Despite its historic role in evolving our understanding of modern molecular genetics, the mechanism governing the bacteriophage T4rII exclusion (Rex) phenotype has remained a mystery for over six decades. The Rex system is thought.....
Keywords: bacteriophage lambda, bacteriophage T4rII, Rex, membrane proteins, sigma factors
Abstract
The T4rII exclusion (Rex) phenotype is the inability of T4rII mutant bacteriophage to propagate in hosts (Escherichia coli) lysogenized by bacteriophage lambda (λ). The Rex phenotype, triggered by T4rII infection of a rex+ λ lysogen, results in rapid membrane depolarization imposing a harsh cellular environment that resembles stationary phase. Rex “activation” has been proposed as an altruistic cell death system to protect the λ prophage and its host from T4rII superinfection. Although well studied for over 60 years, the mechanism behind Rex still remains unclear. We have identified key nonessential genes involved in this enigmatic exclusion system by examining T4rII infection across a collection of rex+ single-gene knockouts. We further developed a system for rapid, one-step isolation of host mutations that could attenuate/abrogate the Rex phenotype. For the first time, we identified host mutations that influence Rex activity and rex+ host sensitivity to T4rII infection. Among others, notable genes include tolA, ompA, ompF, ompW, ompX, ompT, lpp, mglC, and rpoS. They are critical players in cellular osmotic balance and are part of the stationary phase and/or membrane distress regulons. Based on these findings, we propose a new model that connects Rex to the σS, σE regulons and key membrane proteins.
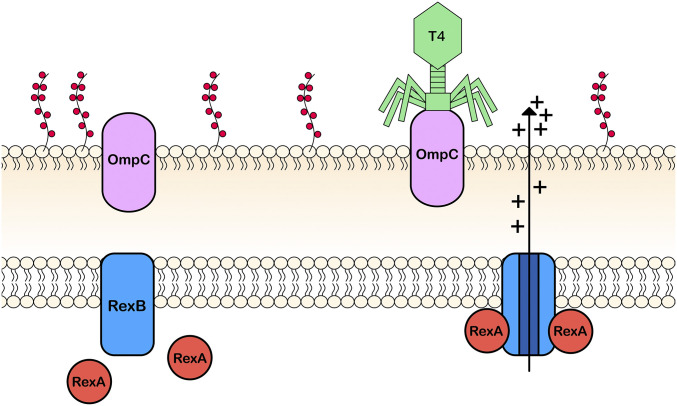
THE T4rII exclusion (Rex) phenotype, first discovered in 1955 by Seymour Benzer (Benzer 1955), is defined as the ability of the rex genes (rexA, rexB) from bacteriophage lambda (λ) to prevent plaque formation by mutant bacteriophage T4rII on λ lysogenized Escherichia coli (Shinedling et al. 1987). It is thought to be a form of defense by λ to protect its host bacterium against other invading bacteriophages. Activation of the Rex phenotype imparts a severe cellular environment that results in cessation of total cellular macromolecular synthesis, depolarization of the cytoplasmic membrane, reduction of the cellular respiration, and death in the majority of cells (Garen 1961; Sekiguchi 1966). Parma et al. (1992) proposed that Rex exclusion occurs by way of a membrane pore (RexB), activated upon interaction with two RexA proteins, establishing a stoichiometric relationship between them (Shinedling et al. 1987; Parma et al. 1992) (Figure 1).
Figure 1.
Upon infection by T4rII, RexA binds to RexB in a 2:1 ratio, activating RexB pore formation and cation efflux. This depolarizes the cell membrane.
In addition to Rex protein stoichiometry, both extra- and intracellular ionic environments mediate Rex regulation. Rex activation is dependent upon monovalent cations such as H+, Na+, K+, NH4+, and Cs+ (Garen 1961; Sekiguchi 1966). In contrast, the presence of divalent cations such as Ca2+ and Mg2+, polyamines, or sucrose can diminish exclusion activity (Garen 1961; Brock 1965; FerroLuzzi-Ames and Ames 1965). Although activation of Rex kills a majority of cells, it still protects ∼1/100 of the “Rex-activated” population (Slavcev and Hayes 2002). Such cells exhibit phenotypic traits characteristic of stationary phase. This quiescent metabolic state is characterized by changes in cellular morphology: spherical appearance, flagellar production, and low cellular proton motive force (Parma et al. 1992). This has led to the hypothesis that Rex somehow triggers an osmotic shift that shunts cells into stationary phase—a metabolic state that is not permissive to the propagation of superinfecting phage such as T4 (Slavcev and Hayes 2003).
Overexpression of RexA was also previously reported to result in “sticky” cells (Hayes and Slavcev 2005), suggesting that the outer membrane may play an important role in the physiological manifestations of Rex and/or its triggering mechanisms. Very similar cellular attributes have been observed in mutants of the tolerance membrane protein TolA and the ion channel outer membrane protein (Omp) OmpA; mutants of both are spherical in shape, leaky, and sensitive to external stresses, including phage infections (Wang and Lin 2001; Lazzaroni et al. 2002). Omps control the influx and the efflux of solutes across the membrane to adapt to external changes, while the Tol system is considered the primary system to import/export micro- and macromolecules to maintain cell structure stability and integrity (Lloubès et al. 2001; Lazzaroni et al. 2002).
In E. coli, most membrane protein expression is under the control of sigma factors. Sigma factors (σ) are small protein subunits required for initiating transcription by binding to the core RNA polymerase and directing transcription at their specific cognate promoter. As such, gene expression may change to adapt to different environmental signals or conditions (Feklístov et al. 2014). The sigma E factor (σE) (rpoE) is activated in response to stress such as hyperosmotic shock, metal ion exposure, and changes in envelope structure. The sigma S factor (σS) (rpoS) maintains cell viability during stationary phase. Mutations to rpoS may also affect the stability of other sigma factors (Battesti et al. 2011). Absence of σS will stimulate an extracellular stress response resulting in elevation of rpoE expression, as well as degradation of Omps and cell lysis (σE-dependent cell lysis; Lima et al. 2013; Kosaka et al. 2017). In turn, small RNAs regulate sigma factors at the transcriptional, translational, and post-translational levels (Schweder et al. 1996; Battesti et al. 2015).
Given the potential role of membrane proteins in combination with the current understanding of the effect of the ionic environment on Rex activity (Garen 1961), we hypothesized that σS- and σE-dependent stress response proteins and their regulators may be involved in the mechanism of Rex. Therefore, we aimed to isolate and identify relevant E. coli host mutations that could influence the Rex phenotype. For the first time, we have linked the manifestation of Rex to genes underlying key host stress responses.
Materials and Methods
E. coli strains and cultures
Bacteria, phages, and plasmids used in this study are described in Table 1. Strains were grown on Luria–Bertani (LB) solid agar at 30°, supplemented with antibiotics [100 μg/ml ampicillin (Ap); kanamycin (Km) 50 μg/ml; 20 μg/ml tetracycline (Tc)]. Liquid cultures were grown in LB at 30° (with Ap for plasmid maintenance). Host cells and mutants were assessed for Rex activity by performing standard relative efficiency of plating (EOP) assays using T4 wild-type (wt) and T4rII stock lysates against an isogenic Rex− parent strain.
Table 1. Bacterial strains, phages, and plasmids used in this study.
| Designation | Relevant characteristics | Source |
|---|---|---|
| Bacterial strains | ||
| DH5α | F−, Δ(argF-lac)169, ϕ80dlacZ58(M15), ΔphoA8, glnV44(AS), λ-,deoR481, rfbC1, gyrA96(NalR), recA1, endA1,thi-1,hsdR17 | E. coli Genetic Stock Collection (CGSC) #12384 |
| W3110 | F−, λ-, IN(rrnD-rrnE)1, rph-1 | CGSC #4474 |
| BW25113 wt | F−, 567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | CGSC #7636 |
| DH5α (λpir) | DH5α lysogenized by phage λ434 (heteroimmune derivative) containing the pir gene | Gift from T. Charles. Kvitko et al. (2012) |
| W3110 (λ) | F−, λ+, λcI578, IN(rrnD-rrnE)1, rph-1 | National BioResource Project (NBRP) #ME6104 (2009) |
| S17-1 (λpir) | TpR SmR recA, thi, pro, hsdR-M+RP4: 2-Tc:Mu: kmR,Tn7, λpir+ | Gift from T. Charles. Matsumoto-Mashimo et al. (2004) |
| JW0427-1 | ΔclpP::kmR, λ– | CGSC #8590 Baba et al. (2006) |
| JW0554-1 | BW25113 isogenic derivative: ΔompT774::kmR | CGSC #8680 Baba et al. (2006) |
| JW0799-1 | BW25113 isogenic derivative: ΔompX786::kmR | CGSC #11794 Baba et al. (2006) |
| JW0912-1 | BW25113 isogenic derivative: ΔompF746::kmR | CGSC #8925 Baba et al. (2006) |
| JW0940-6 | BW25113 isogenic derivative: ΔompA772::kmR | CGSC #8942 Baba et al. (2006) |
| JW1248-2 | BW25113 isogenic derivative: ΔompW764::kmR | CGSC #9125 Baba et al. (2006) |
| JW1312-1 | BW25113 isogenic derivative: ΔompG756::kmR | CGSC #11793 Baba et al. (2006) |
| JW1371-5 | BW25113 isogenic derivative: ΔompN740::kmR | CGSC #9213 Baba et al. (2006) |
| JW2203-1 | BW25113 isogenic derivative: ΔompC768::kmR | CGSC #9781 Baba et al. (2006) |
| JW3368-1 | BW25113 isogenic derivative: ΔompR739::kmR | CGSC #10510 Baba et al. (2006) |
| JW3846-1 | BW25113 isogenic derivative: ΔompL737::kmR | CGSC #10779 Baba et al. (2006) |
| JW5437-1 | BW25113 isogenic derivative: ΔrpoS746::kmR | CGSC #11387 Baba et al. (2006) |
| JW0727-1 | BW25113 isogenic derivative: ΔtolQ786:: kmR | CGSC #8793 Baba et al. (2006) |
| JW0728-1 | BW25113 isogenic derivative: ΔtolR787:: kmR | CGSC #8794 Baba et al. (2006) |
| JW0729-3 | BW25113 isogenic derivative: ΔtolA788:: kmR | CGSC #8795 Baba et al. (2006) |
| JW5100-1 | BW25113 isogenic derivative: ΔtolB789:: kmR | CGSC #11174 Baba et al. (2006) |
| JW5437-1 | BW25113 isogenic derivative: ΔrpoS746:: kmR | CGSC #11387 Baba et al. (2006) |
| JW5503-1 | BW25113 isogenic derivative: ΔtolC732:: kmR | CGSC #11430 Baba et al. (2006) |
| JW0739-1 | BW25113 isogenic derivative: ΔgalM728:: kmR | CGSC #8802 Baba et al. (2006) |
| JW0740-3 | BW25113 isogenic derivative: ΔgalK729:: kmR | CGSC #8803 Baba et al. (2006) |
| JW0741-1 | BW25113 isogenic derivative: ΔgalT730:: kmR | CGSC #8804 Baba et al. (2006) |
| JW0742-1 | BW25113 isogenic derivative: ΔgalE740:: kmR | CGSC #8297 Baba et al. (2006) |
| JW1224-1 | BW25113 isogenic derivative: ΔgalU745:: kmR | CGSC #9110 Baba et al. (2006) |
| JW2027-2 | BW25113 isogenic derivative: ΔgalF731:: kmR | CGSC #9664 Baba et al. (2006) |
| JW2135-1 | BW25113 isogenic derivative: ΔmglC775:: kmR | CGSC #9730 Baba et al. (2006) |
| JW2136-1 | BW25113 isogenic derivative: ΔmglA776:: kmR | CGSC #9731 Baba et al. (2006) |
| JW2137-1 | BW25113 isogenic derivative: ΔmglB777:: kmR | CGSC #9732 Baba et al. (2006) |
| JW2138-1 | BW25113 isogenic derivative: ΔgalS778:: kmR | CGSC #9733 Baba et al. (2006) |
| JW2805-1 | BW25113 isogenic derivative: ΔgalR762:: kmR | CGSC #10192 Baba et al. (2006) |
| JW2910-2 | BW25113 isogenic derivative: ΔgalP789:: kmR | CGSC #10251 Baba et al. (2006) |
| JW3996-1 | BW25113 isogenic derivative: ΔlamB732::kmR | CGSC #10877 Baba et al. (2006) |
| JW1667-5 | BW25113 isogenic derivative: Δlpp-752::kmR | CGSC #9417 Baba et al. (2006) |
| JW0731-1 | BW25113 isogenic derivative: Δpal-790::kmR | CGSC #8796 Baba et al. (2006) |
| Phages | ||
| T4D | Wild-type T4 | Gift from G. Mosig (2009) |
| T4rIIΔ1586 | Δ(rIIA-rIIB) | Gift from G. Mosig (2009) |
| λ (cI-857) | cI[ts]857, (rex+) | NBRP #ME6104 (2009) |
| λF7 | λ, Dam15, imm21, cI[ts]857 | (Gi Mikawa et al. 1996) |
| Plasmids | ||
| pUC19 | High-copy number plasmid; MCS-lacZα, ApR | New England Biolabs (NEB) #N3041S, Whitby, Canada |
| pBSL199 | ori R6K, lacIQ, Tn10, mob (RP4), IS10, ApR, and TcR | NBRP (2009), Alexeyev and Shokolenko (1995) |
| pHA1 | (pUC19) [cI-rexA-rexB]; MCS- lacZα, ApR, pM-cI857-rexA-rexB-timm | This study |
| pHA2 | (pBSL199) [cI-rexA-rexB]; IS10-TcR, pM-cI857-rexA-rexB-timm | This study |
Transformation
Electrocompetent host cells were transformed by 0.5–1.0 μg of plasmid following standard electroporation transformation using the Electroporator 2510 (Eppendorf Canada, Mississauga, CA), and plated on selective LB agar.
Plasmid construction
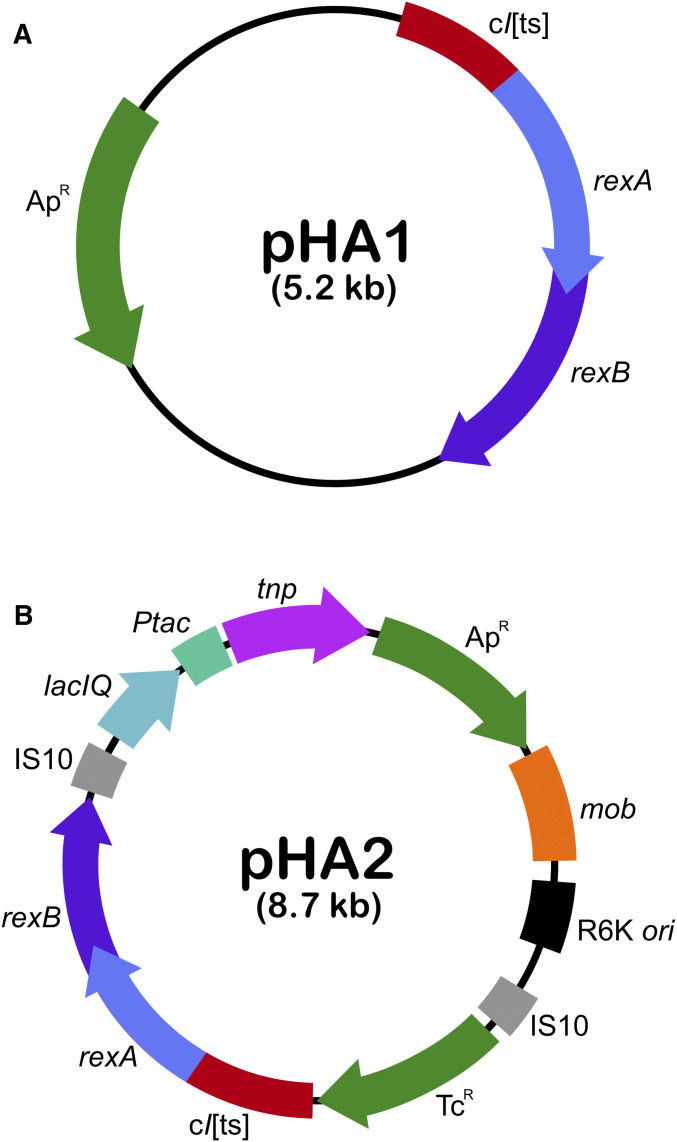
Colony PCR was performed on W3110 (λ) to amplify the λimm (immunity) region with the λimmF (forward) primer: 5′GGGGGGCATTGTTTGGTAGGTGAGAGAT 3′; and the λimmR (reverse) primer: 5′ TTGATCGCGCTTTGATATACGCCGAGAT 3′. Amplification was completed using Phusion polymerase (Thermo Fisher Scientific): initial denaturation at 98° for 30 sec, denaturation at 98° for 10 sec, annealing at 72° for 10 sec, extension at 72° for 3 min, then final extension at 72° for 10 min; repeated 30 times. Reactions were run on 0.8% agarose gel electrophoresis (AGE). The λimm region (2.4 kb) PCR fragment was extracted, purified, and digested by BgIII. The PCR insert (PM-cI857-rexA-rexB-timm rex operon) was isolated and purified using BgIII, which cuts at λ 35,722 bp and λ 38,103 bp, a region that closely flanks the λimm region and the PM-cI857-rexA-rexB-timm rex operon on both sides. Following the digestion of pUC19 by BamHI, the λimm region BgIII-ORPR-PM-cI857-rexA-rexB-timm OLPL--BgIII fragment was cloned into pUC19 to form the rex+ plasmid, pHA1 (Figure 2A and Table 1). XbaI and EcoRI were used to digest pHA1 to excise the XbaI-ORPR-PM-cI857-rexA-rexB-timm-OLPL-EcoRI (2.4 kb) insert fragment. This was subcloned into the pBSL199 suicide vector to yield the suicide “pRex” plasmid, pHA2 (Figure 2B and Table 1). All plasmids were purified using the E.Z.N.A. Plasmid Mini Kit (Omega Bio-Tek). Extracted plasmid was digested to confirm presence of the rex operon, verifying that only a single insert fragment was subcloned per vector and to confirm the expected 5.2 (pHA1) and 8.7 (pHA2) kb vector size, respectively. pUC19 served as the rex− plasmid control for pHA1 and pBSL199 for pHA2.
Figure 2.
(A) Plasmid pHA1 carries rexA and rexB on a high-copy backbone derived from pUC19. (B) pHA2 carries rexA and rexB with a transposable element Tn10 to randomly transpose the cassette into the host genome.
Cell viability assay
Cells harboring pHA1 or pUC19 were assessed for cell viability. E. coli mutants from the Keio collection (Baba et al. 2006), each possessing a single gene deletion, were tested along with their parent strain for Rex activity upon transformation by either plasmid (Table 1). The parent strain transformed with each plasmid, BW25113[pHA1] and BW25113[pUC19], served as controls for Rex activity. The Keio ΔompC mutant (Table 1) precludes T4 adsorption and was used as a negative control for T4 infection. We prepared 1:100 subcultures of cells, as previously described, in LB + Ap and incubated at 30° while shaking at 225 rpm, until A600 = 0.4. 200 μl aliquots of cells were mixed with T4rII lysate at a multiplicity of infection (MOI) of 3. Infected cells were incubated at 30° for 10–15 min, washed twice with 2 ml Tris-NaCl (TN) buffer, and resuspended to a final volume of 1 ml. The suspension was serially diluted in TN buffer. A total of 100 μl aliquots of select dilutions were spread onto LB + Ap agar and incubated at 30° for 48 hr before counting colonies.
Infective center assay
A 1:100 subculture of each Keio mutant carrying pHA1, and BW25113[pUC19] (negative Rex control), was prepared in LB + Ap as previously described, and incubated at 30° with shaking at 225 rpm, until A600 = 0.4. 2 ml of culture was centrifuged at 10,000 × g for 10 min and pelleted cells were resuspended in 1 ml of CaCl2. T4rII phage were added at MOI = 3 and allowed to adsorb to cells for 15–20 min at 37°. Infected cells were washed three times in TN buffer and resuspended in 100 μl TN buffer. A total of 0.3 ml of the original subculture was added to the re-suspended cells, mixed with 3 ml top agar, poured onto prewarmed LB + Ap agar, and incubated overnight at 30°.
Conjugation assay
Overnight cultures of JW0427-1 (ΔclpP, KmR) (recipient) and S17-1(λpir)[pHA2] (rex+ suicide plasmid and donor) were prepared at 30° with shaking at 225 rpm. JW0427-1 (ΔclpP, KmR) was used as a recipient as the rex+ derivative. We previously found that ΔclpP mutants retained full Rex activity, so the gene, to our knowledge, is not involved in Rex (Hayes and Slavcev 2005). Cells were pelleted from 1 ml of each culture, washed twice in 0.5 mM NaCl, and mixed in a 1:2 donor:recipient ratio. Mixtures were pelleted and re-suspended in 80 μl of 0.5 mM NaCl, then added to 100 μl prewarmed LB. After 1–2 hr of incubation and before plating, 0.1 mM IPTG was added and the mixture was incubated for an additional 1–2 hr at 37°. Samples without IPTG (donor cells only) and recipient cells were directly plated and incubated overnight at 30°. Mixtures with IPTG were spot-plated on LB agar and incubated overnight at 30°.
Insertional mutagenesis by transposable rex+ cassette and screening for Rex− mutants
Cells from conjugation assays were diluted in 1 ml LB. A total of 200 μl aliquots were prepared for plating. Prewarmed LB + Tc + Km agar plates were seeded with 105 PFU of T4rII phage diluted in TN buffer to screen for a T4rII-sensitive phenotype (T4rII “biting” of growing colonies). Then, 200 μl of cells were plated and incubated overnight at 30°. The trans-conjugation frequency for Rex− mutants and the frequency of Rex− bitten colonies were determined (data not published).
Phage λ (rex+) lysogenization of Rex− mutants and immunity assay
Overlay plates of isolated Rex− mutants were prepared as follows: 10 μl of 10−4 dilutions of fresh wt λ or cI857 λ in TN buffer were spotted onto LB top agar. After drying, plates were incubated overnight at 30° to generate λ lysogens. Cells within large turbid plaques were isolated to confirm for λ lysogeny. The cI857 λ lysogens were grown at 42° to inactivate the cI repressor, where any lysogens would be induced for phage amplification and lysis of their resident cells. Lysogenized cells able to grow at both 30° and 42° were confirmed for the presence of wt λ lysogens by an immunity assay. Cells were stabbed into a top agar overlay on LB agar containing ∼108 PFU of phage (λimm21) as well as 3 × 108 CFU W3899(λimm21) lysogens, to test for presence of λ immunity to confirm lysogenization. Colonies were stabbed in the overlay top agar and incubated overnight at 30°. Large lysis spots arising from λimm recombinants were visualized the next day.
PCR mapping and sequencing of mini Tn10 insertions in the chromosome
Inverse colony PCR was performed using Taq DNA polymerase. A single fresh colony of each isolated mutant was diluted into 50 μl of ddH2O, where 1 μl was used as a template for the PCR reaction. Four primers were used during two rounds of inverse PCR as described by Nichols et al. (1998). Primers: first round PCR: primer #1 (JEP83) 5′ TTGCTGCTTATAACAGGCACTGAG 3′ and primer #2 (JEP5) 5′ GGCCACGCGTCGACTAGTACNNNNNNNNNNGCTGG 3′; second round PCR: primer #3 (JEP84) 5′ CTTTGGTCACCAACGCTTTTCCCG 3′ and primer #4 (JEP6) 5′ GGCCACGCGTCGACTAGTAC 3′ (Peters and Craig 2000; Shi et al. 2008). The cycling thermal reaction for the first round (primers 1 and 2) proceeds as follows: 95° for 5 min, 95° for 30 sec, 30° for 1 min, 72° for 1 min, repeated 10 times. Next, samples were heated to 95° for 30 sec, 42° for 1 min, and 72° for 1 min, repeated 30 times. A 1:10 dilution of this first PCR reaction was used as a template for a second round (primers 3 and 4), which proceeds as follows: 95° for 5 min, 95° for 30 sec, 50° for 45 sec, and 72° for 1 min, repeated 30 times. Reactions were separated by AGE. The presenting band (∼800 bp) was extracted using the E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek). PCR fragments amplified from primers 3 and 4 were commercially sequenced (Bio-Basic Inc., Markham, Canada).
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Results and Discussion
Despite playing a rich and historic role in evolving our understanding of modern molecular genetics, the mechanism governing the λ T4rII exclusion phenotype has remained a mystery for >60 years. Identification of host genes influencing Rex activity has long been sought to better understand the exclusionary mechanism. Through our work here, we have identified several key groups of host genes and linked them together in a new proposed model.
Assessing Rex activity of host genes suspected to be involved in T4rII exclusion
Based on the current understanding of the interplay of the ionic environment with Rex, we evaluated genes related to transmembrane ion transport, specifically Omps and their regulators. We observed the direct effect of each gene’s deletion on Rex activity in single-gene knockouts. Selected knockout mutants (Baba et al. 2006) (Table 1) were transformed by a high-copy plasmid encoding rexAB (pHA1; Figure 2A), and were assayed for Rex activity (Tables 2 and 3). Overexpression of rexAB has been previously demonstrated without change in the resulting exclusion phenotype so long as the stoichiometric balance between rexA and rexB is maintained (Slavcev and Hayes 2003). By vastly increasing the dosage of rex, we expected only deletions of critical host players to demonstrate significant modulation of Rex activity. We then confirmed these results by examining key deletions with the natural dosage of Rex (Table 4). The wild-type parent strain (BW25113) carrying pHA1 acted as a positive control for Rex, demonstrating complete resistance to T4rII plating and near complete sensitivity to RII suppression of Rex activity by wild-type T4 (Tables 2 and 3). The >106-fold difference in plating between T4 (rII+) and T4rII demonstrates the powerful Rex phenotype imparted by the rex locus encoded on pHA1 and the ability of the rIIA and rIIB genes of T4 to suppress this phenotype.
Table 2. Membrane proteins that attenuate the Rex phenotype.
| Mutation | Rex plasmida | Relative efficiency of platingb | |
|---|---|---|---|
| T4 (rII+) | T4rII (rII−) | ||
| – | + | 1.0 | <3.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔompA | + | N/Ac | N/Ac |
| – | 1.4 | 0.6 | |
| ΔompCd | + | <3.0 × 10−7 | <3.0 × 10−7 |
| – | <3.0 × 10−7 | <3.0 × 10−7 | |
| ΔompF | + | 0.2 | <3.0 × 10−5 |
| – | 1.0 | 0.9 | |
| ΔompG | + | 0.1 | <3.0 × 10−7 |
| – | 1.0 | 0.4 | |
| ΔompL | + | 0.1 | 2.0 × 10−5 |
| – | 1.0 | 1.0 × 10−2 | |
| ΔompN | + | 0.3 | <3.0 × 10−7 |
| – | 1.0 | 0.3 | |
| ΔompR | + | 3.0 × 10−5 | <3.0 × 10−7 |
| – | 1.0 × 10−2 | 0.2 | |
| ΔompT | + | 0.2 | <3.0 × 10−7 |
| – | 1.0 | 1.0 × 10−4 | |
| ΔompW | + | 0.2 | 7.0 × 10−4 |
| – | 1.0 | 0.2 | |
| ΔompX | + | 0.2 | 3.0 × 10−4 |
| – | 1.0 | 0.2 | |
| ΔlamB | + | 1.0 × 10−6 | <3.0 × 10−7 |
| – | 0.7 | 0.8 | |
| Δlpp | + | 4.0 × 10−4 | 3.0 × 10−3 |
| – | 0.8 | 1.0 | |
| ΔtolA | + | 0.3 | 0.8 |
| – | 1.0 | 0.6 | |
| ΔtolB | + | 0.1 | <3.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔtolC | + | 1.0 × 10−2 | <3.0 × 10−7 |
| – | 0.5 | 1.0 | |
| ΔtolQ | + | 8.0 × 10−2 | 4.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔtolR | + | 6.0 × 10−2 | <3.0 × 10−7 |
| – | 0.7 | 0.7 | |
| Δpal | + | 0.7 | <3.0 × 10−7 |
| – | 0.8 | 1.0 | |
The Rex+ plasmid is pHA1, a pUC-derived high-copy plasmid carrying the PM-cI-rexA-rexB-timm cassette.
All assayed strains are isogenic derivatives of Keio collection wild-type BW25113. Relative efficiencies of plating are determined by dividing the number of plaques for the sample by the number of plaques on the 100% control: BW25113 as the 100% control for T4rII plating and BW25113[pHA1] as the 100% control for Rex activity. All results represent the average of three independent plating assays. Strains (rex+) attenuated for Rex activity showed pinpoint plaques compared to rex− counterparts.
Pinpoint plaques were visible but only under high-density plating conditions, which did not make quantification at reasonable dilutions possible.
The ΔompC mutant is a host range mutant that precludes T4 adsorption, so neither phage can infect this strain.
Table 3. Other factors that attenuate the Rex phenotype.
| Mutation | Rex plasmida | Relative efficiency of platingb | |
|---|---|---|---|
| T4 (rII+) | T4rII (rII−) | ||
| – | + | 1.0 | <3.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔmglA | + | 7.0 × 10−2 | <3.0 × 10−7 |
| – | 0.7 | 0.7 | |
| ΔmglB | + | 0.7 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔmglC | + | 0.1 | 2.0 × 10−5 |
| – | 0.7 | 1.0 | |
| ΔgalM | + | 1.0 | <3.0 × 10−7 |
| – | 0.6 | 1.0 | |
| ΔgalK | + | 7.0 × 10−2 | <3.0 × 10−7 |
| – | 0.6 | 1.0 | |
| ΔgalT | + | 8.0 × 10−2 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔgalE | + | 1.0 × 10−2 | <3.0 × 10−7 |
| – | 1.0 | 1.2 | |
| ΔgalU | + | <3.0 × 10−7 | <3.0 × 10−7 |
| – | <3.0 × 10−7 | <3.0 × 10−7 | |
| ΔgalF | + | 1.0 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔgalP | + | 1.0 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔgalS | + | 0.3 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔgalR | + | 1.0 | <3.0 × 10−7 |
| – | 0.7 | 1.0 | |
| ΔrpoS | + | <3.0 × 10−7 | <3.0 × 10−7 |
| – | 0.7 | 0.7 | |
| ΔrssB | + | 0.5 | 3.0 × 10−3 |
| – | 1.0 | 1.0 | |
| ΔrssA | + | 3.0 × 10−3 | 0.2 |
| – | 1.0 | 1.0 | |
| ΔiraP | + | 3.0 × 10−2 | <3.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔiraM | + | 0.5 | <3.0 × 10−7 |
| – | 1.0 | 1.0 | |
| ΔiraD | + | 0.5 | <3.0 × 10−3 |
| – | 1.0 | 1.0 | |
The Rex+ plasmid is pHA1, a pUC-derived high-copy plasmid carrying the PM-cI-rexA-rexB-timm cassette.
All assayed strains are isogenic derivatives of Keio collection wild-type BW25113. EOPs are determined by dividing the number of plaques for the sample by the number of plaques on the 100% control. EOPs of T4/T4rII were determined using BW25113 as the 100% control for T4rII plating and BW25113[pHA1] as the 100% control for Rex activity. All results represent the average of three independent plating assays. Strains (rex+) attenuated for Rex activity showed pinpoint plaques compared to rex− counterparts.
Table 4. Rex activity is influenced by E. coli proteins that affect membrane integrity and maintenance.
| Mutation | Relative EOP of T4rII-infected centersa |
|---|---|
| – | 1.0 |
| – (λ) | <3.0 × 10−7 |
| ΔompT | <3.0 × 10−7 |
| ΔompX | 4.5 × 10−4 |
| ΔompF | 3.3 × 10−5 |
| ΔompA | 5.0 × 10−4 |
| ΔompW | 1.0 × 10−3 |
| ΔompG | <3.0 × 10−7 |
| ΔompN | <3.0 × 10−7 |
| ΔompCb | <3.0 × 10−7 |
| ΔompR | <3.0 × 10−7 |
| ΔompL | <3.0 × 10−6 |
| ΔrpoS | 0.7 |
| ΔtolA | 1.0 |
| ΔrssA | 0.9 |
| ΔrssB | 1.0 |
| ΔiraM | 0.1 |
| ΔiraP | 0.1 |
| ΔiraD | 1.0 |
All assayed strains are rex+ isogenic derivatives of Keio collection wild-type BW25113 lysogenized by λ. EOPs of T4/T4rII were determined using BW25113 as the 100% control for T4rII plating. All mutant-infected centers were permitted to adsorb T4rII for 15 min before washing the infected cells three times, diluting, and plating on the relevant test strain. All results represent the average of three independent plating assays.
The ΔompC mutant is a host-range mutant that precludes T4 adsorption, so neither phage can infect this strain.
Omps:
The Omps all play roles in solute transmembrane transport. Based on this, we expected their absence to impede establishment of the harsh ionic environment that is a hallmark of the Rex phenotype (Figure 1). T4rII infectivity for all Omp deletion (Δomp) mutants carrying the high-copy rex+ pHA1 was observed to be similarly exclusionary with important exceptions (Table 2). To examine the fate of rex+ Δomp mutants following infection by T4rII (a state that normally would confer the onset of Rex cellular phenotypes), we also employed a cell viability assay to determine whether specific Δomp mutants carrying pHA1 (rex+) were as sensitive to T4rII-mediated cell killing following T4rII-infection as wild-type (rex−) (Table 5). All rex+ cells challenged with T4rII generally grew more slowly and colonies were only visible after 48 hr of incubation at 30° compared to the uninfected control strains that were visible within 24 hr, as previously described (Slavcev and Hayes 2002, 2003). The viability of most Δomp mutants harboring the rex+ plasmid was reduced by ∼102-fold compared to that of the rex+ control, indicating that these omp genes exert weak influence over Rex-mediated cellular fate (Table 5).
Table 5. Omp proteins influence rex+ host viability following T4rII infection.
| Mutation | Rex plasmid | EOPa | Cell viabilityb |
|---|---|---|---|
| −1 | – | 3.0 × 10−7 | 0 |
| −1 | + | 1.0 × 10−3 | 1.0 |
| ΔompA | + | 5.0 × 10−4 | 0.5 |
| ΔompCc | + | 0.2 | –3 |
| ΔompF | + | 2.0 × 10−5 | 1 × 10−2 |
| ΔompG | + | 6.0 × 10−4 | 0.6 |
| ΔompL | + | 1.0 × 10−3 | 1 × 10−3 |
| ΔompN | + | 2.0 × 10−4 | 0.5 |
| ΔompR | + | 0.7 | –3 |
| ΔompT | + | 5.0 × 10−4 | 0.5 |
| ΔompW | + | 1.0 × 10−5 | 1 × 10−2 |
| ΔompX | + | 1.0 × 10−5 | 1 × 10−2 |
Efficiency of plating showing CFU arising at 30° after infection by T4rII at an MOI of 3. Average of three trials. A 100% control of T4rII infectivity was BW25113 (rex−); BW25113 (wt) carrying [pHA1] plasmid was employed as the 100% positive control of Rex activity.
Rex-mediated protection of host cells from T4rII challenge. Calculated using BW25113 carrying pHA1 (rex+) plasmid as 100% viability control following T4rII challenge.
The deletion of ompC precludes T4 phage adsorption protecting this strain against infection. Similarly, the ompR mutation inhibits ompC expression precluding T4 phage adsorption protecting this strain against infection by T4.
All Δomp mutants, including the parent strain, were sensitive to T4 (rII+) plating with the exception of the ΔompC derivative that is deficient in the adsorption protein for T4 and is, hence, a host-range mutant (Table 2). In contrast, all nontransformed (rex−) mutants exhibited near complete T4rII plating efficiency, except the ompT outer membrane protease mutant and the ompL porin mutant (Table 2). Below, we discuss key Omps and their roles in Rex.
OmpA:
The hyper-Rex phenotype that similarly inhibited T4 and T4rII plating (Table 2) speaks to the significant role ompA expression plays in Rex (Figure 3); ΔompA also reduced cell viability upon T4rII infection in a rex+ context (Table 5). Interestingly, we found that the ompA mutant carrying the multicopy rex+ pHA1 not only reduced plating of T4rII, but also reduced plating of T4 (rII+), suggesting that OmpA may play a role in RII’s escape of Rex. Upon infection of E. coli cells, T4 normally causes rapid degradation of the messenger RNA (mRNA) of two main membrane proteins, OmpA and Lpp, within ∼1.5 and 2.4 min respectively (Qi et al. 2015); this further supports some interplay between OmpA and RII to avoid Rex.
Figure 3.
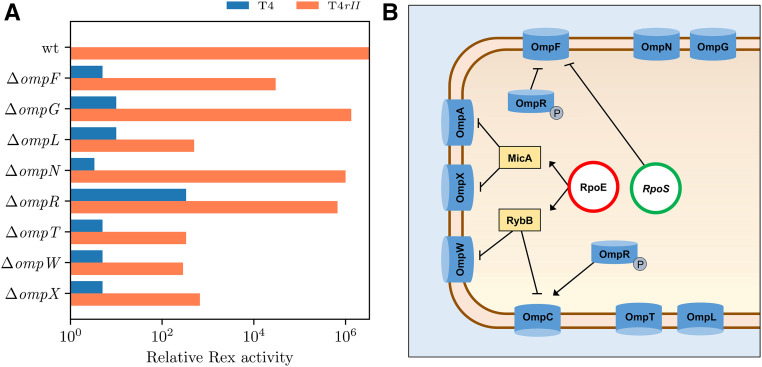
Omps may play a role in Rex, as their deletions affect Rex activity. In particular, ΔompF, ΔompL, ΔompT, ΔompW, and ΔompX attenuate Rex activity. (A) Rex activity is the ability of pHA1-delivered rex genes to reduce plating in the presence or absence of RII. Activity is derived by taking the inverse of the relative EOP of the sample in rex+ conditions (plasmid) compared to rex- conditions (no plasmid). Maximal Rex activity is the complete attenuation of T4rII (rII-) plating in presence of the rex pHA1 in E. coli BW25113 (wt). Absence of Rex activity is full plating of T4 (rII+) in presence of the rex+ pHA1 in E. coli BW25113 (wt). (B) Omps mediate ion transport as regulated by rpoE and rpoS.
Exposure to stress in ΔompA mutants has been shown to induce phenotypic changes in E. coli, whereby cells become spherical in shape, similar to stationary-phase cells (Wang 2002), highlighting the role of OmpA in stimulating the σE stress response. The heightened Rex activity seen in the ompA mutant (Figure 3A) may thus arise due to the predisposition of these host cells to be shunted into osmotic irregularity and a stationary-like phase. Because of the additional osmotic and structural instability caused by the ompA mutation, recovery of the cell’s osmotic rebalance and membrane potential may be irreversible in a rex+ context, which may account for the observed low viability of rex+ ompA mutants (Table 5). Conversely, the inability of ompA mutants to effectively passage and evacuate invading DNA out of the cell (Wang and Lin 2001) during Rex onset could similarly doom the cell to the lethality of the superinfecting DNA. In either case, the irreversible stationary-like state effected by Rex activation in the ompA mutant would also account for the powerful Rex-dependent exclusion of T4rII and T4 (rII+) alike (Snyder and McWilliams 1989; Slavcev and Hayes 2003), as stationary phase prohibits the propagation of T-even species (Bryan et al. 2016). OmpA has also recently been implicated in DNA transformation in E. coli and DNA transfer (Sun et al. 2013). The ability of some cells to recover from Rex activity would mean that the survivors are able to eventually evacuate invading T4rII DNA—a role that may be mediated by OmpA in concert with Rex proteins.
OmpX:
We found that there was a >103-fold increase in T4rII plating in ompX rex+ mutants, compared to the rex+ control (Table 2). Additionally, ΔompX exhibited >102-fold reduced viability on T4rII-infected derivatives carrying the rex+ plasmid compared to the rex+ control, likely due to compromised T4rII exclusion in these rex+ mutants (Table 5). Like OmpA, OmpX has similarly been implicated in cell defense against virulence (Vogt and Schulz 1999). Deletion of ompX sensitizes cells to stressful environments including phage infection and killing (Otto and Hermansson 2004). Otto and Hermansson (2004) found that ΔompX causes significant alterations in cell surface hydrophobicity and negative charge that may also increase phage-bacterial interaction. Small RNAs MicA and RybB regulate expression of ompX and some other membrane proteins (Valentin-Hansen et al. 2007). Their transcripts (micA and rybB), expressed under the control of σE, further downregulate ompA and ompC translation. The consequent disruption of Omp formation enhances σE-dependent cell lysis, as noted by the resultant high protein density in the culture medium (Kabir et al. 2005). Underproduction of OmpX has been noted to reduce σE activity (and vice versa) in E. coli as a “strain-dependent” phenotype (Mecsas et al. 1993), which may help explain the reduction in Rex activity (Figure 3A) in these rex+ mutants.
OmpW:
Another significant attenuator of Rex activity was ΔompW, increasing T4rII plating in a rex+ context by >103-fold (Table 2). OmpW forms porins in the outer membrane, generating long, narrow, hydrophobic channels that serve as ion channels in the transport of small hydrophobic molecules across the membrane (Hong et al. 2006). ΔompW also exhibited 102-fold reduced viability on T4rII-infected derivatives carrying the rex+ plasmid (Table 5). Interestingly, OmpW, along with OmpA and OmpF, has been documented to protect E. coli against environmental stressors including viral superinfection (Wu et al. 2013). The reduction in the viability of ompX, ompW, and ompF mutants (Table 5) supports our observations that these rex+ mutants were attenuated for Rex activity (Figure 3A) and were therefore more sensitive to T4rII infection. As such, they were more readily lysed.
OmpR, OmpC, and OmpF:
As expected, the ΔompC mutants demonstrated complete resistance to T4 and T4rII infections, and therefore serve as the negative controls for T4 infection due to direct prevention of T4 adsorption. Deletion of ompR did not significantly affect T4rII plating, while deletion of ompF increased T4rII plating by >102-fold (Table 2). Therefore, Rex activity is more heavily affected by ΔompF than ΔompR (Figure 3A). The ompF and ompC genes are differentially expressed based on changes in medium osmolarity, as sensed by the membrane-bound EnvR sensor (Srividhya and Krishnaswamy 2004) and carried out by the cytoplasmic transcriptional regulator OmpR, reviewed in depth elsewhere (Mizuno and Mizushima 1990).
The ionic environment is crucial for the Rex phenotype, where monovalent cations are essential for the onset and divalent cations can abrogate the phenotype (Garen 1961; Brock 1965; FerroLuzzi-Ames and Ames 1965; Sekiguchi 1966). OmpF and OmpC function as cation-selective diffusion channels that control cell osmolarity in response to changes to extracellular osmolarity (Cowan et al. 1992; Apirakaramwong et al. 1998). Under high osmotic pressure, ompC expression is upregulated; on the other hand, under low osmotic pressure, ompF expression is upregulated to stabilize cellular osmosis. OmpR regulates transcription of the small RNAs MicC and MicF that stimulate degradation of the ompC and ompF mRNA transcripts, thus reducing their expression in an OmpR-deprived environment (Guillier 2006). Importantly, the expression of ompC can also be stimulated independently of OmpR by sucrose, previously shown to powerfully suppress Rex activity (Schnaitman and McDonald 1984). OmpC also functions as the primary adsorption receptor for T4 (Yu and Mizushima 1982; Washizaki et al. 2016), which we similarly confirmed in this study as ΔompC results in the loss of T4’s ability to infect (Table 2). Because of the prohibitive state of ΔompC toward T4 plating, we cannot definitively conclude any involvement of OmpC in Rex activity. However, based on previous observations of OmpC, OmpF, and OmpR regulation, potential scenarios can logically be envisioned for their roles in Rex activation: (1) as the adsorption receptor for T4, OmpC makes a very logical Rex activation target or sensor, triggered by T4 superinfection; (2) poor T4 infectivity in the ompR mutant could be attributed to reduced expression of ompC, that again, is essential for T4 adsorption; (3) OmpF likely plays an indirect role in Rex, perhaps through osmotic dysregulation and stimulation of a stationary phase-like state; and (4) as OmpC and OmpF are expressed under inverse osmolarity conditions, reduced ompF expression would stimulate ompC transcription leading to increased T4 infection (Srividhya and Krishnaswamy 2004).
OmpL and OmpT:
Rex activity was moderately reduced in the absence of ompL or ompT (Figure 3A). In the presence of rex, T4rII plating increased by 102-fold in the ompL mutant, while no significant changes were observed in the ompT mutant (Table 2). In the absence of rex however, we observed reductions in T4rII plating on both ompT (104-fold reduction) and ompL (102-fold reduction). This is in contrast to T4 (rII+), whose plating does not appear significantly affected by ΔompT or ΔompL regardless of rex presence. It is not currently known what roles, if any, OmpT and OmpL may play in bacteriophage infection. OmpL functions as a low-molecular-weight diffusion porin (Sardesai 2003), while OmpT is a protease that cleaves foreign peptides encountered within the E. coli cell (Stumpe et al. 1998). Absence of ompT or ompL has not previously demonstrated any detriment to bacterial cell growth. However, we did observe that deletion of ompL reduced cell viability 103-fold in a rex+ context (Table 5). T4rII (rII-) plating in general appears to be compromised in ΔompT or ΔompL contexts (Table 2). It is possible that either OmpT or OmpL may serve alternative functions to RII and support T4rII infection in the absence of RII.
Rex and other membrane proteins:
We also examined Rex activity in knockouts of other critical membrane proteins (Figure 4). In general, knockout of tolA, lamB, and to a marginal degree, lpp, tolR, and tolQ, improved T4 (rII+) plating specifically under rex+ conditions. This phenotype was not observed in rex- conditions (Table 2), indicating some connection between these genes and RII. Below, we discuss the effect of these gene knockouts in further detail.
Figure 4.
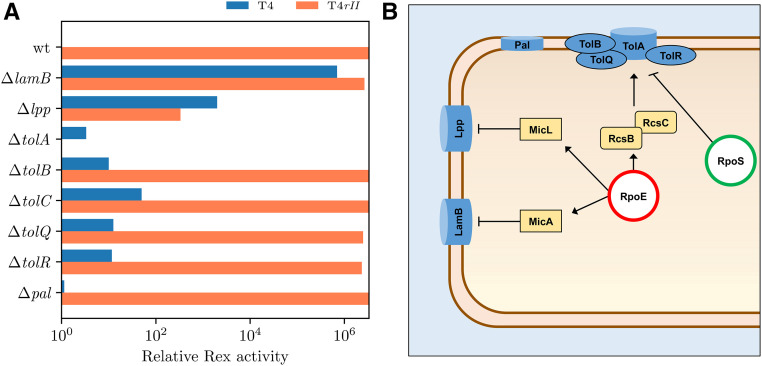
Several other key membrane proteins may play a role in Rex, as their deletions affect Rex activity. In particular, ΔtolA completely restores T4rII plating, thereby eliminating Rex activity. (A) Rex activity is the ability of pHA1-delivered rex genes to reduce plating in the presence or absence of RII. Activity is derived by taking the inverse of the relative EOP of the sample in rex+ conditions (plasmid) compared to rex- conditions (no plasmid). Maximal Rex activity is the complete attenuation of T4rII (rII-) plating in presence of the rex pHA1 in E. coli BW25113 (wt). Absence of Rex activity is full plating of T4 (rII+) in presence of the rex+ pHA1 in E. coli BW25113 (wt). (B) These major membrane proteins are upregulated by rpoE.
LamB:
While knockout of lamB did not improve the plating efficiency of T4rII in a rex+ context, it did greatly reduce the plating efficiency of T4 (rII+) by 106-fold (Table 2). In other words, RII’s ability to circumvent Rex is impaired in absence of lamB (Figure 4A). LamB is mainly responsible for maltose uptake, but also acts as a receptor for λ adsorption (Randall Hazelbauer and Schwartz 1973). A highly abundant protein, LamB, exhibits dynamic spatial localization throughout the outer membrane (Gibbs et al. 2004). Like OmpA, its expression is modulated by σE and by the small RNA MicA. Along with the mal operon, lamB can also be upregulated by σS. Mutations in lamB are very harmful to the cell, causing major defects in the inner membrane, disrupting the proton motive force, and unfolding Omps (Death et al. 1993). It is interesting that ΔlamB showed almost complete abrogation of T4 (rII+) and T4rII plating in a rex+ context, but full plating efficiency in the absence of rex (Table 2). Hence, LamB seems to interact with RII and/or Rex.
Tol, Pal:
Knockout of tolA almost completely abrogated Rex activity (Figure 4A), leading to complete plating of T4rII. Knockouts of tolQ, R, and B showed no effect (Table 2), suggesting the direct involvement of TolA with Rex and/or RII. Interestingly, we observed full plating efficiency with pinpoint plaques of T4rII compared to wild-type T4 on a ΔtolA mutant, but not ΔtolB (Table 2), as previously noted in a different study (Rolfe and Campbell 1977). The Tol proteins play critical roles in maintaining cell membrane stability and integrity (Lazzaroni et al. 1999; Lloubès et al. 2001), including roles in assembly of outer membrane porins and lipopolysaccharide synthesis. Tol normally forms a complex with the membrane-bound Pal lipoprotein (Tol-pal complex) (Cascales et al. 2002). However, knockout of pal surprisingly did not show any effect on Rex (Figure 4A). Alongside Omps, Tol proteins are involved in the import and export of micro- and macromolecules. TolA has three domains that extend to the outer membrane, bridging interactions between outer- and inner-membrane proteins (Levengood-Freyermuth et al. 1993; Derouiche et al. 1996). Transcription of tolA is positively regulated by σE through the activation of the RcsC/B sensor kinase system in response to envelope stress (Dam et al. 2018). Activation of RcsC/B would also stimulate the activation of σS and σE.
TolA indirectly regulates OmpF by increasing ompF expression through downregulation of ompC expression; as such, ompF expression is downregulated in tolA mutants (Lazzaroni et al. 1986; Derouiche et al. 1996). Deletion of tolA also reduces LamB levels, resulting in the onset of cell stress responses (Derouiche et al. 1996). Mutations in tolA changes cell morphology, making bacterial cells very leaky and sensitive to external stresses, including infections, but it also renders them resistant to colicins (Meury and Devilliers 1999; Lloubès et al. 2001; Lazzaroni et al. 2002). Many colicins require the TolQRAB complex, OmpA, and the pore-forming OmpF and OmpC, to translocate into cells. We noted the deletion of any of these demonstrated at least some increase in T4rII plating compared to the rex+ control. There appears to be a strong analogy between the colicin and Rex systems.
Lpp:
In the presence of rex, Δlpp improved T4rII plating 104-fold compared to the rex+ control. In contrast, T4 plating was reduced by 104-fold (Table 2). In addition to the Tol-Pal complex, Lpp is a major prolipoprotein on the inner face of the outer membrane that protects and maintains the structural and functional integrity of the cell membrane (Ozawa and Mizushima 1983). Lpp maintains the network between outer membrane and peptidoglycan layer. Transcription of lpp, like other membrane proteins, is under the regulation of σE, emphasizing its role in stress responses. Mutation of lpp results in loss of the structural link between envelope membranes, consequently releasing periplasmic proteins into the medium and forming vesicles, which is phenotypically similar to mutations in tol-pal (Bernadac et al. 1998). Transcripts of lpp are rapidly degraded after T4 infection (Qi et al. 2015); its deletion does not appear to affect T4 or T4rII plating in the absence of rex genes (Table 2). In contrast, we observed medium plating efficiency for both in a rex+ context, indicating some Lpp involvement in Rex. Since it is not on the outer surface, Lpp might not participate in the onset of Rex, but it may maintain activated Rex throughout the exclusion mechanism. Mutations in lpp do not affect expression of Tol-Pal, but tolA mutations decrease lpp expression (Cascales et al. 2000). Lpp may be an important link between TolA and Omps. Although no direct interaction between TolA and OmpA has yet been found, TolA indirectly interacts with a TolB-Pal-Lpp-OmpA complex as well as the rest of Omps (Lloubès et al. 2001).
Rex and sigma factors:
We next examined Rex activity in sigma factor knockouts (Figure 5) as key Rex-involved membrane proteins are regulated by σS. Most importantly, knockout of rpoS completely abrogated T4 (rII+) and T4rII plating alike in a rex+ context (Table 3). Below, we discuss these knockouts in detail.
Figure 5.
Regulatory small RNAs and rpoS may interact with Rex, as rex genes are able to inhibit T4 (rII+) plating if rpoS is deleted. (A) Rex activity is the ability of pHA1-delivered rex genes to reduce plating in the presence or absence of RII. Activity is derived by taking the inverse of the relative EOP of the sample in rex+ conditions (plasmid) compared to rex- conditions (no plasmid). Maximal Rex activity is the complete attenuation of T4rII (rII-) plating in presence of the rex pHA1 in E. coli BW25113 (wt). Absence of Rex activity is full plating of T4 (rII+) in presence of the rex+ pHA1 in E. coli BW25113 (wt). (B) rpoE and rpoS are regulated by ClpXP activity and respective regulatory small RNAs.
rpoS and regulators:
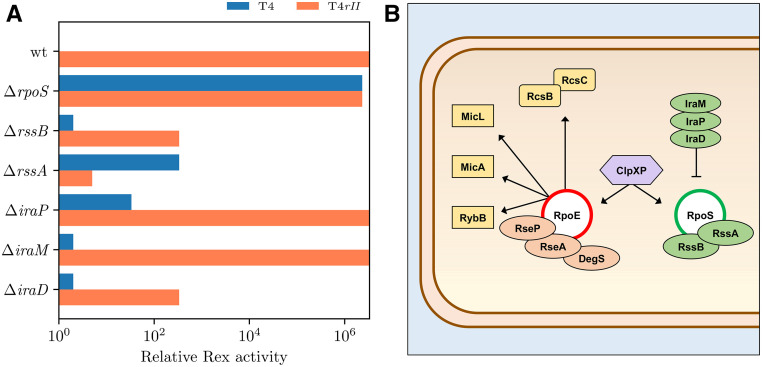
Knockout of rpoS completely abrogated T4 (rII+) and T4rII plating alike in a rex+ context (Table 3). Knockouts of small RNA regulators of σS expression also demonstrated rescue of T4rII plating to various degrees: ΔrssA, ΔrssB, and ΔiraD notably increased T4rII plating (106-fold for ΔrssA, >104 for both ΔrssB and ΔiraD). However, little to no effect on Rex activity was observed in the ΔiraM and ΔiraP mutants in the presence of high rex+ expression (Figure 5A). E. coli cells enter stationary phase upon exposure to extrinsic or intrinsic stress initiating σE activation, where all growth phase genes are switched off and stress response genes are switched on (Chen et al. 2004). σS, encoded by rpoS, is essential for cell survival in stationary phase. It regulates the expression of >10% of all E. coli genes during this phase (Weber et al. 2005). During growth phase, the small RNA protease ClpXP rapidly degrades levels of rpoS mRNA by binding to the rpoS transcript adaptor RssB (Schweder et al. 1996; Battesti et al. 2015). In contrast, the anti-adaptor small RNAs IraD, IraM, and IraP stabilize the rpoS transcript via interactions with RssB during different stress conditions such as the stationary phase. During stationary phase, ClpXP instead increases the expression of rpoE, thereby stimulating the σE response, leading to alteration of Omp stability, which, in turn, leads to altered cell morphology. In a ΔrpoS context, the absence of rpoS transcripts frees ClpXP to elevate σE expression.
Interestingly, while ompF expression is positively controlled by σE through OmpR, its expression is negatively controlled by σS through the small RNA MicF. MicF’s own expression is also downregulated by σS (Pratt and Silhavy 1996; Patten et al. 2004). Expression of ompF has been found to be elevated in rpoS mutants without effect on OmpC levels (Pratt and Silhavy 1996; Battesti et al. 2015). In this study, rpoS mutants were able to fully exclude T4 and T4rII from plating in the presence of rex (Table 3), which might be explained by the overexpression of σE-controlled TolA, LamB, and OmpF and their direct effect/interaction with Rex. Wild-type levels of plating by both T4 and T4rII alike were observed in the absence of rex (Table 3). This super-Rex phenotype was also seen in ompA mutants (Table 2), which could indicate the involvement of rpoS in “triggering” Rex.
rpoE:
Based on our observations of the effect of omps, tolA, and rpoS in Rex activity (Figures 3–5), we believe that the σE pathway may also be intimately connected to Rex. σE is an essential factor that governs transcriptional activation of many downstream genes, including the periplasmic proteins and degradation factors. It has been implicated in a cell lysis pathway entailing small RNAs MicA and RybB that reduce levels of Omps, leading to the eventual disintegration of the outer membrane (Murata et al. 2012). A strikingly similar scenario is when E. coli cells enter stationary phase, where elevation of active σE leads to the decrease in expression of OmpA, OmpC, and OmpW (Kabir et al. 2005). E. coli sigma factors σE and σS share homology with T4 gp-55, the T4 late sigma factor that is responsible for the degradation of the periplasmic peptidoglycan layer 5 min following infection (Kassavetis and Geiduschek 1984; Arisaka 2005). This timing is very close to that recorded for the onset of Rex (7–10 min), which potentially positions σE as a major regulator of Rex activity.
Assessing activity of host genes with natural rex gene dosage
We sought to confirm these effects with the natural gene dosage for T4rII exclusion. We expected that with wild-type rex expression levels, attenuation of Rex would be intensified in absence of key genes. Selected mutants were lysogenized by phage λ and assayed for Rex activity (Table 4). As expected, with reduced gene dosage, attenuation of Rex activity was generally exacerbated. Notable reductions in Rex activity were observed in the following groups of lysogenized knockouts (Table 4): omps, tolA, rpoS, and small RNAs. Deletion of ompL, ompF, ompA, ompX, and ompW improved T4rII plating up to 104-fold compared to the rex+ control. Almost complete abrogation of Rex activity was observed upon lysogenization of the ΔtolA mutant by λ where pinpoint plaques of T4rII were again observed. This was also exhibited in the ΔrpoS(λ) mutant, supporting our suspicion that σS acts in a Rex-dependent manner. Interestingly, almost complete attenuation was observed for λ lysogens of ΔrssA, ΔrssB, and ΔiraD. Together, these findings again suggest that there is a gene dosage-dependent interplay between Rex and small RNAs.
Mutagenesis and isolation of E. coli mutant(s) that influence Rex activity
We also employed a one-step mutagenesis plasmid system (pHA2; Figure 2) that transposes randomly into the E. coli genome while simultaneously expressing λ rexA-rexB. This would ensure expression of rexAB and simultaneously knock out nonessential host genes that could influence Rex. Candidates would be genotypically rex+, but phenotypically Rex− or attenuated for Rex activity. Transposition of the TcR marker into recipient cells (KmR) allowed for selection of successful transposition and integration of the Tn10 transposon carrying the cI857-rexA-rexB cassette. We generated Rex− integrants using conjugative transposition of the pHA2 plasmid into recipient cells by growing exconjugants directly on plates seeded with T4rII as part of the one-step selection for integrants while simultaneously screening for Rex−. We anticipated that Rex+ integrants able to exclude T4rII would be unaffected by the presence of phage and should form regular, circular colonies, while mutants compromised for Rex activity (i.e., able to propagate T4rII) would be characterized by irregular, “bitten” colonies (Gussin and Peterson 1972). Transposition frequency of the rex+ element under inducing conditions generally ranged from 10−3-10−7 of exconjugants (results not shown). The bitten:regular colonies ratio averaged ∼1:1500 in JW0427-1 recipient cells, which is expected considering the >3000 nonessential E. coli protein-encoding genes, most of which would not be expected to influence the Rex phenotype.
We were able to isolate 13 candidate mutants confirmed to be genotypically rex+, while also strongly attenuated or fully abrogated for Rex activity (Table 6). Although these mutants were confirmed to be genotypically rex+, the noted improvement in T4rII plating indicates insertional mutation of a host gene that is either directly or indirectly involved in Rex phenotype. In all cases, abrogated or attenuated Rex activity was not due to a faulty rex cassette, as each isolate was subsequently lysogenized by λ (rex+) to deliver yet another copy of the rex genes and then tested again for Rex activity (Table 6).
Table 6. Isolated integrant mutants attenuated for Rex activity.
| Strain | Wild-type level rex+ expressiona,b | Double rex+ expressionb,c |
|---|---|---|
| Controls | ||
| JW0427-1b | 1.0 | 1.0 |
| JW0427-1(λ)d | <1.0 × 10−7 | <1 × 10−7 |
| Isolates | ||
| JW-HA 1 | 2.3 × 10−5 | 4.0 × 10−6 |
| JW-HA 2 | 7.0 × 10−6 | 5.2 × 10−6 |
| JW-HA 3 | 1.5 × 10−5 | 1.7 × 10−5 |
| JW-HA 4 | 1.0 × 10−3 | 5.0 × 10−4 |
| JW-HA 5 | 1.7 × 10−5 | 5.0 × 10−6 |
| JW-HA 6 | 1.4 × 10−4 | 1.0 × 10−4 |
| JW-HA 9 | 5.0 × 10−6 | 4.0 × 10−6 |
| JW-HA 11 | 1.6 × 10−5 | 1.3 × 10−4 |
| JW-HA 12 | 1.3 × 10−5 | 1.6 × 10−4 |
| JW-HA 19 | 2.0 × 10−4 | 1.5 × 10−4 |
| JW-HA 20 | 8.0 × 10−6 | 6.8 × 10−6 |
| JW-HA 21 | 1.6 × 10−5 | 1.7 × 10−5 |
| JW-HA 25 | 1.8 × 10−5 | 4.0 × 10−6 |
Random insertion of the rexA-rexB transposable cassette into the chromosome renders insertional mutants genotypically rexA+rexB+ (rex+).
E. coli strain JW0427-1 (ΔclpP::kan) (rex−) used as negative control with relative T4rII EOP of 1.0. All values based on averaged EOP from three independent assays.
Transponants1 lysogenized with λ. Hosts carry two copies of rexA-rexB, one from transposon cassette and the second of λ lysogen.
E. coli strain JW0427-1(λ) (ΔclpP::kan) (rex+) used as positive control with relative T4rII EOP of 1.0.
Sequencing of candidate mutants (results not shown) indicated that JW-HA 11 was a 95% match to the nonessential gene mglC, a hydrophobic ABC transporter permease that, when mutated, rescues T4rII plating by >103-fold (Table 6). High expression of rex in a ΔmglC knockout corroborated this finding, demonstrating at least 102-fold improvement in T4rII plating (Table 3). MglC is part of a complex operon expressing Mgl-Gal proteins (Boos et al. 1971; Death et al. 1993; Death and Ferenci 1994). While mglC clearly influences Rex activity, coexpression of mglA may, in tandem, influence RII activity, since mutation of mglA reduces T4 (rII+) plating by >10-fold in a rex+ context (Table 3). Deletion of galK, T, or E reduced T4 (rII+) plating efficiency by up to two orders of magnitude, which may mean that the presence of this operon is needed to enable RII function. Gal proteins may also enhance RII activity in the presence of Rex proteins (Table 3). MglC is likely located on the internal face of the cytoplasmic membrane (Harayama et al. 1983) and is involved in the methyl-galactoside transport system (Hogg et al. 1991). Interestingly, it would therefore share this location with RexB. Furthermore, its internal orientation has the potential for interaction with the cytoplasmic RexA, suggesting much potential for MglC’s role in the activation or mechanism of Rex.
A proposed model for Rex and RII suppression of Rex
Based on the data derived in this work, we attempt to link membrane proteins, sigma factors, and their regulators to the activation, manifestation, and the exclusion mechanism itself (Figure 6). Overall, we propose that in the absence of RII proteins, Rex-mediated abrogation of T4 replication arises from the interplay between σS- and σE-governed regulons.
Figure 6.
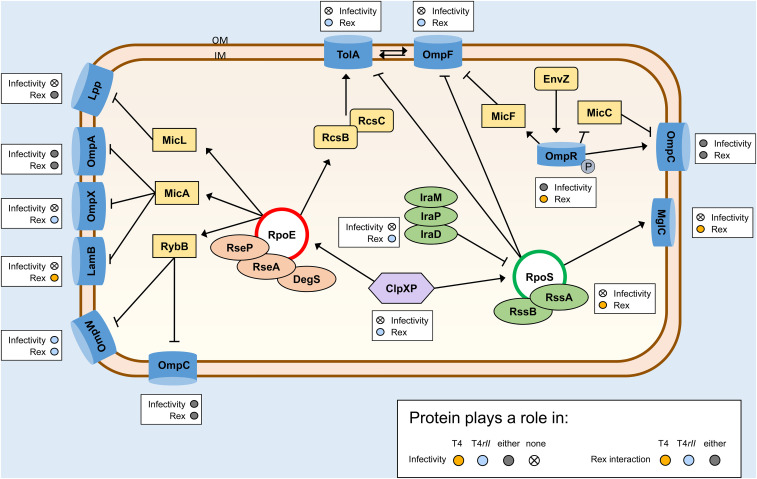
Model of T4 or T4rII infection in a rex+ cell. Upon infection, rpoE/rpoS pathways are activated, depending on the presence of RII. Rex activation may be trigged by TolA, resulting in a physiological cellular shunt into a stationary-like phase. Blue cylinders indicate membrane proteins; green ovals indicate small regulatory RNAs involved in rpoS expression; red ovals indicate regulatory RNAs involved in rpoE expression; yellow boxes indicate small RNAs involved in the expression of membrane proteins. Small circles next to a protein indicate, firstly, its involvement in the infectivity of T4 (yellow), T4rII (blue), both (gray), or neither (X); secondly, postulated interaction with RexA and/or RexB only in the presence of RII (T4) (yellow), only in the absence of RII (T4rII) (blue), or regardless of RII presence (either phage) (gray). Pointed arrowheads indicate stimulation or upregulation, while bars indicate inhibition or downregulation. IM, inner membrane; OM, outer membrane.
T4 infection activates host stress responses:
Upon infection, T4 first adsorbs to OmpC on the outer membrane and injects its DNA into the host cell, causing a disturbance in periplasmic and cytoplasmic osmolarity (Arisaka 2005) and activating membrane sensors such as OmpX and TolA. A σE-mediated stress response is triggered in response to envelope stress (Dam et al. 2018). Membrane and osmotic stress also triggers σS activation and induction of stationary phase genes (Hengge-Aronis et al. 1991, 1993). During growth phase, ClpXP normally outcompetes anti-adaptor small RNAs IraD, IraM, and IraP to bind RssB, minimizing active σS levels (Schweder et al. 1996; Battesti et al. 2015). During T4 infection, activation of σS regulons would instead increase the supply of IraD, IraM, and IraP to outcompete ClpXP, thereby elevating active σS levels (Bryan et al. 2016). Free ClpXP would then be available to elevate σE levels. Generally, in absence of Rex, T4 infection would lead to cell lysis within 15 min (Bryan et al. 2016). Not surprisingly, phage infection is a major stress stimulator; overall, the start of T4 infection is expected to activate stress response pathways, resulting in high expression levels of both σE and σS in absence of Rex.
As OmpA expression is directly linked to σE activation, depleted levels of OmpA in the cell may “prime” rex+ cells for exclusion. The powerful inhibition of T4 and T4rII plating in ΔompA mutants is heavily Rex-dependent as it was not seen in the rex− mutant (Table 2). This suggests that OmpA may be a target for inactivation by Rex protein(s) and that this inactivation may be competitively inhibited by RII proteins. Alternatively, RII suppression of Rex relies upon its interaction with OmpA. Either way, the incomplete abrogation of T4 plating in a ΔompA context suggests that ompA is not the only host gene involved.
Rex onset increases tol and rpoE expression, but reduces rpoS expression:
In a Rex+ environment, onset of Rex is postulated to start with membrane-bound RexB binding with multiple RexA proteins (Parma et al. 1992). This enables RexB-mediated efflux of cations, which would result in further membrane destabilization and depolarization. Activated stress response pathways should replenish the depleted pools of Omps (including OmpX, OmpW, OmpF, or Lpp) in reaction to T4 infection. Moreover, membrane instability also stimulates tol expression to maintain cell integrity. TolA would further stimulate σE activity, leading to increased expression of tol-pal, lamB, lpp, and ompA to restore membrane integrity. We found that only the tolA mutant was able to completely abrogate Rex activity alone (Figure 4A). Thus, we suspect TolA must serve as one of the key controllers that maintains Rex activation and exclusion. Because of its transmembrane position facilitating interaction with both RexA and RexB, we hypothesize that direct TolA interaction with Rex proteins activates formation of the RexB-RexA active complex.
Based on their shared membrane-bound location, RexB could interact with RssB in place of σS, freeing σS from the RssAB-ClpXP complex. This would again free ClpXP to elevate σE levels and expression of the membrane stress regulon. Cytoplasmic RexA could also bind IraD preventing its upregulation of σS. We believe that the activation of Rex would therefore be associated with high σE levels, but growth phase levels of σS.
Rex manifestation is a stationary-like phase:
After Rex-mediated efflux of cytoplasmic cations (K+ efflux), the cell must compensate by stimulating phosphatases and ompF to restore cell osmolarity. Given the observed modulation of Rex activity by both OmpA and OmpW, their regulator σE is expected to direct the harsh physiological cellular conditions associated with Rex via micA and/or rybB expression (Parma et al. 1992). Stress-induced upregulation of ompF would lead to heightened ompA expression and subsequent decreased ompC expression, which reduces T4 adsorption and superinfection. This could explain what has been previously observed upon activation of Rex: cells enter into a temporary growth arrest state, where they become resistant and protected against T4 superinfection (Slavcev and Hayes 2003). Lpp must play an important role in Rex exclusion, since, as we noted, disruption in Lpp expression greatly reduced Rex activity in T4rII (Figure 4A). We postulate that the membrane-bound RexAB complex could be maintained through Lpp, a structural membrane protein, whose own expression is maintained through σE.
This stationary-like phase resembles σE-dependent cell lysis during early stationary phase, where only about 10% of the cell population survives. Prolonged Rex activity eventually starves the cells of necessary nutrients and can eventually lead to cell lysis. From previous (Slavcev and Hayes 2002) and our own results, we see that Rex protects against T4 cell lysis for days, after which the harsh ionic environment could stop Rex, allowing T4 replication to resume and kill the host cell. Any interruption of the Rex system will also disrupt the delicately maintained balance between σE and σS, also leading to σE-mediated death. We postulate that as long as the balance between σE and σS is kept, some surviving Rex cells would eventually escape T4rII infection.
RII attenuates Rex exclusion:
RII localizes to the inner membrane of the host ∼10 min following superinfection (Takacs and Rosenbusch 1975; Mosig et al. 1984), where RexB is also localized (Parma et al. 1992). This coincides in timing with the interaction of small RNAs RssB and RssA with σS to prevent the binding of its anti-adaptors and σS degradation. As we found that both T4 and T4rII alike cannot plate on rex+ ΔrpoS mutants (Table 3), we hypothesize that RII interactions with σS are necessary for T4 infection. In the absence of RII, the combination of RexA and RexB activity may stabilize σS levels, thereby leading to the exclusion phenotype. Conversely if RII is present, it could bind σS to activate it in a similar fashion to IraD, leading to elevated σS pathways. As such, σS-RII interactions would be required to interrupt the onset of the Rex-mediated protective phase. Similarly, LamB, a σE-controlled porin localized in the outer membrane, was also implicated in Rex function in our results. RII may require both proteins to successfully inactivate Rex. As LamB is under the control of σE, it represents a vital link between RII, σE, σS, and Rex.
In conclusion, bacterial cells are highly capable of quickly perceiving and adapting to environmental changes and stresses to survive. The Rex exclusion mechanism is a powerful example of such a protective mechanism against superinfecting T4 mutants. We believe we have now assembled new pieces of the longstanding Rex puzzle. From our findings, we offer an updated model of the λ T4rII exclusion mechanism.
We believe that Rex exclusion arises from the balance of activated σE to σS pathways. Impairment to key players in either pathway attenuates the ability of Rex to exclude T4rII plating. A number of these players are membrane proteins, which is not that surprising given the plethora of structurally observable characteristics associated with the onset of the Rex phenotype. Disruptions in cell membrane permeability have been previously shown to render cells more sensitive to superinfecting T4. Understanding the individual functions of these factors and determining their precise interactions may help to finally unravel the Rex puzzle. Understanding Rex can ultimately provide important clues and strategies for use toward eukaryotic exclusion systems and control mechanisms.
Acknowledgments
This work was supported by the National Sciences and Engineering Research Council of Canada (grant 391457 awarded to RAS); the Saudi Arabian Cultural Bureau in Canada; and the School of Pharmacy, Taibah University, Saudi Arabia. The authors have no conflicts of interest to report.
Footnotes
Communicating editor: M. Rose
Literature Cited
- Alexeyev M. F., and Shokolenko I. N., 1995. Mini-Tn10 transposon derivatives for insertion mutagenesis and gene delivery into the chromosome of Gram-negative bacteria. Gene 160: 59–62. 10.1016/0378-1119(95)00141-R [DOI] [PubMed] [Google Scholar]
- Apirakaramwong A., Fukuchi J., Kashiwagi K., Kakinuma Y., Ito E. et al. , 1998. Enhancement of cell death due to decrease in Mg2+uptake by OmpC (cation-selective porin) deficiency in ribosome modulation factor-deficient mutant. Biochem. Biophys. Res. Commun. 251: 482–487. 10.1006/bbrc.1998.9494 [DOI] [PubMed] [Google Scholar]
- Arisaka F., 2005. Assembly and infection process of bacteriophage T4. Chaos 15: 047502 10.1063/1.2142136 [DOI] [PubMed] [Google Scholar]
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., et al. , 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battesti A., Majdalani N., and Gottesman S., 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65: 189–213. 10.1146/annurev-micro-090110-102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battesti A., Majdalani N., and Gottesman S., 2015. Stress sigma factor RpoS degradation and translation are sensitive to the state of central metabolism. Proc. Natl. Acad. Sci. USA 112: 5159–5164. 10.1073/pnas.1504639112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S., 1955. Fine structure of a genetic region in bacteriophage. Proc. Natl. Acad. Sci. USA 41: 344–354. 10.1073/pnas.41.6.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadac A., Gavioli M., Lazzaroni J. C., Raina S., and Lloubès R., 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180: 4872–4878. 10.1128/JB.180.18.4872-4878.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M. L., 1965. The effects of polyamines on the replication of T4rII mutants in Escherichia coli K12 (λ). Virology 26: 221–227. 10.1016/0042-6822(65)90049-8 [DOI] [PubMed] [Google Scholar]
- Bryan D., El-Shibiny A., Hobbs Z., Porter J., and Kutter E. M., 2016. Bacteriophage T4 infection of stationary phase E. coli: life after log from a phage perspective. Front. Microbiol. 7: 1391 10.3389/fmicb.2016.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., and Unsöld H J, 1971. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur. J. Biochem. 19: 457–470. 10.1111/j.1432-1033.1971.tb01336.x [DOI] [PubMed] [Google Scholar]
- Cascales E., Gavioli M., Sturgis J. N., and Lloubes R., 2000. Proton motive force drives the interaction of the inner membrane TolA and outer membrane Pal proteins in Escherichia coli. Mol. Microbiol. 38: 904–915. 10.1046/j.1365-2958.2000.02190.x [DOI] [PubMed] [Google Scholar]
- Cascales E., Bernadac A., Gavioli M., Lazzaroni J.-C., and Lloubes R., 2002. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol. 184: 754–759. 10.1128/JB.184.3.754-759.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang A., Blyn L. B., and Storz G., 2004. MicC, a second small-RNA Regulator of omp protein expression in Escherichia coli. J. Bacteriol. 186: 6689–6697. 10.1128/JB.186.20.6689-6697.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R. et al. , 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358: 727–733. 10.1038/358727a0 [DOI] [PubMed] [Google Scholar]
- Dam S., Pagès J.-M., and Masi M., 2018. Stress responses, outer membrane permeability control and antimicrobial resistance in Enterobacteriaceae. Microbiology 164: 260–267. 10.1099/mic.0.000613 [DOI] [PubMed] [Google Scholar]
- Death A., and Ferenci T., 1994. Between feast and famine: endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J. Bacteriol. 176: 5101–5107. 10.1128/JB.176.16.5101-5107.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Death A., Notley L., and Ferenci T., 1993. Derepression of LamB protein facilitates outer membrane permeation of carbohydrates into Escherichia coli under conditions of nutrient stress. J. Bacteriol. 175: 1475–1483. 10.1128/JB.175.5.1475-1483.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche R., Gavioli M., Bénédetti H., Prilipov A., Lazdunski C. et al. , 1996. TolA central domain interacts with Escherichia coli porins. EMBO J. 15: 6408–6415. 10.1002/j.1460-2075.1996.tb01032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feklístov A., Sharon B. D., Darst S. A., and Gross C. A., 2014. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu. Rev. Microbiol. 68: 357–376. 10.1146/annurev-micro-092412-155737 [DOI] [PubMed] [Google Scholar]
- FerroLuzzi-Ames G., and Ames B. N., 1965. The multiplication of T4rII phage in E. coli K12(λ) in the presence of polyamines. Biochem. Biophys. Res. Commun. 18: 639–647. 10.1016/0006-291X(65)90834-X [DOI] [Google Scholar]
- Garen A., 1961. Physiological effects of rII mutations in bacteriophage T4. Virology 14: 151–163. 10.1016/0042-6822(61)90190-8 [DOI] [PubMed] [Google Scholar]
- Gi Mikawa Y., Maruyama I. N., and Brenner S., 1996. Surface display of proteins on bacteriophage λ heads. J. Mol. Biol. 262: 21–30. 10.1006/jmbi.1996.0495 [DOI] [PubMed] [Google Scholar]
- Gibbs K. A., Isaac D. D., Xu J., Hendrix R. W., Silhavy T. J. et al. , 2004. Complex spatial distribution and dynamics of an abundant Escherichia coli outer membrane protein. Mol. Microbiol. 53: 1771–1783. 10.1111/j.1365-2958.2004.04242.x [DOI] [PubMed] [Google Scholar]
- Guillier M., 2006. Modulating the outer membrane with small RNAs. Genes Dev. 20: 2338–2348. 10.1101/gad.1457506 [DOI] [PubMed] [Google Scholar]
- Gussin G. N., and Peterson V., 1972. Isolation and properties of rex- mutants of bacteriophage lambda. J. Virol. 10: 760–765. 10.1128/JVI.10.4.760-765.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Bollinger J., Iino T., and Hazelbauer G. L., 1983. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J. Bacteriol. 153: 408–415. 10.1128/JB.153.1.408-415.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., and Slavcev R. A., 2005. Polarity within pM and pE promoted phage lambda cI-rexA-rexB transcription and its suppression. Can. J. Microbiol. 51: 37–49. 10.1139/w04-115 [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R., Klein W., Lange R., Rimmele M., and Boos W., 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173: 7918–7924. 10.1128/JB.173.24.7918-7924.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R., Lange R., Henneberg N., and Fischer D., 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175: 259–265. 10.1128/JB.175.1.259-265.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Voelker C., and Von Carlowitz I., 1991. Nucleotide sequence and analysis of the mgl operon of Escherichia coli K12. Mol. Gen. Genet. 229: 453–459. 10.1007/BF00267469 [DOI] [PubMed] [Google Scholar]
- Hong H., Patel D. R., Tamm L. K., and van den Berg B., 2006. The outer membrane protein OmpW forms an eight-stranded β-barrel with a hydrophobic channel. J. Biol. Chem. 281: 7568–7577. 10.1074/jbc.M512365200 [DOI] [PubMed] [Google Scholar]
- Kabir M. S., Yamashita D., Koyama S., Oshima T., Kurokawa K. et al. , 2005. Cell lysis directed by σE in early stationary phase and effect of induction of the rpoE gene on global gene expression in Escherichia coli. Microbiology 151: 2721–2735. 10.1099/mic.0.28004-0 [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., and Geiduschek E. P., 1984. Defining a bacteriophage T4 late promoter: bacteriophage T4 gene 55 protein suffices for directing late promoter recognition. Proc. Natl. Acad. Sci. USA 81: 5101–5105. 10.1073/pnas.81.16.5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka, T., M. Murata, and M. Yamada, 2017 Survival strategy of Escherichia coli in stationary phase: involvement of σe-dependent programmed cell death, pp. 383–404 in Escherichia coli - Recent Advances on Physiology, Pathogenesis and Biotechnological Applications, edited by A. Samie. InTech Open, London. [Google Scholar]
- Kvitko B. H., Bruckbauer S., Prucha J., McMillan I., Breland E. J. et al. , 2012. A simple method for construction of pir+ enterobacterial hosts for maintenance of R6K replicon plasmids. BMC Res. Notes 5: 157 10.1186/1756-0500-5-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni J. C., Fognini-Lefebvre N., and Portalier R. C., 1986. Effects of lkyB mutations on the expression of ompF, ompC and lamB porin structural genes in Escherichia coli K-12. FEMS Microbiol. Lett. 33: 235–239. 10.1111/j.1574-6968.1986.tb01278.x [DOI] [Google Scholar]
- Lazzaroni J. C., Germon P., Ray M.-C., and Vianney A., 1999. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol. Lett. 177: 191–197. 10.1111/j.1574-6968.1999.tb13731.x [DOI] [PubMed] [Google Scholar]
- Lazzaroni J.-C., Dubuisson J.-F., and Vianney A., 2002. The Tol proteins of Escherichia coli and their involvement in the translocation of group A colicins. Biochimie 84: 391–397. 10.1016/S0300-9084(02)01419-0 [DOI] [PubMed] [Google Scholar]
- Levengood-Freyermuth S. K., Click E. M., and Webster R. E., 1993. Role of the carboxyl-terminal domain of TolA in protein import and integrity of the outer membrane. J. Bacteriol. 175: 222–228. 10.1128/JB.175.1.222-228.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S., Guo M. S., Chaba R., Gross C. A., and Sauer R. T., 2013. Dual molecular signals mediate the bacterial response to outer-membrane stress. Science 340: 837–841. 10.1126/science.1235358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloubès R., Cascales E., Walburger A., Bouveret E., Lazdunski C. et al. , 2001. The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res. Microbiol. 152: 523–529. 10.1016/S0923-2508(01)01226-8 [DOI] [PubMed] [Google Scholar]
- Matsumoto-Mashimo C., Guerout A. M., and Mazel D., 2004. A new family of conditional replicating plasmids and their cognate Escherichia coli host strains. Res. Microbiol. 155: 455–461. 10.1016/j.resmic.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Mecsas J., Rouviere P. E., Erickson J. W., Donohue T. J., and Gross C. A., 1993. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev. 7: 2618–2628. 10.1101/gad.7.12b.2618 [DOI] [PubMed] [Google Scholar]
- Meury J., and Devilliers G., 1999. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol. Cell 91: 67–75. 10.1111/j.1768-322X.1999.tb01085.x [DOI] [PubMed] [Google Scholar]
- Mizuno T., and Mizushima S., 1990. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol. Microbiol. 4: 1077–1082. 10.1111/j.1365-2958.1990.tb00681.x [DOI] [PubMed] [Google Scholar]
- Mosig G., Shaw M., and Garcia G. M., 1984. On the role of DNA replication, endonuclease VII, and rII proteins in processing of recombinational intermediates in phage T4. Cold Spring Harb. Symp. Quant. Biol. 49: 371–382. 10.1101/SQB.1984.049.01.044 [DOI] [PubMed] [Google Scholar]
- Murata M., Noor R., Nagamitsu H., Tanaka S., and Yamada M., 2012. Novel pathway directed by σE to cause cell lysis in Escherichia coli. Genes Cells 17: 234–247. 10.1111/j.1365-2443.2012.01585.x [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Shafiq O., and Meiners V., 1998. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain construction. J. Bacteriol. 180: 6408–6411. 10.1128/JB.180.23.6408-6411.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto K., and Hermansson M., 2004. Inactivation of ompX causes increased interactions of type 1 fimbriated Escherichia coli with abiotic surfaces. J. Bacteriol. 186: 226–234. 10.1128/JB.186.1.226-234.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y., and Mizushima S., 1983. Regulation of outer membrane porin protein synthesis in Escherichia coli K-12: ompF regulates the expression of ompC. J. Bacteriol. 154: 669–675. 10.1128/JB.154.2.669-675.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H., Snyder M., Sobolevski S., Nawroz M., Brody E. et al. , 1992. The Rex system of bacteriophage lambda: tolerance and altruistic cell death. Genes Dev. 6: 497–510. 10.1101/gad.6.3.497 [DOI] [PubMed] [Google Scholar]
- Patten C. L., Kirchhof M. G., Schertzberg M. R., Morton R. A., and Schellhorn H. E., 2004. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol. Genet. Genomics 272: 580–591. 10.1007/s00438-004-1089-2 [DOI] [PubMed] [Google Scholar]
- Peters J. E., and Craig N. L., 2000. Tn7 transposes proximal to DNA double-strand breaks and into regions where chromosomal DNA replication terminates. Mol. Cell 6: 573–582. 10.1016/S1097-2765(00)00056-3 [DOI] [PubMed] [Google Scholar]
- Pratt L. A., and Silhavy T. J., 1996. The response regulator SprE controls the stability of RpoS. Proc. Natl. Acad. Sci. USA 93: 2488–2492. 10.1073/pnas.93.6.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D., Alawneh A. M., Yonesaki T., and Otsuka Y., 2015. Rapid degradation of host mRNAs by stimulation of RNase E activity by Srd of bacteriophage T4. Genetics 201: 977–987. 10.1534/genetics.115.180364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., and Schwartz M., 1973. Isolation of the bacteriophage lambda receptor from Escherichia coli. J. Bacteriol. 116: 1436–1446. 10.1128/JB.116.3.1436-1446.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B. G., and Campbell J. H., 1977. Genetic and physiological control of host cell lysis by bacteriophage lambda. J. Virol. 23: 626–636. 10.1128/JVI.23.3.626-636.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardesai A. A., 2003. The OmpL porin does not modulate redox potential in the periplasmic space of Escherichia coli. EMBO J. 22: 1461–1466. 10.1093/emboj/cdg152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., and McDonald G. A., 1984. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of ompF protein. J. Bacteriol. 159: 555–563. 10.1128/JB.159.2.555-563.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweder T., Lee K. H., Lomovskaya O., and Matin A., 1996. Regulation of Escherichia coli starvation sigma factor (sigma s) by ClpXP protease. J. Bacteriol. 178: 470–476. 10.1128/JB.178.2.470-476.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., 1966. Studies on the physiological defect in rII mutants of bacteriophage T4. J. Mol. Biol. 16: 503–522. 10.1016/S0022-2836(66)80188-2 [DOI] [PubMed] [Google Scholar]
- Shinedling S., Parma D., and Gold L., 1987. Wild-type bacteriophage T4 is restricted by the lambda rex genes. J. Virol. 61: 3790–3794. 10.1128/JVI.61.12.3790-3794.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Parks A. R., Potter B. D., Safir I. J., Luo Y. et al. , 2008. DNA damage differentially activates regional chromosomal loci for Tn7 transposition in Escherichia coli. Genetics 179: 1237–1250. 10.1534/genetics.108.088161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavcev R. A., and Hayes S., 2002. Rex-centric mutualism. J. Bacteriol. 184: 857–858. 10.1128/JB.184.3.857-858.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavcev R. A., and Hayes S., 2003. Stationary phase-like properties of the bacteriophage λ Rex exclusion phenotype. Mol. Genet. Genomics 269: 40–48. 10.1007/s00438-002-0787-x [DOI] [PubMed] [Google Scholar]
- Snyder L., and McWilliams K., 1989. The rex genes of bacteriophage lambda can inhibit cell function without phage superinfection. Gene 81: 17–24. 10.1016/0378-1119(89)90332-6 [DOI] [PubMed] [Google Scholar]
- Srividhya K. V., and Krishnaswamy S., 2004. A simulation model of Escherichia coli osmoregulatory switch using E-CELL system. BMC Microbiol. 4: 44 10.1186/1471-2180-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpe S., Schmid R., Stephens D. L., Georgiou G., and Bakker E. P., 1998. Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli. J. Bacteriol. 180: 4002–4006. 10.1128/JB.180.15.4002-4006.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wang B., Zhu L., Chen M., and Zhan L., 2013. Block and boost DNA transfer: opposite roles of OmpA in natural and artificial transformation of Escherichia coli. PLoS One 8: e59019 10.1371/journal.pone.0059019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs B. J., and Rosenbusch J. P., 1975. Modification of Escherichia coli membranes in the prereplicative phase of phage T4 infection. Specificity of association and quantitation of bound phage proteins. J. Biol. Chem. 250: 2339–2350. [PubMed] [Google Scholar]
- Valentin-Hansen P., Johansen J., and Rasmussen A. A., 2007. Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 10: 152–155. 10.1016/j.mib.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Vogt J., and Schulz G. E., 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 7: 1301–1309. 10.1016/S0969-2126(00)80063-5 [DOI] [PubMed] [Google Scholar]
- Wang Y., 2002. The function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 292: 396–401. 10.1006/bbrc.2002.6657 [DOI] [PubMed] [Google Scholar]
- Wang A., and Lin K., 2001. Effects of N-acetylglucosamine and α-methylglucoside on bacteriophage T4 adsorption to Escherichia coli B23. J. Exp. Microbiol. Immunol. 1: 54–63. [Google Scholar]
- Washizaki A., Yonesaki T., and Otsuka Y., 2016. Characterization of the interactions between Escherichia coli receptors, LPS and OmpC, and bacteriophage T4 long tail fibers. MicrobiologyOpen 5: 1003–1015. 10.1002/mbo3.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Polen T., Heuveling J., Wendisch V. F., and Hengge R., 2005. Genome-wide analysis of the general stress response network in Escherichia coli: S-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187: 1591–1603. 10.1128/JB.187.5.1591-1603.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.-B., Tian L.-H., Zou H.-J., Wang C.-Y., Yu Z.-Q., et al. , 2013. Outer membrane protein OmpW of Escherichia coli is required for resistance to phagocytosis. Res. Microbiol. 164: 848–855. 10.1016/j.resmic.2013.06.008 [DOI] [PubMed] [Google Scholar]
- Yu F., and Mizushima S., 1982. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J. Bacteriol. 151: 718–722. 10.1128/JB.151.2.718-722.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.