doi: 10.1016/j.molcel.2019.09.013. 826–837.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z., Cui W. CRISPR-Cas system for biomedical diagnostic platforms. VIEW. 2020;1(3) doi: 10.1002/VIW.20200008. 1:20200008. [DOI] [Google Scholar]
- 61.Ding X., Yin K., Li Z., Liu C. 2020. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV Virus, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azhar M., Phutela R., Ansari A.H., Sinha D., Sharma N., Kumar M., Aich M., Sharma S., Singhal K., Lad H., Patra P.K., Makharia G., Chandak G.R., Chakraborty D., Maiti S. Rapid, field-deployable nucleobase detection and identification using FnCas9. bioRxiv. 2020 doi: 10.1101/2020.04.07.028167. [DOI] [Google Scholar]
- 63.J. Laguarta, F. Hueto and B. Subirana, COVID-19 artificial intelligence diagnosis using only cough recordings, IEEE Open Journal of Engineering in Medicine and Biology. doi: 10.1109/OJEMB.2020.3026928. [DOI] [PMC free article] [PubMed]
- 64.Quatieri T.F., Talkar T., Palmer J.S. IEEE Open Journal of Engineering in Medicine and Biology. Vol. 1. 2020. A Framework for Biomarkers of COVID-19 Based on Coordination of Speech-Production Subsystems; pp. 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.News M.I.T. Signs of COVID-19 may be hidden in speech signals. https://medicalxpress.com/news/2020-07-covid-hidden-speech.html
- 66.La Marca A., Capuzzo M., Paglia T., Roli L., Trenti T., Nelson S.M. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. BioMed. Online. 2020;41(3):483–499. doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Kasteren P.B., van der Veer B., van den Brink S., Wijsman L., de Jonge J., van den Brandt A., Molenkamp R., Reusken C., Meijer A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2020;128 doi: 10.1016/j.jcv.2020.104412. 104412. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.