Abstract
PURPOSE
In this phase II response-adaptive trial, we investigated the rational application of immune checkpoint blockade in renal cell carcinoma (RCC; ClinicalTrials.gov identifier: NCT03203473).
METHODS
We enrolled patients with metastatic RCC with no prior checkpoint inhibitor exposure. All patients received nivolumab alone with subsequent arm allocation based on response. Patients with a confirmed partial response (PR) or complete response (CR) within 6 months discontinued nivolumab and were observed (arm A). Patients with stable disease or progressive disease (PD) after no more than 6 months of nivolumab received two doses of ipilimumab (arm B). The primary endpoints were the proportion of patients with PR/CR at 1 year after nivolumab discontinuation (arm A) and proportion of nivolumab nonresponders who converted to PR/CR after ipilimumab (arm B).
RESULTS
Overall, 83 patients initiated treatment, of whom 96% had clear-cell histology, 51% were treatment naïve, and 67% had intermediate/poor-risk disease. Median follow-up was 19.5 months. Within 6 months, induction nivolumab resulted in a confirmed PR in 12% of patients (n = 10). Fourteen patients were not allocated to a study arm (seven because of toxicity, seven because of PD). Twelve patients (14%) were allocated to arm A and discontinued nivolumab, of whom five (42%; 90% CI, 18% to 68%) remained off nivolumab at ≥ 1 year. Of 57 patients (69%) allocated to arm B, two patients converted to a confirmed PR (4%; 90% CI, 1% to 11%), and no CRs were observed.
CONCLUSION
In this study, nivolumab followed by two doses of ipilimumab resulted in no CRs and a low PR/CR conversion. The number of patients evaluated for nivolumab discontinuation was too small to assess the value of this approach. Currently, our data do not support a response-adaptive strategy for checkpoint blockade in advanced RCC.
INTRODUCTION
The efficacy of immune checkpoint inhibitors has dramatically revolutionized the treatment paradigm for patients with advanced renal cell carcinoma (RCC). The phase III CheckMate-025 trial (ClinicalTrials.gov identifier: NCT01668784) demonstrated the efficacy of nivolumab, a programmed cell death-protein 1 (PD-1) inhibitor, in patients with advanced RCC having received prior vascular endothelial growth factor (VEGF) targeted therapy.1 In the frontline setting, the phase III CheckMate-214 study (ClinicalTrials.gov identifier: NCT02231749) evaluated the role of combination nivolumab and ipilimumab, a cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitor, compared with sunitinib.2 The immunotherapy combination resulted in improved objective responses (39%), including 11% of patients experiencing a complete response (CR) and prolonged overall survival.3 Currently, frontline treatment options for patients with advanced RCC include immunotherapy combinations of either nivolumab plus ipilimumab,2 pembrolizumab plus axitinib,4 or avelumab plus axitinib,4 and in some scenarios, single-agent VEGF inhibition.
Context
Key Objective
Is it possible to rationally escalate or discontinue treatment with immune checkpoint blockade in patients with metastatic renal cell carcinoma?
Knowledge Generated
Nivolumab followed the addition of two doses of ipilimumab in patients without an objective response to nivolumab monotherapy results in no complete responses and a low response conversion rate. Patients with a complete or partial response to nivolumab monotherapy were eligible to discontinue treatment; however, the number of patients evaluable for maintenance of response after nivolumab discontinuation was too small to assess the value of this approach.
Relevance
A response-based adaptive strategy for nivolumab and ipilimumab is currently not recommended, and upfront dual checkpoint blockade is suggested in patients eligible to receive this treatment.
Despite the marked efficacy observed with immune checkpoint blockade, immune-related adverse events (irAEs) are common and can be life-threatening.5 Unlike adverse events with conventional VEGF targeted therapy, which have a predictable dose-dependent pattern and tend to be reversible, irAEs tend to be variable in onset, presentation, and severity, often require steroids and other immunosuppressive agents for management, and may not be easily reversible.2 With nivolumab monotherapy, grade 3-4 treatment-related adverse events (TRAEs) were observed in 21% of patients, with 8% discontinuing treatment because of toxicity.6 With combination nivolumab and ipilimumab, 47% of patients experienced grade 3-4 TRAEs, with one in five patients requiring treatment discontinuation.3
Immune checkpoint inhibitors, which engage T cells with inherent capacity for memory, have caused us to redefine traditional clinical trial endpoints. Treatment-free survival characterizes antitumor activity and toxicity of the period after cessation of immunotherapy treatment. In patients who discontinued treatment in the CheckMate-214 trial, treatment-free survival was 21% at 24 months since treatment discontinuation, suggesting that a subset of patients may have a durable time off therapy after treatment discontinuation.7
As we optimize therapeutic paradigms for patients with advanced RCC, we questioned whether benefits could be maintained with less intensive treatment. Additionally, at the time our study was launched, there remained uncertainty about the benefit of adding ipilimumab to nivolumab in the frontline setting, given the lack of data on frontline nivolumab. Our trial was designed to shed light on these questions. We specifically hypothesized that an adaptive strategy of treatment intensification and discontinuation based on response would maximize efficacy and limit toxicity for patients with metastatic RCC. We thus designed a multicenter, phase II, response-adaptive trial to investigate the sequential addition of ipilimumab to nivolumab nonresponders and discontinuation of nivolumab in responding patients.
METHODS
Patient Population
This study enrolled patients with histologically confirmed advanced RCC of any histologic subtype. Advanced disease was defined as unresectable, locally recurrent, or metastatic by American Joint Committee on Cancer 7th edition staging. Patients could have received prior therapy, excluding PD-1 pathway or CTLA-4 inhibitors. Other key inclusion criteria included presence of measurable disease per RECIST version 1.1,8 Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, and adequate organ function. Patients with a history of autoimmune disease requiring ≥ 10 mg per day of prednisone or equivalent were excluded. The study was approved by the institutional review board at each institution. All patients provided written informed consent.
Study Design
This was a phase II adaptive trial (Appendix Fig A1, online only). Before therapy initiation, patients underwent a baseline tumor biopsy unless not medically feasible. All eligible patients initially received induction nivolumab with subsequent arm allocation based on RECIST version 1.1 response within 6 months of treatment initiation.8 Nivolumab was administered at 240 mg intravenously every 2 weeks until the protocol was amended, converting nivolumab monotherapy dosing to 480 mg intravenously every 4 weeks. Patients underwent imaging assessments at 8, 16, and 24 weeks. Patients with a partial response (PR) or CR within 6 months of treatment discontinued nivolumab and were observed (arm A). Response confirmation was required before arm allocation. Arm A patients reinitiated nivolumab if they developed progressive disease (PD); two doses of ipilimumab were added to nivolumab if PD persisted or recurred in arm A patients. Once patients were allocated to arm A, they remained in arm A for all analyses. Patients with confirmed stable disease (SD) or PD after a minimum of 2 but no more than 6 months of nivolumab received two doses of ipilimumab with nivolumab continuation (arm B). In combination therapy, patients received nivolumab 3 mg/kg and ipilimumab 1 mg/kg intravenously every 3 weeks for two doses. Dose modifications were not permitted; however, dose delays were allowed. After arm allocation, arm A patients underwent imaging assessments every 8 weeks, and arm B patients underwent imaging after the first 12 weeks and then every 8 weeks.
Primary and Secondary Endpoints
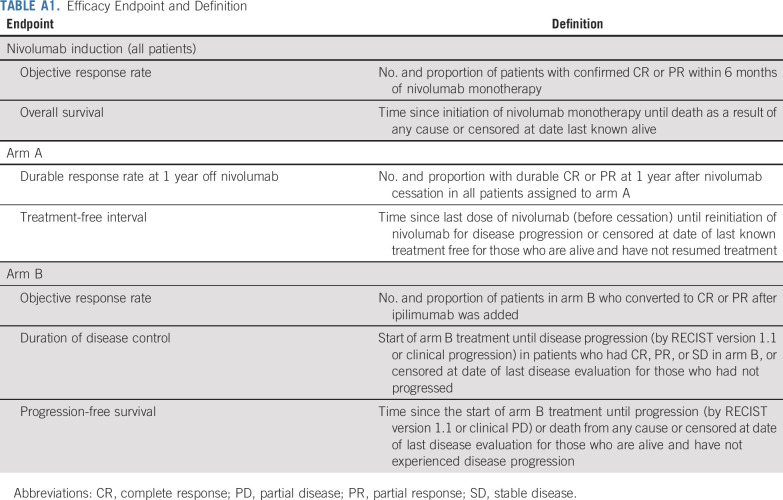
The primary endpoints were the proportion of patients with durable CR/PR at 1 year after nivolumab discontinuation (arm A) and proportion with SD/PD receiving nivolumab who converted to PR/CR after the addition of ipilimumab (arm B). Secondary endpoints included overall survival, treatment-free interval, duration of disease control, progression-free survival (PFS), and toxicity (Appendix Table A1, online only).
Statistical Design
The planned accrual was 83 patients, assuming 23 patients would have a confirmed PR or CR transitioning to arm A, 57 patients with PD or confirmed SD transitioning to arm B, and three patients ineligible or withdrawing (Appendix, online only). With 23 patients in arm A, there would be 94% power to distinguish a true durable PR/CR rate of 35% from 10% for an exact one-sample binomial test (one-sided alpha of .07). With 57 patients in arm B, there would be 92% power for a true PR/CR conversion rate of 20% versus 5% (one-sided alpha of .05) under a Simon’s two-stage design (ie, suspension of arm B if less than or equal to one PR/CR in the first 20 response-evaluable patients). The treatment strategy would be deemed effective if five or more of 23 patients in arm A (observed rate of 22%) had persistent PR/CR at 1 year off nivolumab, and six or more of 57 patients in arm B (observed rate of 11%) converted to PR/CR when ipilimumab was added. Response rates are presented with 90% two-sided exact binomial CIs. Kaplan-Meier methodology was used to assess time-to-event endpoints (Appendix Table A1).
RESULTS
Baseline Characteristics
Eighty-five patients were enrolled between October 2017 and July 2019 at 10 centers in the United States. Two patients never initiated treatment, resulting in 83 patients for analysis. The majority of patients were male (82%; n = 68), had ECOG performance status of 0-1 (99%; n = 82), and had International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate/poor-risk disease9 (67%; n = 56; Table 1). With regard to histology, 96% (n = 80) were clear cell, 8% (n = 7) had sarcomatoid differentiation, and 15% (n = 12) had rhabdoid differentiation. Forty-two patients (51%) were treatment naïve.
TABLE 1.
Baseline Patient and Disease Characteristics
Arm Allocation
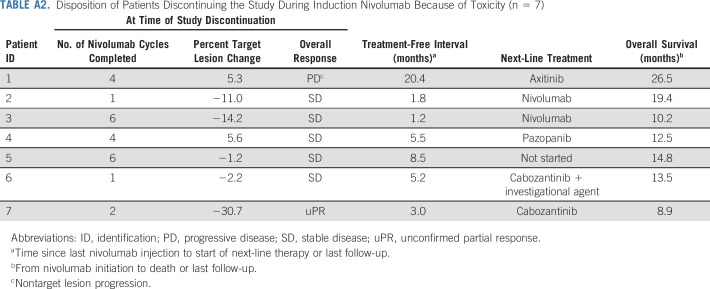
Of the 83 patients who initiated treatment, 69 underwent arm allocation. Fourteen patients did not undergo arm allocation because of PD (n = 7) or toxicity (n = 7; Fig 1; Appendix Table A2, online only). Of the patients who discontinued treatment because of toxicity, the median treatment-free interval was 5.2 months (range, 1.2-20.4 months). Twelve patients were assigned to arm A (10 PR, one unconfirmed PR, and one SD) and 57 patients were assigned to arm B (28 SD, 29 PD). At the time of analysis, three patients in arm A had discontinued the study (two because of toxicity, one because of PD), and 54 patients in arm B had discontinued the study (11 because of toxicity, 37 because of PD, five for other reasons; one death was unrelated to study treatment).
FIG 1.
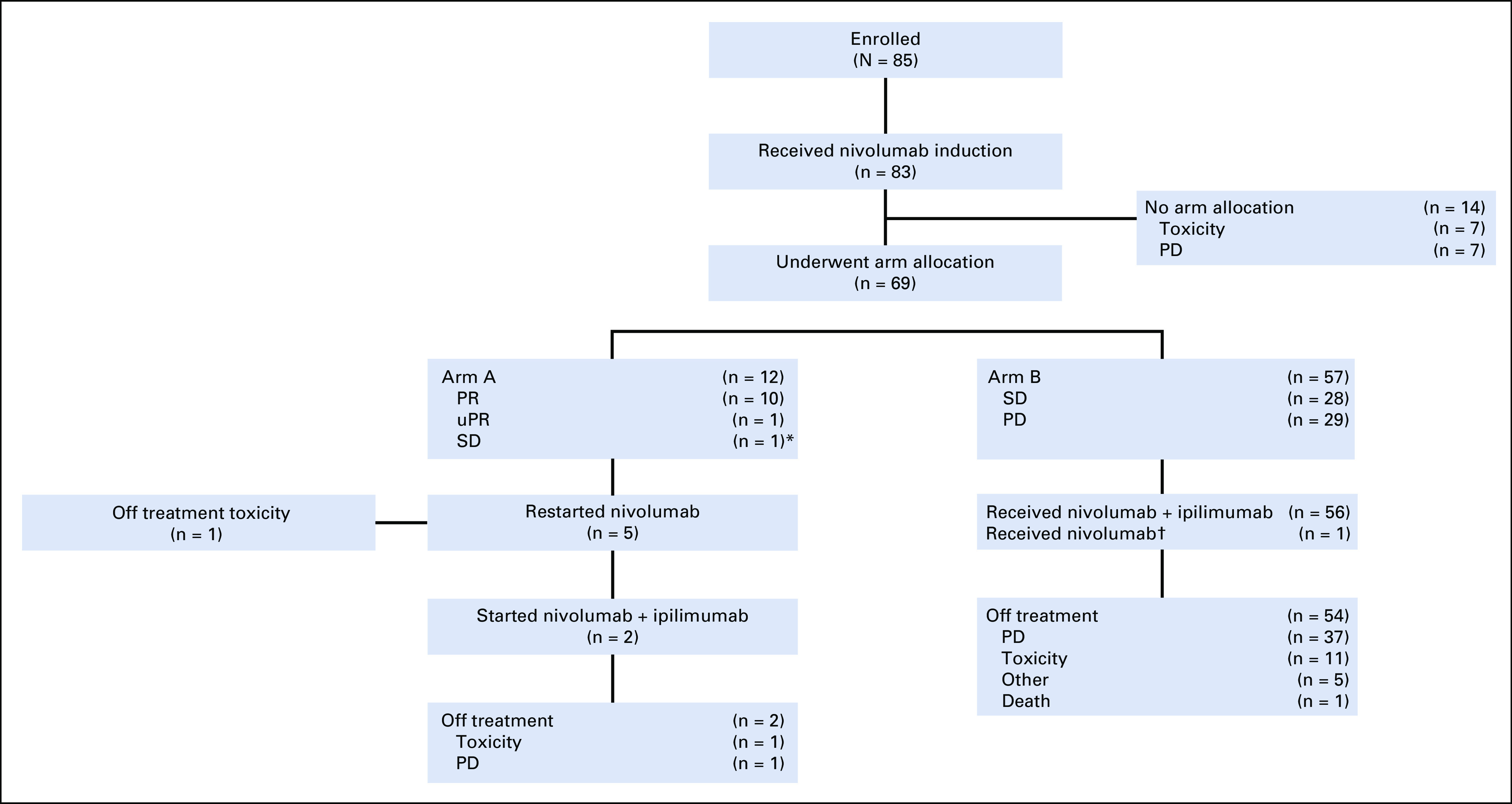
Consort diagram. (*) The non–partial response (PR) patients allocated to arm A represent protocol violations. (†) Received nivolumab alone given infusion reaction to nivolumab, which precluded receipt of ipilimumab. PD, progressive disease; uPR, unconfirmed partial response; SD, stable disease.
Treatment Exposure
The median number of nivolumab induction cycles received was 4 (range, 1-8): median number in arm A patients was 4 (range, 2-8), median number in arm B patients was 4 (range, 2-6), and median number in patients who withdrew from study before arm allocation was 2 (range, 1-6). Of the 57 patients in arm B, 50 received two doses of ipilimumab, six received one dose, and one received no ipilimumab. The median duration of treatment for arm B patients was 3.7 months (range, 1-24 months).
Outcomes of Induction Nivolumab
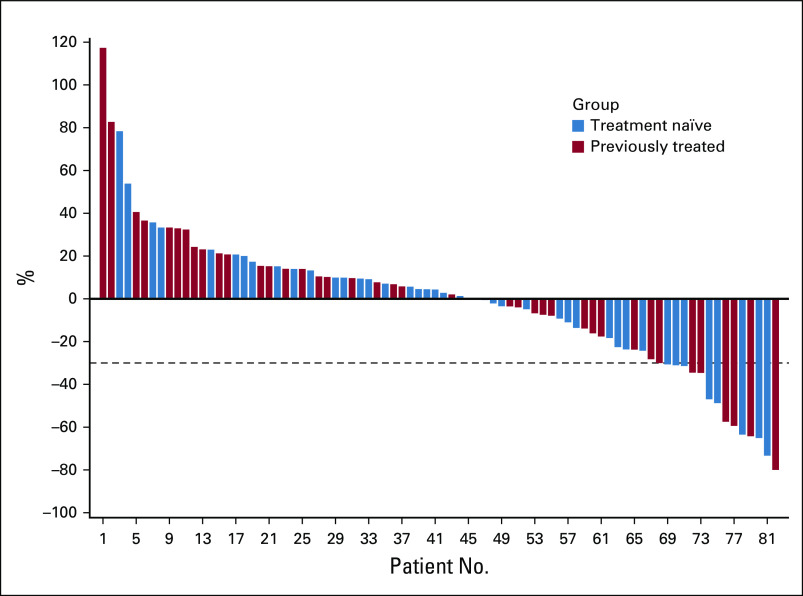
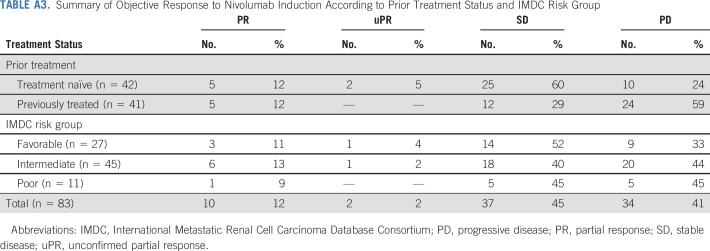
The objective response rate (ORR) including PR and unconfirmed PR within 6 months of nivolumab induction was 14% (n = 12; 90% CI, 9% to 22%; Fig 2; Appendix Table A3, online only). The ORR was 17% (n = 7) in treatment-naïve patients and 12% (n = 5) in previously treated patients. Response rates were similar in patients with IMDC favorable (15%; n = 4) and intermediate/poor-risk disease (14%; n = 8).
FIG 2.
Waterfall plot of maximum percent decline in target lesion during induction nivolumab. Blue, treatment naïve. Red, previously treated.
Outcomes of Arm A Patients
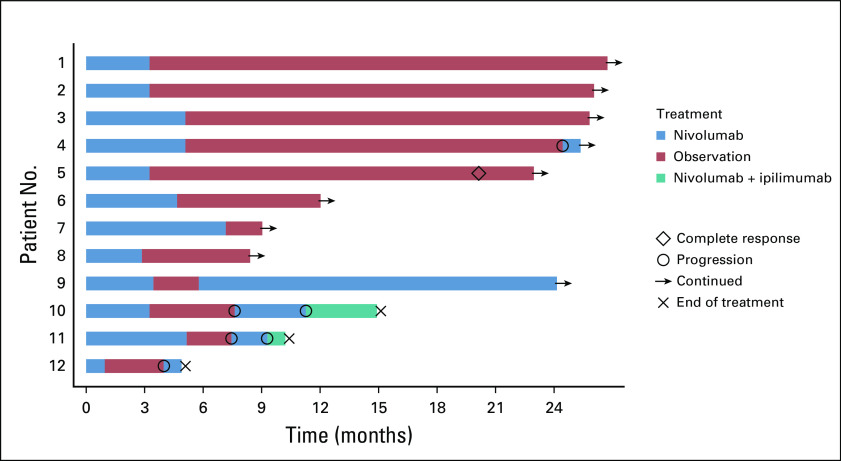
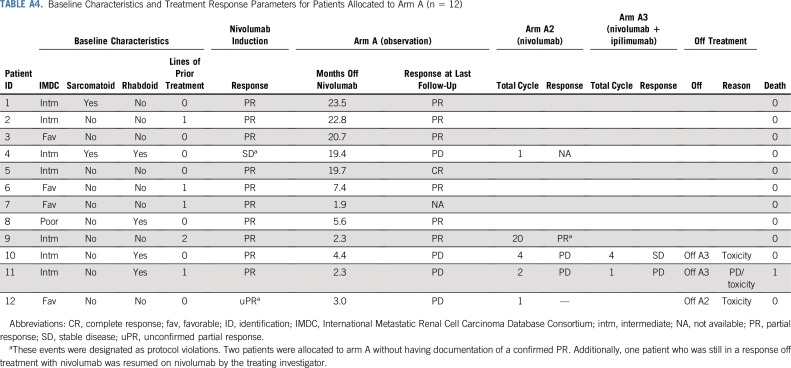
Arm A continued enrollment after the futility assessment, given seven of the first nine patients did not have PD after the first imaging assessment. Of the 12 patients allocated to arm A, five (42%; 90% CI, 18% to 68%) remained off nivolumab at 1 year post-treatment discontinuation (Fig 3; Appendix Table A4, online only). Four patients restarted nivolumab within 6 months after treatment discontinuation (three because of PD; one was still in PR). Three patients who have not reached the 1-year mark are still in active follow-up.
FIG 3.
Swimmer plot of arm A treatment and outcomes. Patient 4 had stable disease with nivolumab induction, and patient 12 had an unconfirmed partial response to nivolumab induction.
Outcomes of Arm B Patients
Of the 57 patients allocated to arm B, two patients (4%) converted to a confirmed PR (90% CI, 1% to 11%), both of whom were previously treated, did not have sarcomatoid/rhabdoid differentiation, and had PD as best response to nivolumab induction (Table 2). Duration of response was 9.2 and 10.9 months, and both patients ultimately discontinued treatment because of PD. Rates of SD and PD as best response were 46% (n = 26) and 40% (n = 23), respectively. The median duration of disease control (maintenance of a PR or SD; n = 28) from arm B start was 10.4 months (95% CI, 5.5 to 13.0 months), and median PFS from arm B start was 4.7 months (n = 45 events/57 patients; 95% CI, 2.7 to 8.3 months; Fig 4A).
TABLE 2.
Arm B Treatment Response
FIG 4.
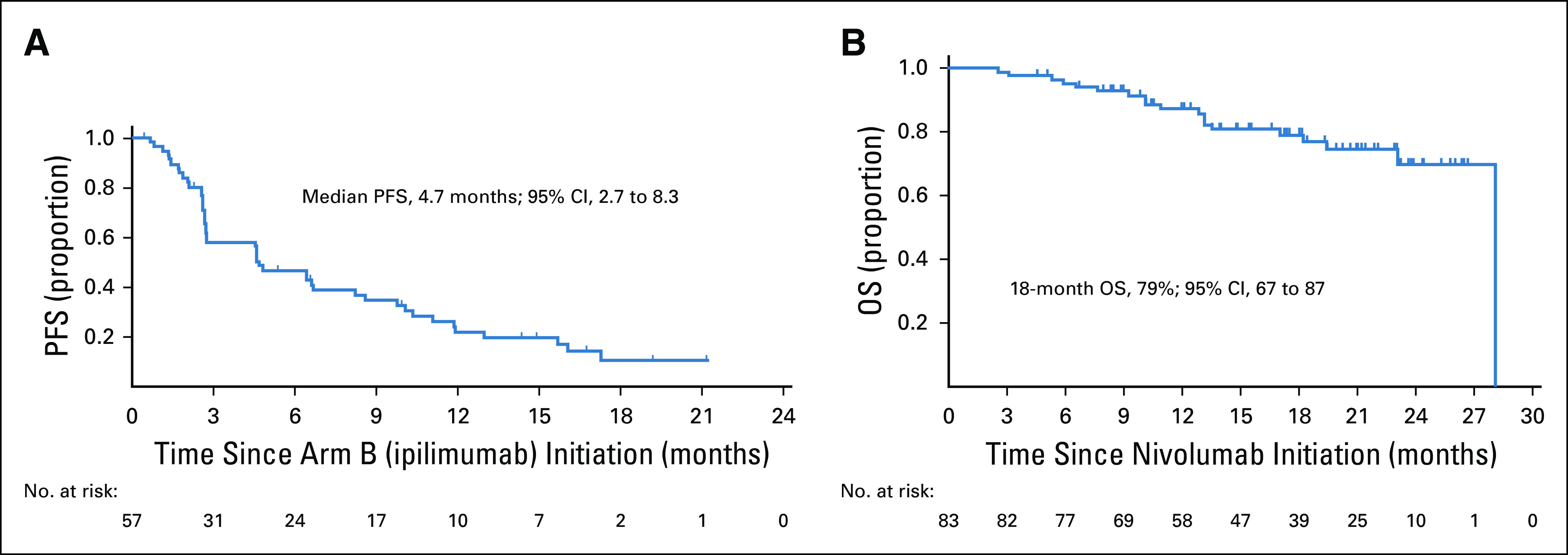
Kaplan-Meier estimate of (A) progression-free survival (PFS) from ipilimumab initiation for arm B patients and (B) overall survival (OS) for the total patient population.
Overall Survival
The median follow-up for overall survival was 19.5 months (range, 2.5-28.1 months). Overall, 19 deaths were observed (arm A [n = 1], arm B [n = 12], and patients who withdrew from the study before arm allocation [n = 6]; Fig 4B). The median overall survival has not been reached. The 18-month overall survival was 79% (95% CI, 67% to 87%).
Adverse Events
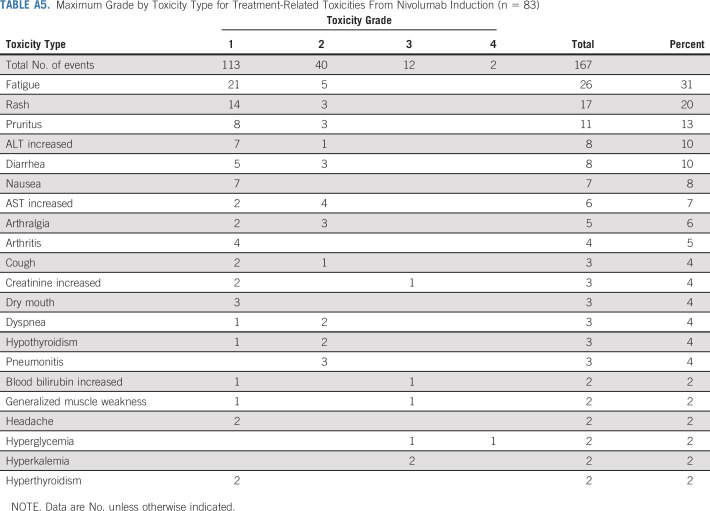
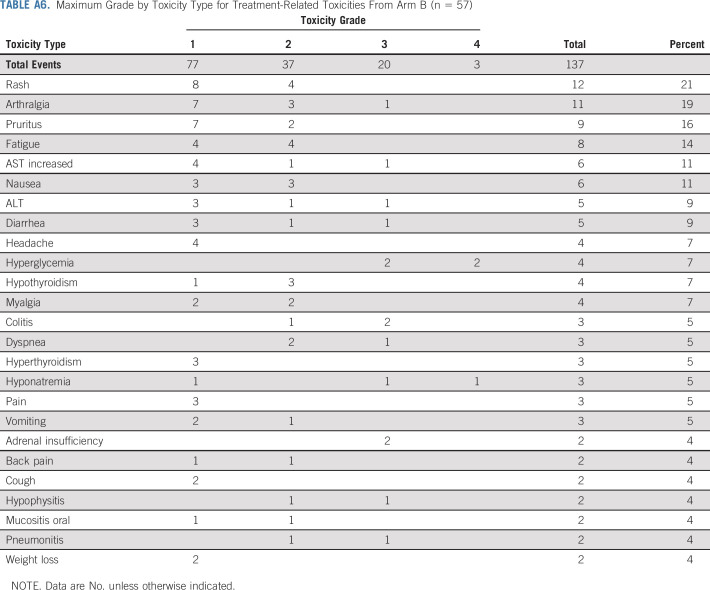
During nivolumab induction, any grade and grade 3-4 TRAEs occurred in 80% (n = 66) and 7% (n = 6) of patients, respectively. Seven patients (8%) required prednisone ≥ 40 mg or the equivalent; seven patients (8%) discontinued treatment because of toxicity, and 12 patients (14%) had a dose delay because of toxicity (Appendix Table A5, online only). In arm B, any grade and grade 3-4 TRAEs occurred post-ipilimumab initiation in 81% (n = 46) and 25% (n = 14) of patients, respectively. Ten patients (18%) required prednisone ≥ 40 mg or the equivalent, 11 patients (19%) discontinued treatment of toxicity, and 18 patients (32%) had a dose delay because of toxicity (Appendix Table A6, online only). There were no treatment-related deaths.
DISCUSSION
In our multicenter, phase II adaptive trial, we addressed several clinically relevant questions for the management of advanced RCC. We investigated the efficacy of nivolumab followed by the addition of two doses of ipilimumab in patients without a PR or CR within 2-6 months of nivolumab. Given the lack of CRs (0%) and low PR/CR conversion rate (4%), our data do not support a strategy of nivolumab followed by two cycles of ipilimumab in nivolumab nonresponders. Furthermore, we investigated nivolumab discontinuation in patients with a confirmed PR/CR within 6 months of nivolumab initiation. Although a subset of patients treated with nivolumab maintained durable responses off treatment at 1 year (42%), the number of patients evaluated for nivolumab discontinuation was too small to assess the value of this approach in nivolumab responders.
Our study used a novel adaptive design to investigate therapy de-escalation strategies in advanced RCC. We did not generate sufficient evidence to overturn the approach of combination nivolumab plus ipilimumab as opposed to sequential immune checkpoint blockade. Furthermore, 17% of patients were not allocated to a treatment arm, affecting the sample size in each treatment arm and further illustrating the limitations of sequential treatment strategies. Although formal comparisons with the phase III CheckMate-214 study cannot be performed given differing patient populations and study design, we demonstrated numerically inferior CR and ORR with our adaptive strategy. Immune checkpoint inhibitors have called for revised clinical endpoints to assess treatment efficacy. Although overall survival remains a gold standard endpoint, depth, durability of response, and treatment-free survival have become important endpoints to consider when selecting therapy options for patients. PD-1 and CTLA-4 inhibition have complementary effects on T-cell activation and prevention of T-cell exhaustion.10 Preclinical models have demonstrated that combined PD-1 and CTLA-4 blockade is superior to either strategy alone.11,12 Studies of RCC evaluating combined upfront versus sequential PD-1 and CTLA-4 blockade are limited. Our study fills an unmet need in the field investigating sequential CTLA-4 blockade in patients not responding to nivolumab.
The role of nivolumab monotherapy in treatment-naïve patients with advanced RCC has yet to be defined. In patients with clear-cell RCC having received one to two prior VEGF targeted therapies, the ORR to nivolumab is 25%, and median time to response was 3.5 months (range, 1.4-24.8 months).1 The KEYNOTE-427 study, which evaluated pembrolizumab in treatment-naïve clear-cell RCC, demonstrated response rates of 36.4% with median time to response of 2.8 months (range, 2.5-12.9 months).13 In our study, in which approximately 50% of patients were treatment naïve, we demonstrated a 6-month response rate to nivolumab of 14%. Our study likely underestimates the response to nivolumab monotherapy given that (1) we mandated arm allocation by 6 months of initiation of nivolumab and thus only captured early responders, and (2) a subset of patients discontinued treatment because of toxicity during induction nivolumab, and we did not follow their response after study discontinuation (n = 7).
It is informative to place our study results in the context of two additional adaptive studies investigating nivolumab monotherapy followed by the addition of ipilimumab. The TITAN RCC trial was a phase II adaptive study in patients with treatment-naïve or previously treated advanced RCC (N = 207).14 This study demonstrated a response to induction nivolumab of 28.7% (n = 31/108) for treatment-naïve patients and 18.2% (n = 18/99) for previously treated patients. Overall, the TITAN RCC study (ClinicalTrials.gov identifier: NCT02917772) demonstrated a low CR rate (2.9%), and the PR/CR conversion rate was approximately 10%. The HCRN-GU-260 study also investigated the role of nivolumab plus ipilimumab in patients without a response to nivolumab monotherapy.15 The response to induction nivolumab (within 48 weeks) was 31.7% (n = 39/123). The overall CR rate was low (5.7% for monotherapy and 0% for salvage therapy), and the PR conversion rate was 13.3%. Furthermore, because of the study design, less than 50% of SD/PD patients were eligible for salvage therapy. Collectively, these data, combined with the results of our study, demonstrate that salvage ipilimumab results in low CR and PR/CR conversion rates. The value of the addition of ipilimumab to nivolumab in treatment-naïve patients will be answered in the phase III CA209-8Y8 study (ClinicalTrials.gov identifier: NCT03873402). Although it is difficult to place the utility of this study in the context of expanding immunotherapy combinations for patients with advanced RCC, this study was mandated by the European Medicines Agency to identify the contribution ipilimumab to frontline nivolumab.
A novel aspect of our study design was to investigate the effect of treatment discontinuation in nivolumab responders. We demonstrated that a subset of patients (42%) maintained durable responses beyond 1 year after nivolumab discontinuation, exceeding the prespecified metric of success for this arm. Longer follow-up is necessary because three patients who remain off treatment have not reached the 1-year assessment timepoint, and they were conservatively included as nonresponders. Although the low number of patients allocated to arm A (n = 12) preclude definitive conclusions about this approach, the data warrant additional investigation, and a randomized discontinuation trial may be merited to formally evaluate this strategy. In the absence of a predictive biomarker to identify patients most likely to benefit from treatment discontinuation and the low number of patients allocated to this, we would not recommend early (4- to 6-month) therapy discontinuation in the absence of toxicity or progression.
With regard to toxicity, rates of grade 3-4 TRAEs and treatment discontinuation for toxicity in our study were within the rates observed with nivolumab from CheckMate-0251 and nivolumab and ipilimumab from CheckMate-214.2 We observed more TRAEs than observed with single-agent nivolumab but fewer than those observed with nivolumab combined with four doses of ipilimumab.
Although this study was a prospective, multicenter adaptive trial, there are several limitations. At the time our study was designed, data in melanoma demonstrated that approximately 40% of patients treated with ipilimumab were not able to receive all four intended doses.16 Additionally, CheckMate-016 investigating differing dosing regimens of nivolumab and ipilimumab in RCC demonstrated differences in toxicity between regimens.17 For this reason, the study only tested the addition of two doses of ipilimumab. The study included a heterogeneous patient population including both treatment-naïve and previously treated patients and patients with nonclear-cell RCC. The study had a small sample size with a limited number of patients allocated to arm A, precluding definitive conclusions about therapy discontinuation. Although the study protocol mandated confirmation of responses, response assessments were investigator assessed. Finally, baseline tissue biomarkers of response, including PD-L1 status and CD8+ T-cell infiltration, are currently underway to better define responding patients.
In summary, our novel, multicenter, phase II trial addressed important clinically relevant questions in the field regarding optimizing the use of immune checkpoint blockade in patients with advanced RCC. Using a response-adaptive design, we investigated the sequential addition of two doses of ipilimumab to nivolumab nonresponders and discontinuation of nivolumab in responding patients. Our data highlight that nivolumab followed by response-based addition of two cycles of ipilimumab results in a lack of CRs and a low PR/CR conversion rate. Additionally, early nivolumab discontinuation in the absence of toxicity resulted in durable responses in a subset of patients. Based on these findings, we cannot recommend a response-based adaptive strategy for nivolumab and ipilimumab, and recommend upfront dual checkpoint blockade in patients eligible to receive this treatment. We are currently investigating novel biomarkers of response and resistance to therapy to better optimize treatment strategies for patients.
ACKNOWLEDGMENT
The authors acknowledge the patients and their families for participating in this study. Additionally, the authors acknowledge the following investigators for their study participation: Neeraj Agarwal (University of Utah), Atish Choudhury (Dana-Farber Cancer Institute), Rohan Garje (University of Iowa), Kerry Kilbridge (Dana-Farber Cancer Institute), Glenn Liu (University of Wisconsin), James W. Mier (Beth Israel Deaconess Medical Center), Matthew Milowsky (University of North Carolina), Mark Pomerantz (Dana-Farber Cancer Institute), Murtuza Rampurwala (University of Chicago), Guru Sonpavde (Dana-Farber Cancer Institute), Grace Suh (University of Chicago), and Randy F. Sweis (University of Chicago). The authors acknowledge all the study team members, including co-investigators, clinical research coordinators at all sites including Dana-Farber/Harvard Cancer Center (John Steinharter, Emanuelle Andrianopoulos), regulatory personnel, and research nurses. The authors acknowledge Michael B. Atkins for his review and editorial support.
Appendix
Study Design
Response was investigator assessed by RECIST version 1.1. In patients who experienced a response, subsequent imaging assessments were used for response confirmation, which was mandated by the study protocol. Confirmation of progressive disease was required unless deemed clinically detrimental by the investigator. Baseline measurements to assess response were reset at the initiation of ipilimumab for arm B. After treatment discontinuation, an optional tumor biopsy was performed. Toxicity was assessed by Common Terminology Criteria for Adverse Events version 4.0.
Hypothesis Selection
The null and alternative hypothesis rates were selected based on historical data at the time the trial was launched. In patients with renal cell carcinoma who were previously treated, the published response rates to nivolumab monotherapy range from 25% to approximately 29% (McDermott D, et al: J Clin Oncol Jun 20;33(18):2013-20., 2015; Motzer R, et al: N Engl J Med Nov 5;373(19):1803-13., 2015). Given that our study included both treatment-naïve and previously treated patients, we assumed 29% (23 of 80) of patients would have a confirmed partial response or complete response transitioning to arm A, the remaining 57 patients with progressive disease or confirmed stable disease transitioning to arm B. The overall enrollment was 83, accounting for 4% early dropout during the induction phase.
For arm A, there were no prior data for this novel treatment approach. The study design was to distinguish a true durable partial response/complete response rate of 35% from 10%, which was considered clinically meaningful from investigators’ perspectives. For arm B, a small phase I (CheckMate 016) reported an objective response rate of 45% in patients treated with different dosing regimens of nivolumab and ipilimumab. Because our study included patients who did not respond to initial nivolumab and patients received salvage therapy with two doses of ipilimumab, a partial response/complete response rate of 20% would be considered promising in this population.
Futility Monitoring
To ensure that discontinuation of nivolumab was not detrimental for patients with initial confirmed partial response/complete response, a futility assessment was planned after nine of 23 arm A patients (39%) had undergone at least one imaging assessment since discontinuing nivolumab. Because we targeted the 1-year remission rate of at least 35% in arm A, an early progressive disease rate of 60% or higher at first scan post-treatment discontinuation would indicate the failure of this treatment approach. Therefore, if we observed five or more of the first nine patients experiencing progressive disease, enrollment of arm A would be suspended. If the true early progressive disease rate is 60% or higher, the probability of observing five or more patients with progressive disease out of nine patients is at least 73% (early stopping probability).
Arm B used a Simon’s two-stage design. If one or fewer partial responses/complete responses are observed in the first 20 patients, arm B will be suspended. If two or more partial responses/complete responses are observed, an additional 37 patients will be enrolled for a total of 57 evaluable patients. The regimen will be declared worthy of additional study if six or more partial responses/complete responses are observed. These decision rules result in a 73% probability of stopping early (at the end of the first stage) if the regimen is inactive. The design yields a 92% probability (statistical power) of declaring the regimen active given a true partial response/complete response rate of 20% or 5% probability of declaring the regimen active given a true partial response/complete response rate of 5% or less.
FIG A1.
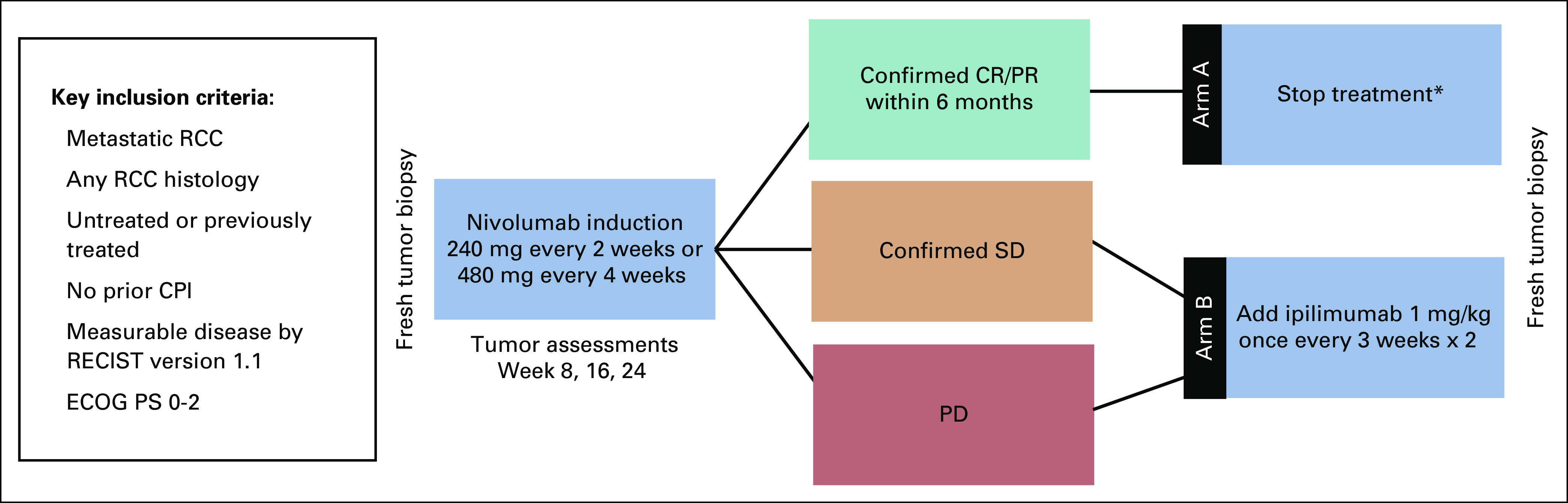
OMNIVORE study schema. (*) If a patient developed progressive disease (PD) after treatment discontinuation, nivolumab was reinitiated; if PD persisted or recurred, ipilimumab 1 mg/kg once every 2 weeks × 2 was added. Futility assessment was performed after the first nine patients enrolled. CPI, checkpoint inhibitor; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; PR, partial response; SD, stable disease; RCC, renal cell carcinoma.
TABLE A1.
Efficacy Endpoint and Definition
TABLE A2.
Disposition of Patients Discontinuing the Study During Induction Nivolumab Because of Toxicity (n = 7)
TABLE A3.
Summary of Objective Response to Nivolumab Induction According to Prior Treatment Status and IMDC Risk Group
TABLE A4.
Baseline Characteristics and Treatment Response Parameters for Patients Allocated to Arm A (n = 12)
TABLE A5.
Maximum Grade by Toxicity Type for Treatment-Related Toxicities From Nivolumab Induction (n = 83)
TABLE A6.
Maximum Grade by Toxicity Type for Treatment-Related Toxicities From Arm B (n = 57)
PRIOR PRESENTATION
Presented at the ASCO 2020 Virtual Annual Meeting, Chicago, IL, May 29-June 2, 2020.
SUPPORT
This study was investigator sponsored with support from Bristol Myers Squibb. R.R.M. is supported in part by the National Cancer Institute (NCI) Grant No. P30CA023100-34. D.A.B. is supported in part by the Dana-Farber/Harvard Cancer Center (DF/HCC) Kidney Cancer Specialized Program of Research Excellence career enhancement program (Grant N9. P50CA101942-15), Department of Defense (DOD)–Congressionally Directed Medical Research Program (Grants No. KC170216, KC190130), and DOD Academy of Kidney Cancer Investigators (Grant No. KC190128). T.L.R. is supported by the Doris Duke Charitable Foundation (Grant No. 2015213) and the NCI at the National Institutes of Health (Grant No. 1K08CA248967-01). T.K.C. is supported in part by the DF/HCC Kidney SPORE and Program P50CA101942, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at the Dana-Farber Cancer Institute.
EQUAL CONTRIBUTION
R.R.M., B.A.M., and W.X. contributed equally to this work. L.C.H. and T.K.C. contributed equally to this work.
CLINICAL TRIAL INFORMATION
NCT03203473 (OMNIVORE)
AUTHOR CONTRIBUTIONS
Conception and design: Rana R. McKay, Wanling Xie, Toni K. Choueiri
Financial support: Toni K. Choueiri
Administrative support: Lauren C. Harshman, Toni K. Choueiri
Provision of study materials or patients: Rana R. McKay, Bradley A. McGregor, Xiao Wei, Christos E. Kyriakopoulos, Tracy L. Rose, Walter M. Stadler, David F. McDermott, Lauren C. Harshman, Toni K. Choueiri
Collection and assembly of data: Rana R. McKay, Bradley A. McGregor, Christos E. Kyriakopoulos, Yousef Zakharia, Benjamin L. Maughan, Tracy L. Rose, Walter M. Stadler, Lauren C. Harshman, Toni K. Choueiri
Data analysis and interpretation: Rana R. McKay, Bradley A. McGregor, Wanling Xie, David A. Braun, Xiao Wei, Christos E. Kyriakopoulos, Yousef Zakharia, Benjamin L. Maughan, Tracy L. Rose, Walter M. Stadler, David F. McDermott, Lauren C. Harshman, Toni K. Choueiri
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Vividion, Bayer, Sanofi, Merck
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Bradley A. McGregor
Consulting or Advisory Role: Bayer, Seattle Genetics/Astellas, Exelixis, AstraZeneca, Astellas Pharma, Genentech, Nextar, Janssen Oncology, Pfizer, EMD Serono, Eisai
Research Funding: Bristol Myers Squibb (Inst), Exelixis (Inst), Calithera Biosciences (Inst), Seattle Genetics/Astellas (Inst)
David A. Braun
Honoraria: LM Education/Exchange Service
Consulting or Advisory Role: Bristol Myers Squibb, Octane Global, Defined Health, Dedham Group, Adept Field Solutions, Slingshot Insights, Blueprint Partnerships, Charles River Associates, Trinity Group, Insight Strategy, Schlesinger Associates
Travel, Accommodations, Expenses: Bristol Myers Squibb
Xiao Wei
Honoraria: OncLive
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Corvus Pharmaceuticals
Christos E. Kyriakopoulos
Consulting or Advisory Role: Exelixis
Research Funding: Sanofi
Yousef Zakharia
Consulting or Advisory Role: Genentech, Eisai, Amgen, Castle Biosciences, Novartis, Exelixis, Pfizer, Cardinal Health, Bayer, Janssen, TTC Oncology, Clovis Oncology
Travel, Accommodations, Expenses: Newlink Genetics
Benjamin L. Maughan
Consulting or Advisory Role: Janssen Oncology, Exelixis, Tempus, Peleton, Bristol Myers Squibb, Astellas Medivation, Bayer
Research Funding: Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Bavarian Nordic (Inst)
Travel, Accommodations, Expenses: Exelixis
Tracy L. Rose
Research Funding: Genentech, GeneCentric, Bristol Myers Squibb, Merck
Walter M. Stadler
Consulting or Advisory Role: CVS Caremark, Sotio, AstraZeneca, Eisai, Bayer, Pfizer, Merck
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Exelixis (Inst), Novartis (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Medivation (Inst), Pfizer (Inst), Merck (Inst), Millennium (Inst), Janssen (Inst), Johnson & Johnson (Inst), AstraZeneca (Inst), AbbVie (Inst), X4 Pharma (Inst), Calithera Biosciences (Inst), Clovis Oncology (Inst), Eisai (Inst), Seattle Genetics (I), Tesaro (Inst), Corvus Pharmaceuticals (Inst), Astellas Medivation (Inst)
Other Relationship: UpToDate, American Cancer Society
David F. McDermott
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Genentech, Pfizer, Exelixis, Novartis, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Eli Lilly, Eisai
Research Funding: Prometheus Laboratories (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Novartis (Inst), Alkermes (Inst), Peloton Therapeutics (Inst)
Other Relationship: BIDMC
Uncompensated Relationships: X4 Pharmaceuticals, AVEO
Lauren C. Harshman
Employment: Surface Oncology
Consulting or Advisory Role: Pfizer, Genentech, Corvus Pharmaceuticals, Merck, Exelixis, Bayer, Novartis, Jounce Therapeutics, EMD Serono, Michael J. Hennessy Associates, Ology Medical Education, Bristol Myers Squibb
Research Funding: Medivation/Astellas (Inst), Bayer (Inst), Sotio (Inst), Genentech (Inst), Dendreon (Inst), Bristol Myers Squibb (Inst), Takeda (Inst), Merck (Inst), Janssen Oncology (Inst), Pfizer (Inst), Endocyte (Inst)
Travel, Accommodations, Expenses: Genentech
Toni K. Choueiri
Employment: Dana-Farber Cancer Hospital
Leadership: Dana-Farber Cancer Hospital, NCCN, KidneyCan, ASCO
Stock and Other Ownership Interests: Pionyr, Tempest Therapeutics
Honoraria: NCCN, UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Healthcare, Kidney Cancer Journal, Exelixis, Prometheus, Lpath, The New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Eli Lilly
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus Laboratories, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Healthcare, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Eli Lilly, European Society for Medical Oncology
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst), Celldex (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Prometheus (Inst), Ipsen (Inst), Corvus Pharmaceuticals (Inst), Cerulean Pharma (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Foundation Medicine (Inst), Roche (Inst), Calithera Biosciences (Inst), Analysis Group (Inst), NCI (Inst), GATEWAY for Cancer Research (Inst), Congressionally Directed Medical Research Programs (DOD; Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430 entitled: Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy; International Patent Application No. PCT/US2018/12209 entitled: PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Journal, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Healthcare, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Eli Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
No other potential conflicts of interest were reported.
REFERENCES
- 1.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tannir NM, McDermott DF, Escudier B, et al: Overall survival and independent review of response in CheckMate 214 with 42-month follow-up: First-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 38, 2020 (suppl; abstr 609) [Google Scholar]
- 4.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17:504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- 6. Motzer RJ, Tykodi SS, Escudier B, et al: Final analysis of the CheckMate 025 trial comparing nivolumab (NIVO) versus everolimus (EVE) with >5 years of follow-up in patients with advanced renal cell carcinoma (aRCC). J Clin Oncol 38, 2020 (suppl; abstr 617) [Google Scholar]
- 7. McDermott DF, Rini BI, Motzer RJ, et al: Treatment-free survival (TFS) after discontinuation of first-line nivolumab (NIVO) plus ipilimumab (IPI) or sunitinib (SUN) in intention-to-treat (ITT) and IMDC favorable-risk patients (pts) with advanced renal cell carcinoma (aRCC) from CheckMate 214. J Clin Oncol 37, 2020 (suppl; abstr 564) [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol. 2020;17:137–150. doi: 10.1038/s41585-020-0282-3. [DOI] [PubMed] [Google Scholar]
- 11.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1371/journal.pone.0161779. Selby MJ, Engelhardt JJ, Johnston RJ, et al: Preclinical development of ipilimumab and nivolumab combination immunotherapy: Mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One 11:e0161779, 2016 [Erratum: PLoS One 11:e0167251, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDermott DF, Lee J-L, Szczylik C, et al: Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): Results from cohort A of KEYNOTE-427. J Clin Oncol 36, 2018 (suppl; abstr 4500) [Google Scholar]
- 14. Grimm M, Schmidinger M, Martinez ID, et al. Tailed immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Annal Oncol 30:v851-v934, 2019 (suppl 5) [Google Scholar]
- 15. doi: 10.1200/JCO.21.02938. Atkins MB, Jegede O, Haas NB, et al: Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J Clin Oncol 38, 2020 (suppl; abstr 5006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1056/NEJMoa1414428. Postow MA, Chesney J, Pavlick AC, et al: Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006-2017, 2015 [Erratum: N Engl J Med 379:2185, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 Study. J Clin Oncol. 2017;35:3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]