Abstract
Alveoli are the gas-exchanging units of the lung, and the alveolar barrier is often a key battleground where pathogens, allergens, and other insults from the environment are encountered. This is seen in the current coronavirus disease 2019 (COVID-19) pandemic, as alveolar epithelium is one of the major targets of SARS-COV-2, the virus that causes COVID-19. Thus, it is essential to understand the mechanisms in order to maintain the integrity of alveoli epithelium. Alveolar type II (AT2) cells behave as tissue stem cells that repair alveoli epithelium during steady-state replacement and after injury. However, not all AT2 cells are equal in their ability for self-renewal or differentiation. Through marker gene identification, lineage tracing, and single-cell RNA-sequencing (scRNA-seq), distinct subpopulations of AT2 cells have been identified that play the progenitor role in a different context. The revelation of AT2 heterogeneity has brought new insights into the role of AT2 cells in various lung disease settings and potentiates the finding of more therapeutics targets. In this mini review, we discuss the recently identified subpopulations of AT2 cells and their functions under steady-state, postinjury, and pathological conditions.
Keywords: alveoli, lung, progenitor, stem cells, type II cells
INTRODUCTION
The gas-exchange units of the lung are composed of millions of saccular structures named alveoli (44). The alveolar epithelium consists of two types of cells: alveolar type I cells (AT1) and alveolar type II cells (AT2) (44). AT1 cells cover ∼95–98% of the alveolar surface area, and they provide a thin interface above the underlining lung microvascular endothelium, forming a structure efficient for gas exchange (44). There are nearly twice as manyAT2 cells as AT1 cells (9); however, because of their cuboidal shape, AT2 occupy only 2–5% of the surface area (28). AT2 cells have multiple functions, including secreting surfactant, transporting sodium and fluids, and modulating immune responses (28). Importantly, AT2 cells also act as adult tissue stem cells in lung homeostatic maintenance, repair, and regeneration (2, 10, 11). Alveoli epithelium is constantly exposed to various insults from the environment (43) and is one of the major targets of SARS-CoV-2, the coronavirus that causes the current COVID-19 pandemic (3, 18, 37, 48). SARS-CoV-2 attacks AT2 through cell surface receptor angiotensin-converting enzyme 2 (ACE2) (16, 18). AT2 also express several other molecules that are critical for SARS-CoV-2 entry, including transmembrane serine protease 2 (TMPRSS2), cathepsin L (CTSL), Basigin (BSG, or CD147) and Furin (42). Thus, there is an urgent need to understand the physiological and pathological response of AT2 toward various environmental stimuli and to identify the mechanisms of alveolar epithelial replacement, repair, and regeneration during steady-state homeostasis, as well as after injury.
The lung epithelium is largely quiescent under normal conditions, but it can acquire remarkable reparative capacity after injury (2, 10). Different from tissues with a faster turnover rate, such as the intestine, the hematopoietic system, and the skin, which utilize a dedicated pool of underdifferentiated stem cells for tissue regeneration (31), the alveoli epithelium mainly relies on facultative progenitor cells, which are differentiated mature cells with specific functions but can be induced to self-renew and/or differentiate into other cell types when needed (17). On the basis of previous in vivo and in vitro studies, AT2 cells have been firmly established as tissue stem cells that repair the injured alveoli epithelium through self-renewal and differentiation into AT1 cells (2, 10–12, 26, 27, 36). Recently, with the development of state-of-the-art technologies, such as lineage tracing and single-cell RNA sequencing (scRNA-seq), AT2 have been shown to be a heterogeneous population, and specific subgroups of AT2 have been identified that may execute the progenitor role in various contexts of homeostasis and repair (30, 47). The identification and characterization of these subpopulations of AT2 cells will help address some of the key questions regarding the mechanisms of alveolar maintenance and repair; for example, what are the intrinsic signals that drive normally quiescent AT2 cells to enter a regenerative program, and what are the niche components that regulate AT2 cell regeneration?
AT2 SUBPOPULATIONS INVOLVED IN HOMEOSTATIC MAINTENANCE
The recently developed lineage tracing mice models allow long-term tracking of the AT2 cell fate. In these mice, Cre recombinase is specifically expressed in AT2 cells through the surfactant protein C (Sftpc) or the lysozyme M (Lyz2) promoters (2, 10). After as much as 16 mo tracking, it was found that in the steady-state condition, AT2 cells are primarily replenished by self-renewal rather than by being derived from other cell types (2, 10). Furthermore, these studies revealed that a subset of the AT2 cells exhibited higher clonogenic potential than others and formed discrete “renewal foci” (2, 10). Only ∼1% of AT2 cells displayed progenitor function by producing small clusters of descendant cells that comprised AT1 and AT2 cells (2, 10). The clusters of renewal cells were more frequently detected at perivascular regions and at the edge of the lungs, which suggest that these discrete locations behave as “hot spots” for alveolar cell renewal (2, 10).
These observations indicate heterogeneity of the AT2 cell population. Consistent with these observations, we have found a small population of CD44high AT2 cells that constitutes ∼3% of the total AT2 population; these are preferentially located at the perivascular regions, resembling the “hotspot” area (6, 10). CD44 is a receptor for hyaluronan and is a surface marker for various stem cells (49). Compared with other AT2, the CD44high AT2 cells exhibit a higher proliferation rate in uninjured lungs. In culture, these cells had a higher potential for giving rise to AT1 and generated larger three-dimensional (3D) organoids than the bulk CD44low AT2 (6). These phenotypes indicate the progenitor potential of these cells. However, since CD44 is expressed in many cell types, a more sophisticated lineage tracing system that labels both CD44 and AT2 markers is needed to further study the in vivo behaviors of these cells.
Wnt signaling is activated in various tissue stem cells, and it is also essential for the development of AT1 and AT2 cells (17). Recently, two elegant studies showed that a group of Axin2+ Wnt-responsive AT2 cells that were sporadically distributed throughout the alveoli were a potential progenitor subpopulation of AT2 cells (30, 47). However, there is some discrepancy between these two studies regarding the dynamics of these cells under steady-state conditions. One study reported that only ∼1% of the total AT2 cells were Axin2+, and this population expanded six-folds over a 12-mo period (30). Whereas in the second study, the authors estimated that Axin2+ cells constitute ∼20% of the AT2, and the number of this population was stable over a 9-mo tracking period (47). Therefore, it is unclear whether the Axin2+ AT2 cells contribute more to the homeostatic replacement compared with bulk AT2 cells. Nonetheless, both studies showed that Wnt signaling is activated in the Axin2+ AT2, and inhibition of Wnt promotes the cells to differentiate into AT1 cells (30, 47). Moreover, a group of fibroblast cells located adjacent to these Axin2+ AT2 were found to produce several Wnt ligands and, thus, may serve as niches of this AT2 subpopulation (30). Furthermore, from gene expression profiling of the Axin2+ AT2 cells, a cell membrane protein TM4SF1 was identified as a potential surface marker of these cells, and this protein was also found to be expressed in ∼30% of the human AT2 cells. The TM4SF1+ AT2 cells in humans were similar to the mice Axin2+ AT2 cells, in that both have enriched expression of several Wnt target genes, and both showed higher clonogenic potential than other bulk AT2 in 3D organoid culture (47), suggesting a superior progenitor potential of these cells.
These studies showed that small subpopulations of AT2 cells have higher progenitor activity and are likely responsible for the steady-state homeostasis. The discrepancy in the cell number and dynamics of the two studies regarding Axin2+ AT2 might exist because AT2 cells have a gradient of Axin2 expression levels, and the two independent studies with slightly different labeling techniques, labeled AT2 with different threshold of Axin2 expression. It would also be interesting to determine whether there is any overlap between the Axin2+ AT2 and the CD44high AT2.
AT2 SUBPOPULATIONS INVOLVED IN REGENERATION AFTER INJURY
In contrast to the tiny percentage of AT2 cells involved in steady-state maintenance, a significantly larger fraction of AT2 can be recruited to exit the quiescence and enter the repair/regeneration programs in response to injury. The percentage of AT2 involved in repair depends on the type and extent of the injury. For example, after injury induced by bacteria Pseudomonas aeruginosa, 30–70% of the AT2 started to express Sca-1 (stem cell antigen 1, or Ly6a) in repair phase (26, 27). The Sca-1+ AT2 cells showed higher proliferation rate and higher potential to differentiate into AT1, thus likely to be the cells involved in repair (26, 27).
It appears that most of the AT2 cells that engaged in repair are “activated” by mechanisms somewhat different from those in steady-state homeostasis. The above mentioned Axin2 lineage tracing studies has also provided insight into the AT2 “activation” mechanism during repair. Nabhan et al. (30) showed that while only ∼1% of AT2 were Axin2+ under normal condition, a majority of AT2 cells started to express Axin2 and proliferate in response to a diphtheria toxin-induced injury (30). Most of the Axin2+ AT2 cells that appeared postinjury were likely induced from bulk AT2 cells that were Axin2− before (30). Moreover, distinct from the steady-state conditions in which the Wnt signaling in Axin2+ AT2 cells were induced by adjacent ligand-expressing niche fibroblasts, AT2 cells postinjury initiated an autocrine or paracrine signal amplification mechanism, in that the AT2 cells themselves started to express Wnt ligands to further activate Wnt signaling in neighboring AT2 cells (30).
Recent development of single-cell RNA sequencing (scRNA-seq) techniques provides an unprecedented tool to characterize tissues at single-cell levels and to detect previously unknown cell populations. From these studies, reparative AT2 subpopulations that only appear during the repair process were identified, and some of these newly identified subpopulations appear to be the intermediate cells at distinct stages during the repair process. A study by Riemondy et al. (35) showed that three AT2 subgroups appeared after LPS-induced lung injury, and these AT2 subsets appeared to undergo distinct reparative steps, i.e., cell proliferation, cell-cycle arrest, and transdifferentiation into AT1, respectively. Intermediate cells were also detected during lung regeneration after pneumonectomy (45).
More recently, three independent studies have been published showing similar intermediate cells by scRNA-seq analysis using the bleomycin injury model (7, 22, 39, 41). In a study by Strunz et al. (39), a longitudinal scRNA-seq study was carried out using epithelial cells collected at six timepoints after bleomycin-induced acute lung injury. A cluster of Krt8+ cells was found to transiently appear after the injury, and these cells are in the trajectory to transit into AT1 cells (39). RNA velocity suggested a dual origin of the Krt8+ cells from AT2 and subsets of club cells (39). In a study by Kobayashi et al. (22), AT2 cells were grown in 3D culture to generate alveolar organoids, scRNA-seq analysis was performed using cells isolated from those organoids and an intermediate cell population that express Cldn4, Ctgf, Krt19, and Sfn were identified. Studies using Ctgf-GFP and Krt19-CreER lineage-labeling mice combined with in vivo injury models further supported that these cells are in the middle of transition from AT2 to AT1 cells (22). In the study by Choi et al. (7), similar transient populations were identified, and in addition, these intermediate populations appear to be activated by IL1β signaling.
The intermediate cells identified from the above three studies were termed Krt8+ alveolar differentiation intermediate (ADI) (39), prealveolar type-1 transitional cell state (22), or damage-associated transition progenitors (7) in different studies. However, all these three groups of cells appear very similar to each other. These cells express low levels of both AT2 and AT1 marker genes. Although rarely detectable in uninjured lungs, their numbers increase significantly after injury. RNA velocity analysis of the scRNA-seq data and lineage tracing studies both supported a trajectory of these intermediate cells toward AT1 cell fate. All three groups of cells expressed very similar sets of signature genes, including Krt8 and Cldn4, and all of these populations showed signs of activated P53, TGFβ, and cell senescence pathways. These studies indicate that these populations represent a largely overlapping, if not identical, population (7, 22, 39, 41). In addition, these cells also appear similar with the “cell cycle arrest subpopulation”, identified by Riemondy et al. (35), or intermediate cells, identified by Wu et al. (45). Furthermore, the Sca-1+ cells that we identified earlier also expressed higher levels of Krt8 and Cldn4 (26), and the ADI cells recently identified showed higher expression of Sca-1 (Ly6a) (39). Thus, there is also likely overlap between these cell populations. One potential limitation in these studies is that type II to type I cell transition likely includes a number of sequential steps and, thus, the intermediate populations identified in these studies may include a mixture of various uncharacterized transient subgroups. Therefore, it would be helpful to precisely synchronize a population of AT2 cells undergoing AT1 cell transition to determine these intermediate steps. In addition, how these intermediate cells are related to distinct subgroups of AT2 cells, for example, the Axin2+ AT2 cells (30, 47), is still unclear.
One important reason to study these “activated” or “intermediate” AT2 cells appearing after injury is to identify genes specifically expressed in these cells, and by determining the function of these genes, the molecular mechanisms that drive AT2 cell-mediated alveolar repair can be elucidated. For example, from studies of genes expressed in the Sca-1+ AT2 cells, we have identified that transcription factor FoxM1 plays an essential role in the reparative type II cells (27). We also found temporally regulated Notch activation by Dlk1 is required for AT2 to AT1 cell transition, and aberrant Notch signaling resulted in an accumulation of intermediate cells in the middle of AT2 to AT1 cell transition (12). These recent scRNA-seq studies have identified more of the mechanisms involved in AT2 to AT1 transition. These include p53 and senescence (22), TGFβ signaling (35), as well as IL-1β/IL-1r1-mediated inflammatory signaling (7). These unbiased high-throughput studies will lead to the discovery of unexpected novel mechanisms in AT2 to AT1 cell transition. In addition, these studies can also give us new mechanistic insight into some of the signaling pathways previously shown to be involved in AT2 cell-mediated repair, for example: bone morphogenetic protein (BMP) (8), Yes associated protein (YAP) (5, 23), TNF/IL1β (21), hypoxia-inducible factor (HIF) (29), as well as those mediated by growth factors such as keratinocyte growth factor and epidermal growth factor (19, 32).
It should also be noted that although the alveoli epithelium relies primarily on AT2 cells for regeneration (2), in response to severe injury, specific populations of airway cells can be mobilized and migrate into the alveoli region to participate in repair. Among these are a group of cells named “distal airway stem cells” or “lineage-negative epithelial progenitors” (LNEPs) that form Trp63+/Krt5+ “pods” to temporarily seal the damaged alveolar barrier (20, 40, 50). Although some of these cells may also form transient cell populations similar to the above described Krt8+ cells (20, 39), the potential for these cells to give rise to AT2 and AT1 cells during repair is unclear. Bronchioalveolar stem cells (BASCs) that coexpress the AT2 marker Sftpc and club cell marker Scgb1a1 residing at the bronchioalveolar duct junction (BADJ) have been shown to have dual-lineage potential to differentiate into alveolar and bronchiolar cells (24, 25, 38), but the contribution of BASCs to alveolar regeneration is difficult to determine because some AT2 cells that reside away from the BADJ region also express Scg1b1a (33).
IMPLICATION OF AT2 HETEROGENEITY IN LUNG DISEASE
The transitional subgroups of AT2 cells identified above in injured mouse lungs were rarely detected in normal mouse or human samples (14, 15, 39) but appear to be abnormally enriched in lungs under various pathological conditions. Signature genes expressed in the intermediate population, including KRT8, CLDN4, and SFN, were detected in lungs of patients with acute respiratory distress syndrome (39), idiopathic pulmonary fibrosis (IPF) (7, 19, 22, 39), adenocarcinoma (7), and most recently coronavirus disease 2019 (COVID-19) (4). In a genetic mouse model of lung fibrosis, in which AT2-specific deletion of Cdc42 resulted in impaired AT2 to AT1 cell transition, elevated mechanical tension and progressive fibrotic development, the number of intermediate cells was found to be increased compared with that of wild-type control (45). Consistently, scRNA-seq studies of patient lungs with pulmonary fibrosis also detected the presence of abnormal epithelial cells, resembling dysregulated AT2 subpopulations (1, 13, 34, 46). In contrast to normal human lungs in which epithelial cells show gene expression profiles indicating differentiated mature cell types, such as AT1 or AT2, in IPF patients, distinct groups of atypical epithelial cells were identified that coexpressed markers of AT1, AT2, and conducting airway cells, suggesting an intermediate state of aberrant differentiation (1, 13, 34, 46). The gene expression of these abnormal cells indicated the activation of TGF-β, YAP, WNT, P53, among others, which are signals implicated in the formation of fibrosis (1, 13, 34, 46). In addition, similar abnormal cells also appear in lung samples from chronic obstructive pulmonary disorder patients (1). These studies have indicated that AT2 cells play a previously underrecognized role in the initiation of lung fibrosis and other chronic lung diseases by undetermined mechanisms.
CONCLUSIONS AND PERSPECTIVES
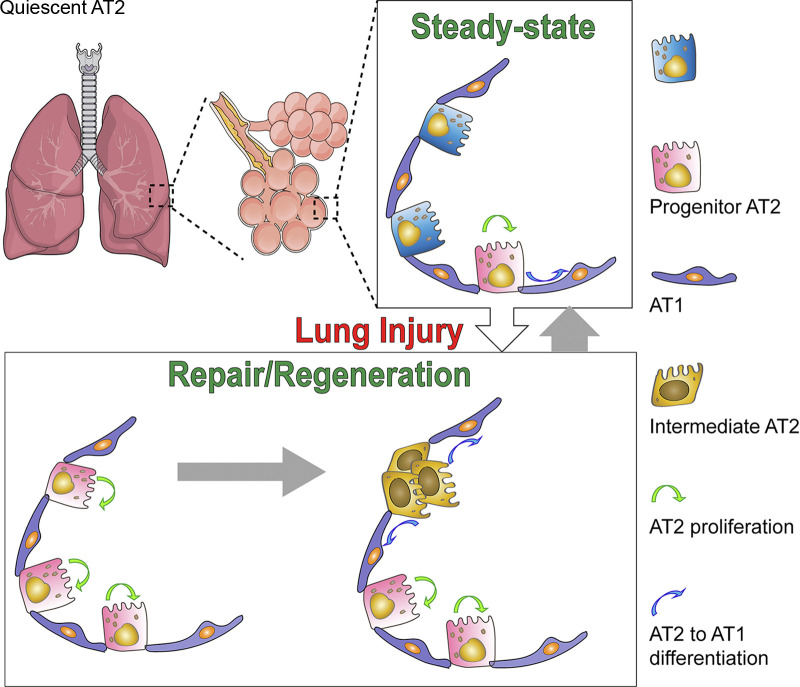
Recent studies revealed that AT2 cells exhibit a high degree of heterogeneity, with subgroups of AT2 cells behaving as progenitor cells and being able to self-renew, differentiate into AT1 cells, and subsequently repair the alveoli. Current results support a model that the AT2 cell subgroups behaving as stem cells during steady-state replacement are likely to be different from those engaging in lung repair after injury. Furthermore, some of the AT2 cells can differentiate into specific subpopulations that form in the middle of AT2 to AT1 transition. The intermediate subpopulations of cells may represent several sequential transitional stages, and most of them will eventually convert into AT1 and repair the injured alveolar epithelium (Fig. 1). The development of novel technologies such as lineage tracing allows tracking the fate of AT2 subgroups. The availability of scRNA-seq now represents a watershed moment in the study of complex interactions between subsets of lung epithelial cells. These scRNA-seq studies through analysis of highly dynamic gene expression changes at single-cell levels have identified novel genes and pathways involved in AT2 progenitor functions. These studies also indicated that the accumulation of aberrant AT2 subpopulations may underlie the pathological mechanisms of various lung diseases.
Fig. 1.
A model. During steady-state homeostasis, only a subgroup of alveolar type II cells (AT2) behave as progenitor cells and are engaged in the replacement of lost alveolar type I cells (AT1) or AT2. After injury, a larger number of AT2 are activated and behave like progenitor cells. Some of the activated AT2 differentiate into specific subpopulations that are in the middle of AT2 to AT1 transition process. Many of these intermediate subpopulations eventually differentiate into AT1 and repair the injured alveolar epithelium.
However, outstanding questions remain, and to answer them requires further improvement of these technologies. For example, the development of dual-marker lineage tracing techniques (25, 38) will allow detailed study of the fate of AT2 subpopulations. While the scRNA-seq technologies are advancing rapidly in both technical procedures and data analysis, the high-noise and low-capture-efficiency nature of these experiments could impose a challenge to detect cells that are rare and only show small differences in gene expression compared with other cells. For instance, the Axin2+ AT2 cells are not clearly clustered into a distinct population in scRNA-seq (47). To move forward, combinatorial approaches, including lineage tracing, scRNA-seq, identification of surface markers, and cell transplantation will be needed to investigate the heterogeneity and function of the AT2 cell subpopulations in normal lungs and in lung diseases.
GRANTS
Q. Chen was supported by the American Heart Association Grant AHA19POST34380566. Y. Liu was supported by National Heart, Lung, and Blood Institute Grant R01 HL105947.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L. prepared figures; Q.C. drafted manuscript; Q.C. and Y.L. edited and revised manuscript; Q.C. and Y.L. approved final version of manuscript.
REFERENCES
- 1.Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, Chu SG, Raby BA, DeIuliis G, Januszyk M, Duan Q, Arnett HA, Siddiqui A, Washko GR, Homer R, Yan X, Rosas IO, Kaminski N. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6: eaba1983, 2020. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396: 320–332, 2020. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Wu H, Yu Y, Tang N. Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res 30: 708–710, 2020. doi: 10.1038/s41422-020-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Rehman J, Chan M, Fu P, Dudek SM, Natarajan V, Malik AB, Liu Y. Angiocrine sphingosine-1-phosphate activation of S1PR2-YAP signaling axis in alveolar type II cells is sssential for lung repair. Cell Rep 31: 107828, 2020. doi: 10.1016/j.celrep.2020.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Suresh Kumar V, Finn J, Jiang D, Liang J, Zhao YY, Liu Y. CD44high alveolar type II cells show stem cell properties during steady-state alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol 313: L41–L51, 2017. doi: 10.1152/ajplung.00564.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Park JE, Tsagkogeorga G, Yanagita M, Koo BK, Han N, Lee JH. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 27: 366–382.e7, 2020. doi: 10.1016/j.stem.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145: dev163014, 2018. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 10.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507: 190–194, 2014. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 12.Finn J, Sottoriva K, Pajcini KV, Kitajewski JK, Chen C, Zhang W, Malik AB, Liu Y. Dlk1-mediated temporal regulation of Notch signaling is required for differentiation of alveolar type II to type I cells during repair. Cell Rep 26: 2942–2954.e5, 2019. doi: 10.1016/j.celrep.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, Peter L, Chung M-I, Taylor CJ, Jetter C, Raju L, Roberson J, Ding G, Wood L, Sucre JMS, Richmond BW, Serezani AP, McDonnell WJ, Mallal SB, Bacchetta MJ, Loyd JE, Shaver CM, Ware LB, Bremner R, Walia R, Blackwell TS, Banovich NE, Kropski JA. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv 6: eaba1972, 2020. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, Huang D, Xu Y, Huang W, Jiang M, Jiang X, Mao J, Chen Y, Lu C, Xie J, Fang Q, Wang Y, Yue R, Li T, Huang H, Orkin SH, Yuan GC, Chen M, Guo G. Mapping the mouse cell atlas by Microwell-seq. Cell 172: 1091–1107, 2018. [Erratum in Cell 173:1307, 2018]. doi: 10.1016/j.cell.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Zhou Z, Fei L, Sun H, Wang R, Chen Y, Chen H, Wang J, Tang H, Ge W, Zhou Y, Ye F, Jiang M, Wu J, Xiao Y, Jia X, Zhang T, Ma X, Zhang Q, Bai X, Lai S, Yu C, Zhu L, Lin R, Gao Y, Wang M, Wu Y, Zhang J, Zhan R, Zhu S, Hu H, Wang C, Chen M, Huang H, Liang T, Chen J, Wang W, Zhang D, Guo G. Construction of a human cell landscape at single-cell level. Nature 581: 303–309, 2020. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15: 123–138, 2014. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH III, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446.e14, 2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang P, Gil de Rubio R, Hrycaj SM, Gurczynski SJ, Riemondy KA, Moore BB, Omary MB, Ridge KM, Zemans RL. Ineffectual type 2-to-type 1 alveolar epithelial cell differentiation in idiopathic pulmonary fibrosis: persistence of the KRT8hi transitional state. Am J Respir Crit Care Med 201: 1443–1447, 2020. doi: 10.1164/rccm.201909-1726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathiriya JJ, Brumwell AN, Jackson JR, Tang X, Chapman HA. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell 26: 346–358.e4, 2020. doi: 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsura H, Kobayashi Y, Tata PR, Hogan BLM. IL-1 and TNFα contribute to the inflammatory niche to enhance alveolar regeneration. Stem Cell Reports 12: 657–666, 2019. doi: 10.1016/j.stemcr.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, Banovich NE, Kropski JA, Tata PR. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol 22: 934–946, 2020. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T, Chapman HA, Morrisey EE, Shen H, Koch WJ, Kosmider B, Wolfson MR, Tian Y. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest 129: 2107–2122, 2019. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156: 440–455, 2014. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W, Qin Z, Li Y, Yang R, Pu W, Zhang L, He L, Zhao H, Yu W, Tang M, Tian X, Cai D, Nie Y, Hu S, Ren T, Qiao Z, Huang H, Zeng YA, Jing N, Peng G, Ji H, Zhou B. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 51: 728–738, 2019. [Erratum in Nat Genet 51:766, 2019]. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Kumar VS, Zhang W, Rehman J, Malik AB. Activation of type II cells into regenerative stem cell antigen-1+ cells during alveolar repair. Am J Respir Cell Mol Biol 53: 113–124, 2015. doi: 10.1165/rcmb.2013-0497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, Zhao YY, Malik AB. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med 208: 1473–1484, 2011. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason RJ. Biology of alveolar type II cells. Respirology 11, Suppl: S12–S15, 2006. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 29.McClendon J, Jansing NL, Redente EF, Gandjeva A, Ito Y, Colgan SP, Ahmad A, Riches DWH, Chapman HA, Mason RJ, Tuder RM, Zemans RL. Hypoxia-inducible factor 1α signaling promotes repair of the alveolar epithelium after acute lung injury. Am J Pathol 187: 1772–1786, 2017. doi: 10.1016/j.ajpath.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359: 1118–1123, 2018. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Post Y, Clevers H. Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell 25: 174–183, 2019. doi: 10.1016/j.stem.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Qiao R, Yan W, Clavijo C, Mehrian-Shai R, Zhong Q, Kim KJ, Ann D, Crandall ED, Borok Z. Effects of KGF on alveolar epithelial cell transdifferentiation are mediated by JNK signaling. Am J Respir Cell Mol Biol 38: 239–246, 2008. doi: 10.1165/rcmb.2007-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4: 525–534, 2009. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, Fernandez R, Akbarpour M, Chen CI, Ren Z, Verma R, Abdala-Valencia H, Nam K, Chi M, Han S, Gonzalez-Gonzalez FJ, Soberanes S, Watanabe S, Williams KJN, Flozak AS, Nicholson TT, Morgan VK, Winter DR, Hinchcliff M, Hrusch CL, Guzy RD, Bonham CA, Sperling AI, Bag R, Hamanaka RB, Mutlu GM, Yeldandi AV, Marshall SA, Shilatifard A, Amaral LAN, Perlman H, Sznajder JI, Argento AC, Gillespie CT, Dematte J, Jain M, Singer BD, Ridge KM, Lam AP, Bharat A, Bhorade SM, Gottardi CJ, Budinger GRS, Misharin AV. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199: 1517–1536, 2019. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, Miller AJ, Spence JR, Gerber AN, Hesselberth JR, Zemans RL. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight 5: e123637, 2019. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27: 493–512, 2011. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 37.Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA, Schipper D, van Run P, Leijten L, Sikkema R, Verschoor E, Verstrepen B, Bogers W, Langermans J, Drosten C, Fentener van Vlissingen M, Fouchier R, de Swart R, Koopmans M, Haagmans BL. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 368:1012–1015, 2020. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, Herold S, Szibor M, Braun T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J 38: e102099, 2019. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, Tsidiridis G, Lange M, Mattner LF, Yee M, Ogar P, Sengupta A, Kukhtevich I, Schneider R, Zhao Z, Voss C, Stoeger T, Neumann JHL, Hilgendorff A, Behr J, O’Reilly M, Lehmann M, Burgstaller G, Königshoff M, Chapman HA, Theis FJ, Schiller HB. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11: 3559, 2020. doi: 10.1038/s41467-020-17358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625, 2015. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verheyden JM, Sun X. A transitional stem cell state in the lung. Nat Cell Biol 22: 1025–1026, 2020. doi: 10.1038/s41556-020-0561-5. [DOI] [PubMed] [Google Scholar]
- 42.Wang A, Chiou J, Poirion OB, Buchanan J, Valdez MJ, Verheyden JM, Hou X, Guo M, Newsome JM, Kudtarkar P, Faddah DA, Zhang K, Young RE, Barr J, Misra R, Huyck H, Rogers L, Poole C, Whitsett JA, Pryhuber G, Xu Y, Gaulton KJ, Preissl S, Sun X. Single nucleus multiomic profiling reveals age-dynamic regulation of host genes associated with SARS-CoV-2 infection (Preprint). bioRxiv doi: 10.1101/2020.04.12.037580. [DOI]
- 43.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 44.Weibel ER. What makes a good lung? Swiss Med Wkly 139: 375–386, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, Shi M, Zhao X, Yuan J, Li J, Yang X, Bin E, Wei D, Zhang H, Zhang J, Yang C, Cai T, Dai H, Chen J, Tang N. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 180: 107–121.e17, 2020. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, Morrisey EE. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555: 251–255, 2018. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH II, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J, Banovich N, Barbry P, Brazma A, Desai T, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Haniffa M, Horvath P, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lafyatis R, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer K, Misharin A, Nawijn M, Nikolic MZ, Ordovas-Montanes J, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rojas M, Rosen O, Saeb-Parsy K, Samakovlis C, Schiller H, Schultze JL, Seibold MA, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann S, Theis F, Tsankov A, van den Berge M, von Papen M, Whitsett J, Xavier R, Xu Y, Zaragosi L-E, Zhang K; HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org; HCA Lung Biological Network . SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11: 254–267, 2011. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 50.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, Wang X, Lim SJ, Vincent M, Lessard M, Crum CP, Xian W, McKeon F. p63+Krt5+ distal airway stem cells are essential for lung regeneration. Nature 517: 616–620, 2015. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]