Abstract
Faithful degradation of mRNAs is a critical step in gene expression, and eukaryotes share a major conserved mRNA decay pathway. In this major pathway, the two rate-determining steps in mRNA degradation are the initial gradual removal of the poly(A) tail, followed by removal of the cap structure. Removal of the cap structure is carried out by the decapping enzyme, containing the Dcp2 catalytic subunit. Although the mechanism and regulation of mRNA decay is well understood, the consequences of defects in mRNA degradation are less clear. Dcp2 has been reported as either essential or nonessential. Here, we clarify that Dcp2 is not absolutely required for spore germination and extremely slow growth, but in practical terms it is impossible to continuously culture dcp2∆ under laboratory conditions without suppressors arising. We show that null mutations in at least three different genes are each sufficient to restore growth to a dcp2∆, of which kap123∆ and tl(gag)g∆ appear the most specific. We show that kap123∆ and tl(gag)g∆ suppress dcp2 by mechanisms that are different from each other and from previously isolated dcp2 suppressors. The suppression mechanism for tL(GAG)G is determined by the unique GAG anticodon of this tRNA, and thus likely by translation of some CUC or CUU codons. Unlike previously reported suppressors of decapping defects, these suppressors do not detectably restore decapping or mRNA decay to normal rates, but instead allow survival while only modestly affecting RNA homeostasis. These results provide important new insight into the importance of decapping, resolve previously conflicting publications about the essentiality of DCP2, provide the first phenotype for a tl(gag)g mutant, and show that multiple distinct mechanisms can bypass Dcp2 requirement.
Keywords: DCP2, mRNA decay, decapping, KAP123, tL(GAG)G
EUKARYOTES share two major messenger RNA (mRNA) decay pathways that are both carried out by exonucleolytic digestion. mRNA degradation is initiated by gradual shortening of the poly(A) tail, followed by Xrn1-mediated 5′ to 3′ decay and RNA exosome-mediated 3′ to 5′ decay (Parker 2012). Because Xrn1 can only degrade RNAs with a 5′-monophosphate (Stevens and Poole 1995; Jinek et al. 2011), removal of the 5′ cap structure by Dcp2 is required in the 5′ to 3′ decay pathway. Importantly, deleting either DCP2 or XRN1 results in stabilization of many yeast mRNAs (Larimer et al. 1992; Dunckley and Parker 1999; He et al. 2003). The stabilization of mRNAs in dcp2 mutants indicates that yeast Dcp2 is the major decapping enzyme, and the 5′ to 3′ pathway is the major mRNA decay pathway. Other enzymes capable of decapping mRNAs have been described both in yeast and other organisms (Jiao et al. 2010; Song et al. 2010; Li et al. 2011; Fujimura and Esteban 2012; Zhou et al. 2015; Grudzien-Nogalska et al. 2016; Doamekpor et al. 2020), but their role in bulk cytoplasmic mRNA degradation has not been fully defined. Consistent with its importance for mRNA decay, deletion of XRN1 causes a slow growth defect, while the phenotype of dcp2∆ is reported inconsistently among different studies. Some studies have reported that dcp2∆ is viable but slow-growing, while others reported that dcp2∆ is lethal (Dunckley and Parker 1999; Giaever et al. 2002; Geisler et al. 2012; He and Jacobson 2015). It has been speculated that this difference between studies is attributable to differences between the strains used (He and Jacobson 2015), but this has not been critically analyzed.
Previously, suppressor screens of budding yeast decapping mutants (dcp1 or dcp2 conditional mutants) have identified EDC1, EDC2, EDC3, SBP1, and DCP2 itself as high-copy suppressors (Dunckley and Parker 1999; Dunckley et al. 2001; Kshirsagar and Parker 2004; Segal et al. 2006). In each case, the improved growth caused by suppressors was correlated with improved decapping activity and mRNA degradation, suggesting that the essential function of the Dcp1-Dcp2 decapping enzyme is indeed mRNA decapping. Although these studies showed that the major function of Dcp1 and Dcp2 is mRNA decapping, they are limited to high-copy suppressor screens of conditional alleles in the decapping enzyme, which may not have revealed the full functions of Dcp2.
To further understand the function of Dcp2, we sought to identify suppressors of the growth defect of a decapping mutant by a complementary experimental evolution of a dcp2 null strain, which can be more powerful in identifying smaller effects and double mutants. Surprisingly, we identified genes that have no obvious connection to mRNA degradation. Among the genes we identified, we focused on the karyopherin KAP123 and the leucine tRNA tL(GAG)G that are recurrently mutated. We showed that a null mutation of each gene is sufficient to suppress the growth phenotype of dcp2∆, and that kap123∆ and tl(gag)g∆ have additive effects. We also show that previously reported viable dcp1∆ and dcp2∆ strains had undetected mutations in KAP123, suggesting that they were mistakenly reported as viable due to the suppressor mutations. Instead, our results suggest that dcp2∆ grows extremely slowly and cannot be continuously cultured under standard conditions. Interestingly, suppression of the growth defect of dcp2∆ is not caused by improved cytoplasmic mRNA decay. Absence of Dcp2 causes a global disturbance of the transcriptome including not only mRNA, but also noncoding RNA, and the suppressor mutations we identified do not restore the transcriptome to normal. However, we do detect a widespread but modest amplitude effect in partially restoring RNA homeostasis. Whether these modest effects on transcripts are a cause or effect of improved growth, or a mixture of both, is not clear. These results indicate that the extremely poor growth of a strain lacking the decapping enzyme can be overcome by several independent mechanisms that have modest effects on the transcriptome compared to the global disruption of the transcriptome caused by dcp2 mutations.
Materials and Methods
Strains, plasmids, and oligonucleotides
The DCP2/dcp2∆ heterozygous diploid in the BY4743 (S288C) background was obtained from Open Biosystems, Huntsville, AL and all other strains (Supplemental Material, Table S1) used are derived from it through standard genetic procedures. Plasmids were generated by standard procedures and are listed in Table S2. Oligonucleotides (Sigma-Aldrich, St. Louis, MO) used in this study are listed in Table S3.
Yeast growth conditions
Yeast was grown either in standard yeast extract peptone (YEP) media containing 2% dextrose or galactose or in synthetic complete media lacking amino acids (Sunrise Science) as required. G418 (0.67 mg/ml), hygromycin B (325 U/ml), or clonNAT (100 µg/ml) was added to YEP plus dextrose media to select for knockouts. Cells were incubated at 30° unless otherwise indicated. The dcp2-7 cultures were incubated for 60 or 90 min at 37° to inactivate the decapping enzyme for the GAL mRNA stability and transcriptome sequencing experiments, respectively.
To induce sporulation, diploid cells were grown in nutrient-depleted media for 4–5 days. Sporulated cells were resuspended in water with Glusulase (Perkin Elmer). This reaction was incubated at 30° for 30 min. Ascus digestion was terminated using water. Haploids were obtained either by tetrad dissection or by random spore isolation (Rockmill et al. 1991). The dcp2∆ spores from the starting haploid formed pinprick-size colonies after 2 weeks of incubation at room temperature.
Experimental evolution was initiated from four haploid dcp2∆ strains, each derived from a different tetrad. Duplicate 5 ml cultures of each of the four haploid dcp2∆ strains were inoculated in YEP containing 2% dextrose, G418 (167 mg/liter), and ampicillin (50 mg/liter). Cultures were grown at 30° until the OD600 of the culture reached > 8.5. Then, 10 µl of this culture (containing on the order of 106 yeast cells) were transferred into 5 ml of fresh media of the same type. This culturing and 500-fold dilution was repeated 30 times. A 500-fold (or 28.97) dilution represents ∼9 doublings. Thus, after 30 cycles of culture and dilution the cultures had gone through ∼270 generations.
For the growth assay on solid media, exponentially growing cells were serially diluted (fivefold) and spotted on the indicated media. For the growth assay in liquid media, exponentially growing cells were diluted to OD600 of 0.1, and transferred to a 96-well plate. Cells were incubated at 30° in a BioTek′s Synergy Mx Microplate Reader. OD600 was measured every 10 min for ∼15 hr. Collected data were processed through Gen5 (BioTek).
Microscopy
To examine cell morphology, exponentially growing cells were analyzed on an Olympus BX60 microscope.
Whole-genome sequencing analysis
Total genomic DNA was isolated from exponentially growing cells using a phenol-chloroform extraction method and further purified with the use of a DNeasy Blood & Tissue Kit (QIAGEN, Valencia, CA) and a MasterPure Yeast DNA Purification Kit (Lucigen). PE150 libraries of the evolved strains were prepared and sequenced by Novogen.
To identify mutations, sequencing reads were trimmed with Trim Sequences (http://hannonlab.cshl.edu/fastx_toolkit/), quality checked with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and mapped with Bowtie2 (Langmead et al. 2009) to Saccharomyces cerevisiae reference genome R64-1-1 (www.ensembl.org). The overall alignment rate was ∼91–99%. Before calling variants, BAM data sets for the individual dcp2Δ strain and heterozygous diploid DCP2/dcp2Δ strain were merged using MergeSamFiles (http://broadinstitute.github.io/picard/). Data sets were further processed for left realignment through BamLeftAlign (https://arxiv.org/abs/1207.3907). To call all the variants, we used FreeBayes (https://arxiv.org/abs/1207.3907) for detection and SnpEff 4.3 (Cingolani et al. 2012) for annotation. Integrated Genome Viewer (https://software.broadinstitute.org/software/igv/download) was used to inspect candidate SNPs. True mutations were differentiated from sequencing errors and preexisting SNPs by being supported by the consensus of the reads in the evolved isolate(s), but not by the reads from the other evolved isolates or the heterozygous diploid DCP2/dcp2Δ strain that we had previously sequenced. The vast majority of preexisting SNPs that we identified in the DCP2/dcp2∆ starting diploid have previously been described in the genome sequences of BY4741 (http://sgd-archive.yeastgenome.org/sequence/strains/BY4741/) and/or BY4742 (http://sgd-archive.yeastgenome.org/sequence/strains/BY4742/). BY4741 and BY4742 are the parents of the diploid BY4743 strain, which in turn is the parent of our DCP2/dcp2∆ starting diploid.
MiModD Deletion Calling (https://sourceforge.net/projects/mimodd/) was used to search for deletions, which identified the ura3∆, his3∆, met15∆, lys2∆, and leu2∆ deletions of BY4743, but no other deletions. MiModD Coverage Statistics (https://sourceforge.net/projects/mimodd/) was used to measure coverage depth by chromosome, to search for aneuploidy.
Protein analysis
Total protein was extracted in IP50 buffer [50 mM Tris-HCl (pH 7.5), 50 mM NaCl, 2 mM MgCl2, 0.1% Triton X-100] with 0.007% β-mercaptoethanol and 0.00174% PMSF, and complete protease EDTA-free mini tablet (Roche) by bead beating and analyzed by Western blot. Blots were probed with anti-Kap123 at 1:5000 (Patel and Rexach 2008; Floch et al. 2015) and anti-Pgk1 at 1:10,000 (Invitrogen, Carlsbad, CA), and developed using Amersham ECL Prime (GE Healthcare). Images were acquired and analyzed using an ImageQuant LAS 4000 biomolecular imager (GE Healthcare) and ImageQuant TL image analysis software.
RNA analysis using Northern blotting
For analyzing the steady-state RNA level, cells exponentially growing at 30° were harvested. For analyzing RNA stability, dcp2-7 mutants were grown in YEP containing 2% galactose at 21° and transferred into a 37° incubator for 1 hr to inactivate the decapping enzyme. Cells were washed with YEP, and dextrose (40% stock solution) was added to a final concentration of 2% to repress transcription of the GAL genes. Although cells were incubated at 37°, samples were collected at the indicated time points and immediately frozen.
For RNA preparation, the harvested cell pellet was lysed by vortexing with glass beads. RNA was purified through two rounds of phenol/chloroform/LET (LiCl-EDTA-Tris HCl, pH8.0) and one additional chloroform extraction, and ethanol precipitated.
Total RNA was analyzed through Northern blotting. Briefly, 10 μg of total RNA was analyzed by electrophoresis on denaturing gels, either 1.3% agarose/formaldehyde gels for mRNA analysis or 6% polyacrylamide (19:1) 8M urea gel for transfer RNA (tRNA) analysis, as indicated. RNA was transferred to a nylon membrane and probed with 32P 5′ end labeled oligonucleotides. For the Northern blots on mutant tRNAs, we prevented differences in detection efficiency by using probes that did not overlap with the mutations. Blots were imaged by phosphorimaging on a Typhoon FLA 7000 (GE Healthcare), and quantitated using ImageQuant software.
Transcriptome analysis
For RNA-sequencing (RNA-seq) analysis, cultures (biological triplicates) of exponentially growing cells were transferred into a 37° incubator and incubated for 90 min to inactivate the decapping enzyme before harvesting. Total RNA was extracted using the hot phenol method (He et al. 2008). For sequencing, poly(A)+ selected RNA was used to construct a library for PE150 Illumina sequencing. Reads were submitted to Sequence Read Archive (SRA) under project number PRJNA626686. Raw reads were trimmed using Trim Galore! (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/), quality checked with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and mapped to reference genome R64-1-1 with TopHat2 (Kim et al. 2013). Gene expression level was determined with featureCounts (Liao et al. 2014) for genes identified in the annotation file from Ensembl (www.ensembl.org) and for XUTs (http://vm-gb.curie.fr/XUT/index.htm) (van Dijk et al. 2011). Differential gene expression was determined through DESeq2 (Love et al. 2014). Gene ontology (GO) analysis was performed through the GO Term Finder (version 0.86).
Finding kap123 mutations in published RNA-seq data
To determine whether previously published RNA-seq experiments inadvertently used kap123 mutant strains and to identify the mutations, we downloaded raw RNA-seq reads from the European Bioinformatics Institute (https://www.ebi.ac.uk/ena). Reads were trimmed with Trim Galore!, quality checked with FastQC, and then aligned with TopHat2 to the R64-1-1 reference genome. Aligned reads were analyzed in Integrated Genome Viewer. This identified the kap123-Y687X in three data sets from a dcp2∆ W303 strain (SRR4163304, SRR4163305, SRR4163306). For the dcp1∆ data sets (SRR4163301, SRR4163302), the TopHat alignment suggested a small deletion, but failed to precisely identify it. Aligning the same data sets with Bowtie2 in very sensitive local mode did precisely identify the deletion as a 21-bp deletion mediated by a GCGGAACC repeat in the wild-type gene. We found no mutations in the dcp1∆ or dcp2∆ strains for any of the other genes that are mutated in our evolved isolates. Similarly, analyzing the dcp2∆ RNA-seq data from reference (Geisler et al. 2012) (SRR364981), we identified the same tl(gag)g mutation as in our evolved isolates 4-1 and 4-2 and a novel kap123 mutation. We did not find kap123 mutations in wild-type controls (SRR4163289, SRR4163290, SRR4163291), xrn1∆ (SRR4163307, SRR4163308, SRR4163309), dcp2-7 (SRR2045250, SRR2045251, our RNA-seq data), dhh1∆ (SRR6362787), pat1∆ (SRR6362781), lsm1∆ (SRR6362784), dcp2-N245 (SRR6362793), dcp2-N245-E153Q (SRR6362796), dcp2-N245-E198Q (SRR6362799), scd6∆ (SRR7162931), caf1∆ (SRR7174202), or dhh1∆ (SRR3493892, SRR4418659) (Radhakrishnan et al. 2016; Wery et al. 2016; Celik et al. 2017; Jungfleisch et al. 2017; He et al. 2018; Webster et al. 2018; Zeidan et al. 2018). This suggests that only very severe decapping defects select for kap123 suppressors.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. RNA-seq data are available at the Sequence Read Archive under project number PRJNA626686. Supplemental data are available at https://doi.org/10.6084/m9.figshare.12985820. All yeast strains and plasmids used are available upon request.
Results
DCP2 is required for normal growth of yeast
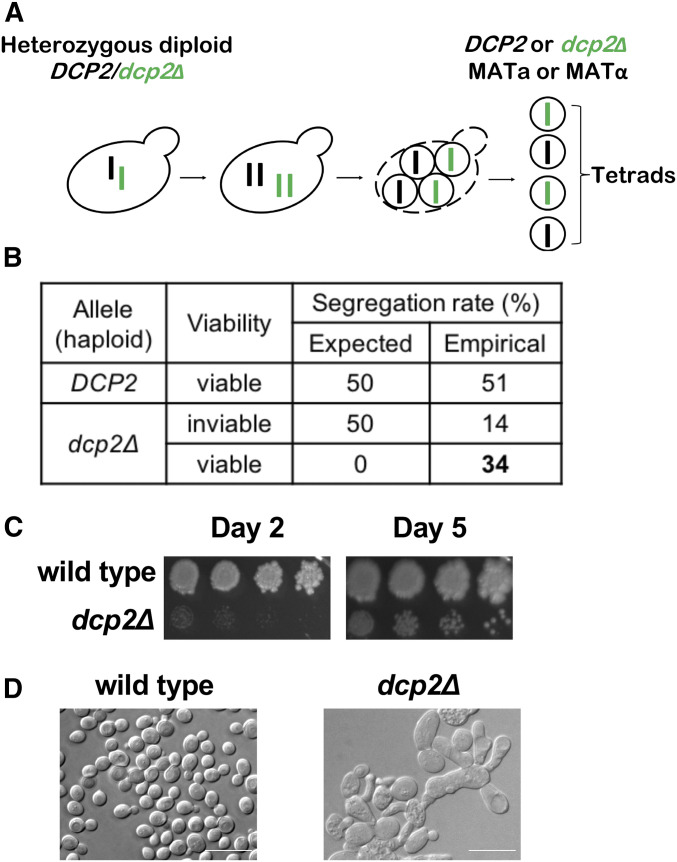
Although DCP2 is annotated as an essential gene in the Saccharomyces Genome Database (yeastgenome.org), other studies reported that DCP2 is not an essential gene (Dunckley and Parker 1999; Giaever et al. 2002; Geisler et al. 2012; He and Jacobson 2015). The genome-wide effort to identify essential genes was based on sporulating a heterozygous diploid DCP2/dcp2∆ strain and attempting to recover haploid dcp2∆ strains. We obtained this same commercially available heterozygous diploid DCP2/dcp2∆ strain and repeated sporulation and tetrad dissection (Figure 1A). We expected that this would produce viable wild-type and inviable dcp2∆ progeny in a 1:1 ratio. However, upon prolonged growth we isolated viable dcp2∆ (34%) along with wild-type (51%) and inviable dcp2∆ progeny (14%) (Figure 1B). Although we were able to recover viable dcp2∆ progeny, these spores formed much smaller colonies even after prolonged incubation.
Figure 1.
Isolation of viable dcp2∆ cells with severe growth and morphological defects. (A) Diagram of tetrad analysis of heterozygous diploid DCP2/dcp2∆ strain. (B) Tetrad dissection results in wild-type and dcp2∆ colonies. If DCP2 is essential as annotated, 50% of the spores would be expected to be inviable. Instead 34% of the spores analyzed were dcp2∆ and viable. (C) dcp2∆ colonies resulting from tetrad dissection grow slowly. Serially diluted wild-type and viable dcp2∆ colonies from B were spotted on YPD solid media and grown at 30° for the indicated times. (D) dcp2∆ cells resulting from tetrad dissection have morphological defects. Cells were grown at 30° until OD600 reaches 0.3–0.4. Samples were diluted in YPD and examined by light microscopy. Bar represents 10 µm.
To further examine the growth and morphology of the recovered dcp2∆ strains, they were serially diluted, spotted on YPD, and cultured at 30°. Although viable, the dcp2∆ strains grow extremely slowly compared to wild type (Figure 1C). Examination through light microscopy revealed irregular and heterogeneous cell morphology, with many elongated cells in clumps (Figure 1D). Additionally, multiple vacuole-like organelles of different sizes accumulated in these cells. Taken together, this suggests that DCP2 is required for normal growth and morphology of budding yeast (see Discussion).
Experimental evolution of dcp2∆ strains results in improved growth and morphology
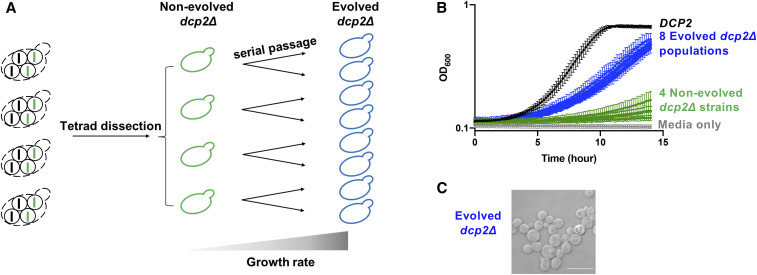
To understand the function of DCP2 that affects cell growth, we decided to identify suppressors of the growth defect of dcp2∆. We used an experimental evolution approach that allows cells to accumulate mutations and enriches for suppressors that are advantageous for fitness in the absence of DCP2. For this, we used four haploid progeny, each derived from a different tetrad (meiosis). Each haploid dcp2∆ strain was used to start duplicate liquid cultures. Once these cultures reached saturation, we diluted them into new media for several iterations (Figure 2A). Throughout the experimental evolution process, growth of all dcp2∆ populations was examined both by spotting serially diluted cultures on solid media (data not shown), and by measuring OD600 of cells growing in liquid media (Figure S1). During the course of the experimental evolution, we observed growth improvement at the 90th generation, and further growth improvement was observed at the 180th generation (Figure S1). However, in most cases, the growth improvement from 180 to 270 generations was minimal (Figure S1). Thus, we stopped the experimental evolution process after ∼270 generations and further analyzed these dcp2∆ populations (evolved dcp2∆). All eight evolved dcp2∆ populations grew better than their parental nonevolved dcp2∆ strain, although not as well as the wild-type strain (Figure 2B). The doubling time of the eight evolved dcp2∆ populations is 1.5- to 2-fold longer than that for the wild-type strain, but much shorter than the doubling time of four nonevolved dcp2∆ strains, which could not be calculated in the 16-hr period of the experiment because of the extremely slow growth. Similar to the growth improvement, the morphological defects in nonevolved dcp2∆ strains are partially restored in evolved dcp2∆ populations (Figure 2C and Figure S2). Evolved dcp2∆ cells had a more homogenous morphology, were less elongated, and less clumped compared to nonevolved dcp2∆ cells. These results suggest that the experimental evolution of dcp2∆ successfully selects suppressor mutations that confer growth improvement on dcp2∆ strains.
Figure 2.
Experimental evolution of dcp2∆ strains results in growth and morphological improvement. (A) Diagram of experimental evolution. Four dcp2∆ strains (middle) isolated from distinct tetrads (left) were subject to serial passage. Two replicate samples of dcp2∆ strains were transferred to new media in iterations until the growth rate increases (right). (B) The dcp2∆ growth defect is partially improved in evolved dcp2∆ populations (blue) compared to nonevolved dcp2∆ strains (green). Cells were grown at 30° and OD600 was measured every 10 min for ∼15 hr. Shown is the average OD600 from replicate cultures and their standard deviations, plotted on a log scale. n = 8 for DCP2, n = 4 for each nonevolved dcp2∆, and n = 2 for each evolved dcp2∆. (C) Morphological defects are partially restored in evolved dcp2∆ populations. A representative microscopic image of an evolved dcp2∆ population is shown. Bar represents 10 µm.
Whole-genome sequence analysis identifies suppressors of dcp2∆ growth defects
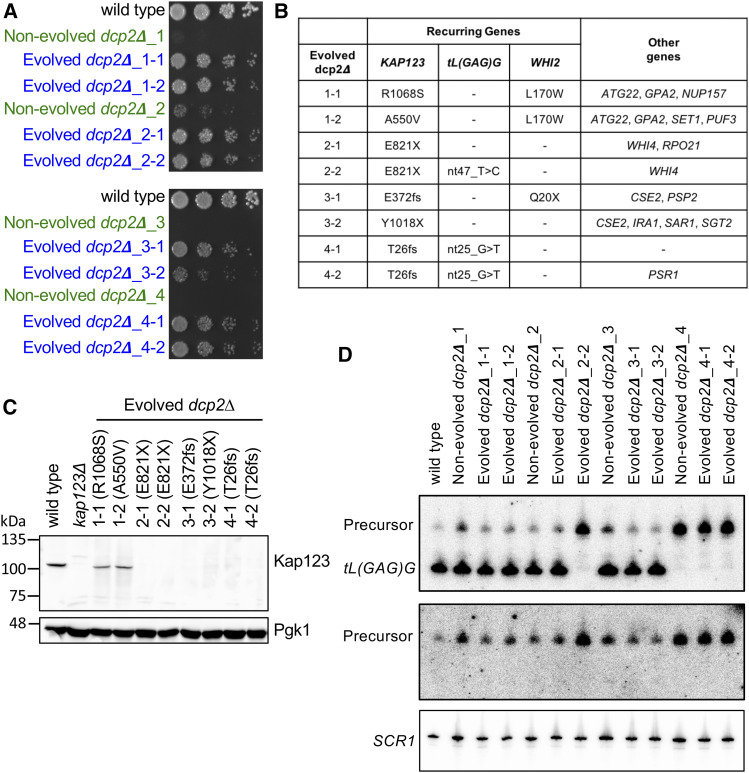
To identify suppressor mutations that confer growth improvement to dcp2∆ we performed whole-genome sequence (WGS) analysis on evolved dcp2∆ strains. We suspected that the evolved populations were genetically heterogeneous, which complicates the analysis and interpretation of WGS. Thus, for each evolved dcp2∆ population, we picked a single colony to generate eight genetically homogeneous evolved dcp2∆ isolates. As we observed in the evolved dcp2∆ populations, all eight evolved dcp2∆ isolates grew better than their nonevolved dcp2∆ counterparts (Figure 3A). We then sequenced the genomes of the eight evolved dcp2∆ isolates as well as the starting diploid DCP2/dcp2∆ strain (average genome coverage 112-fold), and identified mutations that were present in the evolved isolates, but not in the starting diploid (Figure 3B and Figure S3A). Each evolved dcp2∆ isolate contains nonsynonymous mutations in two to six genes that are not present in the heterozygous diploid DCP2/dcp2∆ strain. All of them were point mutations, including substitution and deletion/insertion of a small number of bases. We did not detect any larger deletions or aneuploidy (Figure S3B), which is often seen after the deletion of an essential gene (Liu et al. 2015).
Figure 3.
Whole-genome sequencing identifies multiple null mutations in KAP123 and tL(GAG)G. (A) The growth defect is partially improved in evolved dcp2∆ isolates. A single colony was isolated from each evolved population (blue) and haploid starting strain (green). Each of these genetically homogeneous strains was serially diluted, spotted on YPD solid media, and grown at 30°. Shown is the growth at day 2. (B) Whole-genome sequences were determined for the eight evolved dcp2∆ isolates and compared to the DCP2/dcp2∆ starting diploid. Nonsynonymous mutations that are not present in the starting diploid are listed. (C) Six of the evolved isolates have null mutations in KAP123 that generate a premature stop codon and they do not express Kap123. A representative Western blot analyzing the expression level of Kap123 (top) from the indicated strains is shown. Pgk1 (bottom) is used as loading control. (D) Three of the evolved isolates have null mutations in tL(GAG)G and do not express mature tL(GAG)G tRNA. A representative Northern blot of tL(GAG)G tRNA (top and middle) from the indicated strains is shown. The top panel is probed with an oligonucleotide complementary to mature tL(GAG)G, while the middle panel is probed with an oligonucleotide complementary to 5′ extended precursors of tL(GAG)G. SCR1 is used for loading control (bottom).
Strikingly, all eight evolved dcp2∆ isolates had a mutation in the KAP123 gene, which encodes one of the 14 karyopherins that mediate nucleocytoplasmic trafficking (Chook and Suel 2011; Aitchison and Rout 2012). In total, six different kap123 alleles were identified from the evolved dcp2∆ isolates. Isolates 2-1 and 2-2 are derived from the same haploid spore and both contained the kap123-E821X nonsense mutation. We conclude that this mutation arose very early, before the duplicate cultures were started. Similarly, each pair of evolved isolates shared at least one mutation, which must have arisen early, but also differed from its sister isolate by additional mutations (see Discussion).
Four kap123 mutant alleles have either a nonsense mutation or a frameshift mutation that generates a premature stop codon, and thus are likely loss-of-function mutations. Two of the mutations are missense mutations, A550V and R1068S, that both affect conserved residues that are structurally important (Figure S4A). Western blot analysis showed that the Kap123 protein was not detectable from the six evolved dcp2∆ isolates harboring nonsense or frameshift mutation, implying destabilization of mRNA, protein, or both. In contrast, Kap123-A550V and Kap123-R1068S were expressed (Figure 3C).
In addition to KAP123, we identified multiple alleles of tL(GAG)G and WHI2 in our evolved isolates. WHI2 encodes a protein involved in stress response and the TOR pathway in yeast (Kaida et al. 2002; Chen et al. 2018). One of the whi2 alleles introduces an early stop codon (whi2-Q20X) and thus is likely a loss-of-function allele. The tL(GAG)G gene encodes leucine tRNA with a GAG anticodon, and both of the mutations are predicted to disrupt tRNA folding (Figure S4B). Northern blot analysis indicated that the mutant tRNA was not expressed (Figure 3D). In addition, pre-tRNA with 5′ extensions appeared more abundant in the mutant, suggesting that the structural perturbations in tL(GAG)G interfere with 5′ end processing by RNase P. The nonevolved dcp2∆_4 strain did not produce the mature tRNA (Figure 3D). In addition, the two evolved dcp2∆ strains derived from the dcp2∆_4 had the same allele, implying that this mutation had already arisen in their common ancestor strain, nonevolved dcp2∆_4 (Figure S12A).
Overall, these data suggest that each of the evolved dcp2∆ isolates contains loss-of-function mutations in KAP123, tL(GAG)G, and/or WHI2, as well as other mutations of unclear significance, and that some of these mutations arose very early, whereas others arose later.
Null mutations of KAP123, tL(GAG)G, or WHI2 are sufficient to restore growth of dcp2∆
For genes that were mutated in multiple evolved dcp2∆ isolates, we tested whether a null mutation of each gene is individually sufficient to suppress dcp2∆ growth defects. Because our WGS indicated that dcp2∆ strains quickly accumulated suppressors, we were careful to minimize selection for undesired spontaneous suppressors that would complicate the analysis of the desired potential suppressors [kap123∆, tl(gag)g∆, and whi2∆]. We therefore started with the heterozygous DCP2/dcp2∆ diploid strain that we had sequenced, and that contained wild-type KAP123, WHI2, and tL(GAG)G genes. We then knocked out one of the alleles of KAP123, WHI2, or tL(GAG)G to generate DCP2/dcp2∆ KAP123/kap123∆, DCP2/dcp2∆ WHI2/whi2∆, and DCP2/dcp2∆ tL(GAG)G/tl(gag)g∆ diploids. Each of these three double heterozygous strains was then transformed with a plasmid that carried functional DCP2 and URA3 genes, and haploid progeny were generated and genotyped. Finally, the strains were grown on media lacking uracil (selecting for the DCP2 URA3 plasmid) or media containing 5FOA (counterselecting against the DCP2 URA3 plasmid). Growth on 5FOA-containing media in this assay indicates that the strain is viable in the absence of the DCP2 plasmid. This elaborate experimental setup allowed us to determine growth without inadvertently preselecting for suppressor mutations.
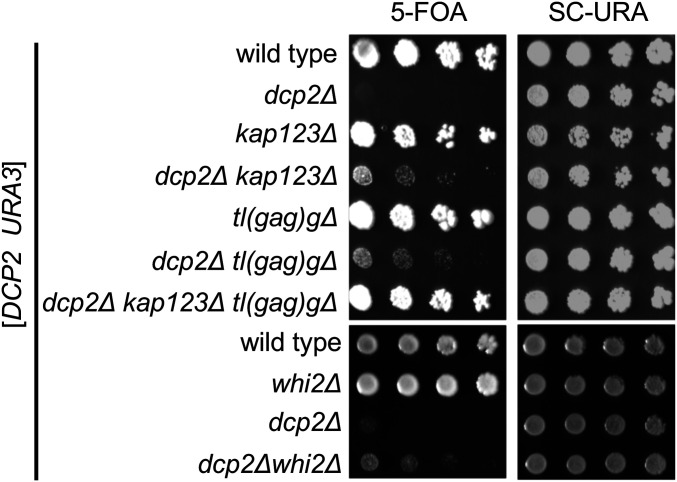
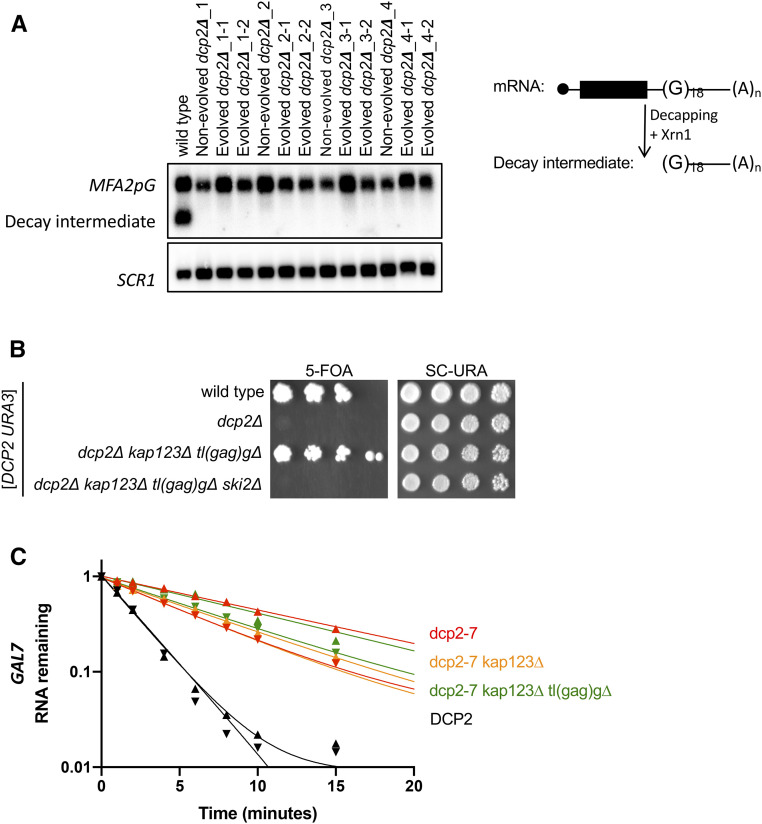
As expected, progeny with a functional chromosomal DCP2 gene derived from each of the double heterozygotes readily formed colonies on 5FOA (Figure 4), indicating that they could grow after losing the DCP2 URA3 plasmid. In contrast, the dcp2∆ progeny derived from each of the double heterozygotes failed to form colonies on 5FOA after up to 5 days of incubation. Importantly, dcp2∆ kap123∆, dcp2∆ whi2∆, and dcp2∆ tl(gag)g∆ strains each formed small colonies on 5FOA-containing media, indicating that they were able to grow after losing the DCP2 plasmid. Thus, each of these null alleles is sufficient to suppress the dcp2∆ growth phenotype.
Figure 4.
Null mutations of KAP123, tL(GAG)G, or WHI2 are sufficient to suppress the growth defect of dcp2∆. Growth assay showing that the extremely slow growth of dcp2∆ is suppressed by kap123∆, tl(gag)g∆, or whi2∆. Null mutations of KAP123 and tL(GAG)G have an additive effect on the growth. Each strain was made by sporulation of a double heterozygous diploid transformed with a URA3 plasmid expressing DCP2. Haploid progeny were serially diluted and spotted on 5FOA and SC-URA (control) solid media. Shown is the growth of a representative haploid strain.
Multiple studies have reported inadvertent mutations of WHI2 in various yeast knockout strains (Lang et al. 2013; Teng et al. 2013; Comyn et al. 2017). In contrast, we could not find any other studies identifying kap123 or tl(gag)g as suppressors. Thus, the effect of whi2∆ on dcp2∆ appears to be less specific than the other two suppressors. Therefore, we decided to focus on KAP123 and tL(GAG)G for further analyses.
To test whether kap123∆ and tl(gag)g∆ suppressed dcp2∆ growth defects through a common mechanism, we generated dcp2∆ kap123∆ tl(gag)g∆ triple mutant with the DCP2 URA3 plasmid. Importantly, the triple mutant grew better on 5FOA plates than either double mutant (Figure 4), indicating that kap123∆ and tl(gag)g∆ act independent of each other to improve dcp2∆ growth. This observation that multiple suppressors further improve growth also explains why the experimental evolution resulted in strains with multiple mutations.
Other viable dcp2∆ or dcp1∆ strains contain similar suppressor mutations
Our WGS data suggest that DCP2 is required for growth under laboratory conditions, but some previous studies have reported that DCP2 is dispensable for survival. Some studies have attributed these differences to the use of different strains, e.g., it has been reported that dcp2∆ is viable in the W303 strain but lethal in S288C (He et al. 2014; He and Jacobson 2015). However, the available genome sequences of W303 and S288C revealed that neither contains obvious null mutations in KAP123, WHI2, or tL(GAG)G. We therefore reanalyzed the strains used in three different studies.
First, we analyzed published RNA-seq data of a dcp2∆ strain in the S288C background to reveal mutations shown as mismatches between the RNA reads and reference genome (Geisler et al. 2012). The dcp2∆ strain used in that study (yJC327 in Figure S12A) was obtained from our laboratory and is derived from the nonevolved strain dcp2∆_4 used in our studies. Our WGS data above indicated that nonevolved strain dcp2∆_4 strain had an early arising tl(gag)g-G25U allele and, as expected, we detected this allele in the RNA-seq reads. This result confirms the Northern blot result above that nonevolved strain dcp2∆_4 lacks mature tL(GAG)G tRNA. In addition, we detected a kap123-T766fs allele in the RNA-seq data that is different from mutations what we identified in WGS analysis. Thus, this strain acquired the tl(gag)g mutation at some point before we shared it, and acquired an additional kap123 mutation at some point before the RNA-seq was performed (Figure S12A).
We also reanalyzed publicly available RNA-seq data for the previously reported viable dcp2∆ and dcp1∆ mutants in the W303 strain background (Celik et al. 2017). Importantly, all of the reads from the dcp2∆ strain that mapped to codon 786 of KAP123 indicated that this codon was a UAA codon instead of a UAC codon (Figure S5A). We conclude that the dcp2∆ strain used in this RNA-seq experiment is a dcp2∆ kap123-Y687X double mutant. Similarly, in the dcp1∆ strain, we detected a 21-nt deletion in KAP123 (kap123-∆510-516; Figure S5B). Moreover, the RNA reads from the control W303 strain indicated that it contained a wild-type KAP123 gene. Thus, the kap123 mutations in the dcp1∆ and dcp2∆ strains are not inherent differences between W303 and S288C, but are instead mutations that inadvertently arose in the mutant strains.
Lastly, much of the initial investigations of decapping were carried out in a yRP strain background, and dcp2∆ was described as viable in this background (Beelman et al. 1996; Dunckley and Parker 1999). We therefore PCR amplified and sequenced the KAP123 gene from this dcp2∆ strain (yRP1346) and detected that the PCR product was a mixture of kap123-G727X and KAP123. Thus, a kap123 mutation appeared to have arisen after this dcp2∆ strain was created.
Overall, these results indicate that many of the previously reported viable dcp2∆ strains contain previously undetected mutations in KAP123 that likely contribute to their growth. We conclude that suppressors of the dcp2∆ slow growth phenotype readily occur in diverse strain backgrounds and have contributed to different conclusions on the essentiality of DCP2 (see Discussion).
Effect of decapping defects on KAP123 expression
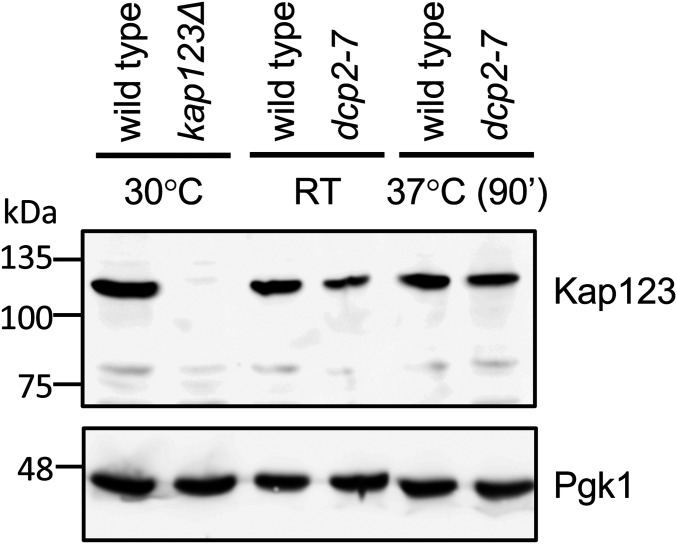
Although most genes in S. cerevisiae can be overexpressed, some “dosage-sensitive” genes cause growth defects upon overexpression. KAP123 is among the most dosage-sensitive genes (Makanae et al. 2013). Thus, we considered the possibility that decapping is critical to maintain the KAP123 mRNA and Kap123 protein at a low nontoxic level. A dcp2∆ strain would thus be lethal because Kap123 is overexpressed to toxic levels, and a kap123 mutation would restore growth. We tested this possibility using two different approaches. Because all of our dcp2∆ strains contain kap123 mutations, we used a dcp2-7 temperature sensitive strain, which has a wild-type KAP123 gene (see Materials and Methods) and performed Western blot analysis. The decapping enzyme in a dcp2-7 strain can be inactivated rapidly by growing it at room temperature and then incubating it at 37° (van Hoof et al. 2000; Dunckley et al. 2001; Schaeffer et al. 2008; Wery et al. 2016) (see Figure 7C and Figure 8 below). This showed that Kap123 was not overexpressed upon Dcp2 inactivation (Figure 5). We also checked the KAP123 levels in the RNA-seq data from dcp2-7 and dcp1∆ strains (Wery et al. 2016; Celik et al. 2017). The dcp2-7 strain contains a wild-type KAP123 allele, and although the dcp1∆ contains a kap123 mutation, this in frame deletion is not expected to affect KAP123 mRNA stability. Consistent with our Western blot, neither decapping mutant strain had an increased KAP123 mRNA level (Figure S6A). These results indicate that suppression of the growth phenotype of Dcp2 is not through reducing KAP123 expression below a toxic level.
Figure 7.
Suppressor mutations of dcp2∆ do not restore mRNA decay. (A) 5′ to 3′ mRNA decay is defective in evolved dcp2∆ strains. Each strain was transformed with an MFA2pG reporter (right). Decapping, or endonucleolytic cleavage, followed by 5′ exonucleolytic decay of MFA2pG produces a decay intermediate that is a sensitive measure of decapping. Total RNA was isolated, and MFA2pG mRNA was analyzed by Northern blotting. A representative blot from three replicates is shown. Top panel was probed for MFA2pG, and the bottom panel for the loading control SCR1. Quantitation of all three replicates is in Figure S7. (B) RNA exosome-mediated decay is required in dcp2∆ suppressor mutants. dcp2∆ kap123∆ tl(gag)g∆ is no longer viable in the absence of SKI2. Each strain was made by sporulation of a quadruple heterozygous diploid transformed with a URA3 plasmid expressing DCP2. Haploid progeny were serially diluted and spotted on 5FOA and SC-URA (control) solid media. Shown is the growth of a representative haploid strain at day 5. (C) The stability of GAL7 mRNA is unaffected by kap123∆ and/or tl(gag)g∆ in DCP2-deficient cells. Cells exponentially growing at 21° in galactose were transferred to 37° for 1 hr. Transcription of GAL7 was repressed by the addition of dextrose at time 0, and cells were harvested at multiple time points. Total RNA was isolated and GAL7 mRNA levels were analyzed by Northern blotting. Plotted is remaining GAL7 mRNA levels relative to SCR1 levels of two biological replicates. Data point triangles are pointing up for one replicate, and down for the other.
Figure 8.
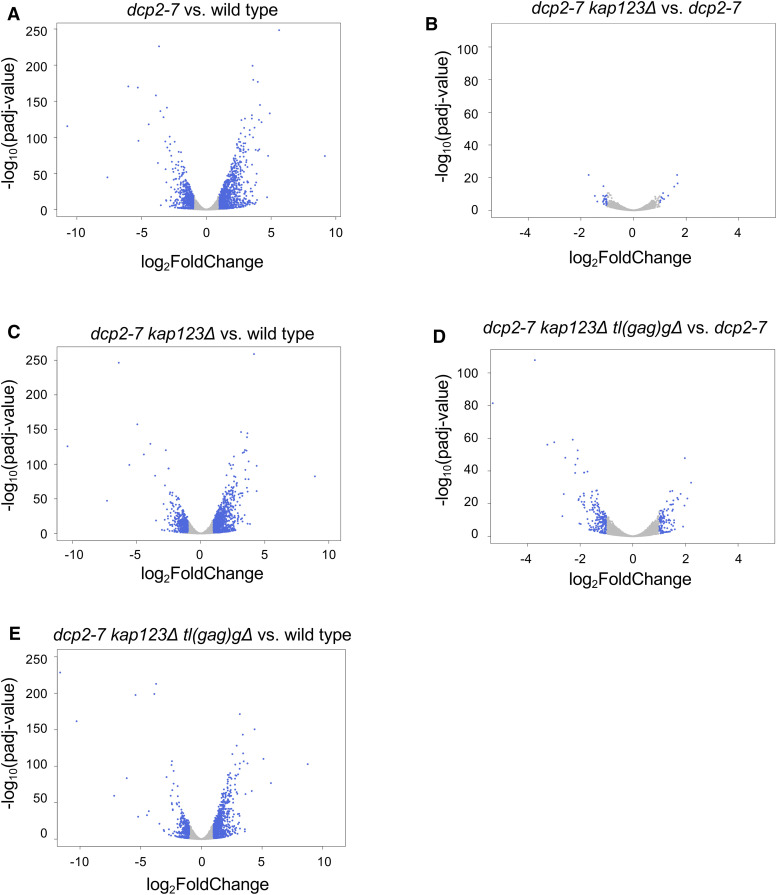
Transcriptome analysis of dcp2 mutants. (A–C) Volcano plots showing differential expression of 7127 annotated genes in dcp2-7 mutants vs. wild type. (D and E) Volcano plots showing differential expression of 7127 annotated genes in dcp2-7 suppressor mutants vs. dcp2-7 strain. (A–E) Transcripts that significantly changed at least twofold (adjusted P-value < 0.05) are in blue. Note the difference in scales between panels A to C and D and E.
Figure 5.
Decapping is not required to maintain Kap123 at a low nontoxic level. Expression of Kap123 is not increased in decapping deficient cells. A representative Western blot analyzing the expression of Kap123 (top) is shown. Pgk1 (bottom) is used a loading control. Lane 1–2: cells were grown at 30°; lane 3–4: cells were grown at room temperature (RT); lane 5–6: cells were grown at RT until OD600 reached 0.6–0.8, then transferred to 37° for 90 min.
The GAG anticodon of tL(GAG)G determines the genetic interaction with DCP2
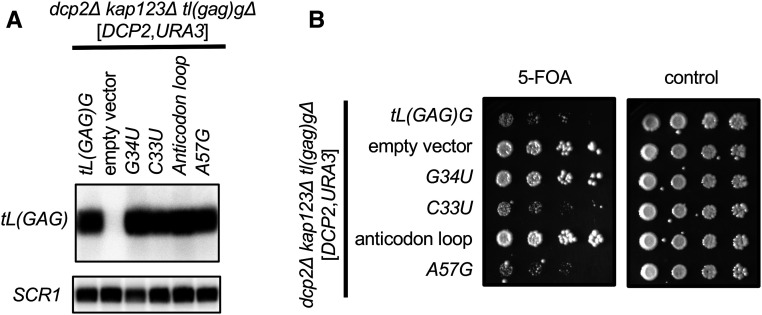
The tl(gag)g∆ that affects growth of dcp2∆ affects one specific tRNA gene out of the 275 tRNA genes in the yeast genome. This suggests that this tRNA must have some unique feature that governs the dcp2∆ genetic interaction. We therefore determined what sequence elements of tL(GAG)G are important for genetic interaction with dcp2∆. We focused on the nucleotides that are different between tL(GAG)G and other leucine tRNAs. There are 12 nucleotides that distinguish tL(GAG)G from other leucine tRNAs. Eight of these replace one base pair with another and seem unlikely to affect tRNA function. The other four are three nucleotides in the anticodon loop (C33, U34, and U38) and A57 in the ψ loop (Figure S4B). Thus, either the anticodon loop or A57 of tL(GAG)G were replaced by the corresponding nucleotides from a different leucine tRNA [tL(UAG)]. Northern blot analysis indicated that both of these mutant tRNAs were expressed similarly to wild-type tL(GAG)G (Figure 6A), but caused different growth phenotypes (Figure 6B). When the A57G mutant was introduced into a dcp2∆ kap123∆ tl(gag)g∆ strain, the growth phenotype was similar to that of wild-type tL(GAG)G. It is important that restoration of tL(GAG)G function reverses the suppression phenotype and thus reduces growth rate. This reduced growth rate is clearly seen for wild-type tL(GAG)G and the A57G mutant, when compared to the empty vector control (Figure 6B). Thus, while A57 distinguishes tL(GAG)G from other Leu tRNAs, it is not relevant to the genetic interaction with dcp2∆. In contrast, the anticodon loop mutant behaved similar to empty vector in this growth assay, and different from the wild-type tL(GAG)G gene. Therefore, the specific sequence of the anticodon stem loop is critical for the genetic interaction with dcp2∆.
Figure 6.
The GAG anticodon of tL(GAG)G affects growth of dcp2 implicating a mechanism that involves translation of CUC and CUU codons. A dcp2∆ kap123∆ tl(gag)g∆ strain harboring a DCP2, URA3 plasmid was transformed with wild-type or mutant tL(GAG)G plasmids, or an empty vector control. (A) Northern blot showing that each tRNA is expressed at similar levels. The blot was probed with a probe that is specific for tL(GAG)G and unaffected by any of the mutations (top) or for SCR1 as a loading control (bottom). (B) The strains were plated on 5-FOA plates (left) showing that the wild-type, C33U, and A57G tRNAs complemented the effect of tl(gag)g∆, but empty vector, G34U, and changing the whole anticodon loop (including G34U) did not. In the presence of DCP2 (right panel), none of the mutations affected growth.
To further narrow down what nucleotides within the anticodon loop are critical, we focused on two striking differences. First, tL(GAG)G is the only tRNA with a C33, the position immediately preceding the anticodon. All 274 other yeast tRNAs have U33, and U33 is important for efficient translation (Santos et al. 1996; Silva et al. 2007), presumably because it is critical to from the “U-turn” three-dimensional structure that makes the anticodon available to base pair with the codon. We thus changed C33 in tL(GAG)G to U33 to match all other tRNAs. This allele was expressed similar to wild type and affected growth of the dcp2∆ kap123∆ tl(gag)g∆ strain, like wild-type tL(GAG)G (Figure 6, A and B). Thus, while C33 is unique to tL(GAG)G, it is also not relevant to the dcp2∆ genetic interaction. The other striking difference between the anticodon loop of tL(GAG)G and other Leu tRNAs is, of course, the anticodon itself. We therefore changed the first anticodon nucleotide (G34) to a U, to match the sequence of the three tL(UAG) genes. Northern blot analysis showed that the level of this mutant tRNAs is comparable to wild-type tL(GAG)G (Figure 6A). Interestingly, when we compared growth, the mutant tRNA with a UAG anticodon behaved similar to an empty vector control and different from the wild-type tL(GAG)G (Figure 6B). Because the GAG is crucial for the genetic interaction with DCP2, we suggest that the mechanism of suppression is to alter translation at (some) CUC or CUU codons.
Suppressor mutations of dcp2∆ do not restore mRNA decay to normal rates
We next used several assays to determine whether the suppressor mutations affected mRNA decay rates or pathways. First, we examined whether the 5′ to 3′ decay pathway is restored in the evolved dcp2∆ isolates. Several other enzymes carry out activities that are similar to Dcp2, including Dcs1, Dxo1, Rai1, and the L-A viral GAG protein. Specifically, Dcs1 cleaves the cap structure that remains after the mRNA is degraded in the 3′ to 5′ direction, Dxo1 and Rai1 digest aberrant caps, and GAG transfers the cap from cellular mRNAs to viral RNA (Blanc et al. 1994; Liu et al. 2002; Xiang et al. 2009; Jiao et al. 2010; Fujimura and Esteban 2011; Chang et al. 2012; Doamekpor et al. 2020). Although none of these enzymes have canonical functions for 5′ to 3′ decay of bulk cytoplasmic mRNA, it appeared possible that our suppressor mutations redirect their activities. To test this possibility, we compared the directionality of mRNA decay in vivo among wild-type, nonevolved, and evolved dcp2∆ isolates. In this assay, we used an mRNA with a G-quadruplex structure in the 3′ UTR. This structure impedes Xrn1, and therefore any decapping and 5′ to 3′ decay results in the accumulation of a decay intermediate that is easily detectable by Northern blotting (Muhlrad et al. 1994). This decay intermediate was undetectable in Northern blots from both the nonevolved and evolved dcp2∆, while this intermediate was abundant in the wild-type control (Figure 7A, quantitated in Figure S7). This result demonstrates that the evolved dcp2∆ strains are still defective in 5′ to 3′ degradation, at least in this widely used model mRNA.
In addition to exonucleases, some endonucleases have been shown to contribute to cytoplasmic mRNA decay in yeast. For example, Ire1 and tRNA splicing endonuclease (TSEN) cleave specific mRNAs (Sidrauski and Walter 1997; Tsuboi et al. 2015). One possibility is that our suppressors increase activity or decrease the specificity of some endonuclease. We also entertained the idea that tl(gag)g mutations could cause stalling of translating ribosomes, which might trigger “no-go” cleavage by Cue2 (Doma and Parker 2006; D’’Orazio et al. 2019). Regardless of the endonuclease involved, endonucleolytic cleavage of the mRNA upstream of the G-quadruplex initiates Xrn1-mediated decay and leads to the same decay 5′ to 3′ decay intermediate as decapping (Doma and Parker 2006; Tsuboi et al. 2015; Cherry et al. 2019; D’Orazio et al. 2019). Therefore, the undetectable level of this decay intermediate indicates that the suppressors do not increase endonucleolytic decay of MFA2pG mRNA.
Second, it has previously been shown that if the 5′ to 3′ decapping pathway is impaired, the RNA exosome and its associated helicase Ski2 become rate limiting for mRNA decay (Anderson and Parker 1998). This is reflected in growth phenotypes. Specifically, ski2∆ does not cause an obvious growth defect if Dcp2 is fully functional, but is lethal if decapping activity is impaired (Anderson and Parker 1998). We therefore combined ski2∆ with dcp2∆ kap123∆ tl(gag)g∆. As before, the dcp2∆ kap123∆ tl(gag)g∆ triple mutant is viable, but the dcp2∆ kap123∆ tl(gag)g∆ ski2∆ quadruple mutant is inviable (Figure 7B), indicating that cytoplasmic RNA exosome activity is required even in the presence of dcp2∆ suppressors. This suggests that the RNA exosome is the major mRNA-degrading activity in the dcp2∆ kap123∆ tl(gag)g∆ mutant. Overall, the MFA2pG assay and the ski2 synthetic lethality indicate that kap123∆ and tl(gag)g∆ do not result in hyperactivation of a novel mRNA degradation pathway.
Third, we tested the effect of kap123∆ and tl(gag)g∆ on stability of three specific mRNAs. Because all our dcp2∆ strains already have suppressor mutations, we again used the temperature-sensitive dcp2-7 allele in this experiment and compared the stability of the GAL7, GAL1, and GAL10 mRNAs in a dcp2-7 strain to their stability in dcp2-7 kap123∆ and dcp2-7 kap123∆ tl(gag)g∆ strains. For this experiment, the expression of the GAL mRNAs was induced by growth in the presence of galactose, the decapping enzyme was then inactivated by incubating the cultures at 37°. Finally, dextrose was added to inhibit transcription from the GAL genes, and RNA was isolated in a time course and analyzed by Northern blotting (Figure S8A). This revealed that the GAL mRNAs were each degraded more slowly in the dcp2-7 strain compared to a DCP2 control, as expected (Anderson and Parker 1998; Dunckley and Parker 1999). However, when comparing the dcp2-7 kap123∆ and dcp2-7 kap123∆ tl(gag)g∆ mutants to dcp2-7, we detected no differences (Figure 7C and Figure S8B). In both of the suppressed strains, the mRNA half-lives were similar to the dcp2-7 single mutant, and more stable than the wild-type control.
Taken together, these results show that unlike previously isolated suppressors (Dunckley and Parker 1999; Dunckley et al. 2001; Kshirsagar and Parker 2004; Segal et al. 2006), our suppressor mutations do not restore decapping or mRNA decay rates to wild-type levels, although we cannot exclude that they have some very small effects on mRNA degradation, or only affect specific mRNAs beyond the ones we tested. Instead, while kap123∆ and tl(gag)g∆ affect the growth of dcp2∆ dramatically they do not have a similar dramatic effect on cytoplasmic mRNA decay.
Suppressors have minor effects on mRNA and noncoding RNA defects in a dcp2 mutant
To gain a better understanding of how dcp2∆ suppressors affect the transcriptome, we performed global gene expression analysis. Biological triplicates of wild type, dcp2-7, dcp2-7 kap123∆, and dcp2-7 kap123∆ tl(gag)g∆ were subjected to RNA-seq analysis after enrichment of poly(A)+ RNAs. As previously reported, dcp2-7 causes widespread disruption of the transcriptome (Wery et al. 2016). In our analysis, the dcp2-7 strain shows 1004 annotated genes significantly upregulated by ≥2-fold (adjusted P-value < 0.05) and 618 annotated genes significantly downregulated by ≥2-fold (Figure 8A). As in previous analysis, similar numbers of genes were up- and downregulated, which we interpret to indicate that the effects are dominated by indirect effects, instead of reflecting altered stability. We did not note any particular gene or group of genes that could explain the morphological and growth phenotypes of dcp2∆. A similar but slightly lower number of genes were affected in the dcp2-7 kap123∆ mutant, where 972 annotated genes were upregulated by ≥2-fold and 550 annotated genes were downregulated ≥2−fold compared to wild type (Figure 8B). Even fewer significant changes in the global transcripts levels were detected in the dcp2-7 kap123∆ tl(gag)g∆ mutant, where 752 annotated genes were upregulated by ≥2-fold and only 340 annotated genes were downregulated by ≥2-fold (Figure 8C). However, even the triple mutant showed widespread defects. We interpret these results to indicate that the suppressors have modest effects on the transcriptome.
To determine whether specific genes were affected by kap123∆ and tl(gag)g∆, we compared the gene expression profile of dcp2-7 kap123∆ and dcp2-7 kap123∆ tl(gag)g∆ to the dcp2-7 strain. Unlike the comparison to wild type (Figure 8, B and C), there are only a few genes differentially expressed. In dcp2-7 kap123∆ mutant, there were only 10 annotated genes upregulated by ≥2-fold and 20 annotated genes downregulated by ≥2-fold (Figure 8D) when compared to the single mutant dcp2-7. In the dcp2-7 kap123∆ tl(gag)g∆ mutant, 102 annotated genes were upregulated by ≥2-fold and 201 annotated genes downregulated by ≥2-fold compared to dcp2-7 (Figure 8E). Thus, suppression of growth defects is accomplished without major effects on the transcriptome.
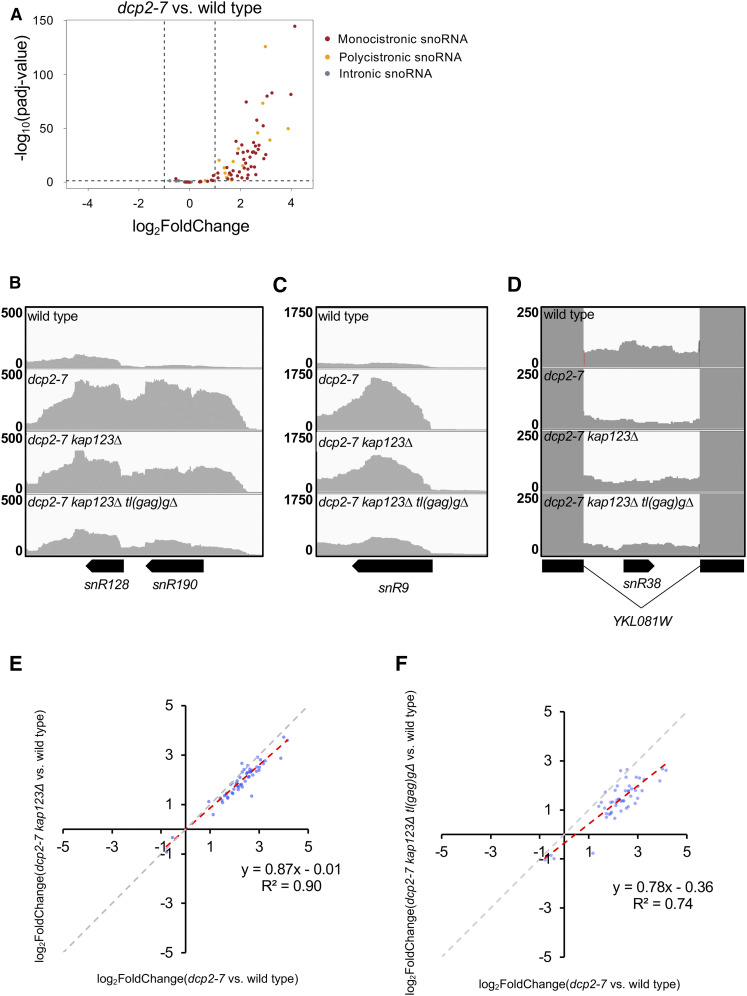
To determine whether the suppression mechanism engages the regulation of certain biological processes or cellular functions, we examined if any specific biological processes are enriched in transcripts whose expression significantly changed at least twofold (adjusted P-value < 0.05 of DESeq2) via GO enrichment analysis (Figures S9 and S10). Interestingly, the only GO category enriched in transcripts downregulated in dcp2-7 kap123∆ tl(gag)g∆ relative to dcp2-7 is ribosomal RNA modification. This was particularly striking because this GO category was upregulated in dcp2-7 compared to wild type. This GO category therefore correlated with the growth effect: it was upregulated in the dcp2-7 mutant, and partially, but not completely, restored in the triple mutant. The genes in this GO category that were affected were mostly small nucleolar RNA (snoRNA) encoding genes. Thus, snoRNA genes are significantly dysregulated in dcp2-7 compared to wild type and this dysregulation is partially alleviated in suppressor strains. This effect on snoRNA genes may be either a cause or an effect of improved growth.
To better understand the effect on snoRNA genes, we looked at subsets of these genes. snoRNAs can be classified by function and conserved elements into C/D box snoRNAs that direct RNA methylation, and H/ACA snoRNAs that direct pseudouridylation of RNA, and both classes of snoRNAs appeared to be affected. snoRNAs can also be divided by gene organization into monocistronic, polycistronic, and intron-encoded snoRNAs. Each of these categories is processed, but Dcp2 is not thought to play a role in snoRNA processing. Instead, the monocistronic snoRNAs are transcribed independently as 7mG capped precursors. These pre-snoRNAs are then 5′ matured into 2,2,7mG-capped snoRNAs by further methylations (Mouaikel et al. 2002), or into a 5′ phosphorylated snoRNA by cleavage by the endonuclease Rnt1 and the exonuclease Rat1 (Chanfreau et al. 1998a; Lee et al. 2003). Similarly, polycistronic precursors are separated into individual snoRNAs by Rnt1, and then 5′ processed by Rat1 (Chanfreau et al. 1998b; Qu et al. 1999). Finally, intron-encoded snoRNAs are processed from the spliced out and debranched intron (Ooi et al. 1998). Both polycistronic (Figure 9, A and B) and monocistronic (Figure 9, A and C) snoRNA loci were upregulated in dcp2-7, but most intron-encoded snoRNA loci were not (Figure 9, A and D). Moreover, inspection of read coverage at snoRNA loci indicated that what we detected was an upregulation of transcripts from snoRNA loci that were 5′ and/or 3′ extended, rather than the mature snoRNAs (Figure 9, B and C). Importantly, the loci for both uncapped (Figure 9B) and capped (Figure 9C) mature snoRNAs were affected. These observations indicate that the defect does not reflect a direct role of Dcp2-mediated decapping in snoRNA processing.
Figure 9.
Suppressors ameliorate expression defects from snoRNA loci in dcp2-7 mutant. (A) The expression of mono- and polycistronic snoRNA loci are upregulated in dcp2-7 mutant. A volcano plot showing differential expression of snoRNA genes in dcp2-7 strain vs. wild type. Monocistronic snoRNA are in red, polycistronic snoRNA genes are in orange, and intronic snoRNA genes are in gray. Dashed lines represent cut-off values for a twofold change in gene expression, and adjusted P-value < 0.05. (B–D) Upregulation of mono- and polycistronic snoRNA loci in dcp2-7 mutant were reduced by kap123∆, and further by kap123∆ tl(gag)g∆. RNA-seq read coverage for the snoRNA gene loci snR128-snR190 (dicistronic), snR9 (monocistronic), and snR38 (intronic) are shown. (E and F) Defective snoRNA expression in dcp2-7 mutant was alleviated by kap123∆ and further by kap123∆ tl(gag)g∆. Plotted is the differential expression of snoRNA loci in suppressor mutants vs. dcp2-7 mutant (relative to wild type). Of 77 snoRNA genes in S. cerevisiae, 53 and 49 transcripts that are statistically significant (adjusted P-value < 0.05 of DESeq2) were plotted, respectively. The gray dashed line with slope 1 is the predicted outcome if kap123∆ and tl(gag)g∆ had no effect. The red dashed line with slope < 1 depicts linear regression analysis results.
To quantitate the effect of the suppressors, we compared the log2 (fold change) of dcp2-7 relative to wild type vs. that in dcp2-7 kap123∆ or dcp2-7 kap123∆ tl(gag)g∆ relative to wild type. Transcripts that were affected in both mutants (relative to wild type) were plotted. Specifically, of the 77 annotated snoRNA genes 53 were significantly affected in both dcp2-7 (relative to wild type) and dcp2-7 kap123∆ (relative to wild type) (Figure 9E). If the kap123∆ had no effect, this should result in data points along a line with a slope of 1 (Figure 9E, gray dashed line). On the other hand, complete restoration of the dcp2-7 defect would result in a line with a slope of 0. Of the 53 analyzed snoRNA transcripts, 87% are in between these two expectations: they were changed less in the dcp2-7 kap123∆ strain (Figure 9E and Figure S11C). On the other hand, 13% were more affected. Linear regression of the data revealed a slope of 0.87 with a high correlation coefficient (R2 = 0.90), indicating that the snoRNA transcripts typically were decreased 13% in the double mutant relative to the dcp2-7 single mutant (Figure 9E). The same analysis of the triple mutant showed a similar but more pronounced effect (Figure 9F and Figure S11C). These results show that snoRNA gene misregulation is less pronounced in the suppressed strains than in the dcp2-7 single mutant cells.
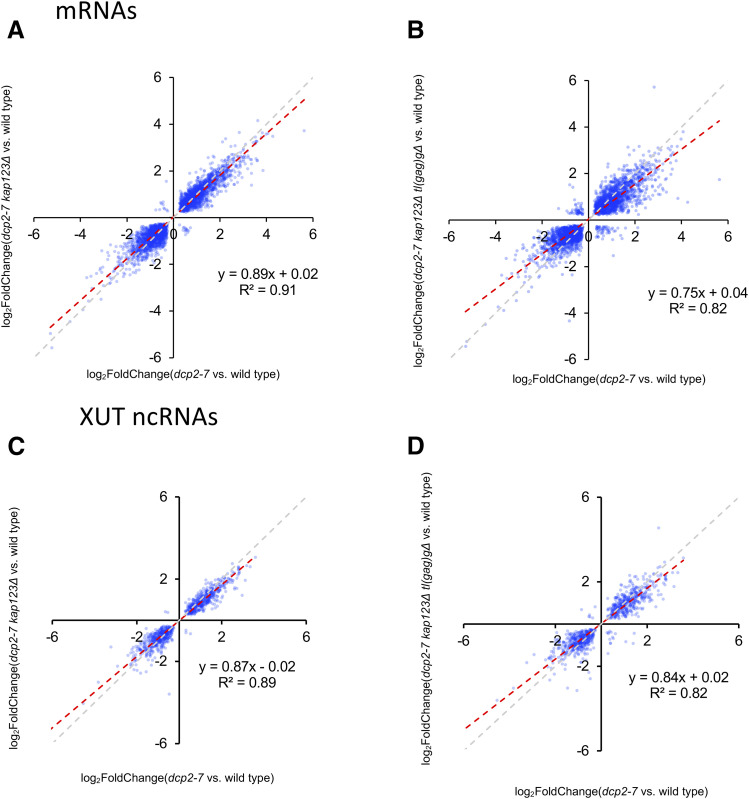
Although snoRNAs were enriched among genes whose expression was increased in dcp2-7 and partially restored in dcp2-7 kap123∆ tl(gag)g∆, we sought to determine whether this effect was restricted to snoRNAs. With this goal in mind we repeated the analysis of log2 (fold change) in dcp2-7 vs. the suppressed strains for annotated genes, which are mostly protein-coding genes (Figure 10, A and B), and for XUTs (Figure 10, C and D). XUTs are noncoding RNAs transcribed by RNA polymerase II and increased in abundance upon xrn1∆ (van Dijk et al. 2011). Because XUTs are transcribed by RNA polymerase II, they should be capped, and thus have to be decapped before they can become Xrn1 substrates. This analysis revealed that the disruption of mRNAs and XUTs that are misregulated in both dcp2-7 and dcp2-7 kap123∆ tl(gag)g∆ strains are less misregulated in the triple mutant. A total of 69% of analyzed mRNA transcripts were less affected in dcp2-7 kap123∆ tl(gag)g∆ than in dcp2 (Figure S11A). Similarly, 66% of analyzed XUTs changed less in the triple mutant than in dcp2-7 compared to wild type (Figure S11B). The double mutant had similar effect of mRNAs and XUTs as the triple mutant, although the effect was slightly reduced.
Figure 10.
Suppressors ameliorate defects in expression of protein coding mRNA and XUT noncoding RNA in dcp2-7 mutant. (A and B) Defective mRNA expression in dcp2-7 mutant was alleviated by kap123∆ and further by kap123∆ tl(gag)g∆. Plotted is the differential expression of mRNA loci in suppressor mutants vs. dcp2-7 mutant (relative to wild type). Of 6691 mRNA genes in S. cerevisiae, 3236 and 3302 transcripts that are statistically significant (adjusted P-value < 0.05 of DESeq2) were plotted, respectively. YBR115C, YCR097W, YEL021W, YNL145W, YER110C, and YPL187W are not plotted. (C and D) Defective XUT expression in dcp2-7 mutant was alleviated by kap123∆, and further by kap123∆ tl(gag)g∆. Plotted is the differential expression of XUT loci in suppressor mutants vs. dcp2-7 mutant relative to wild type. Of 1658 XUT genes in S. cerevisiae, 948 and 898 transcripts that are statistically significant (adjusted P-value < 0.05) were plotted, respectively. (A–D) The gray dashed line with slope 1 is the predicted outcome if kap123∆ and tl(gag)g∆ had no effect. The red dashed line with slope < 1 depicts linear regression analysis results.
Overall, our data showed that dcp2-7 has large effects on a variety of RNAs including both mRNAs and noncoding RNAs such as snoRNAs (which are not Dcp2 substrates) and XUTs (which are Dcp2 substrates), and that the double and triple mutants had similar but slightly less disturbed transcriptomes. Furthermore, since it is hard to envision how a tRNA directly affects noncoding RNAs such as snoRNAs and XUTs, we suspect that the suppressors indirectly affect transcriptome dysregulation.
Discussion
Dcp2 is required for continuous growth
Cytoplasmic mRNA turnover is an important process that regulates gene expression, and Dcp2 carries out a key step. Previous studies have described DCP2 as either an essential gene or a nonessential gene, and in some cases the difference has been attributed to a genetic difference between the two widely used yeast strains, S288C and W303 (Dunckley and Parker 1999; Giaever et al. 2002; Geisler et al. 2012; He and Jacobson 2015). Our studies indicate that very slow-growing dcp2∆ strains can be generated, but that suppressor mutations that improve growth readily occur. We further show that previous studies have inadvertently used dcp1∆ or dcp2∆ strains that contain spontaneous kap123 suppressors. However, similar analysis from other dcp2 alleles (e.g., dcp2-7 and dcp2-N245; Wery et al. 2016; He et al. 2018) and strains with mutations in decapping regulators (lsm1∆, pat1∆, etc.; He et al. 2018) indicated that they contained a wild-type KAP123 gene. This suggests that only very severe decapping defects select for kap123 mutations.
The starting DCP2/dcp2∆ heterozygote used in our tetrad analysis does not contain these suppressors, but a large fraction of the resulting haploid colonies do. It appears implausible that ungerminated and metabolically inactive spores accumulate suppressor mutations to high frequency, without accumulating a large number of other random mutations. Therefore, we conclude that dcp2∆ spores are capable of germinating and dividing for several generations. Strong selective pressure during early generations leads to the selection for suppressors that then allow further growth. The mutation rate in rapidly growing diploid yeast cells has been measured as 1.67 × 10−10 per base pair per cell doubling (Zhu et al. 2014). There are ∼106 cells in a yeast colony, and the KAP123 gene is 3342-bp long. Thus, the chance that a colony formed by a KAP123 cell contains a cell with a kap123 mutation is ∼0.6 (1.67 × 10−10 × 106 × 3342). We suspect that the much longer generation time of dcp2∆ allows for more mutations to occur per generation. It thus appears that a normal or slightly elevated mutation rate, combined with strong selection, is sufficient for an initial suppressor to arise. The need for an initial suppressor for a spore to form a visible colony also explains why ∼30% of dcp2∆ spores did not form visible colonies. Additionally, WGS of one of the nonevolved dcp2∆ strains revealed some reads corresponding to a kap123 mutant allele, while other reads were from a wild-type KAP123 allele (Figure S12A, nonevolved dcp2∆_2). The presence of both alleles in the nonevolved dcp2∆ clearly indicates that this nonevolved population arose from a KAP123 dcp2∆ spore that obtained a kap123 mutation during early growth. Thus, Dcp2 is likely not absolutely required for spore germination and extremely slow growth, but in practical terms it is impossible to continuously culture dcp2∆ under laboratory conditions without suppressors. The question of whether DCP2 should be categorized as an essential gene then becomes dependent on the definition of essential used.
Experimental evolution identifies novel bypass suppressors of dcp2∆
Our experimental evolution approach to identifying suppressors in the decapping enzyme is distinct from previous genetics screens. All previous screens isolated suppressors of a partial defect in the decapping enzyme (dcp2-7 or dcp1-2) and these suppressor mutations restored mRNA decapping by improving the function of the partially defective Dcp1/Dcp2 enzyme (Dunckley and Parker 1999; Dunckley et al. 2001; Kshirsagar and Parker 2004; Segal et al. 2006). Thus, these previous screens were helpful in identifying regulators of Dcp1/Dcp2, but they did not provide much insight into the importance of decapping for growth and cellular homeostasis. We designed our experimental evolution approach to complement these previous studies, reasoning that the use of a complete deletion of the DCP2 gene would preclude selecting suppressors that restored Dcp1/Dcp2 function. Experimental evolution further allows for selection of suppressors with small effects on growth. Small effects on growth may not result in a readily observable effect on colony size in a traditional screen, but are selected slowly but surely in experimental evolution.
We identified nonsynonymous mutations in 16 different genes. Some of these mutations are likely to just have occurred randomly, and may not affect dcp2∆ growth. However, three genes were mutated multiple times and we concentrated our further analysis on those. We show that kap123∆, tl(gag)g∆, and whi2∆ loss-of-function mutations are each sufficient to suppress the growth defect of dcp2∆. One advantage of experimental evolution is that multiple mutations can arise that act additively or even synergistically to further improve growth. This seems to have occurred since six of the eight isolates contain mutations in both KAP123 and either tL(GAG)G or WHI2. Furthermore, we confirmed that the dcp2∆ kap123∆ tl(gag)g∆ triple mutant grew better than either the dcp2∆ kap123∆ or dcp2∆ tl(gag)g∆ double mutant. These observations indicate that the kap123 and tl(gag)g mutations affect dcp2 through different independent mechanisms.
Another advantage of experimental evolution is that it allows multiple mutations to arise where mutation of a particular gene can only improve growth after some other gene is mutated. We did not find clear evidence for this, although this might be the case for some of the mutations we only found once. Although we cannot completely trace the order in which each mutation arose, the comparison of duplicate evolved isolates derived from a common haploid spore indicates that some mutations arose early and others arose later (Figure S12A). This indicates that in lineage 4 a tl(gag)g mutation arose before a kap123 mutation, while in lineage 2 the genes were mutated in the reverse order. The observation that kap123 and tl(gag)g mutations can arise in both orders further confirms that they suppress dcp2∆ by independent mechanisms.
Although we have not formally proven it, several lines of evidence suggest that some of the 13 genes mutated only once also affect dcp2∆ growth. First, evolved isolate 2-1 contains a kap123 mutation but wild-type WHI2 and tL(GAG)G genes, yet grows just as well as some of the isolates with two suppressors. Thus, isolate 2-1 likely contains an additional suppressor. The only nonsynonymous mutation that differs between isolate 2-1 and the nonevolved parent is a single amino acid change in RNA polymerase II (rpo21-R1281C), and thus this may be a suppressor. Second, in lineage 3, both the kap123 and whi2 mutations arose late. This suggests that some other early mutation might have allowed us to recover the nonevolved starting strain. The only nonsynonymous mutation shared between isolates 3-1 and 3-2 is a frameshift in CSE2, which encodes a subunit of the mediator complex. Third, isolate 4-2 contains a mutation in the start codon for Psr1, which forms a complex with Whi2 (Kaida et al. 2002). Further analyses will be required to definitively determine whether these 13 other mutations also affect dcp2∆ growth.
The anticodon of an enigmatic tRNA affects growth of dcp2 strains
Although conservation in related species suggests tL(GAG)G is a functional gene, essentially nothing is known about its function. Previous studies have shown that deletion of tL(GAG)G did not affect growth and thus is not absolutely required for translation of CUC or CUU codons. However, tl(gag)g∆ showed synthetic phenotypes with the loss of tL(UAG) genes, suggesting some contribution to translation (Huang et al. 2012; Bloom-Ackermann et al. 2014). Furthermore, it was shown that mutating the anticodon of tL(GAG)G to UAG resulted in complementation of the lethality of a triple deletion of tL(UAG) genes, and thus this G34U mutant tRNA must be able to translate all 4 CUN codons (Huang et al. 2012). This G34U mutation that allows for translation of all four CUN codons is the same as the one that we show is sufficient to allow viability of dcp2∆. Overall, previous research suggested that tL(GAG)G and tL(UAG) have overlapping functions, which does not explain why tL(GAG)G is conserved. Our results show that tL(GAG)G has a specific function that is not interchangeable with tL(UAG).
It is interesting that no other components of the translation machinery were identified as dcp2∆ suppressors. In addition to tL(GAG)G, there are five other single-gene tRNAs. Four of them are essential, while tR(CCU)J is not. However, tR(CCU)J was not identified as dcp2∆ suppressor mutants. More broadly, none of the translation elongation factors, ribosomal subunits, or aminoacyl synthetases were mutated in our evolved strains. It is possible that these mutations would be found in a larger screen, but it seems unlikely that such a large number of genes would have been missed, while finding two alleles of the small tL(GAG)G gene. This makes it unlikely that a general translation elongation defect improves growth of dcp2∆.
Because the anticodon sequence of tL(GAG)G affects growth of dcp2, but mutations affecting global translation elongation were not isolated, we propose that tL(GAG)G affects translation kinetics or accuracy of CUC or CUU codons, perhaps in some specific context. Further research will be required to determine whether translation at all CUC and CUU codons is affected by tL(GAG)G or whether a specific subset of these codons is affected, how this translation defect affects growth of dcp2 strains, and whether this is related to the effect of codon optimality on mRNA degradation (Presnyak et al. 2015). Similarly, further research on Kap123 is required to fully understand how it affects dcp2∆ growth. Although Kap123 is a karyopherin and has been shown to physically interact with dozens of proteins, its function is largely redundant with other karyopherins, including kap121 (Aitchison and Rout 2012). The only nuclear protein that is known to be mislocalized in kap123∆ is the ribosome biogenesis protein Ecm1 (Yao et al. 2010). In addition, the normally cytoplasmic proteins Srp68 (Grosshans et al. 2001) and Aim44 (Perez and Thorner 2019) become localized to the nucleus if overexpressed, which requires Kap123. The identity of the Kap123 client that affects dcp2∆ growth therefore remains to be determined.
Bypass suppressors of dcp2∆ do not restore mRNA decay rate or direction to normal
Previous genetic screens of suppressors of dcp1 or dcp2 defects have identified “enhancer of decapping” genes that restored decapping to a partially defective Dcp1-Dcp2 complex (Dunckley and Parker 1999; Dunckley et al. 2001; Kshirsagar and Parker 2004; Segal et al. 2006). We expected to either isolate mutations that activate alternative mRNA degradation pathways or mutations that allow survival despite slow mRNA decay. Follow-up experiments indicate that we isolated the latter. We observed that the suppressor mutations isolated here do not appear to restore the 5′ to 3′ decay of mRNA, nor the overall rate of mRNA degradation to wild-type levels. These analyses on select model mRNAs cannot rule out either small effects, or effects on a subset of mRNAs, but we favor the simpler explanation that the suppressor mutations improve growth while maintaining the relatively slow RNA exosome-mediated 3′ to 5′ decay of mRNA.
Bypass suppressors of dcp2∆ do not have a major effect on the transcriptome
Finally, we studied the effect of kap123∆ and tl(gag)g∆ on the transcriptome. Limitations of this study include that we had to use a dcp2-7 strain because dcp2∆ strains could not be cultured without suppressors arising, and thus could not be used as a single mutant control to compare to the double mutants. Although dcp2-7 causes a decapping defect at the restrictive temperature, this defect is not complete. Comparing dcp2-7 to wild type, we observed global disruption in transcriptome homeostasis, confirming previous publications (Geisler et al. 2012; He and Jacobson 2015; Celik et al. 2017). We suspect that the detected changes are dominated by indirect effects and thus cannot be used to identify which transcripts are differentially decapped. Strikingly, GO analysis indicated a significant enrichment of snoRNA loci among the transcripts that were differentially expressed between dcp2-7 and dcp2-7 kap123∆ tl(gag)g∆ (Figure S10B). snoRNA processing pathways are well established and do not involve decapping of snoRNA transcripts. In fact, some mature snoRNAs retain the cap structure while, for other snoRNAs, the Rnt1 endonuclease removes the cap. Both of these classes of snoRNAs were affected. For example, the snR9 snoRNA is well established to retain its cap (Wise et al. 1983) and is not processed by Rnt1 (Chanfreau et al. 1998a), while both snR190 and snR128 are 5′ processed by Rnt1 (Chanfreau et al. 1998b), yet all three are similarly affected by Dcp2 inactivation (Figure 9, B and C). Thus, Dcp2 inactivation appears to indirectly affect transcripts from the snoRNA loci, and the kap123∆ and tl(gag)g∆ suppressors partially suppress this defect. Although this analysis cannot determine whether the partially restored snoRNA misregulation is a cause or an effect of improved growth, the fact that we did not find mutations in either snoRNA genes or genes involved in the snoRNA biogenesis from our WGS analysis suggests that snoRNA expression effects are a consequence of improved growth. Although snoRNAs were enriched among transcripts whose partial restoration upon kap123∆ tl(gag)g∆ reaches statistical significance, we observed by linear regression that mRNAs and XUTs follow a similar trend, but perhaps are not as sensitive to kap123∆ tl(gag)g∆. Although many genes showed a smaller change in the suppressor strains, the effect was small, which likely explains why DESeq2 could not identify any specific genes for which the data reached statistical significance. Our findings that suppressor mutations (partially) alleviate the effect of decapping defects, and that most previous transcriptome-wide studies inadvertently used strains with suppressors imply that most previous studies likely underestimated the transcriptome-wide effects of decapping defects.
Implications
Our results suggest that studies on decapping mutants should be carefully controlled for unanticipated suppressors. Depending on the goals, it might be preferred to compare a dcp2∆ kap123∆ tl(gag)g∆ strain to a kap123∆ tl(gag)g∆ control, thereby reducing the selection for inadvertent suppressors. Finally, the observation that suppressors can readily occur during growth under standard laboratory conditions, and especially during extended culture is not surprising, and is unlikely to be specific for either dcp2 mutations or for S. cerevisiae. We therefore suggest that unexpected phenotypes, or conflicting phenotypes, should be resolved by WGS in organisms such as yeast, where this is feasible for a reasonable cost.
Acknowledgments
This work was supported by National Institutes of Health grant R01-GM099790 to A.v.H. We thank members of the van Hoof laboratory for critical discussions, Kate Travis for performing pilot experiments for the experimental evolution, Lee-Ann Notice for help with analyzing tRNA mutations, and Jaeil Han for insightful comments throughout. Antibodies to Kap123 were a kind gift from Valerie Doye (Institut Jaque Monod) and initially developed by Michael Rexach (University of California, Santa Cruz). The yRP1346 dcp2∆ strain was a kind gift from Roy Parker (University of Colorado). We thank William Margolin (University of Texas Health Science Center at Houston) for microscope usage; Kevin Morano (University of Texas Health Science Center at Houston) for microplate reader usage; and Cesar Arias, Diana Panesso, and An Dihn (University of Texas Health Science Center at Houston) for whole-genome sequencing of the DCP2/dcp2∆ starting diploid strain.
Footnotes
Supplemental material available at figshare: https://doi.org/10.6084/m9.figshare.12985820.
Communicating editor: E. Tran
Literature Cited
- Aitchison J. D., and Rout M. P., 2012. The yeast nuclear pore complex and transport through it. Genetics 190: 855–883. 10.1534/genetics.111.127803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., and Parker R., 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17: 1497–1506. 10.1093/emboj/17.5.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman C. A., Stevens A., Caponigro G., LaGrandeur T. E., Hatfield L. et al. , 1996. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature 382: 642–646. 10.1038/382642a0 [DOI] [PubMed] [Google Scholar]
- Blanc A., Ribas J. C., Wickner R. B., and Sonenberg N., 1994. His-154 is involved in the linkage of the Saccharomyces cerevisiae L-A double-stranded RNA virus Gag protein to the cap structure of mRNAs and is essential for M1 satellite virus expression. Mol. Cell. Biol. 14: 2664–2674. 10.1128/MCB.14.4.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom-Ackermann Z., Navon S., Gingold H., Towers R., Pilpel Y. et al. , 2014. A comprehensive tRNA deletion library unravels the genetic architecture of the tRNA pool. PLoS Genet. 10: e1004084 10.1371/journal.pgen.1004084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A., Baker R., He F., and Jacobson A., 2017. High-resolution profiling of NMD targets in yeast reveals translational fidelity as a basis for substrate selection. RNA 23: 735–748. 10.1261/rna.060541.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanfreau G., Legrain P., and Jacquier A., 1998a Yeast RNase III as a key processing enzyme in small nucleolar RNAs metabolism. J. Mol. Biol. 284: 975–988. 10.1006/jmbi.1998.2237 [DOI] [PubMed] [Google Scholar]
- Chanfreau G., Rotondo G., Legrain P., and Jacquier A., 1998b Processing of a dicistronic small nucleolar RNA precursor by the RNA endonuclease Rnt1. EMBO J. 17: 3726–3737. 10.1093/emboj/17.13.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. H., Jiao X., Chiba K., Oh C., Martin C. E. et al. , 2012. Dxo1 is a new type of eukaryotic enzyme with both decapping and 5′-3′ exoribonuclease activity. Nat. Struct. Mol. Biol. 19: 1011–1017. 10.1038/nsmb.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wang G., Zhang Y., Dayhoff-Brannigan M., Diny N. L. et al. , 2018. Whi2 is a conserved negative regulator of TORC1 in response to low amino acids. PLoS Genet. 14: e1007592 10.1371/journal.pgen.1007592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry P. D., Peach S. E., and Hesselberth J. R., 2019. Multiple decay events target HAC1 mRNA during splicing to regulate the unfolded protein response. eLife 8: e42262 10.7554/eLife.42262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook Y. M., and Suel K. E., 2011. Nuclear import by karyopherin-βs: recognition and inhibition. Biochim. Biophys. Acta 1813: 1593–1606. 10.1016/j.bbamcr.2010.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Patel V. M., Coon M., Nguyen T., Land S. J. et al. , 2012. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Front. Genet. 3: 35 10.3389/fgene.2012.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comyn S. A., Flibotte S., and Mayor T., 2017. Recurrent background mutations in WHI2 impair proteostasis and degradation of misfolded cytosolic proteins in Saccharomyces cerevisiae. Sci. Rep. 7: 4183 10.1038/s41598-017-04525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doamekpor S. K., Gozdek A., Kwasnik A., Kufel J., and Tong L., 2020. A novel 5′-hydroxyl dinucleotide hydrolase activity for the DXO/Rai1 family of enzymes. Nucleic Acids Res. 48: 349–358. 10.1093/nar/gkz1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma M. K., and Parker R., 2006. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440: 561–564. 10.1038/nature04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T., and Parker R., 1999. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 18: 5411–5422. 10.1093/emboj/18.19.5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T., Tucker M., and Parker R., 2001. Two related proteins, Edc1p and Edc2p, stimulate mRNA decapping in Saccharomyces cerevisiae. Genetics 157: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio K. N., Wu C. C., Sinha N., Loll-Krippleber R., Brown G. W. et al. , 2019. The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during no go decay. eLife 8: e49117 10.7554/eLife.49117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floch A. G., Tareste D., Fuchs P. F., Chadrin A., Naciri I. et al. , 2015. Nuclear pore targeting of the yeast Pom33 nucleoporin depends on karyopherin and lipid binding. J. Cell Sci. 128: 305–316. 10.1242/jcs.158915 [DOI] [PubMed] [Google Scholar]
- Fujimura T., and Esteban R., 2011. Cap-snatching mechanism in yeast L-A double-stranded RNA virus. Proc. Natl. Acad. Sci. USA 108: 17667–17671. 10.1073/pnas.1111900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., and Esteban R., 2012. Cap snatching of yeast L-A double-stranded RNA virus can operate in trans and requires viral polymerase actively engaging in transcription. J. Biol. Chem. 287: 12797–12804. 10.1074/jbc.M111.327676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S., Lojek L., Khalil A. M., Baker K. E., and Coller J., 2012. Decapping of long noncoding RNAs regulates inducible genes. Mol. Cell 45: 279–291. 10.1016/j.molcel.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L. et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- Grosshans H., Deinert K., Hurt E., and Simos G., 2001. Biogenesis of the signal recognition particle (SRP) involves import of SRP proteins into the nucleolus, assembly with the SRP-RNA, and Xpo1p- mediated export. J. Cell Biol. 153: 745–762. 10.1083/jcb.153.4.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien-Nogalska E., Jiao X., Song M. G., Hart R. P., and Kiledjian M., 2016. Nudt3 is an mRNA decapping enzyme that modulates cell migration. RNA 22: 773–781. 10.1261/rna.055699.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., and Jacobson A., 2015. Control of mRNA decapping by positive and negative regulatory elements in the Dcp2 C-terminal domain. RNA 21: 1633–1647. 10.1261/rna.052449.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Li X., Spatrick P., Casillo R., Dong S. et al. , 2003. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol. Cell 12: 1439–1452. 10.1016/S1097-2765(03)00446-5 [DOI] [PubMed] [Google Scholar]
- He F., Amrani N., Johansson M. J., and Jacobson A., 2008. Chapter 6. Qualitative and quantitative assessment of the activity of the yeast nonsense-mediated mRNA decay pathway. Methods Enzymol. 449: 127–147. 10.1016/S0076-6879(08)02406-3 [DOI] [PubMed] [Google Scholar]
- He F., Li C., Roy B., and Jacobson A., 2014. Yeast Edc3 targets RPS28B mRNA for decapping by binding to a 3′ untranslated region decay-inducing regulatory element. Mol. Cell. Biol. 34: 1438–1451. 10.1128/MCB.01584-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Celik A., Wu C., and Jacobson A., 2018. General decapping activators target different subsets of inefficiently translated mRNAs. eLife 7: e34409 10.7554/eLife.34409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Yao P., Eriani G., and Wang E. D., 2012. In vivo identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain. Nucleic Acids Res. 40: 10463–10477. 10.1093/nar/gks783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X., Xiang S., Oh C., Martin C. E., Tong L. et al. , 2010. Identification of a quality-control mechanism for mRNA 5′-end capping. Nature 467: 608–611. 10.1038/nature09338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Coyle S. M., and Doudna J. A., 2011. Coupled 5′ nucleotide recognition and processivity in Xrn1-mediated mRNA decay. Mol. Cell 41: 600–608. 10.1016/j.molcel.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungfleisch J., Nedialkova D. D., Dotu I., Sloan K. E., Martinez-Bosch N. et al. , 2017. A novel translational control mechanism involving RNA structures within coding sequences. Genome Res. 27: 95–106 (erratum: Genome Res. 27: 663). 10.1101/gr.209015.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida D., Yashiroda H., Toh-e A., and Kikuchi Y., 2002. Yeast Whi2 and Psr1-phosphatase form a complex and regulate STRE-mediated gene expression. Genes Cells 7: 543–552. 10.1046/j.1365-2443.2002.00538.x [DOI] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R. et al. , 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar M., and Parker R., 2004. Identification of Edc3p as an enhancer of mRNA decapping in Saccharomyces cerevisiae. Genetics 166: 729–739. 10.1534/genetics.166.2.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., and Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G. I., Rice D. P., Hickman M. J., Sodergren E., Weinstock G. M. et al. , 2013. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature 500: 571–574. 10.1038/nature12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer F. W., Hsu C. L., Maupin M. K., and Stevens A., 1992. Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease: sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene 120: 51–57. 10.1016/0378-1119(92)90008-D [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Lee A., and Chanfreau G., 2003. The roles of endonucleolytic cleavage and exonucleolytic digestion in the 5′-end processing of S. cerevisiae box C/D snoRNAs. RNA 9: 1362–1370. 10.1261/rna.5126203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Smyth G. K., and Shi W., 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- Li Y., Song M., and Kiledjian M., 2011. Differential utilization of decapping enzymes in mammalian mRNA decay pathways. RNA 17: 419–428. 10.1261/rna.2439811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rodgers N. D., Jiao X., and Kiledjian M., 2002. The scavenger mRNA decapping enzyme DcpS is a member of the HIT family of pyrophosphatases. EMBO J. 21: 4699–4708. 10.1093/emboj/cdf448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yong M. Y., Yurieva M., Srinivasan K. G., Liu J. et al. , 2015. Gene essentiality is a quantitative property linked to cellular evolvability. Cell 163: 1388–1399. 10.1016/j.cell.2015.10.069 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S., 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanae K., Kintaka R., Makino T., Kitano H., and Moriya H., 2013. Identification of dosage-sensitive genes in Saccharomyces cerevisiae using the genetic tug-of-war method. Genome Res. 23: 300–311. 10.1101/gr.146662.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouaikel J., Verheggen C., Bertrand E., Tazi J., and Bordonne R., 2002. Hypermethylation of the cap structure of both yeast snRNAs and snoRNAs requires a conserved methyltransferase that is localized to the nucleolus. Mol. Cell 9: 891–901. 10.1016/S1097-2765(02)00484-7 [DOI] [PubMed] [Google Scholar]
- Muhlrad D., Decker C. J., and Parker R., 1994. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev. 8: 855–866. 10.1101/gad.8.7.855 [DOI] [PubMed] [Google Scholar]
- Ooi S. L., Samarsky D. A., Fournier M. J., and Boeke J. D., 1998. Intronic snoRNA biosynthesis in Saccharomyces cerevisiae depends on the lariat-debranching enzyme: intron length effects and activity of a precursor snoRNA. RNA 4: 1096–1110. 10.1017/S1355838298980785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., 2012. RNA degradation in Saccharomyces cerevisae. Genetics 191: 671–702. 10.1534/genetics.111.137265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. S., and Rexach M. F., 2008. Discovering novel interactions at the nuclear pore complex using bead halo: a rapid method for detecting molecular interactions of high and low affinity at equilibrium. Mol. Cell. Proteomics 7: 121–131. 10.1074/mcp.M700407-MCP200 [DOI] [PubMed] [Google Scholar]
- Perez A. M., and Thorner J., 2019. Septin-associated proteins Aim44 and Nis1 traffic between the bud neck and the nucleus in the yeast Saccharomyces cerevisiae. Cytoskeleton (Hoboken) 76: 15–32. 10.1002/cm.21500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnyak V., Alhusaini N., Chen Y. H., Martin S., Morris N. et al. , 2015. Codon optimality is a major determinant of mRNA stability. Cell 160: 1111–1124. 10.1016/j.cell.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L. H., Henras A., Lu Y. J., Zhou H., Zhou W. X. et al. , 1999. Seven novel methylation guide small nucleolar RNAs are processed from a common polycistronic transcript by Rat1p and RNase III in yeast. Mol. Cell. Biol. 19: 1144–1158. 10.1128/MCB.19.2.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A., Chen Y. H., Martin S., Alhusaini N., Green R. et al. , 2016. The DEAD-Box protein Dhh1p couples mRNA decay and translation by monitoring codon optimality. Cell 167: 122–132.e9. 10.1016/j.cell.2016.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockmill B., Lambie E. J., and Roeder G. S., 1991. Spore enrichment. Methods Enzymol. 194: 146–149. 10.1016/0076-6879(91)94012-2 [DOI] [PubMed] [Google Scholar]
- Santos M. A., Perreau V. M., and Tuite M. F., 1996. Transfer RNA structural change is a key element in the reassignment of the CUG codon in Candida albicans. EMBO J. 15: 5060–5068. 10.1002/j.1460-2075.1996.tb00886.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer D., Meaux S., Clark A., and van Hoof A., 2008. Determining in vivo activity of the yeast cytoplasmic exosome. Methods Enzymol. 448: 227–239. 10.1016/S0076-6879(08)02612-8 [DOI] [PubMed] [Google Scholar]
- Segal S. P., Dunckley T., and Parker R., 2006. Sbp1p affects translational repression and decapping in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 5120–5130 [corrigenda: Mol. Cell. Biol. 27: 789–790 (2007)]. 10.1128/MCB.01913-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C., and Walter P., 1997. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90: 1031–1039. 10.1016/S0092-8674(00)80369-4 [DOI] [PubMed] [Google Scholar]
- Silva R. M., Paredes J. A., Moura G. R., Manadas B., Lima-Costa T. et al. , 2007. Critical roles for a genetic code alteration in the evolution of the genus Candida. EMBO J. 26: 4555–4565. 10.1038/sj.emboj.7601876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. G., Li Y., and Kiledjian M., 2010. Multiple mRNA decapping enzymes in mammalian cells. Mol. Cell 40: 423–432. 10.1016/j.molcel.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A., and Poole T. L., 1995. 5′-exonuclease-2 of Saccharomyces cerevisiae. Purification and features of ribonuclease activity with comparison to 5′-exonuclease-1. J. Biol. Chem. 270: 16063–16069. 10.1074/jbc.270.27.16063 [DOI] [PubMed] [Google Scholar]
- Teng X., Dayhoff-Brannigan M., Cheng W. C., Gilbert C. E., Sing C. N. et al. , 2013. Genome-wide consequences of deleting any single gene. Mol. Cell 52: 485–494. 10.1016/j.molcel.2013.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Yamazaki R., Nobuta R., Ikeuchi K., Makino S. et al. , 2015. The tRNA splicing endonuclease complex cleaves the mitochondria-localized CBP1 mRNA. J. Biol. Chem. 290: 16021–16030. 10.1074/jbc.M114.634592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E. L., Chen C. L., d’Aubenton-Carafa Y., Gourvennec S., Kwapisz M. et al. , 2011. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature 475: 114–117. 10.1038/nature10118 [DOI] [PubMed] [Google Scholar]
- van Hoof A., Staples R. R., Baker R. E., and Parker R., 2000. Function of the ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell. Biol. 20: 8230–8243. 10.1128/MCB.20.21.8230-8243.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. W., Chen Y. H., Stowell J. A. W., Alhusaini N., Sweet T. et al. , 2018. mRNA deadenylation is coupled to translation rates by the differential activities of Ccr4-Not nucleases. Mol Cell 70: 1089–1100.e8. 10.1016/j.molcel.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wery M., Descrimes M., Vogt N., Dallongeville A. S., Gautheret D. et al. , 2016. Nonsense-mediated decay restricts LncRNA levels in yeast unless blocked by double-stranded RNA structure. Mol. Cell 61: 379–392. 10.1016/j.molcel.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. A., Tollervey D., Maloney D., Swerdlow H., Dunn E. J. et al. , 1983. Yeast contains small nuclear RNAs encoded by single copy genes. Cell 35: 743–751. 10.1016/0092-8674(83)90107-1 [DOI] [PubMed] [Google Scholar]
- Xiang S., Cooper-Morgan A., Jiao X., Kiledjian M., Manley J. L. et al. , 2009. Structure and function of the 5′→3′ exoribonuclease Rat1 and its activating partner Rai1. Nature 458: 784–788. 10.1038/nature07731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Demoinet E., Saveanu C., Lenormand P., Jacquier A. et al. , 2010. Ecm1 is a new pre-ribosomal factor involved in pre-60S particle export. RNA 16: 1007–1017. 10.1261/rna.2012310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan Q., He F., Zhang F., Zhang H., Jacobson A. et al. , 2018. Conserved mRNA-granule component Scd6 targets Dhh1 to repress translation initiation and activates Dcp2-mediated mRNA decay in vivo. PLoS Genet. 14: e1007806 [corrigenda: PLoS Genet. 15: e1008299 (2019)]. 10.1371/journal.pgen.1007806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Bail S., Plasterer H. L., Rusche J., and Kiledjian M., 2015. DcpS is a transcript-specific modulator of RNA in mammalian cells. RNA 21: 1306–1312. 10.1261/rna.051573.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. O., Siegal M. L., Hall D. W., and Petrov D. A., 2014. Precise estimates of mutation rate and spectrum in yeast. Proc. Natl. Acad. Sci. USA 111: E2310–E2318. 10.1073/pnas.1323011111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. RNA-seq data are available at the Sequence Read Archive under project number PRJNA626686. Supplemental data are available at https://doi.org/10.6084/m9.figshare.12985820. All yeast strains and plasmids used are available upon request.