PURPOSE
Relapse is a major cause of treatment failure after allogeneic hematopoietic stem-cell transplantation (allo-HSCT) for high-risk acute myeloid leukemia (HR-AML). The aim of this study was to explore the effect of recombinant human granulocyte colony-stimulating factor (rhG-CSF) combined with minimal-dose decitabine (Dec) on the prevention of HR-AML relapse after allo-HSCT.
PATIENTS AND METHODS
We conducted a phase II, open-label, multicenter, randomized controlled trial. Two hundred four patients with HR-AML who had received allo-HSCT 60-100 days before randomization and who were minimal residual disease negative were randomly assigned 1:1 to either rhG-CSF combined with minimal-dose Dec (G-Dec group: 100 µg/m2 of rhG-CSF on days 0-5 and 5 mg/m2 of Dec on days 1-5) or no intervention (non–G-Dec group). The primary outcome was relapse after transplantation, and the secondary outcomes were chronic graft-versus-host disease (cGVHD), safety of the treatment, and survival.
RESULTS
The estimated 2-year cumulative incidence of relapse in the G-Dec group was 15.0% (95% CI, 8.0% to 22.1%), compared with 38.3% (95% CI, 28.8% to 47.9%) in the non–G-Dec group (P < .01), with a hazard ratio (HR) of 0.32 (95% CI, 0.18 to 0.57; P < .01). There was no statistically significant difference between the G-Dec and non–G-Dec groups in the 2-year cumulative incidence of cGVHD without relapse (23.0% [95% CI, 14.7% to 31.3%] and 21.7% [95% CI, 13.6% to 29.7%], respectively; P = .82), with an HR of 1.07 (95% CI, 0.60 to 1.92; P = .81). After rhG-CSF combined with minimal-dose Dec maintenance, increasing numbers of natural killer, CD8+ T, and regulatory T cells were observed.
CONCLUSION
Our findings suggest that rhG-CSF combined with minimal-dose Dec maintenance after allo-HSCT can reduce the incidence of relapse, accompanied by changes in the number of lymphocyte subtypes.
INTRODUCTION
The prognosis of patients with high-risk (HR) acute myeloid leukemia (AML) continues to worsen after treatment. One potentially curative regimen for HR-AML is allogeneic hematopoietic stem-cell transplantation (allo-HSCT). Over the past decade, although considerable advances have been achieved in therapeutic approaches for allo-HSCT, the best survival rate among adults with HR-AML who have undergone allo-HSCT after achieving complete remission (CR) is only approximately 55%, and relapse remains the leading cause of death in patients after allo-HSCT, accounting for 20%-50% of deaths.1-3 Donor lymphocyte infusion (DLI) is one of the most common interventions for AML relapse because donor lymphocytes are expected to promote the graft-versus-leukemia (GVL) effect.4 However, DLI treatment success in AML relapse has been limited, and the reported overall survival (OS) rates at 3 years are only 10%-20%.5 Accordingly, the development of novel regimens to prevent leukemia relapse remains the highest priority in the treatment of HR-AML after allo-HSCT.
CONTEXT
Key Objective
Relapse remains the leading cause of death in patients with high-risk acute myeloid leukemia (HR-AML) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). This open-label, multicenter, randomized, controlled, phase 2 trial examined whether recombinant human granulocyte colony-stimulating factor (rhG-CSF) combined with minimal-dose decitabine (Dec) have the effect on preventing AML relapse. It was contained 204 patients and, to our knowledge, is the largest prospective, controlled clinical study to date.
Knowledge Generated
In this study, we revealed that rhG-CSF combined with minimal-dose Dec maintenance therapy after allo-HSCT can reduce the incidence of relapse, accompanied by changes in the number of lymphocyte subtypes, leading to the acquisition of graft-versus-leukemia and immune tolerance.
Relevance
Our study demonstrated that rhG-CSF combined with minimal-dose Dec maintenance therapy can be selected as the optimal treatment for HR-AML after allo-HSCT. As a result, this maintenance therapy brings survival benefits by reducing the relapse rate.
Decitabine (Dec) is a hypomethylating agent that is currently approved for the treatment of high-risk myelodysplastic syndrome, chronic myelomonocytic leukemia, and AML.6,7 Recently, retrospective case series studies and small-sample, prospective, single-arm studies have found that the primary effects of hypomethylating treatment for the prevention of AML relapse after allo-HSCT consist of an increase in the number of T regulatory (Treg) cells and the induction of cytotoxic CD8+ T-cell responsiveness to tumor antigens.8-10 Furthermore, these studies explored the appropriate doses and optimal treatment courses of hypomethylating agents.8,9 However, further optimization of the anti-AML effect while reducing the hematologic toxicities of hypomethylating agents must still be addressed.
Importantly, the demethylating effect of Dec has been shown to be cyclin dependent,11 indicating that the combination of Dec with an agent that promotes cell cycle entry could synergistically promote the elimination of AML and prevent relapse. Notably, granulocyte colony-stimulating factor (G-CSF) is a soluble growth factor that interacts with the G-CSF receptor to promote cell entry into the cell cycle.12 Moreover, G-CSF and Dec have been shown to promote the production and function of cytotoxic CD8+ T cells, natural killer (NK) cells. and Treg cells,8,13 which may promote the GVL effect and reduce graft-versus-host disease (GVHD). We hypothesized that recombinant human G-CSF (rhG-CSF) followed by minimal-dose Dec could effectively prevent AML relapse. To test this hypothesis, we conducted a multicenter, open-label, randomized controlled study to investigate the efficacy, safety, and tolerability of G-CSF combined with minimal-dose Dec for prophylaxis against relapse in patients who underwent allo-HSCT.
PATIENTS AND METHODS
Study Design and Participants
This study was a phase II, open-label, multicenter, randomized controlled trial (ChiCTR-IIR-16008182) conducted by the 12 hospitals in Hematopoietic Stem Cell Transplantation–Western China Group. The protocol and all amendments were approved by the China Registered Clinical Laboratory Ethics Committee and the institutional review boards at each of the 12 participating institutions. All of the patients or their legal guardians signed informed consent forms in accordance with the Declaration of Helsinki.
Eligible patients with HR-AML included in the trial were categorized as having AML with poor genetic abnormalities,14 primary refractory AML, relapsed AML, or secondary AML.15,16 Eligible patients met the following criteria: established stable donor hematopoiesis; CR and minimal residual disease (MRD) negative; no acute GVHD (aGVHD) or controlled aGVHD for > 60 days after transplantation; and no uncontrolled infections or severe liver, renal, lung, or heart disease. The exclusion criteria were serious organ dysfunction, leukemia combined with other cancers, active systemic infection, GVHD requiring treatment, inability to schedule follow-up, brain dysfunction or severe mental illness preventing compliance with the study protocol, and hypersensitivity to any components of rhG-CSF and/or Dec.
Sample Size and Power Analysis
Our primary outcome variable was the cumulative incidence of relapse. The sample size was determined using the log-rank test comparing two cumulative incidences. In the preliminary research, the incidences of relapse in the patients who received rhG-CSF plus Dec (G-Dec group) and the patients who received no treatment (non–G-Dec group) were 12.6% and 32.1%, respectively. We assumed 1 year of enrollment and 2 years of follow-up. With a power of 90% and a two-sided test of α = .05 type I error, a total sample size of 204 patients would be needed, with 102 patients in each arm. With this sample size, if there were no deaths without relapses, 46 relapses would be observed, obtaining a 90% power to test a treatment difference between 67.9% and 87.4% with a hazard ratio (HR) of 0.35. See Data Supplement (online only) for details.
Randomization and Procedure
A stratified block randomization method was used in this study. At each research center, consenting eligible participants were stratified by disease status before HSCT (two levels: CR and MRD negative [CRMRD−] and CR and MRD positive [CRMRD+]/partial remission [PR]/no remission [NR]) and randomly assigned in a 1:1 ratio to the G-Dec group or non–G-Dec group using a computer-generated block randomization schedule. The block size was randomly generated among the numbers 4, 6, 8, and 10.
In the G-Dec group, patients received six courses of rhG-CSF combined with minimal-dose Dec (rhG-CSF 100 µg/m2 by subcutaneous injection on days 0-5, and Dec at 5 mg/m2 by infusion on days 1-5). No treatment was given to the patients in non–G-Dec group. rhG-CSF and Dec were administered every 6-8 weeks for up to six courses. The maintenance treatment was discontinued if leukemia relapsed, severe chronic GVHD (cGVHD) occurred, or severe adverse effects of the infusion occurred or upon completion of six cycles of infusion. The observation period extended from enrollment to March 21, 2019 or to the time of the patient’s death, whichever occurred first. Both patients and clinicians were aware of the treatment allocation, but the outcome assessors were blinded to the treatment allocation when assessing all primary and secondary end points.
Statistical Analysis
The primary end point was cumulative incidence of relapse. Secondary end points included the cumulative incidence of cGVHD, cGVHD without relapse, and transplantation-related mortality (TRM); safety of rhG-CSF combined with Dec; OS; and leukemia-free survival (LFS). Exploratory end points were the cell numbers of T, B, NK, and Treg cell subsets during the G-Dec treatment courses. The Mann-Whitney U test, χ2 test, and Fisher’s exact probability test were used to compare the baseline characteristics between the G-Dec and non–G-Dec groups. The competing risk model (Fine and Gray model) was used to estimate 2-year cumulative incidences and HRs with 95% CIs of relapse, cGVHD, and cGVHD without relapse. The Kaplan-Meier method, the log-rank test, and Cox proportional hazard models were used to analyze OS and LFS. Subgroups analysis and interaction P values are shown in a forest plot. In the analysis of T, B, NK, and Treg cell subsets, an analysis of variance of repeated measurement data and t test with Bonferroni corrections were used to assess the differences in the repeated measurement data based on lymphocyte numbers during the G-Dec treatment courses. For details of the statistical analysis, see the Data Supplement.
All reported P values are two-sided. The sample size and power calculations were performed using PASS software (version 11; NCSS Statistical Software, Kaysville, UT). The statistical analyses were performed using IBM SPSS statistics software version 22 (IBM China, Beijing, China) and the R software package version 2.5.0 (http://www.r-project.org).
RESULTS
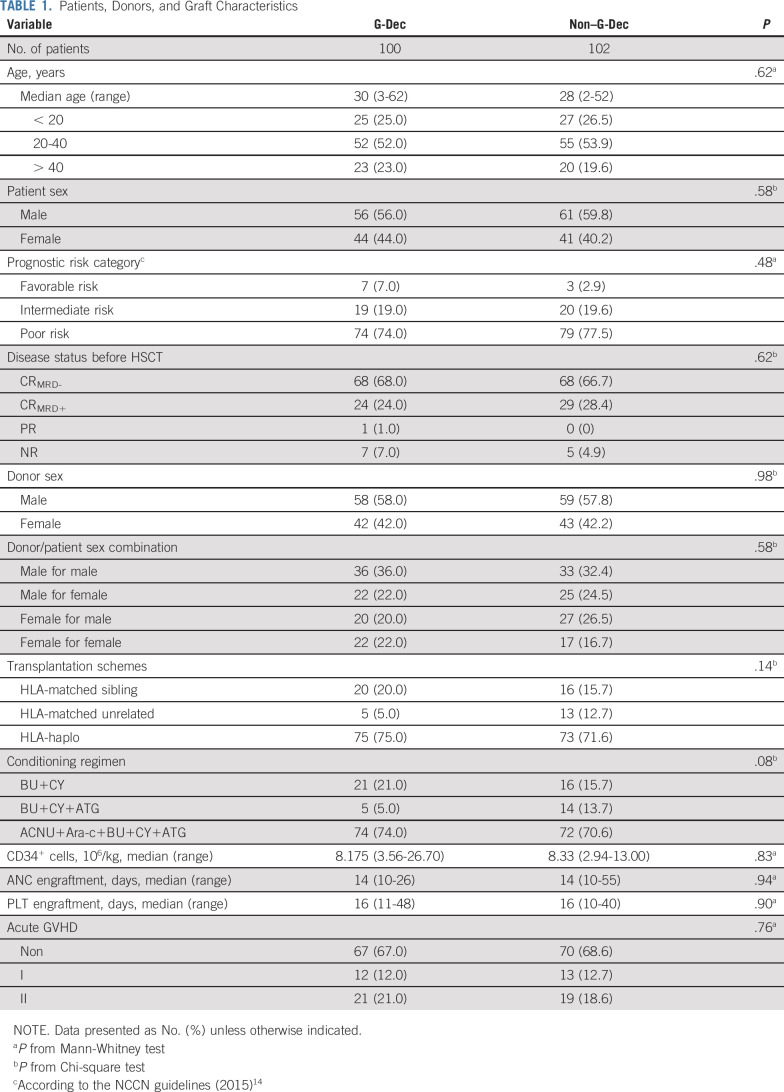
A total of 220 patients from 12 transplantation centers were included in the clinical study from April 5, 2016 to January 16, 2017 (Fig 1). The patient characteristics are provided in Table 1, and other patient characteristics are described in the Data Supplement.
FIG 1.
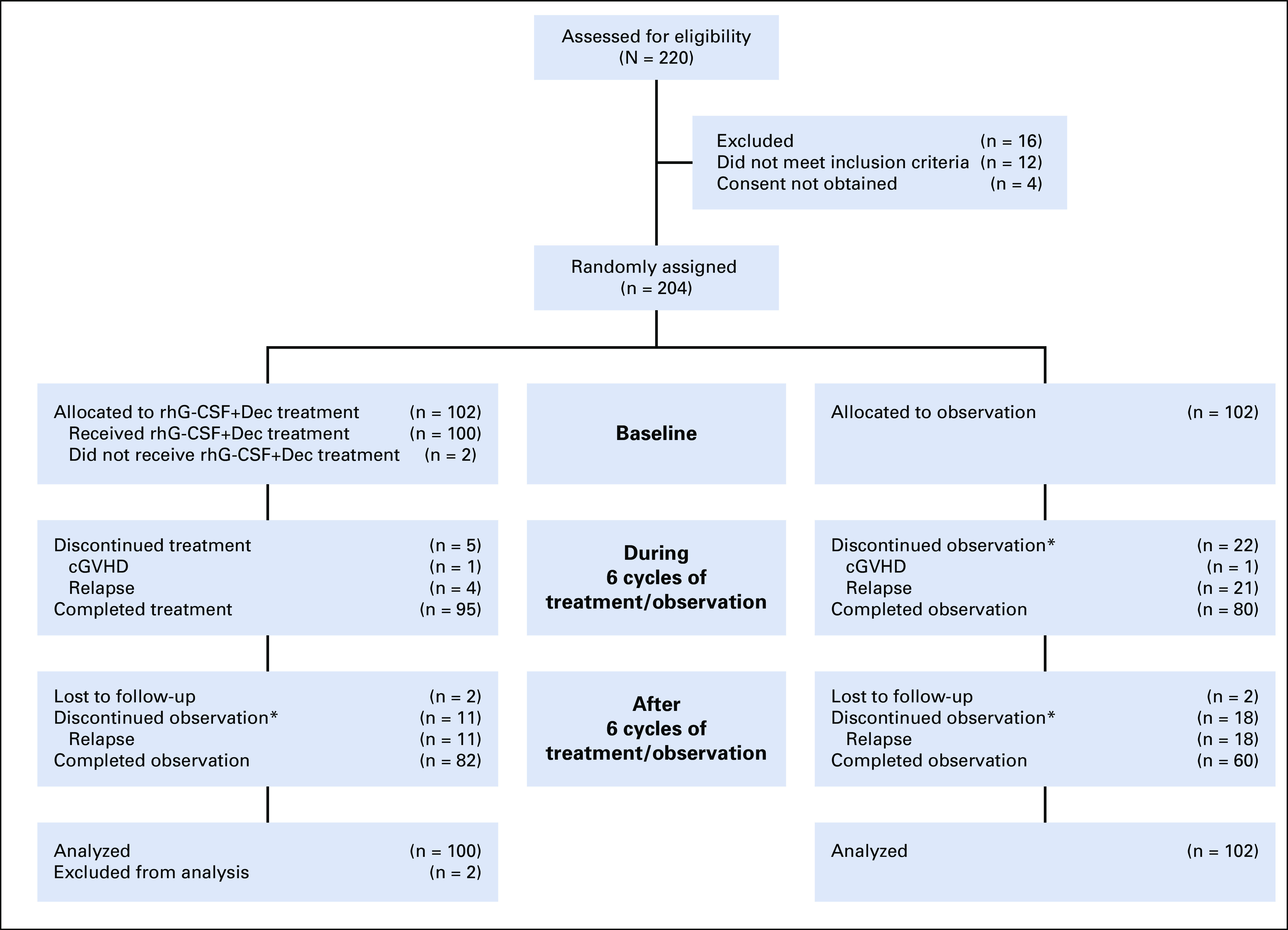
Flowchart of the study participants. (*) Observation was stopped according to the visit schedule, and only disease status and survival of the patients were followed. cGVHD, chronic graft-versus-host disease; Dec, decitabine; rhG-CSF, recombinant human granulocyte colony-stimulating factor.
TABLE 1.
Patients, Donors, and Graft Characteristics
The median follow-up time was 28.0 months (range, 2.8-35.9 months) for the G-Dec group and 26.4 months (range, 2.9-35.7 months) for the non–G-Dec group. We found that relapse occurred in 15 (15.0%) of 100 patients in the G-Dec group and 39 (38.2%) of 102 patients in the control group; the estimated 2-year cumulative incidence rates of relapse were 15.0% (95% CI, 8.0% to 22.1%) and 38.3% (95% CI, 28.8% to 47.9%), respectively, with an HR of 0.32 (95% CI, 0.18 to 0.57; P < .01; Fig 2A) . Both in the univariable competing model and the multivariable model after adjustment for possible confounders, treatment and disease status before HSCT were screened out as influencing factors of relapse. The HRs were 0.31 (95% CI, 0.18 to 0.56; P < .01) and 2.77 (95% CI, 1.60 to 4.79; P < .01) in the multivariable model (Data Supplement). We also performed subgroup analyses based on different clinical characteristics. Among the patients with CRMRD−, the 2-year cumulative incidence of relapse in the G-Dec group was lower than that in the non–G-Dec group (5.9% [95% CI, 0.2% to 11.6%] v 31.0% [95% CI, 19.9% to 42.1%], respectively; P < .01), with an HR of 0.16 (95% CI, 0.06 to 0.48; P < .01). For patients with CRMRD+/PR/NR before transplantation, G-Dec maintenance therapy showed a tendency to reduce the 2-year cumulative incidence of relapse after transplantation compared with no G-Dec (34.5% [95% CI, 17.7% to 51.4%] v 52.9% [95% CI, 35.8% to 70.1%], respectively; P = .05), with an HR of 0.48 (95% CI, 0.23 to 0.99; P = .05). There was no interaction between treatment and disease status before HSCT (P = .10). The other clinical characteristics also showed no interaction with the treatment (Appendix Fig A1, online only).
FIG 2.
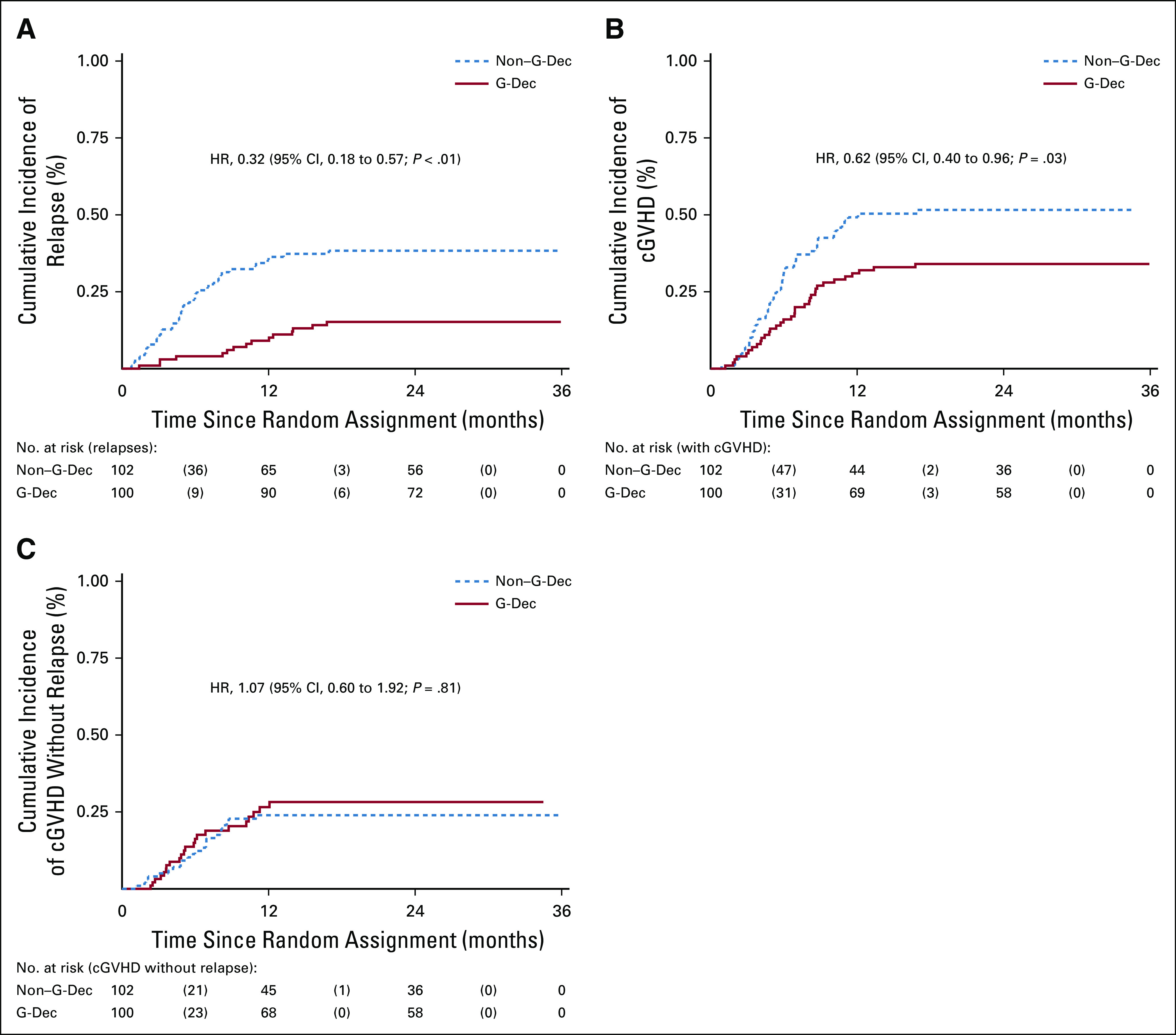
Cumulative incidence of (A) relapse, (B) total chronic graft-versus-host disease (cGVHD), and (C) cGVHD without relapse. G-Dec, recombinant human granulocyte colony-stimulating factor plus decitabine; HR, hazard ratio.
All patients who survived longer than 100 days after HSCT were evaluated to determine the incidence of cGVHD. In the G-Dec group, cGVHD developed in 34 patients (34.0%) as follows: 30 (30.0%) exhibited mild or moderate cGVHD, whereas four (4.0%) exhibited severe cGVHD. In the non–G-Dec group, cGVHD occurred in 49 patients (48.0%) as follows: 45 (44.1%) exhibited mild or moderate cGVHD, and four (3.9%) exhibited severe cGVHD. The 2-year cumulative incidence rates of cGVHD in the G-Dec and non–G-Dec groups were 34.0% (95% CI, 24.7% to 43.3%) and 48.2% (95% CI, 38.4% to 58.0%), respectively (HR, 0.62; 95% CI, 0.40 to 0.96; P = .03; Data Supplement and Fig 2B). Eleven patients in the G-Dec group and 27 patients in the non–G-Dec group developed cGVHD as a result of the discontinuation of immunosuppressants and DLI during recurrence of the disease. Accordingly, no difference was found between the two groups in the incidence of cGVHD without relapse intervention (23.0% [95% CI, 14.7% to 31.3%] in G-Dec group v 21.7% [95% CI, 13.6% to 29.7%] in non–G-Dec group; HR, 1.07 [95% CI, 0.6 to 1.92]; P = .81; Data Supplement and Fig 2C). The clinical manifestations of cGVHD are described in the Data Supplement.
Before the study, 20 patients were randomly identified in each group to examine the effect of the G-Dec treatment on lymphocyte subpopulations. The baseline characteristics and representative analysis of 20 patients in each group are provided in the Data Supplement. We monitored the immune cell subtypes, including T cells, B cells, NK cells, and Treg lymphocytes. No significant changes were observed in the numbers of B cells or CD4+ T cells in either the G-Dec group or the non–G-Dec group (P > .05, Figs 3A and 3B). In contrast, the numbers of CD8+ T, NK, and Treg cells increased by the second to third course of G-Dec treatment (P < .05, Figs 3C-3E). Next, for 20 patients in each group, the numbers of CD8+ T, NK, and Treg cells at the end of the last course of G-Dec treatment were used as covariates in a competing risk model to assess the association between increased effector cell numbers and relapse. The univariable competing model revealed that the increase in CD8+ T, NK, and Treg cells reduced the risk of relapse, and their HRs were 0.99 (95% CI, 0.98 to 1.00; P = .02), 0.97 (95% CI, 0.95 to 0.99; P < .01), and 0.96 (95% CI, 0.92 to 1.00; P = .04), respectively. In the multivariable model, the number of NK cells was found to be an independent factor influencing relapse, with an HR of 0.96 (95% CI, 0.94 to 0.99; P < .01; Data Supplement).
FIG 3.
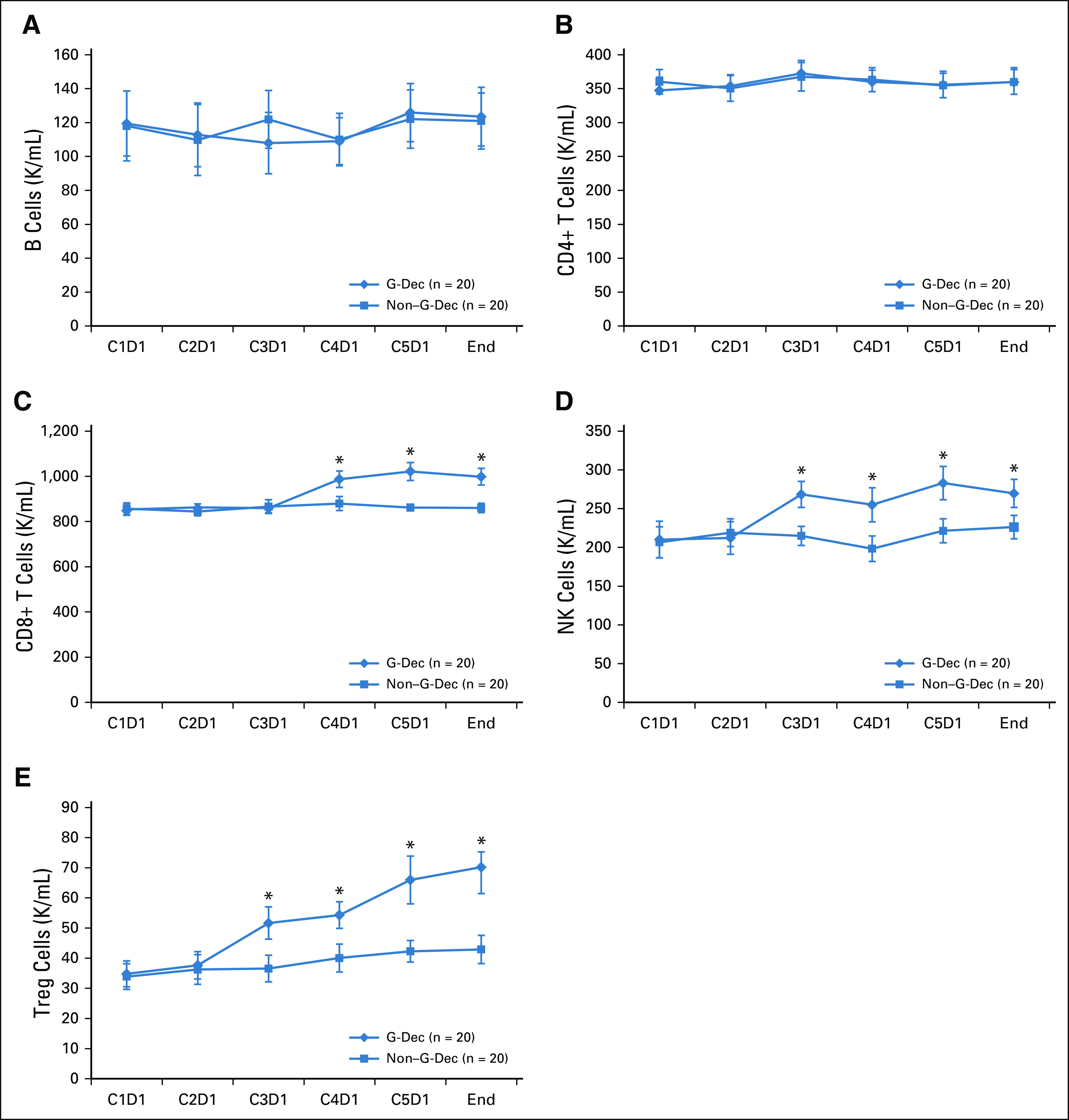
Changes in the mean absolute counts of lymphocyte subsets after recombinant human granulocyte colony-stimulating factor (rhG-CSF) plus decitabine (Dec) maintenance treatment (G-Dec group). The number of patients in both the G-Dec group and the non–G-Dec (no treatment) control group was 20. (A) Natural killer (NK) cells. (B) CD19+ B cells. (C) CD3+CD4+ T cells. (D) CD3+CD8+ T cells. (E) CD4+CD25+FOXP3+ T cells. (*) After Bonferroni correction, P < .001 v non–G-Dec group. C, cycle; C1D1, first day in the first cycle before medication; D, day; K, thousand; Treg, regulatory T cell.
Eighty-three patients in the G-Dec group and 70 patients in the non–G-Dec group were still alive on January 16, 2019. The 2-year cumulative incidence rates of TRM in the G-Dec and non–G-Dec groups were 3.4% (95% CI, 1.1% to 10.3%) and 1.6% (95% CI, 0.2% to 11.1%), respectively, with an HR of 2.4 (95% CI, 0.25 to 22.97; P = .44). The causes of TRM included severe infections in three patients and recurrent episodes of serious GVHD in two patients. The 2-year rates of LFS in the G-Dec and non–G-Dec groups were 81.9% (95% CI, 72.8% to 88.2%) and 60.7% (95% CI, 50.5% to 69.4%), respectively, with an HR of 0.38 (95% CI, 0.22 to 0.66; P < .01; Fig 4A). The 2-year rates of OS in the G-Dec and non–G-Dec groups were 85.8% (95% CI, 77.1% to 91.3%) and 69.7% (95% CI, 59.6% to 77.8%), respectively, with an HR of 0.45 (95% CI, 0.24 to 0.83; P = .01; Fig 4B).
FIG 4.
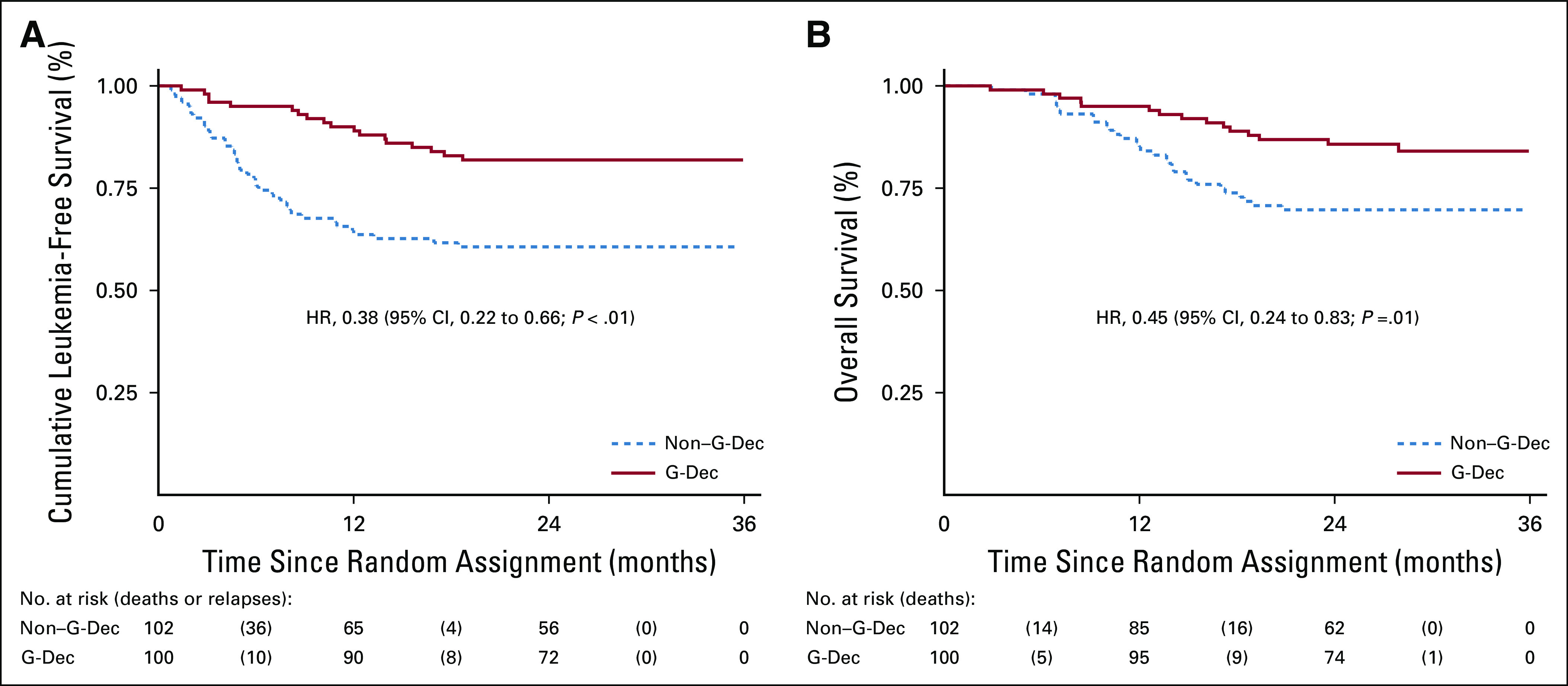
(A) Leukemia-free survival and (B) overall survival among the patients in the two groups. G-Dec, recombinant human granulocyte colony-stimulating factor plus decitabine; HR, hazard ratio.
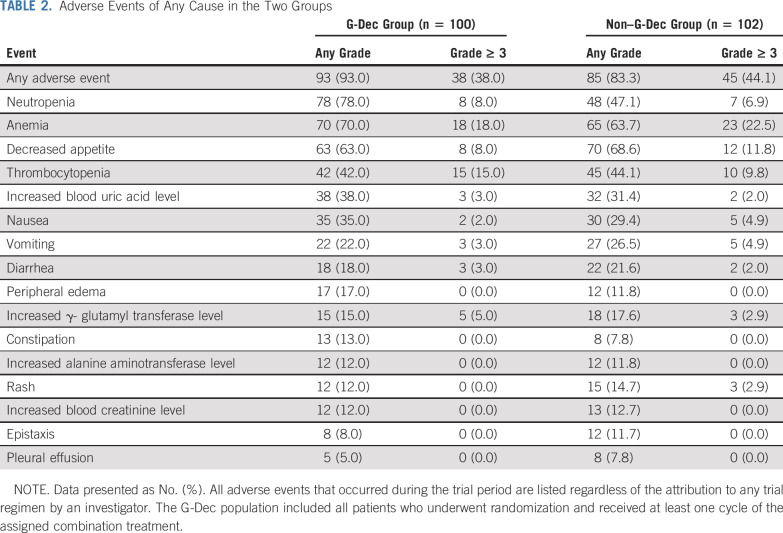
Adverse events of any grade regardless of attribution to a trial regimen by an investigator occurred in 93.0% of the patients in the G-Dec group and 83.3% of the patients in the non–G-Dec group. The most common treatment-emergent adverse event was hypoleukocytosis, which mainly occurred during the first two courses of G-Dec treatment (Data Supplement). The nonhematologic toxicities included nausea, vomiting, diarrhea, peripheral edema, abnormal liver function, and abnormal renal function. Most adverse events were grade 1 or 2 and improved after symptomatic treatment (Table 2). No deaths resulted from lethal organ toxicities as a result of G-Dec maintenance therapy in the study.
TABLE 2.
Adverse Events of Any Cause in the Two Groups
DISCUSSION
In this study, we investigated rhG-CSF combined with minimal-dose Dec as a treatment strategy for HR-AML relapse prophylaxis. The incidence of relapse in the G-Dec group was significantly lower than that in the non–G-Dec group. In addition, the relapse rate in patients with CRMRD− before transplantation was significantly decreased after rhG-CSF combined with minimal-dose Dec treatment, indicating that maintenance treatment has a greater benefit for patients with HR-AML who are MRD negative before transplantation.
Selecting the optimal dose is important when administering hypomethylating agents after allo-HSCT. In our study, approximately 97.0% of patients tolerated at least four cycles, and 96.0% of patients completed all six cycles. Although the main dose-limiting toxicity was myelosuppression, only 13.0% of the patients (13 of 100 patients) experienced grade 3 or 4 neutropenia or thrombocytopenia requiring rhG-CSF or recombinant human thrombopoietin support, and 16.0% of patients (16 of 100 patients) experienced grade 3 or 4 anemia requiring an RBC infusion.
Both our study design and findings differ from those of previous studies using hypomethylation agent maintenance therapy after allo-HSCT. We found that rhG-CSF combined with minimal-dose Dec treatment demonstrated a prominent advantage in the prevention of AML recurrence. rhG-CSF is known to stimulate the production, maturation, and effector functions of granulocytes and co-stimulate early progenitor cells synergistically with several other cytokines.17 Although the use of rhG-CSF in AML has been controversial because it stimulates the in vitro proliferation of leukemic blast cells from most patients with AML,18-20 a large number of clinical studies show that rhG-CSF does not reduce the remission rate of AML chemotherapy.21-25 Furthermore, rhG-CSF combined with Dec and chemotherapy have been proven to improve the remission rate in elderly or refractory patients with AML.26,27 Interestingly, Xiong et al28 found that NK cell populations were expanded in G-CSF–mobilized sources and could also exhibit improved functionality, demonstrating increased GVL capacity. In addition, Dec can enhance NK cell–mediated antibody-dependent cellular cytotoxicity against AML blasts and decrease relapse in patients with AML.29 In our study, the number of NK cells was increased after rhG-CSF combined with Dec treatment. Furthermore, the number of NK cells was found to be an independent factor influencing relapse; with an increasing number of NK cells, the risk of relapse was reduced. This finding suggests that the G-Dec group had a reduced cumulative incidence of relapse as a result of an increase in NK cells. Concomitantly, we found that the number of CD8+ T cells was also increased in patients who received the G-Dec treatment compared with controls. Recently, Ghoneim H.E. et al30 reported that blocking de novo DNA methylation with Dec in activated CD8+ T cells promoted their retention of effector function and inhibited exhaustion. Furthermore, the GVL effects exerted by donor T cells against leukemic-associated antigens also play a crucial role in disease eradication and relapse prevention.31,32 Therefore, we postulate that the effect of G-CSF combined with the Dec maintenance regimen to increase the numbers of NK and CD8+ T cells might be the primary mechanism that reduces relapse in patients with HR-AML after allo-HSCT.
In the current study, G-CSF therapy combined with a Dec maintenance regimen did not increase the incidence of cGVHD in patients with HR-AML after allo-HSCT. The lymphocyte subset analysis showed that the number of Treg cells significantly increased after the second cycle of treatment. This finding is in agreement with preclinical animal models, in which Treg cells have been shown to suppress GVHD without decreasing the GVL effect.33 Dec and azacytidine (another demethylation drug) have been shown to expand immunomodulatory Treg cells in animal models and in phase I and II clinical trials.8,34 Recently, in vitro experiments have shown that the exposure of T cells to azacytidine leads to demethylation of the FOXP3 promoter, FOXP3 overexpression, and expansion of Treg cells.35 Moreover, G-CSF can promote the expansion and function of Treg cells without diminishing their function, cytokine profiles, or phenotypic characteristics.36-38
In terms of limitations, the small sample size in some subgroups might have resulted in low test power, and the open-label design might have exaggerated the treatment effect in the G-Dec group. The results require validation in a multicenter, randomized, controlled, double-blind trial with a larger sample size. In addition, only 20 patients were randomly identified in each group to examine the effect of G-Dec treatment on lymphocyte subpopulations and the relationship between lymphocyte subpopulations and relapse. The representativeness of 20 patients is relatively limited. Further studies of lymphocyte subpopulations and functional studies should be performed to show the alloreactivity or anti-AML efficacy in the entire cohort.
Altogether, to our knowledge, our findings demonstrate that rhG-CSF combined with minimal-dose Dec treatment after allo-HSCT could significantly reduce the incidence of HR-AML relapse. The reduction in relapse is associated with increased numbers of NK and CD8+ T cells, which likely promote the GVL effect, and increased numbers of Treg cells, which likely control the development of GVHD. Future studies are required to investigate combinations of other drugs or treatment regimens with minimal-dose Dec for AML relapse prophylaxis in patients who are MRD positive before transplantation.
ACKNOWLEDGMENT
We thank the patients and their families and all investigators and site personnel.
APPENDIX
FIG A1.
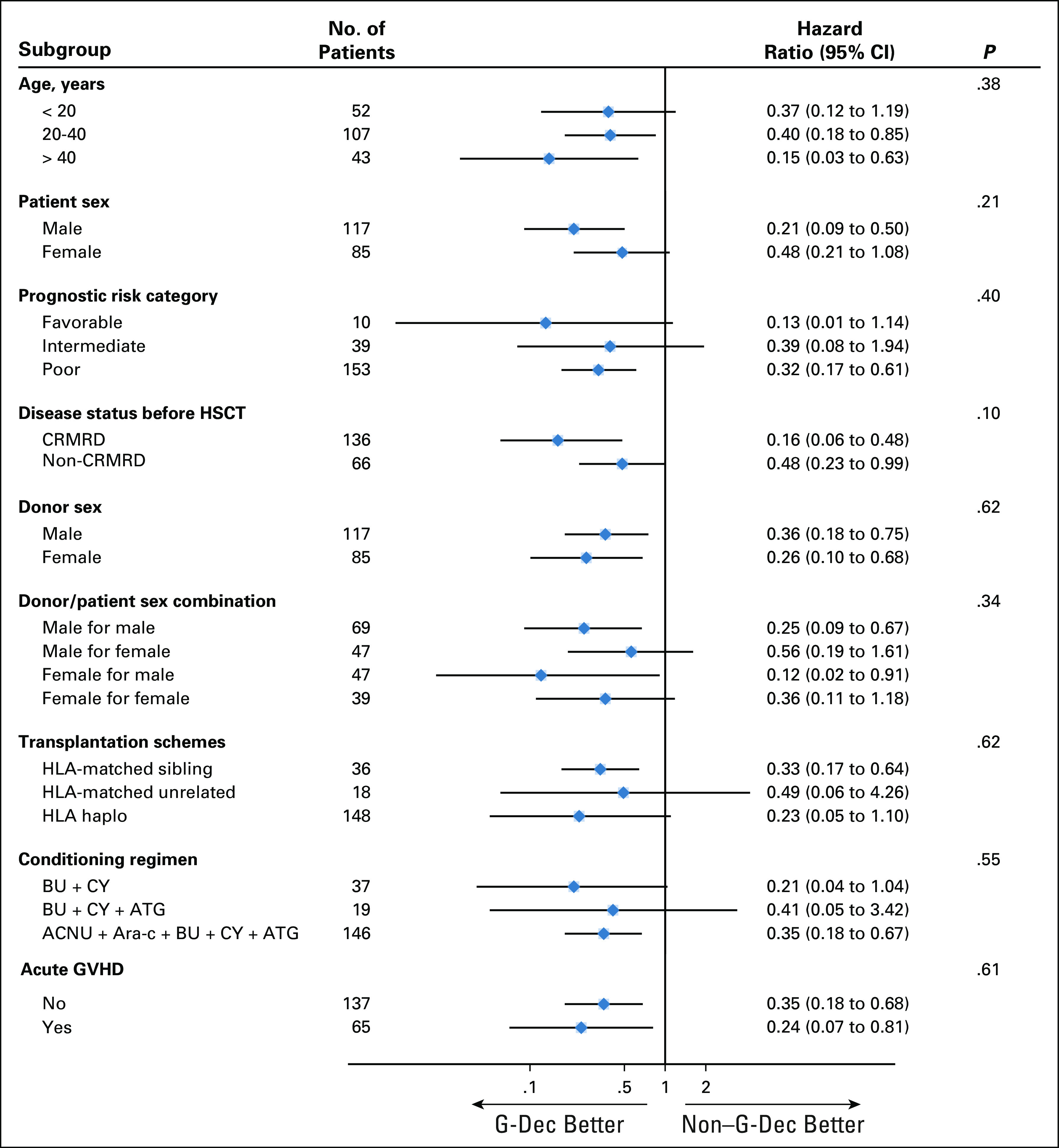
Forest plot summarizing hazard ratios for the G-Dec group versus the non-G-Dec group in the subgroup analyses with a test for interaction. ACNU, nimustine; Ara-C, cytarabine; ATG, thymoglobulin; BU, busulfan; CRMRD−, complete remission and minimal residual disease negative; CY, cyclophosphamide; HSCT, hematopoietic stem-cell transplantation.
SUPPORT
Supported by the National Key Research and Development Program of China (Grants No. 2016YFA0202104 and 2017YFA0105502), the Chinese National Natural Science Foundation (Grants No. 81370593, 81570131, 81570097, and 81873424), the Chongqing Social Undertakings, People’s Livelihood Guarantee and Technology Innovation Foundation (Grant No. cstc2016shms-ztzx10003), the Chongqing Key Project of Basic and Frontier Research Program (Grant No. cstc2015jcyjBX0077), the Chongqing National Natural Science Key Foundation (Grant No. cstc2019jcyj-zdxmX0023), the Research Fund from the Clinical Foundation of Army Medical University (Grants No. 2018JSLC0034 and 2018XLC1006), and Xinqiao Hospital (Grant No. 2018YQYLY007). This article is the authors’ own work and not an official position of the institution or funder.
CLINICAL TRIAL INFORMATION
Lei Gao, Yanqi Zhang, and Sanbin Wang contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Lei Gao, Yanqi Zhang, Xi Zhang
Financial support: Lei Gao, Xi Zhang
Administrative support: Lei Gao, Xi Zhang
Provision of study materials or patients: Lei Gao, Sanbin Wang, Peiyan Kong, Yi Su, Jiong Hu, Ming Jiang, Hai Bai, Tao Lang, Li Liu, Tonghua Yang, Xiaobin Huang, Fang Liu, Shifeng Lou, Yao Liu, Cheng Zhang, Hong Liu, Li Gao, Jia Liu, Lidan Zhu, Qin Wen, Ting Chen, Ping Wang, Min Mao, Cunbang Wang, Xianlin Duan, Le Luo, Xiangui Peng, Xi Zhang
Collection and assembly of data: Lei Gao, Sanbin Wang, Peiyan Kong, Yi Su, Jiong Hu, Ming Jiang, Hai Bai, Tao Lang, Jishi Wang, Li Liu, Tonghua Yang, Xiaobin Huang, Fang Liu, Shifeng Lou, Yao Liu, Cheng Zhang, Hong Liu, Li Gao, Jia Liu, Lidan Zhu, Qin Wen, Ting Chen, Ping Wang, Jun Rao, Min Mao, Cunbang Wang, Xianlin Duan, Le Luo, Xiangui Peng, Xi Zhang
Data analysis and interpretation: Lei Gao, Yanqi Zhang, Kaniel Cassady, Jiang F. Zhong, Xi Zhang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of rhG-CSF Combined With Decitabine Prophylaxis on Relapse of Patients With High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kaniel Cassady
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ruggeri A Labopin M Sanz G, et al. : Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia 29:1891-1900, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Tsai SB Rhodes J Liu H, et al. : Reduced-intensity allogeneic transplant for acute myeloid leukemia and myelodysplastic syndrome using combined CD34-selected haploidentical graft and a single umbilical cord unit compared with matched unrelated donor stem cells in older adults. Biol Blood Marrow Transplant 24:997-1004, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Xu L Chen H Chen J, et al. : The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China: Recommendations from the Chinese Society of Hematology. J Hematol Oncol 11:33, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y Chen H Chen J, et al. : The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett 438:63-75, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Schmid C Labopin M Nagler A, et al. : Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol 25:4938-4945, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Yun S Vincelette ND Abraham I, et al. : Targeting epigenetic pathways in acute myeloid leukemia and myelodysplastic syndrome: A systematic review of hypomethylating agents trials. Clin Epigenetics 8:68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J Chen Y Zhu Y, et al. : Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget 6:6448-6458, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodyear OC Dennis M Jilani NY, et al. : Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 119:3361-3369, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Pusic I Choi J Fiala MA, et al. : Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 21:1761-1769, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S Kim YJ Lee J, et al. : Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol 8:118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen XM Lin J Yang J, et al. : Double CEBPA mutations are prognostically favorable in non-M3 acute myeloid leukemia patients with wild-type NPM1 and FLT3-ITD. Int J Clin Exp Pathol 7:6832-6840, 2014 [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L Wen Q Chen X, et al. : Effects of priming with recombinant human granulocyte colony-stimulating factor on conditioning regimen for high-risk acute myeloid leukemia patients undergoing human leukocyte antigen-haploidentical hematopoietic stem cell transplantation: A multicenter randomized controlled study in southwest China. Biol Blood Marrow Transplant 20:1932-1939, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Franzke A: The role of G-CSF in adaptive immunity. Cytokine Growth Factor Rev 17:235-244, 2006 [DOI] [PubMed] [Google Scholar]
- 14. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology (NCCN guidelines): Acute myeloid leukemia. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 15.Estey EH: How to manage high-risk acute myeloid leukemia. Leukemia 26:861-869, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Schiller GJ: High-risk acute myelogenous leukemia: Treatment today ... and tomorrow. Hematology Am Soc Hematol Educ Program 2013:201-208, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Yong KL: Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: Comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF). Br J Haematol 94:40-47, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Welte K Gabrilove J Bronchud MH, et al. : Filgrastim (r-metHuG-CSF): The first 10 years. Blood 88:1907-1929, 1996 [PubMed] [Google Scholar]
- 19.Nara N Murohashi I Suzuki T, et al. : Effects of recombinant human granulocyte colony-stimulating factor (G-CSF) on blast progenitors from acute myeloblastic leukaemia patients. Br J Cancer 56:49-51, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usuki K Iki S Endo M, et al. : Granulocyte colony-stimulating factor in acute myeloid leukemia. Stem Cells 13:647-654, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Heil G Hoelzer D Sanz MA, et al. : A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. Blood 90:4710-4718, 1997 [PubMed] [Google Scholar]
- 22.Dombret H Chastang C Fenaux P, et al. : A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia. N Engl J Med 332:1678-1683, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Godwin JE Kopecky KJ Head DR, et al. : A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: A Southwest Oncology Group study (9031). Blood 91:3607-3615, 1998 [PubMed] [Google Scholar]
- 24.Bradstock K Matthews J Young G, et al. : Effects of glycosylated recombinant human granulocyte colony-stimulating factor after high-dose cytarabine-based induction chemotherapy for adult acute myeloid leukaemia. Leukemia 15:1331-1338, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Goldstone AH Burnett AK Wheatley K, et al. : Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 98:1302-1311, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Li L Zhang X Yu H, et al. : Low-dose hypomethylating agent decitabine in combination with aclacinomycin and cytarabine achieves a better outcome than standard FLAG chemotherapy in refractory/relapsed acute myeloid leukemia patients with poor-risk cytogenetics and mutations. Onco Targets Ther 11:6863-6870, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J Hong M Zhu Y, et al. : Decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin is as effective as standard dose chemotherapy in the induction treatment for patients aged from 55 to 69 years old with newly diagnosed acute myeloid leukemia. Leuk Lymphoma 59:2570-2579, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y Mouginot M Reppel L, et al. : Modification of NK cell subset repartition and functions in granulocyte colony-stimulating factor-mobilized leukapheresis after expansion with IL-15. Immunol Res 65:1130-1138, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Vasu S He S Cheney C, et al. : Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood 127:2879-2889, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoneim HE Fan YP Moustaki A, et al. De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 170: 142-157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rücker-Braun E Link CS Schmiedgen M, et al. : Longitudinal analyses of leukemia-associated antigen-specific CD8+ T cells in patients after allogeneic stem cell transplantation. Exp Hematol 44:1024-1033.e1, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Ni X Song Q Cassady K, et al. : PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest 127:1960-1977, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Papa B Ruggeri L Urbani E, et al. : Clinical-grade-expanded regulatory T cells prevent graft-versus-host disease while allowing a powerful T cell-dependent graft-versus-leukemia effect in murine models. Biol Blood Marrow Transplant 23:1847-1851, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Wang L Liu Y Beier UH, et al. : Foxp3+ T-regulatory cells require DNA methyltransferase 1 expression to prevent development of lethal autoimmunity. Blood 121:3631-3639, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper ML Choi J Karpova D, et al. : Azacitidine mitigates graft-versus-host disease via differential effects on the proliferation of T effectors and natural regulatory T cells in vivo. J Immunol 198:3746-3754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald KPA Le Texier L Zhang P, et al. : Modification of T cell responses by stem cell mobilization requires direct signaling of the T cell by G-CSF and IL-10. J Immunol 192:3180-3189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Aveni M Rossignol J Coman T, et al. : G-CSF mobilizes CD34+ regulatory monocytes that inhibit graft-versus-host disease. Sci Transl Med 7:281ra42, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Ukena SN Velaga S Goudeva L, et al. : Human regulatory T cells of G-CSF mobilized allogeneic stem cell donors qualify for clinical application. PLoS One 7:e51644, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]