Abstract
Cell-to-cell fusion is crucial for the development and propagation of most eukaryotic organisms. Despite this importance, the molecular mechanisms mediating this process are only poorly understood in biological systems. In particular, the step of plasma membrane merger and the contributing proteins and physicochemical factors remain mostly unknown. Earlier studies provided the first evidence of a role of membrane sterols in cell-to-cell fusion. By characterizing different ergosterol biosynthesis mutants of the fungus Neurospora crassa, which accumulate different ergosterol precursors, we show that the structure of the sterol ring system specifically affects plasma membrane merger during the fusion of vegetative spore germlings. Genetic analyses pinpoint this defect to an event prior to engagement of the fusion machinery. Strikingly, this effect is not observed during sexual fusion, suggesting that the specific sterol precursors do not generally block membrane merger, but rather impair subcellular processes exclusively mediating fusion of vegetative cells. At a colony-wide level, the altered structure of the sterol ring system affects a subset of differentiation processes, including vegetative sporulation and steps before and after fertilization during sexual propagation. Together, these observations corroborate the notion that the accumulation of particular sterol precursors has very specific effects on defined cellular processes rather than nonspecifically disturbing membrane functioning. Given the phenotypic similarities of the ergosterol biosynthesis mutants of N. crassa during vegetative fusion and of Saccharomyces cerevisiae cells undergoing mating, our data support the idea that yeast mating is evolutionarily and mechanistically more closely related to vegetative than sexual fusion of filamentous fungi.
Keywords: ergosterol, cell fusion, plasma membrane fusion, mating, Neurospora crassa
Fusion between genetically and developmentally identical or different cells is a common process in the growth and development, of eukaryotic organisms. It serves a plethora of biological functions including gamete merger during sexual propagation, muscle and organ development, neuronal repair, or the formation of multinucleate cells, such as macrophage-derived osteoclasts or giant cells. In filamentous fungi, cell-to-cell fusion promotes multicellular growth and the formation and functioning of mycelial colonies. In many ascomycete species, the cell tips of germinating vegetative spores (conidia) mutually attract each other during colony establishment and fuse into supracellular networks (germling fusion) that further develop into the hyphal colony (Roca et al. 2005). This cooperation of genetically identical individuals likely promotes competitiveness and allows the coordinated conquering of a specific habitat by clonal spores (Richard et al. 2012). Within mature mycelial colonies, fusion between hyphal branches (hyphal fusion) increases the interconnectivity within the cellular network, promoting the exchange of water and nutrients throughout the colony, and supporting its coordinated growth and propagation.
In addition, the sexual reproduction of outcrossing fungal species involves the merger of two genetically different mating partners. Fertilization is typically achieved through the fusion of specialized reproduction structures or cells to enable subsequent nuclear fusion and genetic recombination (Bistis 1981).
Despite the broad occurrence and high relevance of cell-to-cell fusion, the molecular mechanisms mediating this process remain only poorly understood in any biological system. In particular, the last step of the cell-to-cell fusion process, the merger of the plasma membranes, remains enigmatic. In recent years, the red bread mold Neurospora crassa has advanced as a model system for studying the molecular mechanisms of eukaryotic cell-to-cell fusion in general, and fungal colony initiation and development in particular (Herzog et al. 2015; Fischer and Glass 2019). In vegetative germling fusion, N. crassa employs an unusual communication mechanism, in which the two fusion partners take turns in signal sending and receiving. As a result of this cellular dialog, the germlings direct their growth toward each other until physical contact is achieved (Fleissner et al. 2009a; Fleißner and Herzog 2016). While the molecular factors governing these cellular interactions are starting to unfold, the mechanisms mediating the fusion process after cell-to-cell contact remain mostly elusive. Fusion pore formation requires cell wall remodeling, followed by adherence and subsequent fusion of the plasma membranes. So far, no bona fide fusogen, a protein required and sufficient for plasma membrane merger, has been identified in fungi (Aguilar et al. 2013). However, transmembrane proteins promoting plasma membrane fusion during vegetative and sexual cell merger have been described in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and N. crassa. In all species, a significant fraction of fusion pairs lacking the PRM1 protein fail to merge the plasma membranes and remain with tightly adhered lipid bilayers (Heiman and Walter 2000; Fleissner et al. 2009b; Curto et al. 2014). A similar but less-severe defect is observed in Δlfd-1 mutants of N. crassa, in which about one-fifth of fusion pairs fail in membrane merger (Palma-Guerrero et al. 2014). PRM1 and LFD-1 mediate both vegetative and sexual fusion, suggesting that both are part of a general fusion machinery. In addition to the membrane merger defect, all of the described S. cerevisiae and N. crassa mutants exhibit significantly increased lysis of fusion pairs. The onset of lysis coincides with the moment of cytoplasmic mixture, suggesting that it is caused by the engagement but aberrant functioning of the fusion machinery (Aguilar et al. 2007; Palma-Guerrero et al. 2014).
In addition to fusion-mediating proteins, plasma membrane merger is also influenced by the composition of the lipid bilayer itself. Crucial lipid constituents of eukaryotic cell membranes are sterols, which have essential roles for membrane properties and the functioning of membrane-associated proteins (Dufourc 2008). Different S. cerevisiae and N. crassa mutants deficient in the biosynthesis of ergosterol, the prevalent sterol in fungal cell membranes, are affected in cell fusion-related cellular communication or plasma membrane merger (Jin et al. 2008; Aguilar et al. 2010; Weichert et al. 2016). Strikingly, specific phenotypic deficiencies of some ergosterol biosynthesis (erg) mutants correlate with specific structural features of the accumulating sterol precursors. For example, a defect in the last step of ergosterol biosynthesis mediated by the sterol C-24(28) reductase ERG-2 results in the accumulation of a precursor carrying a conjugated double bond in its aliphatic side chain. As a consequence, signaling through the MAK-1 mitogen-activated protein kinase (MAPK) cascade, which is essential for germling communication and cell-to-cell contact recognition in N. crassa, is strongly affected, while other related membrane-associated signaling processes, including a second MAPK pathway (MAK-2), remain functional (Weichert et al. 2016). These observations indicate that changes in the sterol structure have specific rather than general effects on cell functioning.
In S. cerevisiae, mutants accumulating ergosterol precursors with an altered double-bond configuration in the B ring (Δerg2 or Δerg3, Figure 1A) exhibit a Δprm1-like phenotype, with a significant percentage of mating pairs remaining in the stage of closely adhered plasma membranes. Despite the morphological similarities of the Δprm1 and erg mutants, both defects act independently, indicated by additive effects in the respective double mutants (Jin et al. 2008). However, so far the molecular processes affected by the accumulation of intermediates of ergosterol biosynthesis in these erg mutants remain unknown.
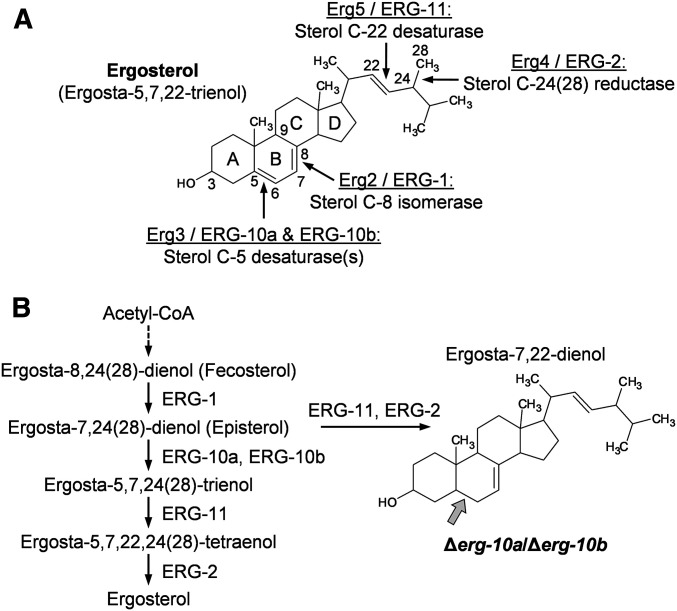
Figure 1.
The sterol C-5 desaturases ERG-10a and ERG-10b have a redundant function in ergosterol biosynthesis in N. crassa. (A) Structure of ergosterol. Enzymes and their functions mediating double-bond arrangements in ergosterol precursors are depicted (S. cerevisiae/N. crassa). Numbers along ergosterol denote the position of selected carbon atoms, and letters indicate the carbon rings. (B) Late steps in the hypothetical biosynthesis pathway of ergosterol in N. crassa showing the reactions catalyzed by the enzymes presented in (A). ERG-10a and ERG-10b mediate the same reaction, whose block results in the accumulation of a sterol intermediate with an altered ring structure (gray arrow).
Here, we show that structural changes in the ring system of ergosterol lead to membrane fusion deficiencies during vegetative cell-to-cell fusion in N. crassa, indicating functional conservation between yeast mating and vegetative fusion in the filamentous fungus. However, sexual cell-to-cell merger remains unaffected in the erg mutants of N. crassa, indicating that the altered membrane structure does not compromise the general basic fusion machinery. Consistent with these findings, we could pinpoint the sterol-dependent defect in plasma membrane merger to a step prior to the engagement of the fusion machinery, suggesting a novel role of membrane structure in the signaling events preceding the initiation of plasma membrane fusion.
Materials and Methods
Strains and growth conditions
The strains of N. crassa used and generated in this study are listed in Supplemental Material, Table S1. Strains were routinely grown at 30° in agar slant tubes containing Vogel’s minimal medium (MM) (Vogel 1956) as previously described in Schürg et al. (2012). Crosses were conducted on Westergaard’s medium (Westergaard and Mitchell 1947) as described before (Serrano et al. 2018). Spores were obtained from 1-week-old cultures through harvesting in sterile distilled water. Spore suspensions were filtered through Miracloth (Calbiochem, San Diego, CA) and counted with a hemocytometer.
Linear hyphal growth rates were determined as described in Fleissner et al. (2005). To quantify sporulation, the strains were first grown in MM slant tubes for 7 days at 26° with a 12-hr light–dark rhythm. Conidia were harvested in 2 ml of sterile distilled water and purified from hyphal fragments by filtration through Miracloth. The spore numbers per tube were determined with a hemocytometer. To determine the lengths of aerial hyphae, 2 ml of liquid MM in a long reaction tube inoculated with 104 spores were incubated for 5 days at 30° in the dark.
To compare the sensitivity of strains to nystatin, agar plates with sorbose-rich medium, which induces colonial growth in N. crassa (Tatum et al. 1949), were spotted with 5-µl droplets of 10-fold serial dilutions of spore suspensions, resulting in 105–10 conidia per spot. The media were supplemented with freshly prepared solutions of nystatin dihydrate (100 µg/ml in DMSO; Roth). Photos of the plates were captured after incubation at 30° for 72 hr.
Strain construction
Complementation of Δerg-10a/Δerg-10b:
To complement the double mutant Δerg-10a/Δerg-10b, strain MW_126 was transformed with plasmids containing either the gene erg-10a (NCU06207) or erg-10b (NCU04983), including 1000 bp upstream and downstream of these sequences. To construct the plasmids, both genes were PCR-amplified from genomic DNA of the wild-type (WT) strain FGSC (Fungal Genetics Stock Center) 2489 using the primer pairs 1445/1446 and 1447/1448, respectively (Table S2). The PCR products were cloned via PacI and NotI into vector 728 (a pMF272 [Freitag et al. 2004] derivative lacking the gfp sequence), enabling the site-specific integration at the his-3 locus of the N. crassa genome (Freitag and Selker 2005). The resulting plasmids 745 (erg-10a) and 746 (erg-10b) were transformed into the histidine-auxotrophic double mutant MW_126 as described in Margolin et al. (1997).
Construction of erg-1 gene deletion strains:
For the deletion of erg-1 in N. crassa, a gene replacement cassette for homologous recombination was constructed using yeast recombinational cloning (Raymond et al. 1999) as described in Colot et al. (2006). The 5′ and 3′ untranslated regions of the erg-1 gene were PCR-amplified from genomic DNA of strain FGSC 9719 with primer pairs 103/104 and 105/106, respectively (Table S2). Using the S. cerevisiae strain FY834 for the yeast transformation (Winston et al. 1995), these flanks were assembled with the hygromycin resistance gene amplified with primers 82/83 from plasmid pCSN44 (Staben et al. 1989) and the EcoRI/XhoI-linearized yeast vector pRS426 (Christianson et al. 1992) as described before (Colot et al. 2006). The plasmid DNA extracted from the yeast colonies was used as a template in a PCR with primers 103 and 106 to amplify the assembled erg-1 gene deletion cassette. Transformation of N. crassa with the gene knockout cassette was performed as previously described (Fleissner and Glass 2007), using the mus-52::bar deletion mutant FGSC 9719 as the recipient strain for efficient homologous recombination (Colot et al. 2006). Transformants were confirmed by Southern blot analysis and purified by backcrossing with the WT strain. To generate Δerg-1 strains expressing cytosolic GFP and mCherry, a mutant auxotrophic for histidine (MW_347) was transformed with plasmids pMF272 (Freitag et al. 2004) and pMFcherry (Schürg et al. 2012), respectively. Accordingly, a histidine-auxotrophic double deletion strain lacking both erg-1 and Prm1 (MW_450) was transformed in the same way to generate ΔPrm1/Δerg-1 strains expressing GFP and mCherry, respectively.
Construction of erg mutants lacking Prm1:
Mutants lacking erg genes and Prm1 were obtained via sexual crosses (data not shown). The strains lacking erg-10a, erg-10b, and Prm1, and expressing cytosolic GFP and mCherry, respectively, were constructed by transforming a histidine-auxotrophic triple mutant (MW_277.1) with the vectors pMF272 or pMFcherry.
Cell-to-cell fusion assays
Vegetative spore germling fusion assays were conducted as previously described (Fleissner et al. 2009b). In brief, conidial suspensions were spread on MM agar plates, incubated at 30° for 4 hr and analyzed by brightfield and fluorescence microscopy. Agar blocks of ∼10 × 10 mm were cut from the plates, covered with coverslips, and analyzed by brightfield and fluorescence microscopy. The number of germlings undergoing directed growth toward each other was determined from microscopic images with comparable cell densities as previously described (Weichert et al. 2016). The quantitative analysis of cell-to-cell fusion between germlings expressing green- and red-fluorescing proteins was performed as described in Fleissner et al. (2009b). For live-cell imaging of SO-GFP and MAK-2-GFP in interacting germlings, samples were prepared as described above and analyzed by fluorescence microscopy (Fleissner et al. 2009a). Sexual fusion between trichogynes and microconidia was assessed as described in Fleissner et al. (2005).
Analysis of fruiting body development
The phenotypic characterization of fruiting bodies and progeny was performed with a stereomicroscope as described in Weichert et al. (2016). To determine the number of protoperithecia per area unit, strains were grown on crossing plates for 7 days as described above. The quantification of the protoperithecia was based on representative images captured from at least three independent cultures. The average number of protoperithecia per 1 mm2 was determined using the software Fiji (https://fiji.sc/). Images of perithecia were taken 10–14 days after fertilization, either as viewed from above the crossing plates or at an angle of ∼45° relative to the camera. Rosettes of asci from crosses were prepared by placing isolated perithecia into a droplet of water on a glass slide and gently squeezing them under a coverslip. At least 1 month after fertilization, ejected ascospores were harvested in 2 ml of sterile distilled water from the lids of the crossing plates, heat-activated at 59° for 20 min, spread on MM plates, and incubated at room temperature for 16–20 hr. Germinating ascospores were analyzed with a stereomicroscope and isolated in MM slant tubes for growth into vegetative mycelia.
Staining of the plasma membrane and cell wall of germling pairs
The staining of cell membranes with FM4-64 was performed as described in Hickey et al. (2002). FM4-64 was prepared as a solution of 100 µg in 1 ml of sterile distilled water, which was further diluted to working solutions of 25 µM.
To simultaneously stain the cell membrane and the cell wall in germling pairs, samples were treated with 10 µl of a mixture of FM4-64 and calcofluor white (CFW, fluorescence brightener 28). First, CFW was prepared as a stock solution of 7 mg/ml in DMSO and then diluted 1:100 in sterile distilled water. Next, one volume of the working solution of CFW was mixed with nine volumes of the working solution of FM4-64, which were immediately used for fluorescence microscopy of the double-stained germling pairs.
Analysis of cell lysis in germling pairs
Fusion-induced cell lysis was quantified as previously described in Schumann et al. (2019), using MM with normal and reduced Ca2+ concentrations (0.68 and 0.34 mM, respectively).
Visualization of sterol distribution in germlings and trichogynes
To determine the distribution of plasma membrane sterols in interacting germlings, samples were prepared as described above and treated with 10 μl containing 100 μg/ml of filipin III (Sigma [Sigma-Aldrich], St. Louis, MO) in 1% (v/v) DMSO. Trichogynes were obtained from crosses as described in Fleissner et al. (2005), followed by the treatment of samples (agar blocks of 5 × 5 mm) with 5 µl of the filipin III solution between 24 and 48 hr after incubation. Cells were analyzed within 15 min after filipin staining by fluorescence microscopy using a DAPI filter setup.
Brightfield and fluorescence microscopy
Images of germlings were captured with an Axiophot 2 microscope (Zeiss [Carl Zeiss], Jena, Germany) coupled to a pixelfly 270 XS (pco) camera, using 63×/1,40 oil plan-apochromat and 100×/1,30 oil plan-neofluar objectives and DIC optics. The double-staining of germlings with FM4-64 and CFW was performed using an Axio Observer.Z1 inverse microscope (Zeiss) using light-emitting diodes for the excitation light. Brightfield images of fruiting bodies and asci were captured with an M60 stereomicroscope (Leica) coupled to a DFC295 camera.
Sterol extraction and analysis
The extraction and analysis of sterols was performed as described in our previous study (Weichert et al. 2016). In brief, sterols were isolated from frozen, ground mycelia using a 3:1 (v/v) mixture of chloroform and methanol, subsequent saponification with 10% (w/v) potassium hydroxide in methanol, followed by extraction in n-hexane. Sterol extracts were derivatized with 2,2,2-trifluoro-N-methyl-N-(trimethylsilyl) acetamide and analyzed by gas chromatography/mass spectrometry (GC/MS) as described in (Nawrath et al. 2010). The sterol biosynthesis intermediates were identified by comparing their mass spectra with data available from databases and the literature.
Statistical analysis
Statistical data analysis was performed with GraphPad Prism (version 8.3.1) using analysis of variance (ANOVA) with Tukey’s or Dunnett’s multiple comparison tests, or unpaired, two-tailed Student’s t-tests, to evaluate statistically significant differences in fungal growth, cell-to-cell fusion, and cell lysis.
Data availability
Strains and plasmids are available upon request. The authors affirm that all reagents, software, and data that are necessary for drawing the conclusions presented in this study are fully represented within this article, and its tables and figures. Supplemental material available at figshare: https://doi.org/10.25386/genetics.13050176.
Results
The absence of the redundant sterol C-5 desaturases ERG-10a and ERG-10b affects specific differentiation steps in the life cycle of N. crassa
In earlier studies, we reported that the sterol C-5 desaturases ERG-10a and ERG-10b, encoded by the gene loci NCU06207 and NCU04983, respectively, have a redundant function in ergosterol biosynthesis in N. crassa (Weichert et al. 2016; Herzog et al. 2020). They introduce a double bond between C-5 and C-6 of the B ring in the sterol molecule (Figure 1A). Their absence results in the accumulation of ergosta-7,22-dienol, which differs from ergosterol only by the lack of this specific double bond (Figure 1B).
To test if this metabolic defect influences growth and development of the fungus, we characterized respective gene knockout mutants. Consistent with the redundant function of ERG-10a and ERG-10b, mutants lacking the individual genes (FGSC 20057 and N4-30, respectively) were macroscopically indistinguishable from the WT reference strain FGSC 2489 (Figure 2A). However, a Δerg-10a/Δerg-10b double mutant (N4-38) showed a strong decrease in asexual spore differentiation, whereas the overall growth rate was only slightly affected (Figure 2B) and aerial hyphae formation was WT-like (data not shown). In contrast to the WT or single mutants, cultures of the double mutant also accumulated a brownish pigment (Figure 2A). Reintegration of either the erg-10a or erg-10b gene into the double mutant restored the WT phenotype, confirming the redundant functions of the encoded enzymes (Figure 2, A and B).
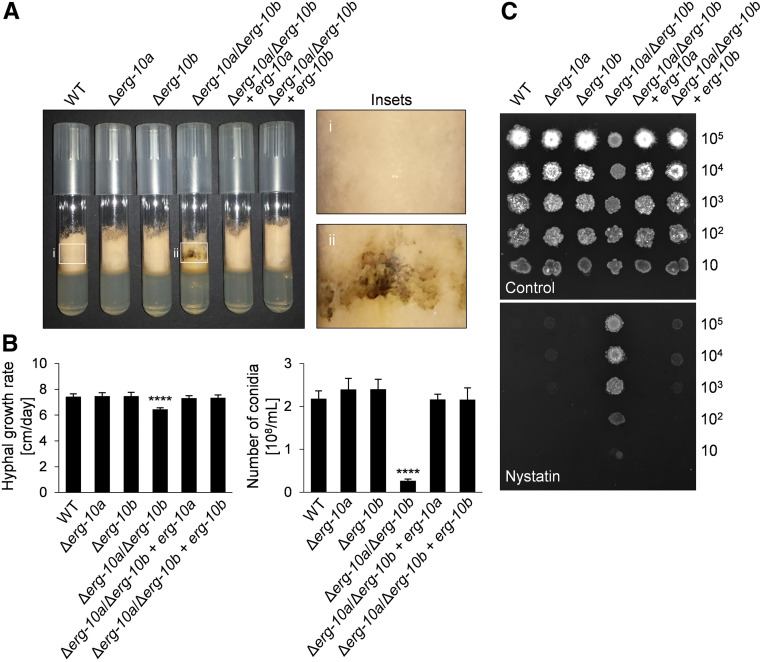
Figure 2.
Impact of Δerg-10a and Δerg-10b on vegetative growth of N. crassa. (A) Growth of WT (FGSC 2489), Δerg-10a (FGSC 20057), Δerg-10b (N4-30), Δerg-10a/Δerg-10b (N4-38), Δerg-10a/Δerg-10b + erg-10a (MW_630), and Δerg-10a/Δerg-10b + erg-10b (MW_631) in MM tubes. Insets: in contrast to WT (i), Δerg-10a/Δerg-10b (ii) typically produces dark pigments along the glass wall of the tube. (B) Quantification of linear hyphal extension rates and sporulation. Values represent the mean ± SD from at least three independent experiments per strain (****P < 0.0001, ANOVA with Tukey’s post hoc test). (C) Ten-fold serial dilutions of conidia of the indicated strains were spotted onto sorbose-rich medium in the absence or presence of the polyene antifungal compound nystatin. FGSC, Fungal Genetics Stock Center; MM, minimal medium; WT, wild-type.
Due to their lack of ergosterol, classical erg mutants of N. crassa generated by UV mutagenesis show increased resistance to ergosterol-binding antifungals called polyenes (Grindle 1973, 1974). The Δerg-10a/Δerg-10b isolate was also less sensitive to the polyene nystatin than the WT, the single-gene deletion mutants, or the complemented strains (Figure 2C), consistent with a block in ergosterol biosynthesis in the double mutant. Taken together, the presence of either ERG-10a or ERG-10b sufficiently supports WT-like growth of N. crassa, while the absence of both sterol C-5 desaturases results in a specific deficiency in spore differentiation.
The lack of ERG-10a and ERG-10b results in plasma membrane fusion deficiencies during vegetative germling fusion
Our earlier study revealed that Δerg-2 germlings interact at a significantly reduced frequency and are unable to arrest growth after cell-to-cell contact. In contrast, directed growth of Δerg-10a/Δerg-10b germlings is comparable to WT (Weichert et al. 2016). The interaction of WT germlings includes membrane recruitment of the MAPK MAK-2 and the fungal-specific signaling factor SO (Fleissner et al. 2009a). As expected, the membrane recruitment of both proteins was also unaffected in the Δerg-10a/Δerg-10b double mutant (Figure S1). The structural differences of ergosta-7,22-dienol and ergosterol therefore have no effect on the membrane-associated signaling processes preceding cell-to-cell contact, in contrast to the changed aliphatic side chain of the sterol accumulating in Δerg-2.
To test for potential fusion defects after cell-to-cell contact, germlings from WT or Δerg-10a/Δerg-10b strains expressing either cytosolic GFP or mCherry were mixed, and incubated to allow cell-to-cell interactions. Cell pairs consisting of a red and a green cell were analyzed by fluorescence microscopy. WT cell pairs mostly exhibited both fluorescent markers in either fusion partner, indicating cytoplasmic mixing as a result of cell-to-cell fusion (Figure 3A). In contrast, >30% of Δerg-10a/Δerg-10b pairs failed to exchange their cytoplasm, although the cells were tightly adhered to one another (Figure 3, B and C). We also analyzed the Δerg-11 and Δerg-2/Δerg-11 mutants, which interact in a WT-like manner (Weichert et al. 2016). These isolates were not affected in their fusion frequencies (88.2 ± 0.8% and 87.4 ± 1.5%, respectively), indicating that the Δerg-10a/Δerg-10b fusion deficiency represents a novel phenotype among all of the erg mutants of N. crassa tested so far.
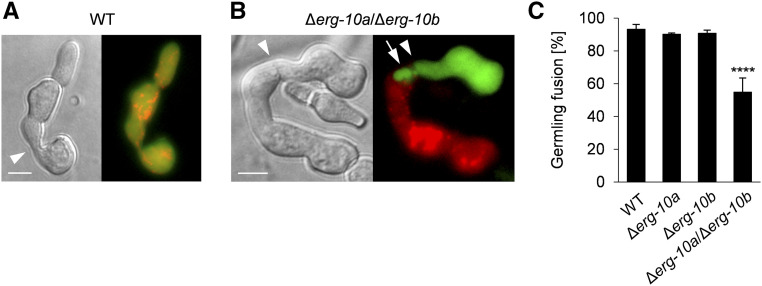
Figure 3.
Germlings of ∆erg-10a/∆erg-10b are impaired in cell-to-cell fusion. (A) Microscopic analysis of cell-to-cell fusion in a pair of WT germlings expressing cytosolic GFP and Cherry Red (N3-06 + N3-07) after physical contact (arrowhead), resulting in mixing of green and red fluorescence. (B) A pair of Δerg-10a/Δerg-10b germlings expressing cytosolic GFP or Cherry Red (MW_175 + MW_179). Note the lack of cytoplasmic mixing and the extrusion extending from one cell into the other (arrow). Bar in (A and B), 5 μm. (C) Quantification of germling fusion in cell pairings of WT (N3-06 + N3-07), Δerg-10a (N5-01 + N5-02), Δerg-10b (MW_103 + MW_105), and Δerg-10a/Δerg-10b (MW_175 + MW_179). Values represent the mean ± SD from at least three independent cell populations with 87–109 germling pairs per replicate (****P < 0.0001, ANOVA with Tukey’s post hoc test). WT, wild-type.
In nonfused Δerg-10a/Δerg-10b cell pairs, one cell often formed a finger-like structure, which reached into the cytoplasm of the opposing cell (Figure 3B). This phenotype strongly resembled the earlier reported membrane fusion defect of S. cerevisiae or N. crassa Δprm1 mutants. In both species, 50% of mutant cell pairs fail to merge their tightly opposed plasma membranes after successful cell wall deconstruction (Heiman and Walter 2000; Fleissner et al. 2009b). The comparison of green- and red-fluorescing cell pairs of Δerg-10a/Δerg-10b and ΔPrm1 readily revealed comparable finger- or bubble-like structures in both mutants (Figure S2). This striking phenotypic similarity suggested that the Δerg-10a/Δerg-10b mutant is also defective in plasma membrane merger after cell wall remodeling. Costaining of the cell wall and the plasma membrane confirmed that unfused ΔPrm1 and Δerg-10a/Δerg-10b germling pairs both showed a WT-like opening in the cell wall, but no subsequent fusion pore formation (Figure 4A). Complementation of the double mutant with either the erg-10a or erg-10b gene fully restored WT-like fusion pore formation (Figure S3). These observations indicate that the altered sterol composition in the Δerg-10a/Δerg-10b mutant specifically affects plasma membrane fusion after cell wall degradation.
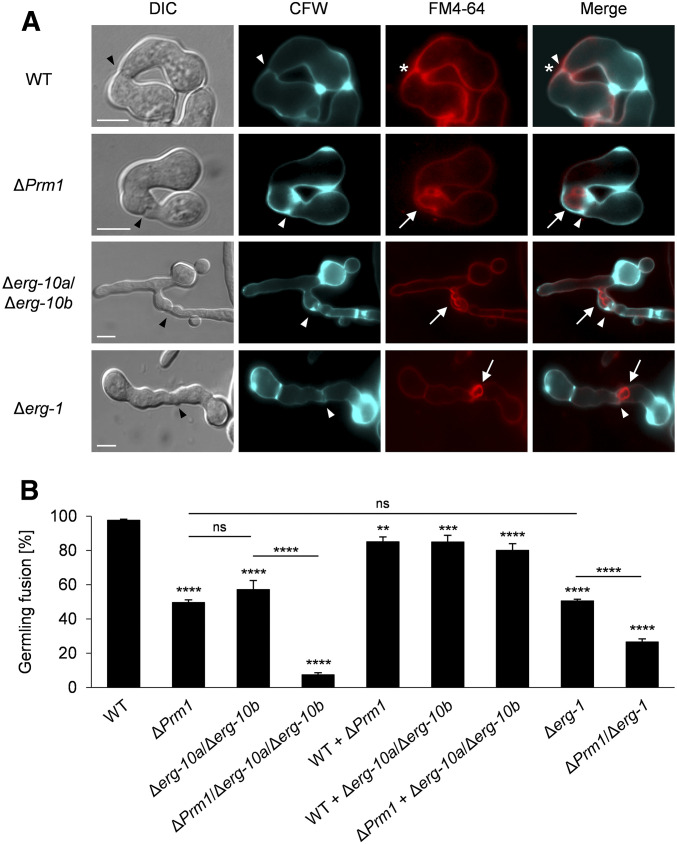
Figure 4.
Germlings of the mutants Δerg-10a Δerg-10b and Δerg-1 are defective in plasma membrane merger during cell-to-cell fusion. (A) Costaining with CFW and FM4-64 to visualize the cell wall and the plasma membrane, respectively. Black arrowheads indicate cell contacts. Note that WT (FGSC 2489) pairings form both an opening in the cell wall (white arrowhead) and a fusion pore in the membrane (asterisk). The mutants ΔPrm1 (A32), Δerg-10a/Δerg-10b (N4-38), and Δerg-1 (MW_308) form membrane invaginations (arrows) that stretch through the cell wall opening from one into the other cell (white arrowheads). Bar, 5 μm. (B) Quantification of germling fusion of erg mutants carrying the additional deletion of Prm1. Values represent the mean ± SD from three independent experiments per set of strains with a range of 40–211 germling pairs per population (**P < 0.01, ***P < 0.001, ****P < 0.0001; ANOVA with Tukey’s post hoc test). See Figure S2 for images of representative germling pairs from these experiments. CFW, calcofluor white; ns, not significant; WT, wild-type.
The germling fusion defects of Δerg-10a/Δerg-10b are independent of PRM1 and LFD-1
The striking resemblance of the membrane fusion defects of the ΔPrm1 and Δerg-10a/Δerg-10b mutants suggested that accumulation of the ergosterol precursor could specifically impair the function of the PRM1 protein. To test this hypothesis, we employed classical genetics and analyzed membrane fusion in a ΔPrm1/Δerg-10a/Δerg-10b triple mutant. As expected, the deletion of all three genes also caused membrane invaginations similar to those found in cell pairs of ΔPrm1 or Δerg-10a/Δerg-10b (Figure S2). However, the rate of successful fusion dropped in the triple mutant to <10% compared with ∼50% and 60% in ΔPrm1 and Δerg-10a/Δerg-10b, respectively (Figure 4B). These data indicate that the fusion defects of ΔPrm1 and Δerg-10a/Δerg-10b are independent, suggesting that the altered sterol composition of this erg mutant does not impact PRM1 functions.
To further investigate this question, we quantified fusion in heterotypic germling pairs. Consistent with previous observations (Heiman and Walter 2000; Fleissner et al. 2009b), the fusion frequencies of ΔPrm1 and Δerg-10a/Δerg-10b cells approached almost normal levels (∼85%) when one of the interaction partners was WT (Figure 4B). Similarly, mixed germling pairs consisting of a Δerg-10a/Δerg-10b and a ΔPrm1 cell exhibited 80% fusion success, indicating that the mutants can mutually compensate their deficiencies.
In N. crassa, the LFD-1 protein possesses a redundant role that is independent of PRM1 during plasma membrane merger (Palma-Guerrero et al. 2014). Consistent with these earlier studies, we also observed the formation of membrane invaginations after cell-to-cell contact with a relatively mild effect on membrane fusion in a Δlfd-1 mutant (Figure S4, A–C). A triple mutant lacking lfd-1, erg-10a, and erg-10b showed slightly further reduced fusion frequencies compared to Δerg-10a/Δerg-10b (Figure S4C). These observations suggest that LFD-1 is rather a supporting factor during plasma membrane merger that, similarly to PRM1, acts independently of the altered sterol composition of Δerg-10a/Δerg-10b.
Structural alterations in the sterol ring system in the Δerg-1 mutant also disturb plasma membrane fusion between germlings
Our earlier study revealed that the structural features of the main sterol accumulating in erg mutants can very specifically correlate with distinct developmental and cell biological defects (Weichert et al. 2016). Since the membrane fusion defect of Δerg-10a/Δerg-10b germlings was unique among all of the N. crassa ergosterol biosynthesis mutants tested so far, we hypothesized that this phenotype correlates with specific structural features of the sterol precursor formed by this mutant. Of all previously described erg mutants, Δerg-10a/Δerg-10b is the only one to accumulate ergosta-7,22-dienol (Weichert et al. 2016), which differs from ergosterol only by a missing double bond in the ring system (Figure 1, A and B). Therefore, we decided to analyze mutants of the gene erg-1 (NCU04156), which encodes a sterol C-8 isomerase homologous to Erg2 of S. cerevisiae, that contributes to the structure of the sterol ring system (Figure 1A). Earlier studies indicated that a classical erg-1 mutant obtained by random mutagenesis (FGSC 2721) accumulates sterols with a double bond between positions C-8 and C-9, instead of C-7 and C-8 (Grindle 1973, 1974; Grindle and Farrow 1978). We confirmed this mutant by sequencing of the erg-1 gene, which revealed a single point mutation that results in a Gly to Asp substitution at position 66 of the protein. Interestingly, the macroscopic appearance of the erg-1 mutant resembled the Δerg-10a/Δerg-10b strain, including the characteristic pigment production (Figure 2A and Figure S5A). Germling fusion pairs of erg-1 also showed membrane invaginations at the cell-to-cell contact zone similar to those observed in Δerg-10a/Δerg-10b (Figures S3A and S5B). For further analysis, we created a Δerg-1 gene knockout strain (Figure S5, C and D). Backcrossing of a primary transformant with WT revealed that the deletion of erg-1 significantly delayed growth and development of the mutant progeny (Figure S6). Mycelia of Δerg-1 exhibited developmental defects similar to the classical erg-1 mutant and the Δerg-10a/Δerg-10b isolate (Figure 2, A and B and Figure S5D). Consistent with the sterol composition reported for the classical erg-1 mutant and an independent deletion strain (Grindle and Farrow 1978; Hu et al. 2018), our GC/MS analysis of Δerg-1 not only confirmed a block in ergosterol biosynthesis, but also the accumulation of mainly three sterol intermediates that all contained a double bond between positions C-8 and C-9, instead of C-7 and C-8, of the ring system (Figure 1A and Figure S7, A and B). Two of these intermediates (ergosta-8,22-dienol and fecosterol) also lacked the double bond between C-5 and C-6 (Figure S7B), comparable with the sterol accumulating in Δerg-10a/Δerg-10b (Figure 1B) (Weichert et al. 2016). In summary, mutations in the genes erg-1 and erg-10a/erg-10b cause altered double bond arrangements in the B ring of the sterol core and result in comparable developmental defects.
While the frequency of interactions between Δerg-1 germlings (MW_308, 81.7 ± 0.9%) was comparable to WT (Weichert et al. 2016), cell pairs of this mutant typically formed membrane invaginations after physical contact reminiscent of Δerg-10a/Δerg-10b, ΔPrm1, and Δlfd-1 (Figure 4A and Figure S4A). Similar to Δerg-10a/Δerg-10b, the fusion frequency in Δerg-1 germlings was reduced by ∼50% (Figure 4B). Knockout of the Prm1 gene in Δerg-1 further reduced this number by another 50%, supporting the notion that the fusion defects caused by an altered sterol composition are independent of PRM1 (Figure 4B). While the triple mutant Δlfd-1/Δerg-10a/Δerg-10b had also shown an additive fusion defect, the frequency of germling fusion in the Δlfd-1/Δerg-1 double mutant (62%) was surprisingly in between those of the corresponding Δerg-1 (39%) and Δlfd-1 (87%) strains (Figure S4C). Although these observations show differences between both erg mutants with regard to LFD-1, their phenotypic similarities and additive fusion defects with ΔPrm1 indicate that their altered sterol composition affects similar molecular processes. Since PRM1 and LFD-1 have independent and redundant roles during plasma membrane merger in N. crassa (Palma-Guerrero et al. 2014), both transmembrane proteins are probably differentially affected by an altered membrane composition. In conclusion, the structural similarities of the accumulating sterols in Δerg-10a/Δerg-10b and Δerg-1 (Figure 1B and Figure S7B) cause comparable specific macroscopic and microscopic phenotypes, but appear to differentially impact membrane-associated proteins.
Membrane fusion in Δerg-10a/Δerg-10b and Δerg-1 is affected before engagement of the fusion machinery
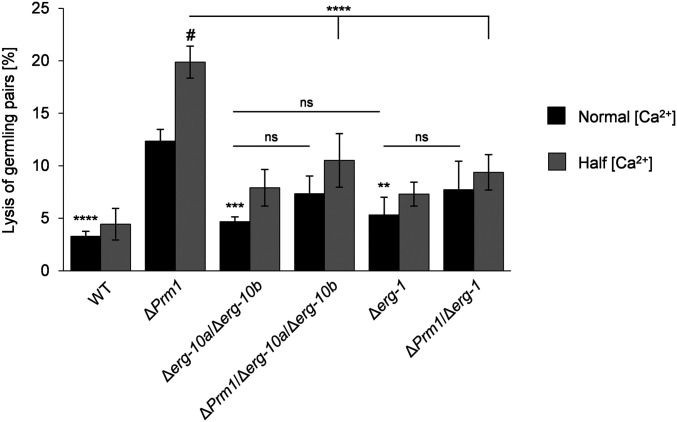
Cells of S. cerevisiae and N. crassa lacking PRM1 homologs are prone to lysis during mating and germling fusion, respectively. Lysis correlates with cytoplasmic mixture, indicating that aberrant engagement of the fusion machinery results in membrane rupture and cell death (Jin et al. 2004; Aguilar et al. 2007). In both fungi, fusion-induced lysis is enhanced by low levels of extracellular calcium ions (Ca2+), suggesting that Ca2+-dependent repair mechanisms counteract this deficiency (Aguilar et al. 2007; Palma-Guerrero et al. 2014). Since germling pairs of Δerg-10a/Δerg-10b and Δerg-1 have a ΔPrm1-like defect in plasma membrane fusion (Figure 4A), we tested whether these erg mutants also undergo fusion-induced cell lysis. Consistent with previous reports (Palma-Guerrero et al. 2014), ∼12% of ΔPrm1 cell pairs and 3% of WT pairings underwent lysis on regular MM (Figure 5). This frequency increased to ∼20% for ΔPrm1 germling pairs when the Ca2+ content was reduced by one-half, compared with no significant increase in WT. In contrast to ΔPrm1 and similar to WT, cell integrity of Δerg-10a/Δerg-10b and Δerg-1 fusion pairs was not compromised on regular medium, and only a slight increase in lysis was observed on reduced Ca2+. Interestingly, introducing the deletion of erg-10a/erg-10b or erg-1 into the ΔPrm1 strain partially suppressed its lysis phenotype to a level of 8% on regular medium and 10% on reduced Ca2+ (Figure 5). Taken together, these data indicate that in the Δerg-10a/Δerg-10b and Δerg-1 mutants, membrane fusion is blocked at a stage before PRM1 is required and therefore before engagement of the fusion machinery.
Figure 5.
The various plasma membrane fusion mutants differ in the frequency of fusion-induced cell lysis. Quantitative analysis of cell lysis in germling pairs of WT (FGSC 2489) and mutants lacking erg genes and/or Prm1 on MM, with normal and reduced levels of Ca2+. Values represent the mean ± SD from three independent populations of 100–125 germling pairs per replicate, strain, and growth condition. Statistical analysis with ANOVA and Tukey’s post hoc test: **P < 0.01, ***P < 0.001, ****P < 0.0001 for comparisons with ΔPrm1 at normal Ca2+ concentration; horizontal lines denote additional comparisons between strains (***P < 0.001); # denotes a significant difference (P < 0.001) for comparisons of ΔPrm1 between normal and half Ca2+ concentration, with no significant differences for any of the other strains between these two growth conditions. FGSC, Fungal Genetics Stock Center; MM, minimal medium; ns, not significant; WT, wild-type.
In contrast to ΔPrm1, the deletion of erg genes only affects vegetative fusion but not mating
A major advantage of using N. crassa as a model system for studying cell-to-cell fusion is the occurrence of different experimentally amenable fusion events within its life cycle. These include vegetative fusion between genetically identical spore germlings, but also nonself sexual fusion between cells of different mating types (Fischer and Glass 2019). During sexual development of this heterothallic fungus, prefruiting bodies (protoperithecia) of the female partner form specialized receptive hyphae called trichogynes, which grow toward and fuse with pheromone-secreting male cells of the opposite mating type (Raju 1980; Bistis 1983).
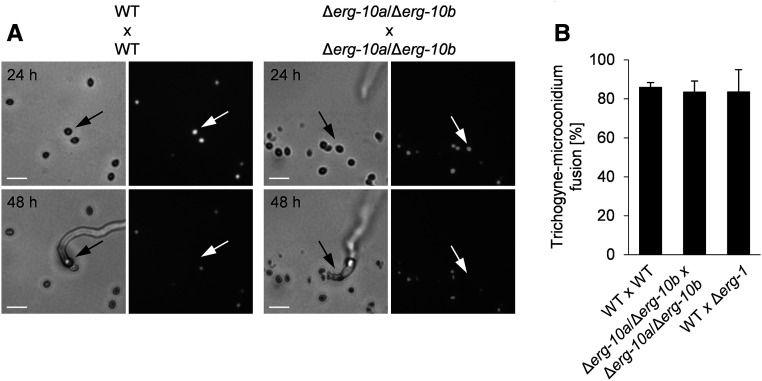
The absence of PRM1 in N. crassa reduces successful cell-to-cell fusion in both vegetative and sexual cell merger by 50%, suggesting that the protein is part of a basic, general membrane fusion machinery (Fleissner et al. 2009b). Similarly, the erg2 and erg3 mutants of S. cerevisiae show significant reduced membrane fusion during mating (Jin et al. 2008). Based on the phenotypic similarity of ΔPrm1 to Δerg-10a/Δerg-10b and Δerg-1 during germling fusion of N. crassa (Figure 4A), we hypothesized that mating might also be affected in these erg mutants. To test this notion, protoperithecia of the mat a strain of WT or erg mutants were fertilized with uninuclear microconidia from mat A strains, whose nuclei were labeled by histone-1 (H1)-GFP (Figure 6A). Successful fusion of the mating partners resulted in transport of the male nucleus through the trichogyne and could be observed as disappearance of the GFP signal (Fleissner et al. 2009b). As a result, the Δerg-10a/Δerg-10b mutant was not impaired in sexual fusion, reaching trichogyne–microconidium fusion rates comparable with WT crosses (Figure 6, A and B). Since the Δerg-1 mutant formed only low numbers of protoperithecia (Figure S6, B and C), we could only analyze the impact of this gene deletion on male cells in heterozygous crosses with WT. Here too, no effect on mating fusion was observed (Figure 6B). Taken together, these data indicate that in N. crassa, the accumulation of membrane sterols with altered ring system structures specifically affects vegetative cell fusion (Figure 4B), but not mating fusion.
Figure 6.
The deletion of erg-10a/erg-10b does not impact mating fusion in N. crassa. (A) Representative images of mating pairs in homozygous crossings of WT (FGSC 988 × N2-12) and Δerg-10a/Δerg-10b (N4-37 × MW_181). Microconidia (black arrows) expressing H1-GFP were observed before (24 hr) and after (48 hr) contact with a trichogyne of the opposite mating type (mat a × mat A). The disappearance of the nuclear fluorescence in the spores (white arrows) indicates cell-to-cell fusion events, resulting in the transition of nuclei via the trichogynes into the fruiting bodies. Bar, 10 μm. (B) Quantification of fusion between trichogynes and microconidia. Values represent the mean ± SD from three to six independent crossings for each combination with 39–122 mating pairs per replicate (no statistically significant differences were detected between these crosses: P > > 0.05, ANOVA with Dunnett’s post hoc test). FGSC, Fungal Genetics Stock Center; WT, wild-type.
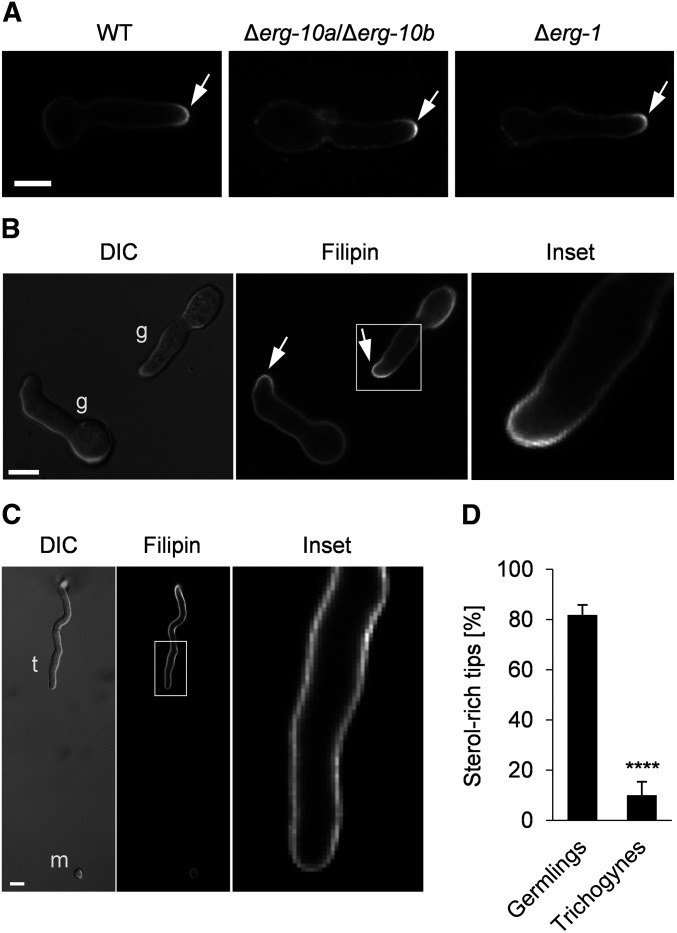
Based on these striking findings, we hypothesized that the sterol distributions in spore germlings and trichogynes might differ from each other. Staining with the sterol-binding dye filipin revealed sterol accumulation at the cell tips of spore germlings (Figure 7A), consistent with the occurrence of sterol-rich domains in the plasma membrane of various types of polarized fungal cells (Athanasopoulos et al. 2019). No differences in sterol distribution were observed among WT, Δerg-10a/Δerg-10b, and Δerg-1 germlings (Figure 7A). In interacting WT germlings, both partner cells typically accumulated sterols at the plasma membrane while they were redirecting their growth toward each other (Figure 7B). While ∼80% of interacting germ tubes possessed sterol-rich tips, sterol-rich membrane domains were remarkably almost absent in trichogynes growing toward a male cell (Figure 7, C and D). In contrast, an intense fluorescent signal was present along the entire plasma membrane of these receptive hyphae (Figure 7C). These data suggest fundamental differences in sterol distribution between vegetative and sexual cells of N. crassa.
Figure 7.
Membrane sterol distribution differs between vegetative and sexual cells of N. crassa. Germlings and trichogynes were stained with filipin. (A) Accumulation of sterols at the polarized cell tips of WT (FGSC 2489), Δerg-10a/Δerg-10b (N4-38), and Δerg-1 (MW_308) germlings. (B) Arrows indicate sterol-rich domains in interacting WT germlings (g). The white square marks the area of the enlarged image to the right. (C) Trichogyne (t)–microconidium (m) interaction. The white rectangle indicates the position of the enlarged image to the right. Bar in (A–C), 5 μm. (D) Quantification of sterol-rich cell tips in germlings and trichogynes during cell-to-cell interactions. Values represent the mean ± SD from three and two independent experiments with ∼100 germling pairs and up to 40 trichogynes per replicate (****P < 0.0001, unpaired, two-tailed Student’s t-test). FGSC, Fungal Genetics Stock Center; WT, wild-type.
Postfertilization events and fruiting body development are deficient in Δerg-10a/Δerg-10b and Δerg-1
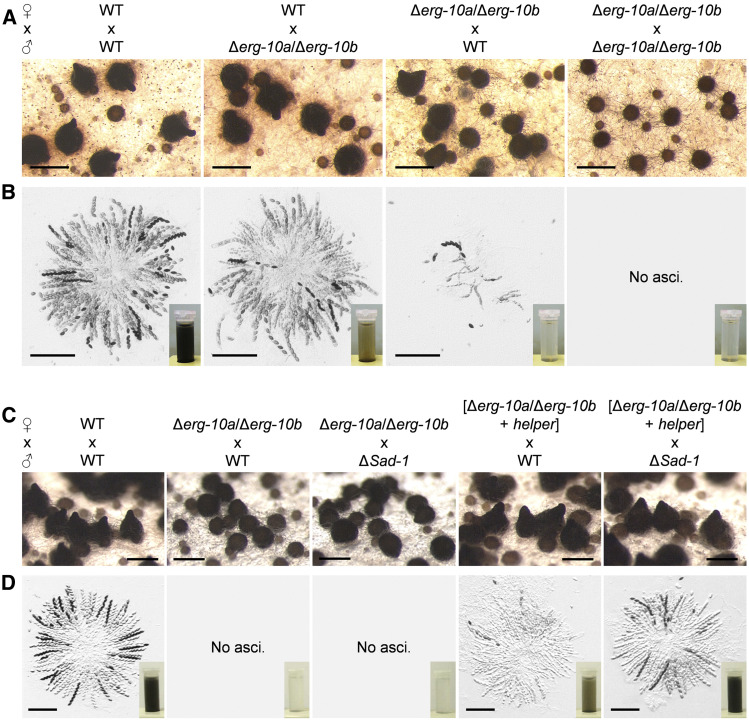
Compared with yeast, the more complex sexual life cycle of N. crassa allows study of the impact of erg gene deletions during multicellular development, such as fruiting body formation (Raju 2009). Despite the normal mating fusion of Δerg-10a/Δerg-10b crossing partners (Figure 6), subsequent fruiting body development was strongly affected (Figure 8). In heterozygous crosses between WT as the female and Δerg-10a/Δerg-10b as the male partner, the number of mature asci and ascospores was moderately decreased compared with WT reference crosses. However, when Δerg-10a/Δerg-10b served as the female, fertility was strongly impaired, typically resulting in immature perithecia that harbored only a few or no asci, and produced barely any ascospores (Figure 8, A and B). To pinpoint this defect, we created a heterokaryon of the double mutant with the so-called helper strain (FGSC 4564) by vegetative fusion. The helper strain carries an inactive MAT locus and rescues only fertility defects that arise before karyogamy in the heterokaryotic perithecia (Perkins 1984). Using the Δerg-10a/Δerg-10b/helper heterokaryon as a female largely restored perithecial maturation and asci formation; however, only reduced numbers of ascospores were ejected (Figure 8, C and D). This observation indicates that the absence of erg-10a and erg-10b affects both pre- and postfertilization events in fruiting body development. Notably, the partially restored fertility of the Δerg-10a/Δerg-10b/helper heterokaryon phenocopied heterozygous crosses between the single mutants Δerg-10a and Δerg-10b (Figure S8A), which were originally pursued to construct the double knockout strain. While homozygous crosses between the single mutants were indistinguishable from the WT control, heterozygous crosses between Δerg-10a and Δerg-10b resulted in mature perithecia with full rosettes of asci that only produced limited amounts of black ascospores, which were not rescued in a heterokaryon with the helper strain (Figure S8, A and B). In the case of the heterozygous cross between Δerg-10a and Δerg-10b, one nucleus was WT for erg-10a and the other one for erg-10b. During meiosis, these genes cannot pair with their homologous copy because it is missing in the crossing partner. These mismatches within the pairs of homologous chromosomes result in MSUD (meiotic silencing of unpaired DNA), a temporary gene inactivation mechanism in N. crassa (Shiu et al. 2001). To test a potential role of MSUD in the observed fertility defects, we analyzed heterozygous crosses between Δerg-10a and Δerg-10b in which the male crossing partner carried a deletion of Sad-1, a gene that codes for an RNA-dependent RNA polymerase essential for MSUD (Shiu et al. 2001). Consistent with our hypothesis, ΔSad-1 fully restored the amount of mature ascospores in the asci of the heterozygous crosses between Δerg-10a and Δerg-10b (Figure S8C). By extending this approach to Δerg-10a/Δerg-10b, we observed that the combination of the helper strain and ΔSad-1 fully restored the fertility of the double mutant (Figure 8, C and D). These results indicate that MSUD contributes to the poor fertility of crosses involving the deletions Δerg-10a and Δerg-10b after karyogamy, probably by temporarily blocking ergosterol biosynthesis at the level of C-5 sterol desaturation within the developing asci.
Figure 8.
Fertility of the Δerg-10a/Δerg-10b mutant is affected by pre- and postfertilization deficiencies. (A) Fruiting body development in homozygous and heterozygous crosses (mat A × mat a) of WT (FGSC 2489 and FGSC 988) and Δerg-10a/Δerg-10b (N4-38 and N4-37). (B) Microscopic analysis of rosettes of asci isolated from the fruiting bodies shown in (A). Inset images show the amount of ascospores harvested from one representative plate per crossing. (C and D) Analysis of perithecia, asci, and ascospores (insets) from crosses with a strain carrying the suppressor mutation ΔSad-1 (FGSC 11151) and/or involving the helper strain (FGSC 4564). Note that only the presence of the helper and the ΔSad-1 mutation fully restore fertility of the crosses. Bars in A and C, 500 μm; B and D, 200 μm. FGSC, Fungal Genetics Stock Center; WT, wild-type.
Similar to Δerg-10a/Δerg-10b, the Δerg-1 mutant was also poorly fertile as the female crossing partner, which could only partially be rescued in a heterokaryon with the helper strain. However, by introducing ΔSad-1 into these crosses, the fertility of Δerg-1 was completely restored (Figure S8D). Taken together, these results indicate that the accumulation of sterols with an altered double-bond arrangement in the ring system specifically impairs asci development and ascospore formation. Thus, the sterol composition in the membranes of diverse cell types involved in multicellular development differentially and specifically affects cellular behavior and function.
Discussion
Sterols are essential components of eukaryotic cell membranes and impact the properties of the lipid bilayer, as well as the function of membrane-associated proteins. In this study, we corroborate the hypothesis that specific structural sterol features have a distinct impact on specific cellular processes. While ergosterol precursors carrying a conjugated double bond in their aliphatic side chain, such as those accumulating in the Δerg-2 mutant, strongly affect cell-to-cell communication and cell contact recognition in germinating spores of N. crassa (Weichert et al. 2016), sterols with an altered ring system accumulating in the Δerg-10a/Δerg-10b and Δerg-1 mutants still promote these processes, but hinder subsequent plasma membrane merger. In a population of Δerg-10a/Δerg-10b or Δerg-1 spore germlings, about one-third of fusion pairs fail to merge, but two-thirds still undergo fusion. This indicates that the accumulating precursors can still support the fusion-related functions, but only to a limited extent. In addition, these B ring-modified sterol precursors are also generally able to support colony growth and development, but specifically affect the differentiation of vegetative spores and fruiting bodies. Notably, the marked drop in asexual sporulation of Δerg-10a/Δerg-10b and Δerg-1 as such (Figure 2B and Figure S5D) contrasts with normal conidiation of Δerg-2 and ΔPrm1 cultures (Fleissner et al. 2009b; Weichert et al. 2016), suggesting that the cell fusion defects are independent of the deficiencies in colony differentiation.
The highly specific effect of sterol structure on cellular differentiation is particularly illustrated by the opposing outcomes in vegetative vs. sexual cell fusion in the Δerg-10a/Δerg-10b and Δerg-1 mutants. While plasma membrane merger during vegetative spore germling fusion is highly reduced, it is normal during sexual mating. The defect during vegetative fusion is strikingly similar to the phenotype of the ΔPrm1 and Δlfd-1 mutants, which lack transmembrane proteins mediating plasma membrane fusion. Interestingly, however, the absence of these proteins affects vegetative and sexual fusion alike (Fleissner et al. 2009b; Palma-Guerrero et al. 2014), supporting the hypothesis that they are part of a general plasma membrane fusion machinery that mediates various fusion events within the fungal life cycle. However, so far our understanding of plasma membrane fusion in fungi remains extremely limited, in part because no fusogen has yet been identified in fungi (Aguilar et al. 2013). Normal fusion during mating of the Δerg-10a/Δerg-10b or Δerg-1 strains indicates that the accumulation of the sterol precursors does not affect this proposed core fusion machinery, or its delivery to the plasma membrane, raising the question of where their deleterious effects come into play. A significant proportion of ΔPrm1 or Δlfd-1 cell-to-cell pairs undergo lysis at the moment the fusion machinery is engaged. This observation has led to the notion that PRM1 and LFD-1 control the fidelity of the fusion process, probably by confining plasma membrane merger to a spatially restricted surface area (Aguilar et al. 2007; Palma-Guerrero et al. 2014). However, introduction of the Δerg-10a/Δerg-10b or Δerg-1 mutations into ΔPrm1 or Δlfd-1 suppresses their lysis phenotype. Therefore, we hypothesize that in the erg mutants the block of the fusion process occurs before engagement of the fusion machinery, thereby making PRM1 and LFD-1 dispensable in those genetic backgrounds. So far, the molecular processes mediating the steps between cell-to-cell contact recognition and engagement of the fusion machinery remain elusive. Our data indicate that, even at these late stages of the fusion process, differences between vegetative and sexual fusion exist.
Vegetative and sexual cell-to-cell communication and fusion of filamentous fungi share core conserved factors with yeast mating. However, mounting evidence indicates that the homologs of these factors are differently wired during mating and germling fusion in N. crassa. While the upstream conserved pheromone receptors are only involved in trichogyne–conidium interactions, homologs of the downstream yeast pheromone response pathway are central for germling interaction in N. crassa and other molds (Daskalov et al. 2017; Fischer and Glass 2019). Surprisingly, several factors homologous to proteins involved in yeast mating are only essential for germling fusion but dispensable for mating of the mold, including the here analyzed ERG-10a/ERG-10b and ERG-1, but also ERG-2, the polarity factor BEM-1, or components of the striatin-interacting phosphatase and kinase (STRIPAK) signaling complex (Simonin et al. 2010; Schürg et al. 2012; Weichert et al. 2016). The role of the conserved pheromone response MAPKs in mating of N. crassa remains so far cryptic, since the respective mutants do not form prefruiting bodies or trichogynes (Maerz et al. 2008). However, the findings of this study support the hypothesis that mating of S. cerevisiae is mechanistically and evolutionarily more closely related to vegetative than sexual fusion of filamentous fungi. Together, these examples illustrate how conserved molecular building blocks have likely been recombined and repurposed during evolution.
Although mating fusion is unaffected in Δerg-10a/Δerg-10b or Δerg-1, fruiting body formation and postfertilization events are deficient in these isolates. Partial complementation by the helper strain and rescue after inactivation of MSUD indicates independent effects of the altered sterol composition before and after karyogamy. Numerous cell fusion mutants are also affected in fruiting body formation, probably because cell-to-cell fusion contributes to the development of these multicellular structures (Lichius et al. 2012). Meiosis after karyogamy occurs in N. crassa in specialized cells, the so-called asci. Formation of each individual ascus also involves a cell-to-cell fusion event, the so-called crozier fusion, which could be hindered by the presence of the sterol precursors. However, testing of this hypothesis is cumbersome because these fusion events are not experimentally amenable. It will be of great interest to identify the molecular functions and pathways specifically affected at various steps of the vegetative and sexual life cycle of the erg mutants.
While the molecular targets affected by the accumulation of the ergosterol precursors lacking a double bond at position C-5 or containing a C-8 desaturation (Figure 2 and Figure S7) remain elusive, their impact on membrane structure can be predicted. The structure of the sterol ring system determines the tilting angle of these molecules within the lipid bilayer and their interaction with other lipids (Róg et al. 2009; Mannock et al. 2010). As a consequence, alterations in this part of the sterols impair lipid packaging, and therefore fluidity and permeability of the membrane, as well as the formation of sterol-rich membrane domains. The sterol ring system also impacts membrane curvature, which is crucial for the fusion of lipid bilayers to surpass the high-energy barrier required for membrane merger, including vesicle fusion (Podbilewicz 2014; Yang et al. 2016). Similar to the germling fusion defects of Δerg-10a/Δerg-10b and Δerg-1 (Figure 4), the corresponding erg3 and erg2 mutants impaired in yeast mating also accumulate ergosterol precursors with an altered ring system (Heese-Peck et al. 2002; Jin et al. 2008). While the same plasma membrane fusion defect in another S. cerevisiae mutant, Δerg6, has been attributed to the altered side-chain structure in the accumulating zymosterol (Jin et al. 2008), this sterol also features a double-bond arrangement in the ring system identical to fecosterol (Heese-Peck et al. 2002), one of the sterols accumulating in the absence of C-8 isomerase function (Figure 2B and Figure S7B). Therefore, we conclude that the ring system structure of sterols is a major determinant of plasma membrane fusion, possibly by impacting lipid–lipid interactions. In humans, the accumulation of 7-dehydrocholesterol, a precursor of cholesterol that contains one additional double bond at C-7 in the B ring, causes a severe developmental disorder known as Smith-Lemli-Opitz syndrome (Porter and Herman 2010). This sterol intermediate shows reduced interactions with the acyl chains of the phospholipids in the lipid bilayer, thereby reducing the order of the membranes and increasing the lateral mobility of the molecules within the lipid bilayer (Tulenko et al. 2006; Chen and Tripp 2012). Thus, structural alterations in sterols at the level of individual double bonds can have detrimental outcomes for cells, possibly paired with the depletion of the end product of sterol biosynthesis. However, the set of erg mutants available in N. crassa underscores that some sterol intermediates fully compensate for the loss of ergosterol (Weichert et al. 2016), highlighting that specific phenotypes caused by a block in sterol biosynthesis can exclusively correlate with the structure of the accumulating sterol species.
Acknowledgments
We are grateful to Louise Glass for sharing of N. crassa strains and Ralf Schnabel and Christian Hennig for continuous assistance in developing our microscopy. This work has been partially supported by funding from the German Research Foundation (Deutsche Forschungsgemeinschaft grant FL706/2-1 and FL706/3-1) to A.F.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.13050176.
These authors contributed equally to this work.
Communicating editor: M. Freitag
Literature Cited
- Aguilar P. S., Engel A., and Walter P., 2007. The plasma membrane proteins Prm1 and Fig1 ascertain fidelity of membrane fusion during yeast mating. Mol. Biol. Cell 18: 547–556. 10.1091/mbc.e06-09-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P. S., Heiman M. G., Walther T. C., Engel A., Schwudke D. et al. , 2010. Structure of sterol aliphatic chains affects yeast cell shape and cell fusion during mating. Proc. Natl. Acad. Sci. USA 107: 4170–4175. 10.1073/pnas.0914094107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P. S., Baylies M. K., Fleissner A., Helming L., Inoue N. et al. , 2013. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29: 427–437. 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos A., André B., Sophianopoulou V., and Gournas C., 2019. Fungal plasma membrane domains. FEMS Microbiol. Rev. 43: 642–673. doi: 10.1093/femsre/fuz022 [DOI] [PubMed] [Google Scholar]
- Bistis G. N., 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73: 959–975. 10.1080/00275514.1981.12021425 [DOI] [Google Scholar]
- Bistis G. N., 1983. Evidence for diffusible, mating-type-specific trichogyne attractants in Neurospora crassa. Exp. Mycol. 7: 292–295. 10.1016/0147-5975(83)90051-8 [DOI] [Google Scholar]
- Chen C., and Tripp C. P., 2012. A comparison of the behavior of cholesterol, 7-dehydrocholesterol and ergosterol in phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1818: 1673–1681. 10.1016/j.bbamem.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., and Hieter P., 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. 10.1016/0378-1119(92)90454-W [DOI] [PubMed] [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M. et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357 (erratum: Proc. Natl. Acad. Sci. USA 103: 16614). 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M.-A., Sharifmoghadam M. R., Calpena E., De Leon N., Hoya M. et al. , 2014. Membrane organization and cell fusion during mating in fission yeast requires multipass membrane protein Prm1. Genetics 196: 1059–1076. 10.1534/genetics.113.159558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalov A., Heller J., Herzog S., Fleißner A., and Glass N. L., 2017. Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi, pp. 215–229 in The Fungal Kingdom, edited by Heitman J., Howlett B., Crous P., Stukenbrock E., James T., et al American Society of Microbiology (ASM), Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourc E. J., 2008. Sterols and membrane dynamics. J. Chem. Biol. 1: 63–77. 10.1007/s12154-008-0010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M. S., and Glass N. L., 2019. Communicate and fuse: how filamentous fungi establish and maintain an interconnected mycelial network. Front. Microbiol. 10: 619 10.3389/fmicb.2019.00619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., and Glass N. L., 2007. SO, a protein involved in hyphal fusion in Neurospora crassa, localizes to septal plugs. Eukaryot. Cell 6: 84–94. 10.1128/EC.00268-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., Sarkar S., Jacobson D. J., Roca M. G., Read N. D. et al. , 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 4: 920–930. 10.1128/EC.4.5.920-930.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., Leeder A. C., Roca M. G., Read N. D., and Glass N. L., 2009a Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc. Natl. Acad. Sci. USA 106: 19387–19392. 10.1073/pnas.0907039106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., Diamond S., and Glass N. L., 2009b The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions. Genetics 181: 497–510. 10.1534/genetics.108.096149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleißner A., and Herzog S., 2016. Signal exchange and integration during self-fusion in filamentous fungi. Semin. Cell Dev. Biol. 57: 76–83. 10.1016/j.semcdb.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Freitag M., and Selker E. U., 2005. Expression and visualization of red fluorescent protein (RFP) in Neurospora crassa. Fungal Genet. Newsl. 52: 14–17. 10.4148/1941-4765.1124 [DOI] [Google Scholar]
- Freitag M., Hickey P. C., Raju N. B., Selker E. U., and Read N. D., 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41: 897–910. 10.1016/j.fgb.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Grindle M., 1973. Sterol mutants of Neurospora crassa: their isolation, growth characteristics and resistance to polyene antibiotics. Mol. Gen. Genet. 120: 283–290. 10.1007/BF00267158 [DOI] [PubMed] [Google Scholar]
- Grindle M., 1974. The efficacy of various mutagens and polyene antibiotics for the induction and isolation of sterol mutants of Neurospora crassa. Mol. Gen. Genet. 130: 81–90. 10.1007/BF00270520 [DOI] [PubMed] [Google Scholar]
- Grindle M., and Farrow R., 1978. Sterol content and enzyme defects of nystatin-resistant mutants of Neurospora crassa. Mol. Gen. Genet. 165: 305–308. 10.1007/BF00332531 [DOI] [PubMed] [Google Scholar]
- Heese-Peck A., Pichler H., Zanolari B., Watanabe R., Daum G. et al. , 2002. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 13: 2664–2680. 10.1091/mbc.e02-04-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M. G., and Walter P., 2000. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 151: 719–730. 10.1083/jcb.151.3.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S., Schumann M. R., and Fleißner A., 2015. Cell fusion in Neurospora crassa. Curr. Opin. Microbiol. 28: 53–59. 10.1016/j.mib.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Herzog S., Brinkmann H., Vences M., and Fleißner A., 2020. Evidence of repeated horizontal transfer of sterol C-5 desaturase encoding genes among dikarya fungi. Mol. Phylogenet. Evol. 150: 106850 10.1016/j.ympev.2020.106850 [DOI] [PubMed] [Google Scholar]
- Hickey P. C., Jacobson D. J., Read N. D., and Glass N. L., 2002. Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 37: 109–119. 10.1016/S1087-1845(02)00035-X [DOI] [PubMed] [Google Scholar]
- Hu C., Zhou M., Wang W., Sun X., Yarden O. et al. , 2018. Abnormal ergosterol biosynthesis activates transcriptional responses to antifungal azoles. Front. Microbiol. 9: 9 10.3389/fmicb.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Carlile C., Nolan S., and Grote E., 2004. Prm1 prevents contact-dependent lysis of yeast mating pairs. Eukaryot. Cell 3: 1664–1673. 10.1128/EC.3.6.1664-1673.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., McCaffery J. M., and Grote E., 2008. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell Biol. 180: 813–826. 10.1083/jcb.200705076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichius A., Lord K. M., Jeffree C. E., Oborny R., Boonyarungsrit P. et al. , 2012. Importance of MAP kinases during protoperithecial morphogenesis in Neurospora crassa. PLoS One 7: e42565 10.1371/journal.pone.0042565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerz S., Ziv C., Vogt N., Helmstaedt K., Cohen N. et al. , 2008. The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa. Genetics 179: 1313–1325. 10.1534/genetics.108.089425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N. A. H., McMullen T. P. W., and McElhaney R. N., 2010. The effect of variations in phospholipid and sterol structure on the nature of lipid-sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 163: 403–448. 10.1016/j.chemphyslip.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Margolin B. S., Freitag M., and Selker E. U., 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44: 34–36. 10.4148/1941-4765.1281 [DOI] [Google Scholar]
- Nawrath T., Dickschat J. S., Kunze B., and Schulz S., 2010. The biosynthesis of branched dialkylpyrazines in myxobacteria. Chem. Biodivers. 7: 2129–2144. 10.1002/cbdv.201000158 [DOI] [PubMed] [Google Scholar]
- Palma-Guerrero J., Leeder A. C., Welch J., and Glass N. L., 2014. Identification and characterization of LFD1, a novel protein involved in membrane merger during cell fusion in Neurospora crassa. Mol. Microbiol. 92: 164–182. 10.1111/mmi.12545 [DOI] [PubMed] [Google Scholar]
- Perkins D., 1984. Advantages of using the inactive-mating-type am1 strain as a helper component in heterokaryons. Fungal Genet. Newsl. 31: 41–42. [Google Scholar]
- Podbilewicz B., 2014. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 30: 111–139. 10.1146/annurev-cellbio-101512-122422 [DOI] [PubMed] [Google Scholar]
- Porter F. D., and Herman G. E., 2010. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52: 6–34. 10.1194/jlr.R009548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju N. B., 1980. Meiosis and ascospore genesis in Neurospora. Eur. J. Cell Biol. 23: 208–223. [PubMed] [Google Scholar]
- Raju N. B., 2009. Neurospora as a model fungus for studies in cytogenetics and sexual biology at Stanford. J. Biosci. 34: 139–159. 10.1007/s12038-009-0015-5 [DOI] [PubMed] [Google Scholar]
- Raymond C. K., Pownder T. A., and Sexson S. L., 1999. General method for plasmid construction using homologous recombination. Biotechniques 26: 134–141. 10.2144/99261rr02 [DOI] [PubMed] [Google Scholar]
- Richard F., Glass N. L., and Pringle A., 2012. Cooperation among germinating spores facilitates the growth of the fungus, Neurospora crassa. Biol. Lett. 8: 419–422. 10.1098/rsbl.2011.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M. G., Read N. D., and Wheals A. E., 2005. Conidial anastomosis tubes in filamentous fungi. FEMS Microbiol. Lett. 249: 191–198. 10.1016/j.femsle.2005.06.048 [DOI] [PubMed] [Google Scholar]
- Róg T., Pasenkiewicz-Gierula M., Vattulainen I., and Karttunen M., 2009. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta 1788: 97–121. 10.1016/j.bbamem.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Schumann M. R., Brandt U., Adis C., Hartung L., and Fleißner A., 2019. Plasma membrane integrity during cell-cell fusion and in response to pore-forming drugs is promoted by the penta-EF-hand protein PEF1 in Neurospora crassa. Genetics 213: 195–211. 10.1534/genetics.119.302363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürg T., Brandt U., Adis C., and Fleissner A., 2012. The Saccharomyces cerevisiae BEM1 homologue in Neurospora crassa promotes co-ordinated cell behaviour resulting in cell fusion. Mol. Microbiol. 86: 349–366. 10.1111/j.1365-2958.2012.08197.x [DOI] [PubMed] [Google Scholar]
- Serrano A., Illgen J., Brandt U., Thieme N., Letz A. et al. , 2018. Spatio-temporal MAPK dynamics mediate cell behavior coordination during fungal somatic cell fusion. J. Cell Sci. 131: jcs213462 10.1242/jcs.213462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu P. K., Raju N. B., Zickler D., and Metzenberg R. L., 2001. Meiotic silencing by unpaired DNA. Cell 107: 905–916. 10.1016/S0092-8674(01)00609-2 [DOI] [PubMed] [Google Scholar]
- Simonin A. R., Rasmussen C. G., Yang M., and Glass N. L., 2010. Genes encoding a striatin-like protein (ham-3) and a forkhead associated protein (ham-4) are required for hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 47: 855–868. 10.1016/j.fgb.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Staben C., Jensen B., Singer M., Pollock J., Schechtman M. et al. , 1989. Use of a bacterial Hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 36: 79–81. 10.4148/1941-4765.1519 [DOI] [Google Scholar]
- Tatum E. L., Barratt R. W., and Cutter V. M., 1949. Chemical induction of colonial paramorphs in Neurospora and Syncephalastrum. Science 109: 509–511. 10.1126/science.109.2838.509 [DOI] [PubMed] [Google Scholar]
- Tulenko T. N., Boeze-Battaglia K., Mason R. P., Tint G. S., Steiner R. D. et al. , 2006. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 47: 134–143. 10.1194/jlr.M500306-JLR200 [DOI] [PubMed] [Google Scholar]
- Vogel H. J., 1956. A convenient growth medium for Neurospora. Microb. Genet. Bull. 13: 42–46. [Google Scholar]
- Weichert M., Lichius A., Priegnitz B.-E., Brandt U., Gottschalk J. et al. , 2016. Accumulation of specific sterol precursors targets a MAP kinase cascade mediating cell–cell recognition and fusion. Proc. Natl. Acad. Sci. USA 113: 11877–11882. 10.1073/pnas.1610527113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard M., and Mitchell H. K., 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34: 573–577. 10.1002/j.1537-2197.1947.tb13032.x [DOI] [Google Scholar]
- Winston F., Dollard C., and Ricupero-Hovasse S. L., 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11: 53–55. 10.1002/yea.320110107 [DOI] [PubMed] [Google Scholar]
- Yang S.-T., Kreutzberger A. J. B., Lee J., Kiessling V., and Tamm L. K., 2016. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 199: 136–143. 10.1016/j.chemphyslip.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all reagents, software, and data that are necessary for drawing the conclusions presented in this study are fully represented within this article, and its tables and figures. Supplemental material available at figshare: https://doi.org/10.25386/genetics.13050176.