ABSTRACT
Effective prophylactic and therapeutic interventions are urgently needed to address the coronavirus disease 2019 (COVID‐19) pandemic. Various antiviral drugs have recently been tested. Type I interferon (IFN) is a regulatory protein involved in the innate immune response, with broad‐spectrum antiviral activities and the ability to directly block viral replication and support the immune response to eliminate virus infection. Insufficient virus‐induced type I IFN production is characteristic of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, because SARS‐CoV‐2 suppresses the IFN response by interacting with essential IFN signaling pathways. Exogenous type I IFN is recommended for treating COVID‐19. Unexpectedly however, angiotensin converting enzyme‐2 (ACE2) receptor, which acts as a SARS‐CoV‐2 receptor, was shown to be stimulated by IFN, raising doubts about the suitability of IFN use. However, further studies have excluded concerns regarding IFN administration. Type I IFNs, including IFN‐α1b, have been used clinically as antiviral drugs for many years and have shown strong antiviral activity against SARS‐CoV‐2 in vitro. Preliminary clinical studies of type I IFNs, especially when delivered via aerosol inhalation, have demonstrated efficacy for the treatment and prevention of COVID‐19. Randomized controlled trials of IFN for COVID‐19 treatment are ongoing.
Keywords: Interferon, COVID‐19, SARS‐CoV‐2, ACE2, Treatment
This review summarizes SARS‐CoV‐2 antagonize the innate immune response key component type I interferon and the efficacy and safety of type I interferon clinical studies on treatment and prevention of COVID‐19.
1. Introduction
The novel coronavirus disease 2019 (COVID‐19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) poses a serious threat to human populations globally. According to the World Health Organization (WHO), more than 42 million people worldwide had been confirmed with SARS‐CoV‐2 infection up to 24 October 2020, and more than 1 million people had died of COVID‐19. 1 Numerous clinical trials are currently ongoing to explore effective antiviral drugs for the treatment of COVID‐19. Type I interferons (IFN‐I), as a key component of innate antiviral response, have been tested for the treatment and prevention of COVID‐19.
2. IFN‐I is a key component of the innateresponse
The clinical manifestations and pathology of COVID‐19 are similar to those of SARS, but the severities of the diseases differ. 2 Unlike severe SARS infection, SARS‐CoV‐2 infection shows a wide range of clinical features, ranging from asymptomatic, mild, and moderate to severe and critical. The clinical manifestations of virus infection largely depend on virus–host interactions. Asymptomatic outcomes of SARS‐CoV‐2 infection might thus be attributed to strong host innate antiviral defense, resulting in significantly faster virus turnover than in symptomatic patients. The innate IFN‐I induction and response provide the first line of defense against viral infection. 3 IFN‐I includes IFN‐α, IFN‐β, and other IFN subtypes. IFN‐α/β expression can be induced by virus binding to cell surface receptors following recognition by pattern recognition receptors, including the retinoic acid‐inducible gene 1 (RIG‐1)/mitochondrial antiviral signaling (MAVS)/TANK‐binding kinase 1 (TBK1)/interferon regulatory factor 3 (IRF3), Toll‐like receptor 3/TBK1/IRF3, and cyclic GMP‐AMP synthase/stimulator of interferon genes/TBK1/IRF3 signaling pathways, IRF3 phosphorylation, and nuclear translocation. 3 , 4 IFN‐α/β then initiates activation of the Janus kinase/signal transducer and activator of transcription pathway resulting in the expression of IFN‐stimulated genes (ISGs) to accomplish their antiviral function. An abnormal innate IFN response state has been shown in SARS‐CoV‐2‐infected patients, resulting in a different degree of suppression compared with SARS. 5
3. Insufficient IFN‐I response is characteristic of SARS‐CoV‐2 infection
A study using human lung tissue explants ex vivo found that SARS‐CoV‐2 showed more efficient in infection and replication compared with SARS‐CoV. 6 SARS‐CoV‐2 RNA levels peaked within 5 days after onset, at levels 1000 times higher than the peak of SARS‐CoV RNA at 7–10 days after onset. 7 The main reason for this may be IFN production is blocked and is insufficient to repress SARS‐CoV‐2 replication, as indicated by inefficient and delayed IFN‐I responses in SARS‐CoV‐2‐infected cells, as well as in COVID‐19 patients. 8 Analysis of virus dynamics showed that SARS‐CoV‐2 virus replication in the oropharynx/nasopharynx peaked in most patients, but then gradually decreased after the symptoms appeared, with the peak of virus replication thus occurring before the upper respiratory tract symptoms (i.e. during the asymptomatic infection stage). 8 Sub‐optimal activation of the innate immune response, especially reduced IFN‐I induction, would allow SARS‐CoV‐2 to replicate actively to high levels before the onset of clinical symptoms. IFN has thus been recommended to prevent SARS‐CoV‐2 infection in susceptible individuals and to treat patients in the early stage of infection. Given that the viral load in respiratory secretions from COVID‐19 patients peaked early at the time of symptom onset, the innate immune response profiles characterized by a delayed and depressed IFN response provide the pharmaceutical basis for the use of IFN for COVID‐19 treatment.
A recent study showed that the severity of COVID‐19 disease was associated with restrained innate IFN‐I levels, as well as reduced ISG expression. 9 Plasma IFN‐α2 levels in critical COVID‐19 patients were remarkably lower than in patients with mild‐to‐moderate disease, and IFN‐β was undetectable in all patients with mild to critical disease. This suggests that reduced IFN‐I responses to SARS‐CoV‐2 infection might be related to COVID‐19 pathogenicity and progression. Blocking of IFN‐α production by SARS‐CoV‐2 might be one reason why COVID‐19 patients progress to a severe or critical state. Another clinical study showed that the innate IFN response was repressed, presenting as a sustained IFN‐I absence, in about 20% of critically ill COVID‐19 patients, and patients without IFN‐α production had a poorer prognosis. 9 However, ISG levels were increased after IFN‐α stimulation, indicating that IFN downstream signaling pathways were not impaired in COVID‐19 patients. 10 Exogenous IFN could thus supplement the virus‐inhibited low IFN‐I levels and normalize the innate IFN response in COVID‐19 patients.
4. Molecular mechanism of SARS‐CoV‐2 antagonized the IFN‐I response
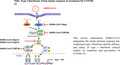
Viruses have evolved different molecular mechanisms to overcome virus‐induced IFN‐I expression and signaling, allowing them to survive the innate immune response. Viruses may encode proteins that target intermediary protein kinases involved in IFN‐I inducible antiviral responses. SARS‐CoV‐2 and SARS‐CoV are both β‐coronaviruses with 79%–82% nucleotide sequence homology. 11 , 12 The SARS‐CoV‐2 genome comprises 12 putative functional open reading frames (ORF) and 16 putative non‐structural proteins (Nsp), with no notable differences from SARS‐CoV. 12 The mechanism by which SARS‐CoV‐2 inhibits IFN‐I to interfere with the innate immune response is mostly preserved (Figure 1). Evidence suggests that SARS‐CoV‐2 uses several conserved virulent genes to antagonize the IFN response, including ORF9b, Nsp13 (helicase), ORF3b, and Nsp1. 13 , 14 , 15 SARS‐CoV‐2 ORF9b associates with Tom70, indirectly suppressing the IFN signaling adaptor MAVS. 13 Nsp13 blocks IFN‐induced expression by interacting with the IFN signaling intermediate TBK1. Nsp15 represses IFN expression through interacting with TBK1 and the IRF3 activator ring finger protein 41/neuregulin receptor degradation protein 1. 13 SARS‐CoV‐2 ORF3b may block the final step of the IFN‐induction signaling pathway, IRF3 phosphorylation and nuclear translocation. SARS‐CoV‐2 ORF3b encodes a novel short protein, showing more potent suppression of IFN induction than SARS‐CoV ORF3b, as shown by ORF3b isolated from two severe COVID‐19 cases. 12 , 14 Nsp1 effectively blocks the RIG‐1‐dependent IFN‐inducing pathway and innate IFN antiviral responses via associating with ribosomes to inhibit translation of RIG‐1 and ISGs. 15 Further studies are needed to compare the IFN‐antagonizing and ‐stimulation functions of other SARS‐CoV‐2 ORF proteins with the functions of their orthologs in SARS‐CoV. 2 , 12 SARS‐CoV‐2 may be predicted to harbor other molecular mechanisms to interfere with IFN induction and signaling and with antiviral ISGs.
FIGURE 1.

SARS‐CoV‐2 encodes multiple different proteins that target different intermediary protein kinases of the IFN‐I‐inducible antiviral response. MDA5, melanoma differentiation factor 5; RIG‐1, retinoic acid‐inducible gene 1; MAVS, mitochondrial antiviral signaling; TBK1, TANK‐binding kinase 1, IKKε, IκB kinase ε; IRF, interferon regulatory factor; IFN, interferon.
5. Potential of IFN‐I to inhibit SARS‐CoV‐2
IFN‐I is a cytokine with a pivotal role in inducing an antiviral response to a wide range of viruses. 3 A recent preclinical study demonstrated that SARS‐CoV‐2 was highly sensitive to IFN‐I treatment in cultured cells. 16 IFN‐α significantly reduced the SARS‐CoV‐2 virus titer in Vero cells at a concentration of 50 IU/mL, with EC50 values for IFN‐α and IFN‐β in Vero cells infected with SARS‐CoV‐2 of 1.35 IU/mL and 0.76 IU/mL, respectively. SARS‐CoV‐2 is thus more sensitive to human IFN‐I than many other human pathogenic viruses, including SARS‐CoV. 16
Recombinant human IFN‐α1b demonstrated even more promising antiviral effects against SARS‐CoV‐2 in two separate studies using Vero cells. One study showed that recombinant human IFN‐α1b effectively inhibited SARS‐CoV‐2 replication in vitro, with an EC50 of 0.059 U/mL and therapeutic index (TI) >1 694 915. 17 Comparative assessment of its anti‐viral activity and cytotoxicity showed that IFN‐α1b was more effective and safer against SARS‐CoV‐2 in vitro than remdesivir and ganciclovir. 17 Another study demonstrated that IFN‐α1b had a stronger antiviral effect (>5 times higher; MIC <0.001 ng/mL) than two other IFN drugs IFN‐α2b and novaferon (unpublished data). Meanwhile, IFN‐α1b also demonstrated good safety (TI >3125; unpublished data). The demonstrated ability of IFN‐α1b to inhibit SARS‐CoV‐2 may thus inform the IFN antiviral treatment strategy.
6. Concern of recombinant IFN for anti‐SARS‐CoV‐2 treatment
Angiotensin converting enzyme 2 (ACE‐2) has been recognized as a SARS‐CoV receptor, 18 and also acts as a receptor for SARS‐CoV‐2 viruses. 19 Binding of the S1 domain of the SARS coronavirus spike protein to ACE‐2 initiates viral entry into the host cell. 20 , 21 , 22 This interaction between SARS‐CoV‐2 and ACE‐2 has recently become an area of intense interest in terms of developing treatments against COVID‐19. 23
Unexpectedly, ACE2 was also recognized as an ISG, 24 raising doubts about the suitability of IFN treatment in SARS‐CoV‐2‐infected patients, because of the possibility that the IFN‐stimulated increase in ACE2 levels might increase the entry of SARS‐CoV‐2. High expression of ACE2 might thus be a double‐edged sword. This is also relevant in relation to the treatment of COVID‐19 patients with hypertension, given that ACE inhibitors (ACEI) and angiotensin II receptor blockers (ARB) are widely used as antihypertensive agents and increase the expression of ACE2. However, a clinical study showed that the unadjusted COVID‐19 mortality rate was significantly lower in patients receiving ACEI/ARBs compared with those without ACEI/ARBs (3.7% vs. 9.8%), 25 suggesting that ACE2 generally has a positive role against SARS‐CoV‐2. At a molecular level, ACE2 is necessary but not sufficient for SARS‐CoV‐2 entry, and other co‐receptors or enzymes, such as transmembrane serine protease 2 and furin, may be also needed for efficient SARS‐CoV‐2 binding and entry. 19 , 26 Cholesterol has also been reportedly related to SARS‐CoV‐2 entry, and SARS‐CoV‐2 cannot attach to the ACE2 receptor on the membrane in the absence of cholesterol, even at sub‐saturating levels of SARS‐CoV‐2. 27 Notably, ACE2 protein expression levels were increased in young children compared with adults in that study, but the symptoms of children infected with SARS‐CoV‐2 were generally milder than those in adults, providing indirect evidence to support the use of IFN‐I treatment for COVID‐19.
Interestingly, IFN‐β and IFN‐γ increase ACE 2 expressi on, 28 , 29 whil e IFN‐α doe s not stimulate the expression of ACE2, 24 suggesting that different IFN subtypes play different roles against certain viruses. Furthermore, the antiviral actions of different IFNs against SARS‐CoV‐2 might counterbalance any proviral effects of ACE2 induction and thus restrict the virus. 30
7. Clinical studies of IFN‐I for COVID‐19
An open‐label, randomized, phase II trial showed that COVID‐19 patients treated with triple combination therapy (IFN‐β1b, lopinavir/ritonavir, and ribavirin) had a shorter median time from the start of treatment to negative virus detection (−5 days), shorter time to symptom alleviation (−4 days), and shorter hospital stay (−5.5 days) compared with control patients. 31 Meanwhile, there was no difference in common adverse events between the combination treatment and control groups. Subgroup comparison suggested that IFN‐β1b played a key role in the combination treatment, although there were fewer patients in the IFN‐β1b subgroup. Significant differences in outcomes suggest that IFN might be an effective therapeutic agent for SARS‐CoV‐2. 31 The efficacy of IFN‐I against COVID‐19 was further supported in a case study. 32
8. Nebulized IFN treatment for COVID‐19
IFN‐I has been widely prescribed for various virus infections, including new virus pathogens. Clinical evidence of the antiviral effects of IFN treatment can usually be extrapolated to apply to phylogenetically closely related viruses. A randomized, double‐blind, placebo‐controlled, multicenter clinical study of aerosolized IFN‐α1b inhalation in patients with adult viral pneumonia, including some patients with coronavirus, showed that the clinical symptoms of expectoration, lung rales, and respiratory rate were significantly improved, especially on days 5–7 after treatment, and the clinical symptoms improved significantly from 66% to 77% on the 7th day. 33 This study supports the option for the emergency use of IFN‐α to treat the COVID‐19 pandemic. IFN‐α aerosol inhalation was recommended as the first antiviral treatment of COVID‐19 in the “Diagnosis and treatment protocol for COVID‐19 patients” released by the National Health Commission of the People’s Republic of China, and in the recommendations for the treatment of children with COVID‐19. 34 Nebulized IFN‐α2b, with or without Arbidol treatment, significantly reduced the duration of detectable SARS‐CoV‐2 virus in the upper respiratory tract and the duration of elevated blood levels of the inflammatory markers interleukin‐6 and C‐reactive protein. 35 Nebulized IFN‐α2b treatment could quickly reduce SARS‐CoV‐2 carriage. 36 Liu et al 37 reported that nebulized IFN‐α2b, combined with low‐dose systemic corticosteroids and lopinavir/ritonavir, contributed to the zero mortality rate in COVID‐19 patients. Nebulized IFN was also shown to decrease mortality in COVID‐19 patients in other studies. 38 , 39 Fu et al 40 showed that nebulized IFN‐k plus trefoil factor 2 (TFF2) was associated with clinical improvement in COVID‐19 patients and their consequent early discharge from hospital. A study involving about 100 COVID‐19 patients (NCT04385095) reported that nebulized IFN‐β1a might be highly effective, with a 79% lower risk of developing severe disease.
9. IFN‐α for prevention of COVID‐19
A study of the distribution and infection pattern of SARS‐CoV‐2 infection showed that it started in nasal epithelial cells, 41 suggesting that IFN should be administered as a spray or drops delivered to the nose or throat. A prospective open‐label study to investigate the efficacy and safety of IFN‐α1b nasal drops for COVID‐19 prevention found that 2944 medical staff members were all protected from SARS‐CoV‐2 infection during a 28‐day period of using nasal IFN‐α1b, while 3062 medical staff in the same area without IFN‐α1b preventive medication were infected with COVID‐19. 42 IFN‐α1b nasal drops can effectively protect medical personnel at risk of SARS‐CoV‐2 infection, thus demonstrating potential for their preventive use in susceptible healthy people. 42
10. Outlook
An impaired innate immune response characterized by insufficient IFN‐I induction is key to SARS‐CoV‐2 infection. Exogenous IFN‐I has been recommended as a candidate treatment for COVID‐19. Clinical studies of IFN‐I, either alone or combined with other drugs, have shown positive results in controlling the COVID‐19 pandemic. 43 Nebulized inhalation of recombinant human IFN‐α1b showed confirmed antiviral effects and good safety, especially in children. 44 Registered clinical studies of recombinant human IFN‐α1b for the prevention and treatment of COVID‐19 are ongoing and promising. A clinical study examining the effects of type III IFN against SARS‐CoV‐2 has also been registered. International cooperation among different clinical IFN trials has initiated the repurposing of the classical antiviral drug IFN for the COVID‐19 pandemic.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Lin F, Shen K. Type I interferon: From innate response to treatment for COVID‐19. Pediatr Invest. 2020;4:275‐280. 10.1002/ped4.12226
REFERENCES
- 1. WHO coronavirus disease (COVID‐19) dashboard. Geneva: World Health Organization, 2020. https://covid19.who.int/. Accessed October 24, 2020. [Google Scholar]
- 2. Xie M, Chen Q. Insight into 2019 novel coronavirus ‐ An updated interim review and lessons from SARS‐CoV and MERS‐CoV. Int J Infect Dis. 2020;94:119‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNab F, Mayer‐Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4:914‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei L, Ming S, Zou B, Wu Y, Hong Z, Li Z, et al. Viral invasion and type I interferon response characterize the immunophenotypes during COVID‐19 infection. 2020; Available at SSRN 3555695.
- 6. Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS‐CoV‐2 and SARS‐CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID‐19. Clin Infect Dis. 2020;71:1400‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 8. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181:1036‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trouillet‐Assant S, Viel S, Gaymard A, Pons S, Richard J, Perret M, et al. Type I IFN immunoprofiling in COVID‐19 patients. J Allergy Clin Immunol. 2020;146:206‐208.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Péré H, et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid‐19 patients. Science. 2020;369:718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon DE, Jang GM, Bouhaddou M, Xu JW, Obernier K, White KM, et al. A SARS‐CoV‐2‐human protein interaction map reveals drug targets for drug repurposing. Nature. 2020;583:459‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, et al. SARS‐CoV‐2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS‐CoV‐2. Science. 2020;369:1249‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS‐CoV‐2 infection. Antiviral Res. 2020;179:104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen KD, Shi DR, Lu XY, Cheng LF, Weng TH, Liu FM, et al. Recombinant human interferon α1b effectively inhibit the SARS‐CoV‐2 in vitro, Engineering. 2020. (submitted).
- 18. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181:281‐292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581:215‐220. [DOI] [PubMed] [Google Scholar]
- 22. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581:221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS‐CoV‐2 and COVID‐19. Pharmacol Res. 2020;157:104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS‐CoV‐2 in human airway cells. Life Sci Alliance. 2020;3:e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Yuan Z, Pavel MA, Hansen SB. Cholesterol and COVID19 lethality in elderly. bioRxiv. 2020;www.biorxiv.org/content/10.1101/2020.05.09.086249v2. [Google Scholar]
- 28. Shibabaw T, Molla MD, Teferi B, Ayelign B. Role of IFN and complements system: Innate immunity in SARS‐CoV‐2. J Inflamm Res. 2020;13:507‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuang MW, Cheng Y, Zhang J, Jiang XM, Wang L, Deng J, et al. Increasing host cellular receptor—angiotensin‐converting enzyme 2 expression by coronavirus may facilitate 2019‐nCoV (or SARS‐CoV‐2) infection. J Med Virol. 2020; 10.1002/jmv.26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Busnadiego I, Fernbach S, Pohl MO, Karakus U, Huber M, Trkola A, et al. Antiviral activity of type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS‐CoV‐2. mBio. 2020;11:e01928‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta‐1b, lopinavir– ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: An open‐label, randomised, phase 2 trial. Lancet. 2020;395:1695‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gemcioglu E, Davutoglu M, Ozdemir EE, Erden A. Are type 1 interferons treatment in multiple sclerosis as a potential therapy against covid‐19? Multi Scler Relat Dis. 2020;42:102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang R, Han B, Song M, Xue B, Zhang YX, Ding YY, et al. Efficacy and safety of aerosol inhalation of recombinant human interferon α1b (IFNα1b) injection for noninfluenza viral pneumonia, a multicenter, randomized, double‐blind, placebo‐controlled trial. J Inflamm (Lond). 2020;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen KL, Yang YH, Wang TY, Zhao DC, Jiang Y, Jin RM, et al. Diagnosis, treatment, and prevention of 2019 novel cornavirus in children: Experts’ consensus statement. World J Pediatr. 2020;16:223‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X, Wang X, et al. Interferon‐α2b Treatment for COVID‐19. Front Immunol. 2020;11:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID‐19. J Zhejiang Univ (Med Sci). 2020;49:215‐219. (in Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Y, Li J, Feng Y. Critical care response to a hospital outbreak of the 2019‐nCoV infection in Shenzhen, China. Crit Care. 2020;24:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mary A, Hénaut L, Schmit JL, Lanoix JP, Brazier M. Therapeutic options for coronavirus disease 2019 (COVID‐19)‐modulation of type I interferon response as a promising strategy? Front Public Health. 2020;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu W, Liu Y, Liu L, Hu H, Cheng X, Liu P, et al. An open‐label, randomized trial of the combination of IFN‐k plus TFF2 with standard care in the treatment of patients with moderate COVID‐19. EClinicalMedicine. 2020;27:100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH III, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429‐446.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meng ZJ, Wang TY, Chen L, Chen XH, Li LT, Qin XQ, et al. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. medRxiv. 2020; 10.1101/2020.04.11.20061473. [DOI] [PubMed] [Google Scholar]
- 43. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer‐Smadja N. Type 1 interferons as a potential treatment against COVID‐19. Antiviral Res. 2020;178:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen KL, Zhang GC, Shang YX, Li XW, Xu XW, Chen Q, et al. Specialists consensus on pediatric clinical application of recombinant human interferon‐α1b. Chin J Appl Clin Pediatr. 2015;30:1214‐1219. (in Chinese) [Google Scholar]


