Abstract
BACKGROUND:
The utility of cerebrospinal fluid drainage (CSFD) for prevention of spinal cord ischemia (SCI) after thoracic endovascular aortic repair (TEVAR) remains unclear. We previously published our institutional algorithm restricting preoperative CSFD to patients deemed high risk for SCI. Since that publication, our algorithm has evolved with preoperative CSFD avoided in all patients undergoing isolated descending +/− arch TEVAR. This study evaluates the updated algorithm in a contemporary cohort.
METHODS:
Patients who underwent TEVAR for descending aortic +/− arch pathology between 2/2012 - 9/2018 at a single center were identified from an institutional aortic surgery database. The algorithm includes left subclavian artery (LSA) revascularization in cases of coverage with no preservation of antegrade flow, permissive hypertension, and use of evoked potential monitoring. The primary endpoints were SCI or postoperative CSFD.
RESULTS:
N=225 patients underwent descending +/− arch TEVAR during the study interval. 2 patients (0.9%) had CSFD prior to TEVAR in violation of the algorithm and were excluded from the study cohort. 81% had endograft coverage below T6. The LSA was fully covered in 100 patients (47%), all of whom underwent LSA revascularization. Following the updated algorithm, the incidence of temporary or permanent SCI was 0%. No patient required postoperative CSFD.
CONCLUSIONS:
A restrictive lumbar CSFD algorithm including permissive hypertension and LSA revascularization in the setting of descending +/− arch TEVAR appears safe with a 0% incidence of SCI in 223 consecutive patients treated over a 6.5-year interval. We recommend consideration of further prospective study to evaluate this algorithm.
Classifications: TEVAR, aneurysm, aortic dissection, spinal cord ischemia, cerebrospinal fluid drainage
Graphical Abstract
Thoracic endovascular aortic repair (TEVAR) has become the dominant approach to descending aortic pathology in anatomically-amenable situations. National studies indicate the use of TEVAR has increased by 100-400%, depending on indication, since the turn of the 21st century,1-4 and endovascular approaches to complex pathologies previously thought to require open surgery have proliferated.
Spinal cord ischemia (SCI) occurs less frequently with TEVAR than with open surgery, but remains a devastating complication that can lead to permanent paraparesis or paraplegia in up to 5.7% of TEVAR patients per recent systematic reviews.5,6 Spinal cord perfusion pressure (SCPP) is the difference between the systolic (SBP) or mean arterial pressure (MAP) and intraspinal canal pressure.7 SCI risk after TEVAR is primarily due to decreased SCPP, due to systemic hypotension and/or reduced local perfusion through occlusion of the great anterior radicular artery (artery of Adamkiewicz) as well as important collateral blood supplies via the left subclavian artery (LSA).8
Historically, cerebrospinal fluid drainage (CSFD) has been used to decrease the intraspinal canal pressure, thereby increasing SCPP. CSFD use in TEVAR was justified primarily using data from open aortic surgery, in which blood pressure lability is more frequent and extreme. While its use in select high SCI-risk TEVAR patients has been associated with decreased SCI,5,9 it has also been associated with significant complications and even death.10,11
We have previously published results with a selective CSFD protocol, whereby the use of preoperative CSFD was limited to patients considered high-risk for SCI. This included those with a history of prior open or endovascular thoracic, abdominal, or thoracoabdominal (TAAA) repair plus planned long-segment thoracic aortic coverage, as well as those undergoing hybrid Crawford Extent I-III TAAA repair.12 In this prior series of 381 patients undergoing TEVAR over a nearly 10-year period, 81 patients were preoperatively drained and the permanent SCI rate was 7.4% among the drained patients versus 0.3% in the undrained patients. The results suggested that CSFD could be safely avoided in a large proportion of patients, and the data further suggested that our selection criteria for preoperative CSFD may have been too inclusive, as 39% of patients who met selection criteria for CSFD did not receive a prophylactic drain and none suffered a neurologic complication.
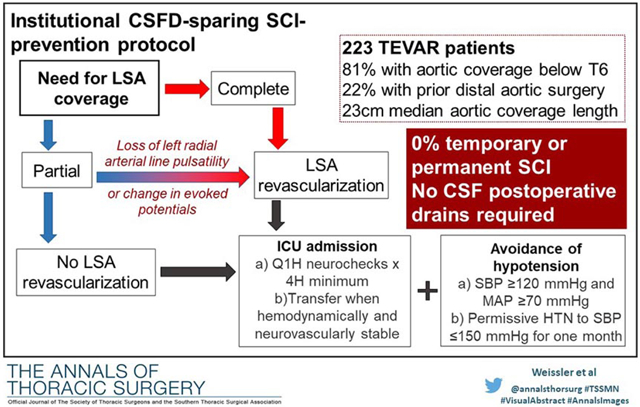
Consequently, in the subsequent 6.5 years, we have further refined our institutional algorithm to eliminate preoperative CSFD entirely for all patients undergoing isolated descending +/− arch TEVAR, with preoperative drains now limited to patients undergoing hybrid or endovascular TAAA repair.13 This updated algorithm (Figure 1) recognizes the importance of the LSA to spinal cord blood flow and mandates LSA revascularization in all patients with complete LSA coverage with no preservation of antegrade pulsatile flow.8 In addition, the updated algorithm utilizes permissive hypertension in the first month after surgery, with preoperative anti-hypertensive medications only resumed for persistent SBP >150 mm Hg. The purpose of the current paper is to evaluate results with this updated algorithm in a contemporary cohort of patients undergoing isolated descending +/− arch TEVAR.
Figure 1:
Institutional TEVAR spinal cord ischemia-prevention algorithm. All patients with complete LSA coverage undergo LSA revascularization as do select patients (Figure 2) with partial LSA coverage. Hypotension is avoided in all patients, regardless of LSA coverage, and all are admitted to the cardiothoracic ICU for at least four hours or until blood pressure and neurologic status are stable and acceptable without requiring intervention. HTN, hypertension; ICU, intensive care unit; LSA, left subclavian artery; MAP, Mean arterial pressure; SBP, systolic blood pressure.
Patients and Methods
Patients and Data Source
All TEVAR procedures performed at Duke University Medical Center (Durham, NC) for descending aortic pathology (no distal landing zone beyond zone 5) with or without arch involvement between February 2012 and September 2018 were identified from a prospectively maintained institutional aortic surgery database. The current study interval begins immediately after our prior selective CSFD protocol publication (study end date 1/2012).11 Patients with preoperative SCI (n=4) were excluded. Pre-operative, intraoperative, and post-operative data were abstracted from the database with patient electronic health record review used to supplement missing data as needed. The Duke University Institutional Review Board approved this study. The need for individual patient consent was waived.
Lumbar CSF Drainage, Left Subclavian Artery Revascularization, and Spinal Cord Management
Selection of patients for TEVAR, techniques of device delivery and deployment, and postoperative surveillance have been previously described.14 Under the updated institutional algorithm (Figure 1), all patients with complete LSA coverage with no preservation of antegrade pulsatile flow undergo surgical revascularization as previously described15 or have flow preserved via LSA branch stent as part of an IDE clinical trial.
Bilateral radial arterial lines are placed preoperatively in all cases where either full or partial LSA coverage is planned.16 In cases with partial coverage, the left radial arterial line tracing is used to confirm preservation of pulsatility after endograft deployment. Intraoperative neurophysiologic monitoring with motor- and sensory-evoked potentials is likewise used,17 and the evoked potentials should remain intact after partial LSA endograft coverage. If either of these criteria are not met, then we proceed to revascularize the LSA in the partial coverage setting (Figure 2). Somatosensory and motor evoked potential monitoring is used in all elective cases and when feasible pending patient stability in urgent/emergent cases.
Figure 2:
Indications for revascularization of partially covered left subclavian artery with TEVAR. All patients undergoing TEVAR with planned complete or partial LSA coverage have bilateral radial arterial lines placed. Motor- and sensory-evoked potentials are used in all elective cases and when feasible pending patient stability in urgent/emergent cases. If either the evoked potentials or left radial arterial line show significant abnormality in cases of partial LSA coverage, the patient undergoes LSA revascularization. LSA, left subclavian artery; TEVAR, thoracic endovascular aortic repair.
All patients are admitted to the cardiothoracic surgical intensive care unit post-operatively for hourly neurovascular checks. Blood pressure is maintained at an SBP of ≥120 mm Hg and MAP of ≥70 mm Hg using fluid and/or vasopressor medications as needed. Patients remain in the ICU until hemodynamic goals are met without the need for intervention and their neurologic exam is intact for a minimum of 4 hours post-operatively. Once transferred to the step-down unit, neurological checks continue every four hours until patients meet discharge criteria. Permissive hypertension is used in the 30-day postoperative period with preoperative anti-hypertensive medications only being resumed for persistent SBP >150 mm Hg. Further, patients are contacted 72 hours after discharge to review home blood pressure readings, and home health referrals are placed for patients with questionable ability to monitor blood pressure in the ambulatory setting.
Variables and Outcomes and Statistical analysis
Clinical events and complications were defined in accordance with the Society of Thoracic Surgeons Adult Cardiac Surgery Database definitions (available at www.sts.org). Patient and procedural characteristics were reported using percentages for categorical variables and medians [interquartile range] for continuous variables. The primary study endpoints included incidence of temporary or permanent SCI or need for postoperative CSFD either in-hospital or within 30 days. All follow-up assessments were done at the Duke University Center for Aortic Disease, and a dedicated nurse practitioner contacted all patients to ensure clinical follow-up appointments were maintained as previously described.14
Results
Two hundred twenty-five patients underwent descending +/− arch TEVAR during the study interval of 2/2012 - 9/2018. Of these, 2 (0.9%) had a CSF drain placed prior to TEVAR in violation of the algorithm, while the remaining 223 constitute the study cohort. Patient demographics are detailed in Table 1. Median age was 67 years and the most common comorbidities were hypertension, hyperlipidemia, and tobacco abuse. Procedural details are listed in Table 2. Indications for TEVAR included degenerative aneurysm in 50% and acute or chronic dissection in 40%; nearly 25% of cases were non-elective. The vast majority of cases had features considered high risk for SCI in the literature such as long-segment (>20 cm) coverage or prior distal aortic repair;18-20 specifically, 22% of patients had undergone prior open or endovascular descending or abdominal aortic repair, the extent of distal pavement was below T6 (zone 5) in 81%, nearly all of whom were within several centimeters of the celiac axis, and the median length of aortic coverage was 23 cm. The LSA was partially or fully covered in 71% (n=159) of patients, of whom 100 (44.8%) had full coverage and underwent LSA revascularization via either bypass (n= 94) or LSA branch stent (n=6).
Table 1:
Demographics and comorbidities
| Overall N=223(%) |
|
|---|---|
| Gender (male) | 146 (65.5%) |
| White | 129 (57.8%) |
| Black | 81 (36.3%) |
| Hypertension | 200 (89.7%) |
| Hyperlipidemia | 149 (66.8%) |
| Current or former smoker | 128 (57.4%) |
| Diabetes | 39 (17.5%) |
| CAD | 42 (18.8%) |
| Prior CVA | 23 (10.3%) |
| COPD | 48 (21.5%) |
| CKD | 40 (17.9%) |
| PAD | 50 (22.4%) |
| CHF | 20 (9.0%) |
| Prior MI | 7 (3.1%) |
| Median [Q1 - Q3] | |
| Age (years) | 67.0 [56.5 - 74.0] |
| BMI | 26.7 [23.9 - 31.5] |
CAD: coronary artery disease, CHF: congestive heart failure, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, CVA: cerebrovascular accident, PAD: peripheral artery disease, MI: myocardial infarction
Table 2:
Procedural characteristics
| Overall | |
|---|---|
| (n=223) | |
| Indications for surgery and prior history | |
| Prior aortic surgery | 92 (41.3%) |
| Prior distal aortic repair | 50 (22.4%)a |
| Prior open/endovascular DTA repair | 29 (13%) |
| Prior open/endovascular abdominal aortic repair | 18 (8%) |
| Prior open/hybrid TAAA repair | 9 (4%) |
| Degenerative aneurysm | 112 (50.2%) |
| Acute dissection | 15 (6.7%) |
| Chronic dissection | 74 (33.2%) |
| Trauma | 17 (7.6%) |
| Other | 5 (2.2%) |
| Urgency | |
| Elective | 168 (75.3%) |
| Urgent | 36 (16.1%) |
| Emergent | 19 (8.5%) |
| Procedural characteristics and coverage | |
| Distal landing zone | |
| 4 | 42 (18.8%) |
| 5 | 181 (81.2%) |
| Length of aortic coverage (cm), median [Q1-Q3] | 23.0 [15.6 - 30.0] |
| LSA covered | |
| Not covered | 64 (28.7%) |
| Covered | 159 (71.3%) |
| Complete LSA coverage | 100 (44.8%) |
| LSA revascularization | 100 (44.8%) |
Some patients had more than one form of distal aortic repair
DTA, descending thoracic aorta; TAAA, thoracoabdominal aortic aneurysm
In regards to the study endpoints, no patient suffered temporary or permanent SCI and no patient required CSFD postoperatively. Further, no patient suffered bilateral loss of somatosensory and motor evoked potentials suggestive of spinal cord ischemia during the procedure. There were five deaths (2.2%) in-hospital or within 30 days, all of which occurred in patients treated urgently (n=4) or emergently, and none of whom had SCI; the stroke rate was 1.3%. Median length of stay following TEVAR was 3 days (IQR 2-6).
Comment
CSFD has been widely used for spinal cord protection following TEVAR despite a lack of strong evidence, including no randomized controlled trials, with current practice based mainly on expert opinion (level C evidence)18-20 and single center or non-comparative cohorts.21,22 In 2013, our group published results with a selective CSFD protocol, whereby the use of preoperative CSFD was limited to patients considered high-risk for SCI,11 including those with a history of distal aortic repair plus planned long-segment thoracic aortic coverage or hybrid TAAA repair, and demonstrated that CSFD could safely be avoided in a large proportion of patients. The data also suggested that our selection criteria for preoperative CSFD may have been too inclusive, as 39% of patients who met selection criteria for CSFD did not receive a prophylactic drain and none suffered a neurologic complication.
As a result, since that prior publication, we have further refined our institutional algorithm to eliminate preoperative CSFD entirely for all patients undergoing isolated descending +/− arch TEVAR, with preoperative drains now limited to patients undergoing hybrid or endovascular TAAA repair.13 This updated algorithm (Figure 1) recognizes the importance of the LSA to spinal cord blood flow and mandates LSA revascularization in all patients with LSA coverage with no preservation of antegrade pulsatile flow.8 Likewise, the updated algorithm utilizes permissive hypertension in the first month after surgery, with preoperative anti-hypertensive medications only resumed for persistent SBP >150 mm Hg. The current report details the results of this updated algorithm and demonstrates the more restrictive approach to be safe and effective with no temporary or permanent SCI or need for postoperative CSFD in 223 consecutive patients, the vast majority of whom had features considered high risk for SCI, undergoing descending +/− arch TEVAR over a 6.5-year period. Based on these results, we feel prophylactic CSFD can likely be avoided in nearly all patients undergoing TEVAR limited to the descending thoracic aorta (zone 5 or more proximally). Of note, a recent report from the Mayo Clinic examining CSFD complications in patients undergoing fenestrated-branched endovascular aortic repair came to a similar conclusion with no SCI in patients undergoing first-stage TEVAR.22 Based on their results, including an “alarming” rate of severe complications following prophylactic CSFD, the Mayo group has changed their institutional practice and no longer recommends CSFD during first-stage TEVAR or for patients with pararenal or Extent IV TAAA undergoing endovascular repair. Further, prophylactic CSFD use is individualized for endovascular Extent III TAAA repair cases, and likewise similar to our protocol, still used routinely for Extent I and II endovascular TAAA repairs. A recent study from the Vascular Quality Initiative (VQI) did find benefit for CSFD in the setting of endovascular TAAA repair, although the study suffers from a number of limitations including low rates of LSA revascularization (a mandatory part of our algorithm in the setting of LSA coverage with no preservation of antegrade flow), data granularity limitations of an administrative data set, and low overall use of CSFD in the entire population.23 As such, the VQI data suggest the need for further prospective study of CSFD in the TAAA population rather than representing convincing evidence of benefit.
The most comprehensive data to date on CSFD complications, a systematic review and meta-analysis of 4,714 patients and 34 studies found a pooled event rate of 6.5% for overall complications and a drainage-related mortality of nearly 1%.21 Given the results with TEVAR for descending pathology continue to improve with very low permanent SCI rates of 1-2% or less in many reports,5,14 it is likely that prophylactic CSFD may cause as many neurological complications as it potentially prevents. For example, in the aforementioned recent study from the Mayo Clinic,22 the authors found a 9% rate of CSFD-related complications, including severe, potentially life-threatening complications in 4%, and one-third of the SCIs in their study were actually caused by CSFD. Further, as the current paper highlights, if an algorithm recognizing the importance of maintaining pulsatile LSA perfusion and permissive hypertension is strictly followed, the incidence of SCI approaches zero in the absence of prophylactic CSFD.
With regards to the two main components of our institutional algorithm, preservation of antegrade LSA flow is of vital importance for several anatomic reasons including that the anterior spinal artery supplying the anterior two-thirds of the spinal cord is formed from the vertebral artery branches of the subclavian arteries, as well as the fact that the LSA represents the primary source of collateral pathways to the great anterior radicular artery (artery of Adamkiewicz) outside of the spinal column via the thoracodorsal artery and internal thoracic artery.8 To this point, case reports document spinal cord rescue after zone 2 TEVAR via urgent LSA revascularization when CSFD has failed.24,25 As for the permissive hypertension component, a recent report by Sandhu et al from the University of Texas Houston group of 1,059 patients undergoing open descending or TAAA repair over an 11 year period found the mean SBP and MAP at the time of onset of delayed SCI were 107 mm Hg and 68 mm Hg, respectively, and that 90% of delayed SCI events occurred when the SBP was <130 mm Hg with no patient developing SCI with a SBP >150 mm Hg.7 These numbers, which are derived from a much higher risk cohort of patients undergoing open descending and TAAA repair versus the descending-only TEVAR cohort of the current report, fit nicely with our current algorithm where we maintain SBP >120 mm Hg and MAP >70 mm Hg and only consider instituting anti-hypertensive medications for sustained SBP >150 mm Hg.
There are several limitations of the current study. First, as a retrospective study from a single high-volume academic center, the results are potentially subject to sampling bias and suffer from a limited cohort size. Further, the results may not be generalizable to lower volume community hospitals. Also, granular data on pre- and postoperative blood pressures were not collected as part of the analysis, and therefore more patient-specific blood pressure targets cannot be extrapolated from the data presented. The study also lacks a control group of similar patients undergoing prophylactic CSFD, although the event rate was zero in the cohort presented and therefore the control group could at best be non-inferior. Also, emergent CSFD placement is available 24/7 in our institution, which allows the decision to avoid prophylactic CSFD less complicated than in centers where this expertise may not be available. Further, data from the aforementioned VQI registry paper suggested rescue CSFD to be less effective than prophylactic placement, although it is unknown whether this is true in the setting of concomitant LSA revascularization or permissive HTN as these data were not detailed in the VQI dataset.23 Regardless, given the known potentially life-threatening risks of CSFD, the decision regarding its prophylactic use should at a minimum be made, as suggested by Rong et al,21 via multidisciplinary discussion that weighs the risks of CSFD against the risk of SCI, which based on the data presented is exceedingly low if the proposed algorithm is strictly followed.
In conclusion, a restrictive lumbar CSFD algorithm, which avoids the known potentially serious risks of drain placement, and includes permissive hypertension and LSA revascularization in the setting of endograft coverage for descending +/− arch TEVAR appears safe with a 0% incidence of SCI in 223 consecutive patients treated over a 6.5-year interval. We recommend consideration of further prospective study to evaluate this algorithm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grigorian A, Spencer D, Donayre C, et al. National trends of thoracic endovascular aortic repair versus open repair in blunt thoracic aortic injury. Ann Vasc Surg. 2018;52:72–78. [DOI] [PubMed] [Google Scholar]
- 2.Ultee KHJ, Zettervall Sl, Soden PA, et al. the impact of endovascular repair on management and outcome of ruptured thoracic aortic aneurysms. J Vasc Surg. 2017;66:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DW, Goodney PP, Nolan BW, et al. National trends in utilization, mortality, and survival after repair of type B aortic dissection in the Medicare population. J Vasc Surg. 2014;60:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao JM, Bakaeen FG, Cornwell LD, et al. Nationwide trends and regional/hospital variations in open versus endovascular repair of thoracocabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144:612–616. [DOI] [PubMed] [Google Scholar]
- 5.Dijkstra ML, Vainas T, Zeebregts CJ, Hooft L, van der Laan MJ. Editor’s Choice – Spinal cord ischaemia in endovascular thoracic and thoraco-abdominal aortic repair: Review of preventative strategies. Eur J Vasc Endovasc Surg. 2018;55:829–841. [DOI] [PubMed] [Google Scholar]
- 6.Huang Q, Chen XM, Yang H, Lin QN, Qin X. Effect of left subclavian artery revascularisation in Thoracic Endovascular Aortic Repair: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2018;56:644–651. [DOI] [PubMed] [Google Scholar]
- 7.Sandhu HK, Evans JD, Tanaka A, et al. Fluctuations in spinal cord perfusion pressure: A harbinger of delayed paraplegia after thoracoabdominal aortic repair. Semin Thoracic Surg.2017;29:451–459. [DOI] [PubMed] [Google Scholar]
- 8.Hughes GC. Commentary: Left subclavian artery revascularization during zone 2 thoracic endovascular aortic repair: Bypass versus transposition? Just do it! J Thorac Cardiovasc Surg.2019;1–3. [DOI] [PubMed] [Google Scholar]
- 9.Maier S, Shcherbakova M, Beyersdorf F, et al. Benefits and risks of prophylactic cerebrospinal fluid catheter and evoked potential monitoring in symptomatic spinal cord ischemia low-risk thoracic endovascular aortic repair. Thorac Cardiovasc Surg. 2019;67:379–384. [DOI] [PubMed] [Google Scholar]
- 10.Yang GK, Misskey J, Arsenault K, Gagnon J, Janusz M, Faulds J. Outcomes of a spinal drain and intraoperative neurophysiologic monitoring protocol in thoracic endovascular aortic repair. Ann Vasc Surg.2019;1–10. [DOI] [PubMed] [Google Scholar]
- 11.Wynn MM, Mell MW, Tefera G, et al. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg.2009;49:29–34. [DOI] [PubMed] [Google Scholar]
- 12.Hanna JM, Anderson ND, Aziz H, Shah AA, McCann RL, Hughes GC. Results with selective peroperative lumbar drain placement for thoracic endovascular aortic repair. Ann Thor Surg. 2013;95:1968–1975. [DOI] [PubMed] [Google Scholar]
- 13.Benrashid E, Wang H, Andersen ND, Keenan JE, McCann RL, Hughes GC. Complementary roles of open and hybrid approaches to thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2016;6:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranney DN, Cox ML, Yerokun BA, Benrashid E, McCann RL, Hughes GC. Long-term results of endovascular repair for descending thoracic aortic aneurysms. J Vasc Surg. 2018;67:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voigt SL, Bishawi M, Ranney D, Yerokun B, McCann RL, Hughes GC. Outcomes of carotid-subclavian bypass performed in the setting of thoracic endovascular aortic repair. J Vasc Surg. 2019;69:701–709. [DOI] [PubMed] [Google Scholar]
- 16.Lee TC, Andersen ND,Williams JB, Bhattacharya SD, McCann RL, Hughes GC.Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg. 2011;92:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain AM, Swaminathan M, McCann RL, Hughes GC. Neurophysiologic intraoperative monitoring during endovascular stent graft repair of the descending thoracic aorta. J Clin Neurophysiol. 2007;24:328–335. [DOI] [PubMed] [Google Scholar]
- 18.Riambau V, Böckler D, Brunkwall J, et al. Editor’s choice – Management of descending thoracic aorta diseases: Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4–52. [DOI] [PubMed] [Google Scholar]
- 19.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic disease of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 20.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. [DOI] [PubMed] [Google Scholar]
- 21.Rong LQ, Kamel MK, Rahouma M, et al. Cerebrospinal-fluid drain-related complications in patients undergoing open and endovascular repairs of thoracic and thoraco-abdominal aortic pathologies: A systematic review and meta-analysis. Br J Anaesth. 2018;120:904–913. [DOI] [PubMed] [Google Scholar]
- 22.Kärkkäinen JM, Cirillo-Penn NC, Sen I, et al. Cerebrospinal fluid drainage complications during first stage and completion fenestrated-branched endovascular aortic repair. J Vasc Surg. 2019; in press. [DOI] [PubMed] [Google Scholar]
- 23.Suarez-Pierre A, Zhou X, Gonzalez JE, et al. Association of preoperative spinal drain placement with spinal cord ischemia among patients undergoing thoracic and thoracoabdominal endovascular aortic repair. J Vasc Surg. 2019;70:393–403. [DOI] [PubMed] [Google Scholar]
- 24.Borghese O, Sbenaglia G, Giudice R. Late-onset paraplegia after endovascular repair of type B aortic dissection managed by urgent left subclavian artery revascularization: a case report. Ann Vasc Surg. 2019;58:384.e9–14. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura E, Nakamura K, Furukawa K, et al. Left subclavian artery revascularization for delayed paralysis after thoracic endovascular aortic repair. Ann Vasc Dis. 2019;12:233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]




