Abstract
PURPOSE
Desmoplastic small round cell tumor (DSRCT), a rare sarcoma of adolescents/young adults primarily involving the peritoneum, has a long-term survival of < 20% despite aggressive multimodality treatment. B7H3 is expressed on DSRCT cell surface, providing a target for antibody-based immunotherapy.
PATIENTS AND METHODS
In this phase I study, we evaluated the safety, pharmacokinetics, and biodistribution of intraperitoneal (IP) radioimmunotherapy (RIT) with the anti-B7H3 murine monoclonal antibody 131I-omburtamab in patients with DSRCT or other B7H3-expressing tumors involving the peritoneum. After thyroid blockade, patients received 131I-omburtamab as a single IP injection at escalated activities from 1.11 to 3.33/GBq/m2. A prior tracer dose of IP 74 MBq124I-omburtamab was used for radioimmuno–positron emission tomography imaging. Each injection was followed by IP saline infusion.
RESULTS
Fifty-two patients (48, three, and one with DSRCT, peritoneal rhabdomyosarcoma, and Ewing sarcoma, respectively) received IP 131I-omburtamab administered on an outpatient basis. Maximum tolerated dose was not reached; there were no dose-limiting toxicities. Major related adverse events were transient: grade 4 neutropenia (n = 2 patients) and thrombocytopenia (n = 1), and grade 1 (10%) and grade 2 (52%) pain lasting < 2 hours related to saline infusion. Hypothyroidism was not observed, and antidrug antibody was elicited in 5%. Mean (± SD) projected peritoneal residence time was 22.4 ± 7.9 hours. Mean projected absorbed doses for 131I-omburtamab based on 124I-omburtamab dosimetry to normal organs were low and well within tolerable limits. More than 80% 131I remained protein bound in blood 66 hours after RIT. On the basis of peritoneal dose and feasibility for outpatient administration, the recommended phase II activity was established at 2.96 GBq/m2. Patients with DSRCT receiving standard whole-abdominal radiotherapy after RIT did not experience unexpected toxicity.
CONCLUSION
IP RIT 131I-omburtamab was well tolerated with minimal toxicities. Radiation exposure to normal organs was low, making combination therapy with other anticancer therapies feasible.
INTRODUCTION
Desmoplastic small round cell tumor (DSRCT), a rare neoplasm of adolescents and young adults, typically presents with widespread intra-abdominal tumors usually arising from the peritoneum and rarely from other serosal surfaces.1 DSRCT is characterized by the presence of the t(11;22)(p13:q12) chromosomal translocation,2 which leads to an EWS-WT1 fusion.3,4 Optimal therapy is not well established: tumors are only moderately chemosensitive, and gross total resection (GTR) of disease, although challenging, is necessary for long-term survival.5 External-beam whole abdominopelvic radiotherapy (WAP-RT) seems to improve outcomes.6,7 However, despite aggressive multimodality therapy, reported long-term progression-free survival (PFS) is < 20%,8,9 with treatment failures resulting primarily from intraperitoneal (IP) recurrences caused by a failure to eradicate microscopic residual disease.10 Results from experimental approaches such as myeloablative chemotherapy with autologous stem-cell transplantation11 and hyperthermic IP chemotherapy have been disappointing12,13 and often associated with significant toxicity,14 warranting consideration of other therapies. Peritoneal involvement is extremely rare in other pediatric solid tumors but has been reported for carcinomas,15 mesothelioma,16 germ cell tumors,17 gliomas,18 neuroblastoma,19 melanoma, and rhabdomyosarcoma (RMS)20 and is often associated with a poor prognosis. Furthermore, curative or palliative treatments for malignant ascites remain major unmet needs.21
CONTEXT
Key Objective
To determine the safety, pharmacokinetics, and biodistribution of intraperitoneal (IP) radioimmunotherapy (RIT) with anti-B7H3 murine monoclonal antibody 131I-omburtamab in patients with desmoplastic small round cell tumor (DSRCT) or other B7H3-expressing tumors. The absence of an effective therapy and the poor long-term survival among patients with DSRCT and other tumors with peritoneal involvement require the development of novel therapies for these rare tumors.
Knowledge Generated
Fifty-two patients (48, three, and one with DSRCT, peritoneal rhabdomyosarcoma, and Ewing sarcoma, respectively) received IP 131I-omburtamab administered as outpatient therapy. Therapy was well tolerated, and there were no dose-limiting toxicities. 124I-omburtamab-mediated radioimmuno–positron emission tomography permitted the assessment of dosimetry and biodistribution. Recommended phase II activity was established at 2.96 GBq/m2, and outpatient administration was feasible.
Relevance
Radiation exposure to normal organs was low, making combination therapy of IP RIT with other anticancer therapies feasible.
B7H3, a cell surface glycoprotein antigen related to immune checkpoint molecules, inhibits natural killer cells and T cells22,23 and regulates tumor cell migration and invasion.24 Whereas B7H3 transcript is expressed ubiquitously in tumors and normal tissues by quantitative reverse transcription-polymerase chain reaction, B7H3 protein was found only on tumors and not on most normal tissues by both Western blot and immunohistochemistry.5,25,26 We developed the murine monoclonal immunoglobulin (Ig) G1 antibody omburtamab (previously called 8H9) for clinical use. Omburtamab binds to cell surface B7H3 on most pediatric solid tumors, including 96% of DSRCT27 and in > 80% of other pediatric malignancies that involve the peritoneum; binding to normal tissues is restricted.28,29 It has a slow dissociation rate (koff), which is crucial for sustained in vivo drug efficacy.29,30 Radioiodinated omburtamab targets and suppresses RMS xenografts in mouse models.31
Compartmental administration of radioimmunotherapy (RIT) achieves favorable dosimetry and higher tumor-to-nontumor ratios when compared with systemic administration and has the potential to reduce systemic radioisotope-related toxicity.32 In animal experiments, the IP compartment permits a long peritoneal residence time and incomplete transfer of therapeutic agents into the systemic circulation.33 Intravenous 131I-omburtamab showed liver uptake despite the low level of hepatic expression (ClinicalTrials.gov NCT00582608), suggesting possible sequestration. In a phase I/II study of intraventricular RIT of CNS and leptomeningeal metastases (ClinicalTrials.gov identifier: NCT00089245), omburtamab labeled with the novel positron-emitting isotope 124I allowed the acquisition of radioimmuno–positron emission tomography (PET) images for detailed biodistribution and pharmacokinetic data in patients.34 These indicated a favorable radiation dose to the cerebrospinal fluid compartment treated with RIT when compared with blood or other normal tissues.35 The addition of 131I-omburtamab to a multimodality therapeutic regimen has changed the natural history of neuroblastoma metastatic to the CNS with long-term survival rates of > 50% being achieved in what hitherto was a uniformly lethal disease.36 124I-omburtamab is also being investigated as a theranostic agent for disseminated intrapontine glioma via intrapontine injection using convection-enhanced delivery (ClinicalTrials.gov identifier: NCT01502917).37
We hypothesized that IP RIT with 131I-omburtamab could achieve favorable dosimetry in the peritoneal compartment, have tolerable systemic toxicity, and enhance local control by targeted delivery of radiation to micrometastases, which are poorly accessible by other treatment modalities. We tested this hypothesis in a phase I study in which we investigated the safety of 131I-omburtamab. We studied pharmacokinetics and biodistribution using the radioimmunoconjugate 124I-omburtamab.
PATIENTS AND METHODS
Patients
Patients with DSRCT with peritoneal involvement, or other refractory or relapsed B7H3-expressing tumors involving the peritoneum with < 20% chance of long-term survival, were eligible. Cell surface expression of B7H3 was confirmed by immunohistochemistry for non-DSRCT tumors using methods described previously.27 Other salient inclusion criteria included age > 1 year, ability to comply with radiation safety restrictions, and availability of 2×106 CD34+ cryopreserved cells/kg for infusion. Key exclusion criteria included the presence of dense peritoneal adhesions preventing adequate IP distribution and grade ≥ 2 toxicities evaluated by National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0, with the exception of myelosuppression.
Study Design
The protocol was approved by the Memorial Sloan Kettering Institutional Review Board (IRB). Written consent was obtained from patients or guardians before study procedures. After high-dose neoadjuvant chemotherapy, patients underwent debulking surgery and adhesiolysis, followed by insertion of an indwelling IP catheter. In general, if abdominopelvic debulking surgery was not deemed feasible, patients were not offered participation in the trial. Five to 7 days before protocol therapy, oral saturated solution of potassium iodide (5-7 drops orally three times a day) and liothyronine (25-75 μg orally per day) was commenced for thyroid protection and continued for a total of 42 days. Thyroid blockade was confirmed by a subnormal thyroid-stimulating hormone (TSH) level before IP injection. Initially, a 74 MBq tracer activity of 124I-omburtamab was injected IP for dosimetry and pharmacokinetic studies. A single therapeutic dose of 131I-omburtamab (radiolabeled at a specific activity of 0.74-1.11 GBq/mg31) was administered IP 3 days later. Each activity of RIT was followed by IP infusion of 1.2 L/m2 saline to expand the peritoneal cavity and facilitate dispersion of RIT. Patients were premedicated with acetaminophen and diphenhydramine. Institutional radiation safety precautions including lead shielding, patient and caregiver education, and portable radiation detectors for dose-rate monitoring were strictly implemented. Radiation exposure dose to family caregivers was maintained at < 500 mrem in compliance with applicable regulations. Toxicity was monitored clinically at weekly clinic visits and biochemically by CBC and complete chemistry. A standard 3+3 phase I design was implemented for escalation of 131I-omburtamab with a starting activity of 1.11 GBq/m2. Escalation in increments of 0.37 GBq/m2 was planned if zero of three or one of six patients at each level experienced dose-limiting toxicity (DLT) for a period of 35 days after 131I-omburtamab. DLTs were defined as any grade ≥ 3 toxicity, with the exception of myelosuppression, fever, rash, and hypotension. Autologous stem-cell rescue was mandated if absolute neutrophil count (ANC) was < 500/μL for three consecutive evaluations 28 to 35 days after IP 131I-omburtamab or at any time for life-threatening infection in the presence of ANC < 500/μL. After the recommended phase II dose was established, an additional 24 patients were treated at that activity but were monitored for toxicity for 14 days or until additional therapy was initiated, whichever was later. Immunogenicity was evaluated by measuring human antimouse antibody (HAMA) titers using a previously described enzyme-linked immunosorbent assay.34 The protocol schema is outlined in Table 1. For patients with measurable disease, response was assessed between days 24 and 38 using RECIST criteria. After mandated observation was completed, patients received additional therapy at the discretion of their primary physicians. Most patients received WAP-RT after IP RIT. PFS and overall survival (OS) were calculated using Kaplan-Meier methodology from the day of 131I-omburtamab administration and were censored on March 1, 2020.
TABLE 1.
Treatment Schema
Pharmacokinetics and Radiation Dosimetry
Blood clearance for 124I-omburtamab or 131I-omburtamab was measured using serial venous blood samples drawn at baseline and approximately 1, 2, 8, 18, 24, 30, 42, 66, 90, and 144 hours after IP injection. Measured aliquots of blood were assayed in duplicate, and activity concentrations were derived as % injected activity/gram. Biologic and effective clearance was derived by fitting time-activity concentration data, with decay corrected to the time of administration, to a biexponential function. Mean blood absorbed dose was calculated by multiplying the blood cumulated activity concentration by 124I or 131I equilibrium dose constant for nonpenetrating radiation. Blood clearance was compared for 124I-omburtamab and 131I-omburtamab. In addition, 30 and 66 hours after 131I-omburtamab injection, blood samples were evaluated by trichloroacetic acid precipitation for free radioiodine content.
Whole-body PET scans, using the same scanner and uniform acquisition parameters (Fig 1), were performed within 2 to 4 hours and approximately 24, 48, 120, and 168 hours after 124I-omburtamab injection. Normal organ and whole-body absorbed doses for IP 131I-omburtamab were derived on the basis of 124I-omburtamab imaging using region-of-interest analysis to extract time-activity data of uptake in major organs (heart, liver, spleen, kidneys, GI tract, and peritoneal cavity) and whole body. Cumulated activity concentrations and residence times in each organ were derived. Whole-body clearance, mean organ absorbed doses, effective dose equivalent, and effective dose were calculated using the Organ Level Internal Dose Assessment/EXponential Modeling (OLINDA/EXM) radiation dosimetry program34 for 131I radionuclide and the Reference Man anatomic model closest in whole-body mass to that of the patient. A peritoneal cavity model was also used.38 Detailed statistical analysis is described in the Protocol (online only).
FIG 1.
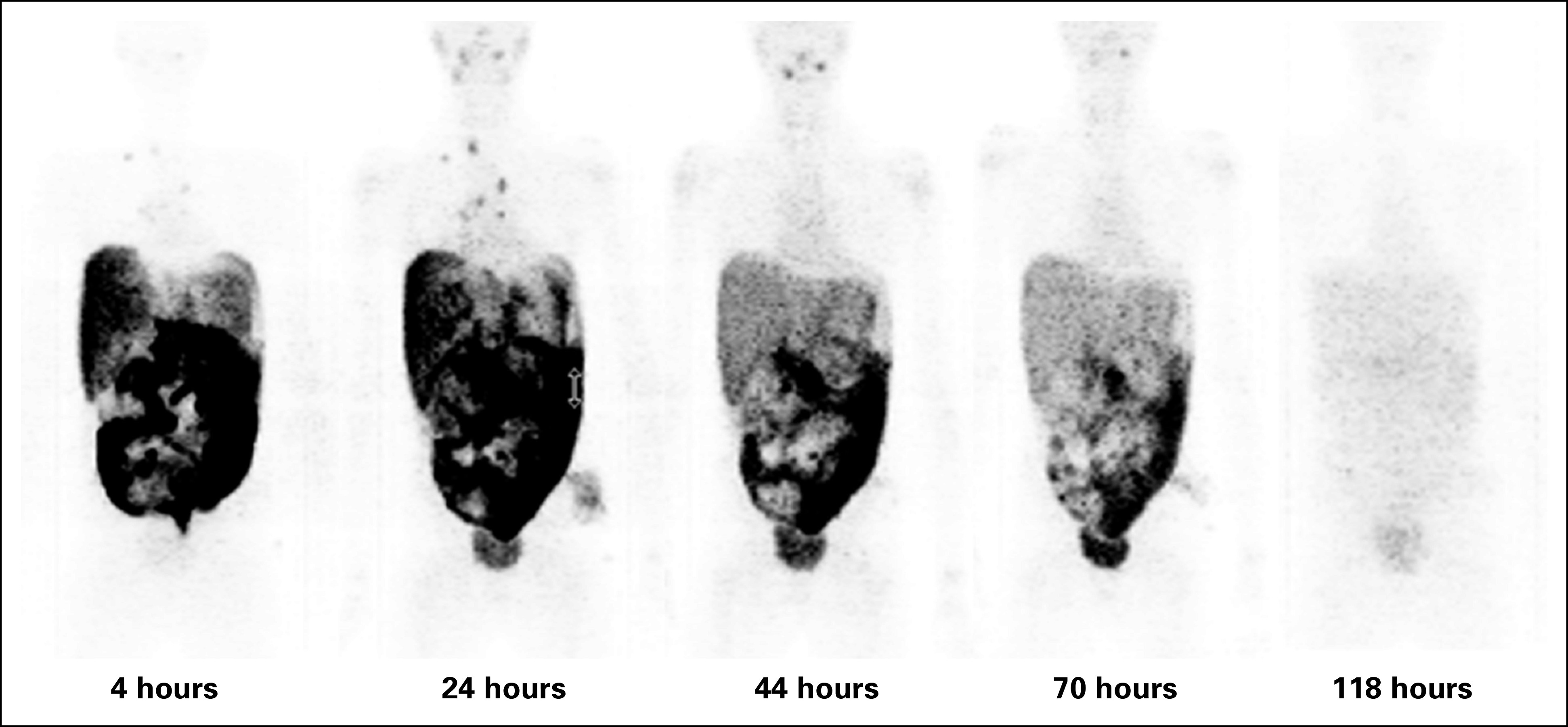
Serial positron emission tomography scans of a study patient, used for organ dosimetry. Numbers indicate hours after IP 124I-omburtamab administration.
RESULTS
Patient Demographics
Fifty-two patients (41 males and 11 females) received IP RIT with 131I-omburtamab as outpatients (except for the first three patients for whom the IRB required inpatient observation) between 2010 and 2019. These included five patients who were treated on a single-patient use basis: four because they had pre-existing hypothyroidism and could not be evaluated for thyroid toxicity, and one on a compassionate basis after IRB approval. In addition, six enrolled patients could not receive RIT because of blockage of the IP catheter. Forty-eight treated patients had the diagnosis of DSRCT; three and one had B7H3-expressing RMS and Ewing sarcoma, respectively. Median age at RIT was 18.5 years (range, 2.9-38 years), and median time from diagnosis to RIT was 9.9 months (range, 5.1-56.3 months). Median time from catheter insertion to RIT was 17 days (range, 9-32 days). Thirty-five of the 48 patients with DSRCT underwent GTR before RIT; twenty-nine of the 35 without experiencing prior disease progression. Patients with RMS and Ewing sarcoma underwent complete cytoreductive surgery before RIT, although all had relapsed previously.
Escalation of 131I-Omburtamab Activity
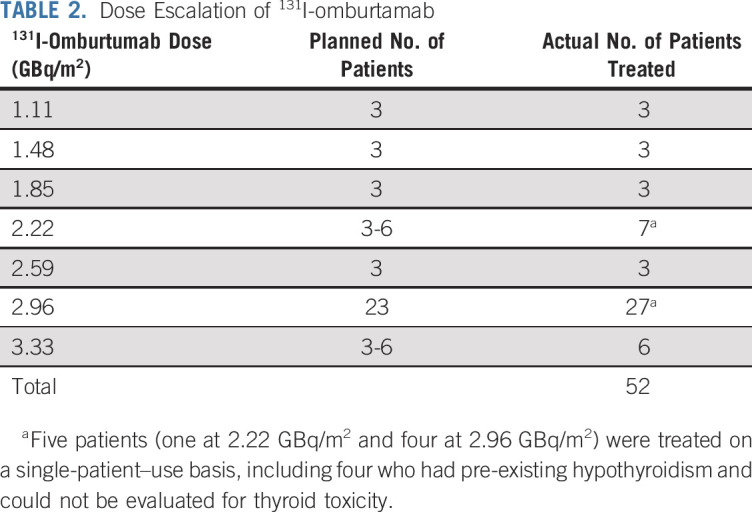
Initially, the administered activity of 131I-omburtamab was planned to be escalated from 1.11 to 2.22 GBq/m2/treatment, with six candidates treated at 2.22 GBq/m2. Because the treatment was well tolerated and no DLT was encountered, 131I-omburtamab administered activity was further escalated to 3.33 GBq/m2. Again, no DLT was encountered. The recommended phase II activity was established at 2.96 GBq/m2 (80 mCi/m2) to allow outpatient administration of 131I-omburtamab while meeting the requirements for the safe administration of radionuclides (typically, 7.4 GBq/dose for 131I). After the recommended phase II activity was established, an additional 24 patients were treated at 2.96 GBq/m2/dose to acquire further safety data before the opening of a follow-up phase II study. The number of patients treated at each dose level is listed in Table 2. Actual administered activity and protein mass ranged from 1.25 to 7.61 GBq and 1.7 to 10.2 mg, respectively.
TABLE 2.
Dose Escalation of 131I-omburtamab
Toxicity
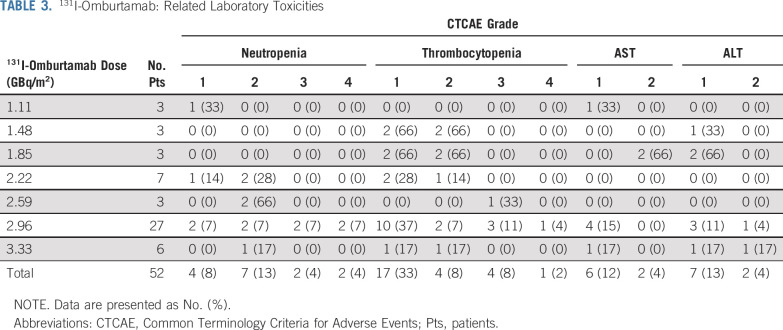
Both dosimetry and diagnostic administrations were well tolerated at all dose levels. No DLTs were encountered. Acute toxicities attributable to RIT were related to the large volume of saline flush and were transient (lasting < 2 hours). These included grade 2 pain (52%) and grade 1 (10%) or 2 (10%) vomiting. Two patients experienced grade 1 fatigue lasting < 1 week after IP RIT. Laboratory abnormalities are detailed in Table 3. No intermediate or long-term toxicities attributable to IP RIT were observed. Despite all patients having received high doses of chemotherapy before IP RIT, grade > 2 myelosuppression was uncommon and was encountered only at doses > 2.22 GBq/m2. Myelosuppressive changes were self-limiting without intervention in general; one patient each required filgrastim (1 dose) and platelet transfusion (single aliquot). No patient required autologous stem cell rescue after RIT. Hypothyroidism, as assessed by TSH measurements after discontinuation of iodine drops and liothyronine, was not seen in any patient (n = 37 of 49 evaluable). HAMA developed in two of 44 tested patients (5%).
TABLE 3.
131I-Omburtamab: Related Laboratory Toxicities
Pharmacokinetics and Dosimetry
124I-omburtamab–based dosimetry was studied for the first 33 patients. Blood radiation exposure was low, and pharmacokinetics exhibited a biphasic pattern for both 124I-omburtamab (n = 29) and 131I-omburtamab (n = 36). This consisted of an initial rising phase with a mean (± SD) half-time of 49.6 ± 65.6 hours and a subsequent falling phase with a mean half-time of 70 ± 118.4 hours for 124I-omburtamab. Calculations that included four patients in whom low-level activity persisted in the blood contributed to the wide SD: respective early- and late-phase half-times after discounting these outliers were 27.9 ± 21.2 hours and 49.2 ± 25.9 hours. For 131I-omburtamab, respective early- and late-phase half-times were 17.3 ± 21.5 hours and 69.1 ± 33.4 hours. Using 124I-omburtamab data, calculated median projected blood exposure for 131I-omburtamab was 0.67 ± 0.34 mGy/MBq; this compared well with actual blood exposure for 131I-omburtamab, which was 0.84 ± 0.46 mGy/MBq. In 24 patients, for whom both 124I-omburtamab and 131I-omburtamab kinetics data were available, a positive correlation was observed between estimates for the two groups (r = 0.43). Radioactivity in the circulation was largely blood bound: whole-blood free iodine content at 30 and 66 hours was only 6.9% ± 3.9% and 4.2% ± 3%, respectively (n = 43).
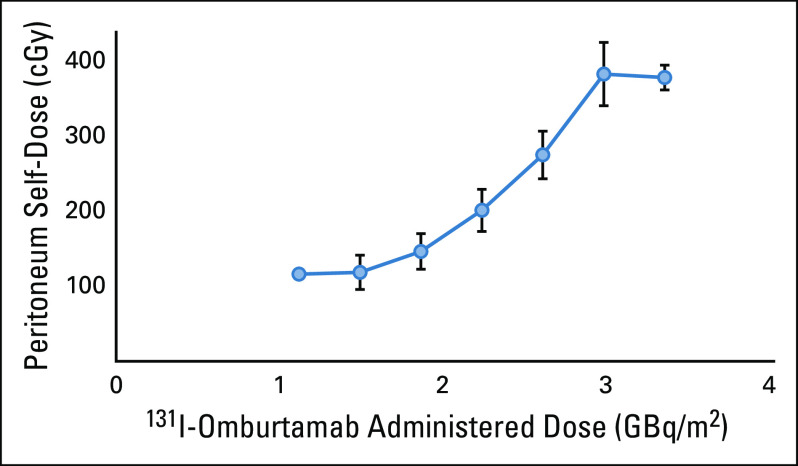
124I-omburtamab–based whole-body and organ dosimetry for 131I-omburtamab (n = 33 patients) is reported in Table 4. Mean whole-body clearance half-time and residence times were 44.4 ± 9.2 hours, and 51.7 ± 8.6 hours, respectively. Peritoneal residence time was 22.4 ± 7.9 hours. In general, radiation exposure to normal organs was low, with the highest projected absorbed doses noted for the thyroid, urinary bladder wall, uterus, liver, pancreas, and stomach wall. The projected absorbed dose to the peritoneum was 0.65 ±0.21 mGy/MBq. Although the total absorbed dose to the peritoneum increased as administered activity was increased, it seemed to plateau at 2.96 GBq/m2 (Fig 2). Of the 33 patients undergoing dosimetry studies, only one patient had unresected abdominopelvic disease at the time of 124I-omburtamab PET scans. This single gastrohepatic node was visualized on PET at 21 hours after injection and the projected 131I-omburtamab dose was calculated as 0.59 mGy/MBq. γ-single-photon emission computerized tomography (SPECT) scans after IP 131I-omburtamab generally showed good peritoneal distribution for all patients.
TABLE 4.
Mean Organ-Absorbed Doses of 131I-Omburtamab (mGy/MBq) on the Basis of 124I-Omburtamab Dosimetry (n = 33 patients)
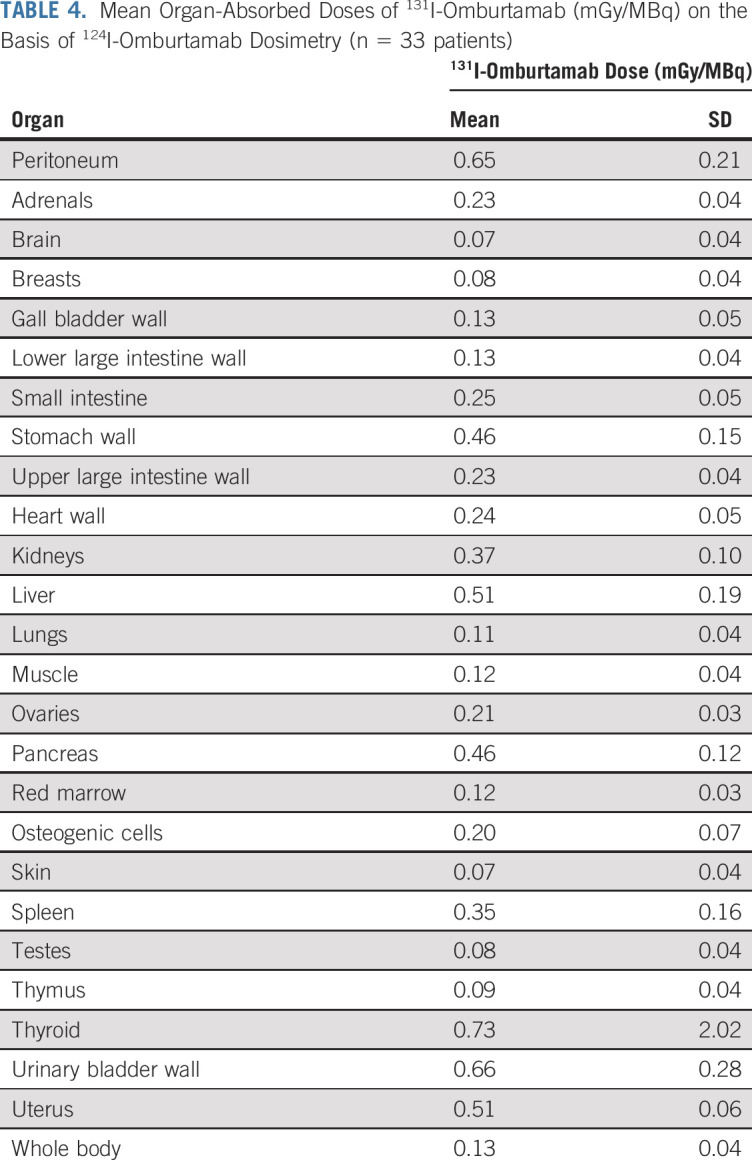
FIG 2.
Relation of mean peritoneal absorbed dose to injected activity. Error bars represent SEM.
Responses, Post-RIT Therapy, and Survival in Patients With DSRCT
At study entry, 13 patients had measurable disease before IP RIT, whereas 35 had no evaluable DSRCT. As expected with RIT designed to target micrometastases, no responses were observed in patients with measurable disease. Immediate post-RIT therapy included 3,000 cGy WAP-RT (n = 30) administered at a median of 35 days (range, 16-74 days) after RIT, chemotherapy (n = 12), allogeneic transplantation (n = 3), and no therapy at all (n = 3). Of the 30 patients undergoing WAP-RT, 18 received ASCR after completing WAP-RT, either electively (n = 6) or to reduce the duration of thrombocytopenia to permit additional therapy (n = 12). All patients undergoing stem-cell infusions had hematopoietic reconstitution.
Survival was significantly worse for patients with residual disease receiving IP RIT compared with those treated after R1 resection; median PFS and OS (statistical analysis for survival described in Protocol) for the two groups was 8.6 ± 2.1 months versus 15.2 ± 0.9 months and 16.9 ± 4.3 months versus 54.1 ± 9.2 months, respectively (P < 0.01 for both). Of the 23 patients receiving IP RIT after GTR at the recommended phase II dose or higher, nine remain alive and disease free at a median follow-up of 42 months after RIT, and only four (17%) developed their initial relapse in the abdominopelvic compartment. Patients receiving < 2.96 GBq/m2 (the recommended phase II dose; n = 12) after GTR had a poorer (although statistically nonsignificant) median abdominal PFS (15 ± 9.1months) than did the 23 who received ≥ 2.96 GBq/m2 (25.7 ± 6.7 months; P = .2).
Outcome in Patients Without DSRCT
None of the three patients with RMS had measurable disease at the time of IP RIT. Of these, two remain alive without recurrence at ≥ 29.4 and ≥ 97.4 months after RIT. The sole patient with disseminated abdominopelvic Ewing sarcoma had no response to IP RIT and developed rapidly progressive disease.
DISCUSSION
Intracompartmental delivery is an attractive approach for RIT when the targeted disease is restricted mainly to a particular body compartment, as in DSRCT. The intracompartmental approach is particularly suited to RIT with the murine IgG1 monoclonal antibody omburtamab, which has limited recruitment of human effectors but has a high affinity to the target antigen B7H3. Because B7H3 is also expressed on common malignancies such as carcinomas,39 131I-omburtamab–mediated IP RIT has potential in the management of carcinoma-associated malignant ascites, a complication for which there are no established curative or palliative therapies. In this first phase I trial of IP-administered 131I-omburtamab, we established its safety; related toxicities were mild and transient, with the main adverse event being short-lasting abdominal pain and distension related to IP saline infusion after RIT. Myelosuppression was grade < 4 in almost all patients, and only one patient required a blood product (platelet support). Outpatient administration meeting all radiation safety regulatory limits was feasible when activity of 2.96 GBq/m2 was administered to both children and adults. An additional reason for establishing 2.96 GBq/m2 as the recommended phase II activity was the plateau in peritoneal dose, a critical factor in effective RIT delivery.
Omburtamab retained binding to 131I after it transitioned from the peritoneum to the blood, with < 10% free iodine being detected in the blood. This could explain the relatively mild myelosuppression encountered despite patients having previously received high-dose chemotherapy typically administered for DSRCT and high-risk RMS. The lack of hypothyroidism and a low projected thyroid uptake of < 500 rads were remarkable for a radioiodinated antibody, indicating the success of the thyroid blockade regimen. The low incidence of antidrug antibody (HAMA) could be a result of immune suppression mediated by alkylator-containing chemotherapy5 received by patients before RIT. Low blood and systemic radiation exposures have also been observed in other intracompartmental therapies with radioiodinated omburtamab.35
Although other radioimmunoconjugates have been used in the past for IP RIT, primarily to target surface antigens on ovarian carcinoma,40-42 to our knowledge ours is the first in a predominantly pediatric/adolescent population. Organ biodistribution for other radioimmunoconjugates, because of the choice of radioisotope used (eg, α emitters) or era of trials (predominantly γ emitters), could only be estimated by relatively less precise methods such as γ-SPECT scans43 or external radiation probes.44 The availability of the positron-emitting radioimmunoconjugate 124I-omburtamab allowed us to derive precise organ exposures for therapeutic 131I-omburtamab. To our knowledge, this is the first study to use radioimmuno-PET to assess dosimetry and biodistribution for IP RIT. The strong correlation in blood pharmacokinetics between 124I-omburtamab and 131I-omburtamab strengthened the validation of this approach. Calculated organ exposure was well within tolerated limits for all organs, corroborated by the observed lack of toxicities. The calculated radiation dose to the peritoneum was comparable to that reported in the only other IP RIT study reporting on dosimetry in humans.43 For most patients treated with the proposed phase II activity of 131I-omburtamab, the peritoneal dose was < 500 cGy, although as with other studies, we could not calculate the dose to the tumor because most patients did not have grossly evaluable or measurable disease at study entry or had disease in other body compartments not accessible to IP RIT. The relatively modest whole-peritoneal radiation dose permitted the safe administration of 3,000 cGy whole-abdominal radiotherapy after IP RIT to most patients with DSRCT. Patients with RMS had received WAP-RT before IP RIT and did not experience unforeseen adverse events.
As expected for a therapeutic modality targeted at microscopic or minimal disease, we did not see tumor responses in measurable IP or extraperitoneal disease. Survival evaluation for patients with DSRCT was not the main objective of this phase I study. However, we deemed it important to analyze because we wanted to evaluate the impact of 131I-omburtamab when added to multimodality therapy (high-dose chemotherapy, GTR of tumor, and WAP-RT), which is considered standard-of-care at our institution. Patients receiving IP RIT in addition to standard therapy had superior PFS and OS compared with historical data on patients who received a similar multimodality regimen without IP RIT (median OS of 54 months for the former v 36 months for the latter).10 In addition, two of three patients with recurrent RMS in the peritoneal compartment are long-term relapse-free survivors after receiving WA-IMRT followed by IP RIT. Based on the favorable toxicity profile of 131I-omburtamab and the encouraging preliminary data generated from this phase I trial, we have initiated a phase II trial of IP RIT in patients with DSRCT with 131I-omburtamab as a single dose of 2.96 GBq/m2 followed 2 to 4 weeks later by WA-IMRT at 3,000 cGy to the whole abdomen, with the goal of improving survival (ClinicalTrials.gov identifier: NCT04022213). Patients with relapsed RMS and other high-risk B7H3-expressing tumors involving the peritoneum will also be eligible. In addition, preclinical testing of 177Lu177-omburtamab, a β-emitting radioimmunoconjugate that might be more conducive to IP RIT, is underway.
ACKNOWLEDGMENT
We thank the nuclear medicine research nurses, surgery and in-patient nurse practitioners, and the radiation safety team for patient care. We thank Joe Olechnowicz for editorial assistance. Researchers at Memorial Sloan Kettering Cancer Center (“MSK”) developed omburtamab, which is exclusively licensed by MSK to Y-mAbs. As a result of this licensing arrangement, MSK has institutional financial interest in the compound and in Y-mAbs.
SUPPORT
Supported by Taybandz, the Steven Vanover Foundation, and Sarcoma Specialized Program of Research Excellence (SPORE) and by Cancer Center Core Grant Nos. NIH P30 CA008748 and NIH P35CA232130 (J.S.L.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Shakeel Modak, Jorge A. Carrasquillo, Michael P. LaQuaglia, Nai-Kong V. Cheung, Neeta Pandit-Taskar
Financial support: Shakeel Modak
Administrative support: Shakeel Modak, Jason S. Lewis, Neeta Pandit-Taskar
Provision of study material or patients: Shakeel Modak, Pat Zanzonico, Emily K. Slotkin, Serge K. Lyashchenko,Todd Heaton, Michale P. LaQuaglia, Neeta Pandit-Taskar
Collection and assembly of data: Shakeel Modak, Pat Zanzonico, Milan Grkovski, Emily K. Slotkin, Jorge A. Carrasquillo, Serge K. Lyashchenko, Jason S. Lewis, Irene Y. Cheung, Todd Heaton, Neeta Pandit-Taskar
Data analysis and interpretation: Shakeel Modak, Milan Grkovski, Jorge A. Carrasquillo, Irene Y. Cheung, Neeta Pandit-Taskar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shakeel Modak
Consulting or Advisory Role: Y-mAbs Therapeutics, Illumina RP
Patents, Royalties, Other Intellectual Property: Two patents pending, no financial benefit
Pat Zanzonico
Patents, Royalties, Other Intellectual Property: Y-mAbs
Emily K. Slotkin
Research Funding: Eli Lilly (Inst)
Jorge A. Carrasquillo
Consulting or Advisory Role: Regeneron, Y-mAbs Therapeutics
Research Funding: Regeneron
Serge K. Lyashchenko
Leadership: Evergreen Theragnostics
Stock and Other Ownership Interests: Evergreen Theragnostics
Consulting or Advisory Role: Evergreen Theragnostics, Y-mAbs Therapeutics, Clarity Pharmaceuticals
Jason S. Lewis
Leadership: Philip
Stock and Other Ownership Interests: Philip, Telix Pharmaceuticals, Evergreen Theragnostics, Summit Biomedical Imaging
Consulting or Advisory Role: Varian Medical Systems, Clarity Pharmaceuticals, TPG Capital, Invicro LLC
Research Funding: Merck, Lilly, MabVax, Curadel
Patents, Royalties, Other Intellectual Property: Elucida Oncology, Macrocyclics, Theragnostics
Travel, Accommodations, Expenses: CSRA
Irene Y. Cheung
Stock and Other Ownership Interests: Y-mAbs Therapeutics (I), Eureka Therapeutics (I), Abpro (I)
Patents, Royalties, Other Intellectual Property: Methods for detecting minimal residual disease (Inst); dectin-1 (CLEC7A) single-nucleotide polymorphism as a biomarker for predicting antibody response when using β-glucan as a vaccine adjuvant (Inst)
Nai-Kong V. Cheung
Stock and Other Ownership Interests: Y-mAbs Therapeutics, Abpro, Eureka
Consulting or Advisory Role: Abpro, Eureka Therapeutics
Research Funding: Y-mAbs Therapeutics (Inst), Abpro (Inst)
Patents, Royalties, Other Intellectual Property: Single-chain variable fragment (scFv) constructs of anti-disialoganglioside GD2(GD2) antibodies (Inst); therapy-enhancing glucan (Inst); use of monoclonal antibody (mAb) 8H9 (Inst); methods for preparing and using scFv (Inst); GD2 peptide mimics (Inst); methods for detecting MRD (Inst); anti-GD2 antibodies (Inst); generation and use of human leukocyte antigen-A2–restricted peptide-specific mAbs and chimeric antigen receptors (CARs) (Inst); high-affinity anti-GD2 antibodies (Inst); multimerization technologies (Inst); bispecific human epidermal growth factor 2 (HER2) and cluster of differentiation 3 (CD3) binding molecules (Inst); affinity matured humanized 8H9 (Inst); anti–chondroitin sulfate proteoglycan 4 antibodies and uses thereof (Inst); receptor tyrosine kinase like orphan receptor 2 (ROR2) antibodies (Inst); T-cell receptor–like antibody agents specific for Epstein-Barr virus (EBV) latent membrane protein 2A peptide presented by human HLA (Inst); anti-CD33 antibody agents (Inst); anti-killer cell immunoglobulin like receptor, three Ig domains and long cytoplasmic tail 1 (KIR3DL1) antibodies (Inst); modular self-assembly disassembly (SADA) technologies (Inst); A33-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product (Inst); anti–L1-cell adhesion molecule (CAM antibodies) and uses thereof (Inst); anti-A33 antibodies and uses thereof (Inst); 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) BsAb for new humanized next-generation anti-glycoprotein (GP) A33 antibodies with Fc-enhanced function or bispecific properties (Inst); herceptin-C825 conjugate for pretargeted radioimmunotherapy and application as a theranostic product (Inst); anti-polysialic acid antibodies and uses thereof (Inst); methods of enhancing immunogenicity of poorly immunogenic anti-specific vaccines using oral yeast b-glucans (Inst); small molecule hapten chelates for pretargeted radioimmunotherapy with anti-DOTA (lanthanide) bispecific antibodies (proteus; Inst); An N-acetylgalactosamino dendron-clearing agent for DOTA-pretargeted radioimmunotherapy (Inst); heterodimeric tetravalency and specificity (HDTVS) antibody compositions and uses thereof (Inst); multimerization of interleukin (IL)-15/IL-15 receptor a complexes to enhance immunotherapy (Inst); CD22 antibodies and methods of using the same (Inst); CD33 antibodies and methods of using the same to treat cancer (Inst); CD19 antibodies and methods of using the same (Inst); anti-CD33 antibodies for treating cancer (Inst); anti–six transmembrane epithelial antigen of the prostate (STEAP)-1 antibodies and uses thereof (Inst); anti-glypican 3 antibodies and uses thereof (Inst); multimodal fluorine-Cyanin 3/5/7-DOTA-hapten compositions, diagnostics, fluorescence-guided surgery, and radioimmunotherapy (Inst); anti-CD3 antibodies and uses thereof; anti-CD3 antibodies and uses thereof (Inst); anti-GD2 SADA conjugates and uses thereof (Inst); anti-GD2 antibodies and uses thereof (Inst); dectin-1 (CLEC7A) single nucleotide polymorphism as a biomarker for predicting antibody response when using beta-glucan as a vaccine adjuvant (Inst)
Travel, Accommodations, Expenses: Partners Therapeutics
Neeta Pandit-Taskar
Honoraria: AstraZeneca/MedImmune, Actinium Pharmaceuticals
Consulting or Advisory Role: Progenics, Illumina
Speakers' Bureau: Actinium Pharmaceuticals
Research Funding: Imaginab, Regeneron, Bristol Myers Squibb, Janssen, Clarity, Bayer Health
Travel, Accommodations, Expenses: Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499–513. [PubMed] [Google Scholar]
- 2.Sawyer JR, Tryka AF, Lewis JM. A novel reciprocal chromosome translocation t(11;22)(p13;q12) in an intraabdominal desmoplastic small round-cell tumor. Am J Surg Pathol. 1992;16:411–416. doi: 10.1097/00000478-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–2840. [PubMed] [Google Scholar]
- 4.Gerald WL, Haber DA. The EWS-WT1 gene fusion in desmoplastic small round cell tumor. Semin Cancer Biol. 2005;15:197–205. doi: 10.1016/j.semcancer.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: Prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14:1526–1531. doi: 10.1200/JCO.1996.14.5.1526. [DOI] [PubMed] [Google Scholar]
- 6.Desai NB, Stein NF, LaQuaglia MP, et al. Reduced toxicity with intensity modulated radiation therapy (IMRT) for desmoplastic small round cell tumor (DSRCT): An update on the whole abdominopelvic radiation therapy (WAP-RT) experience. Int J Radiat Oncol Biol Phys. 2013;85:e67–e72. doi: 10.1016/j.ijrobp.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Osborne EM, Briere TM, Hayes-Jordan A, et al. Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol. 2016;119:40–44. doi: 10.1016/j.radonc.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Scheer M, Vokuhl C, Blank B, et al. Desmoplastic small round cell tumors: Multimodality treatment and new risk factors. Cancer Med. 2019;8:527–542. doi: 10.1002/cam4.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbiah V, Lamhamedi-Cherradi SE, Cuglievan B, et al. Multimodality treatment of desmoplastic small round cell tumor: Chemotherapy and complete cytoreductive surgery improve patient survival. Clin Cancer Res. 2018;24:4865–4873. doi: 10.1158/1078-0432.CCR-18-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lal DR, Su WT, Wolden SL, et al. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005;40:251–255. doi: 10.1016/j.jpedsurg.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Forlenza CJ, Kushner BH, Kernan N, et al. Myeloablative chemotherapy with autologous stem cell transplant for desmoplastic small round cell tumor. Sarcoma. 2015;2015:269197. doi: 10.1155/2015/269197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes-Jordan AA, Coakley BA, Green HL, et al. Desmoplastic small round cell tumor treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Results of a phase 2 trial. Ann Surg Oncol. 2018;25:872–877. doi: 10.1245/s10434-018-6333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honoré C, Atallah V, Mir O, et al. Abdominal desmoplastic small round cell tumor without extraperitoneal metastases: Is there a benefit for HIPEC after macroscopically complete cytoreductive surgery? PLoS One. 2017;12:e0171639. doi: 10.1371/journal.pone.0171639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiles ZE, Murphy AJ, Anghelescu DL, et al. Desmoplastic small round cell tumor: Long-term complications after cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2020;27:171–178. doi: 10.1245/s10434-019-07339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reingruber B, Boettcher MI, Klein P, et al. Hyperthermic intraperitoneal chemoperfusion is an option for treatment of peritoneal carcinomatosis in children. J Pediatr Surg. 2007;42:E17–E21. doi: 10.1016/j.jpedsurg.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Milano E, Pourroy B, Rome A, et al. Efficacy of a combination of pemetrexed and multiple redo-surgery in an 11-year-old girl with a recurrent multifocal abdominal mesothelioma. Anticancer Drugs. 2006;17:1231–1234. doi: 10.1097/01.cad.0000236312.50833.a7. [DOI] [PubMed] [Google Scholar]
- 17.Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: An intergroup study. J Pediatr Surg. 2004;39:424–429, discussion 424-429. doi: 10.1016/j.jpedsurg.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Hill DA, Dehner LP, White FV, et al. Gliomatosis peritonei as a complication of a ventriculoperitoneal shunt: Case report and review of the literature. J Pediatr Surg. 2000;35:497–499. doi: 10.1016/s0022-3468(00)90221-5. [DOI] [PubMed] [Google Scholar]
- 19.Kaste SC, Marina N, Fryrear R, et al. Peritoneal metastases in children with cancer. Cancer. 1998;83:385–390. doi: 10.1002/(sici)1097-0142(19980715)83:2<385::aid-cncr25>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Chung CJ, Bui V, Fordham LA, et al. Malignant intraperitoneal neoplasms of childhood. Pediatr Radiol. 1998;28:317–321. doi: 10.1007/s002470050363. [DOI] [PubMed] [Google Scholar]
- 21.Hodge C, Badgwell BD. Palliation of malignant ascites. J Surg Oncol. 2019;120:67–73. doi: 10.1002/jso.25453. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Vale M, Leung E, et al. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728–1734. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 23.Flies DB, Chen L. The new B7s: Playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 24.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404–413. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Richards S, Prasad DV, et al. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 26.Chapoval AI, Ni J, Lau JS, et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 27.Modak S, Gerald W, Cheung NK. Disialoganglioside GD2 and a novel tumor antigen: Potential targets for immunotherapy of desmoplastic small round cell tumor. Med Pediatr Oncol. 2002;39:547–551. doi: 10.1002/mpo.10151. [DOI] [PubMed] [Google Scholar]
- 28.Modak S, Kramer K, Gultekin SH, et al. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61:4048–4054. [PubMed] [Google Scholar]
- 29.Xu H, Cheung I, Guo HF, et al. MicroRNAmiR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Cheung I, Cheung NK. 4Ig-B7H3: a target for monoclonal antibody of human solid tumors. Cancer Res. 2008;68:9s. (suppl; abstr 2139) [Google Scholar]
- 31.Modak S, Guo HF, Humm JL, et al. Radioimmunotargeting of human rhabdomyosarcoma using monoclonal antibody 8H9. Cancer Biother Radiopharm. 2005;20:534–546. doi: 10.1089/cbr.2005.20.534. [DOI] [PubMed] [Google Scholar]
- 32.Meredith RF, Buchsbaum DJ, Alvarez RD, et al. Brief overview of preclinical and clinical studies in the development of intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2007;13:5643s–5645s. doi: 10.1158/1078-0432.CCR-07-0985. [DOI] [PubMed] [Google Scholar]
- 33.Quadri SM, Malik AB, Tang XZ, et al. Preclinical analysis of intraperitoneal administration of 111In-labeled human tumor reactive monoclonal IgM AC6C3-2B12. Cancer Res. 1995;55:5736s–5742s. [PubMed] [Google Scholar]
- 34.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandit-Taskar N, Zanzonico PB, Kramer K, et al. Biodistribution and dosimetry of intraventricularly administered 124I-omburtamab in patients with metastatic leptomeningeal tumors. J Nucl Med. 2019;60:1794–1801. doi: 10.2967/jnumed.118.219576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19:1040–1050. doi: 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson EE, Stabin MG, Davis JL, et al. A model of the peritoneal cavity for use in internal dosimetry. J Nucl Med. 1989;30:2002–2011. [PubMed] [Google Scholar]
- 39.Fauci JM, Straughn JM, Jr, Ferrone S, et al. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol Oncol. 2012;127:420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Hallqvist A, Bergmark K, Bäck T, et al. Intraperitoneal α-emitting radioimmunotherapy with 211at in relapsed ovarian cancer: Long-term follow-up with individual absorbed dose estimations. J Nucl Med. 2019;60:1073–1079. doi: 10.2967/jnumed.118.220384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oei AL, Verheijen RH, Seiden MV, et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez RD, Huh WK, Khazaeli MB, et al. A phase I study of combined modality (90)Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8:2806–2811. [PubMed] [Google Scholar]
- 43.Cederkrantz E, Andersson H, Bernhardt P, et al. Absorbed doses and risk estimates of (211)At-MX35 F(ab’)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys. 2015;93:569–576. doi: 10.1016/j.ijrobp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Meredith R, Torgue J, Shen S, et al. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med. 2014;55:1636–1642. doi: 10.2967/jnumed.114.143842. [DOI] [PMC free article] [PubMed] [Google Scholar]