PURPOSE
Many patients with HR+, HER2− early breast cancer (EBC) will not experience recurrence or have distant recurrence with currently available standard therapies. However, up to 30% of patients with high-risk clinical and/or pathologic features may experience distant recurrence, many in the first few years. Superior treatment options are needed to prevent early recurrence and development of metastases for this group of patients. Abemaciclib is an oral, continuously dosed, CDK4/6 inhibitor approved for HR+, HER2− advanced breast cancer (ABC). Efficacy and safety of abemaciclib in ABC supported evaluation in the adjuvant setting.
METHODS
This open-label, phase III study included patients with HR+, HER2−, high-risk EBC, who had surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes, or one to three nodes and either tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20%, were eligible and randomly assigned (1:1) to standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years). The primary end point was invasive disease-free survival (IDFS), and secondary end points included distant relapse–free survival, overall survival, and safety.
RESULTS
At a preplanned efficacy interim analysis, among 5,637 randomly assigned patients, 323 IDFS events were observed in the intent-to-treat population. Abemaciclib plus ET demonstrated superior IDFS versus ET alone (P = .01; hazard ratio, 0.75; 95% CI, 0.60 to 0.93), with 2-year IDFS rates of 92.2% versus 88.7%, respectively. Safety data were consistent with the known safety profile of abemaciclib.
CONCLUSION
Abemaciclib when combined with ET is the first CDK4/6 inhibitor to demonstrate a significant improvement in IDFS in patients with HR+, HER2− node-positive EBC at high risk of early recurrence.
INTRODUCTION
More than 90% of patients with breast cancer are diagnosed with early-stage disease, of whom approximately 70% have cancers that are hormone receptor positive (HR+) and human epidermal growth factor receptor 2 negative (HER2−).1,2 Standard treatment varies depending on risk of recurrence but includes combinations of surgery, radiotherapy, adjuvant/neoadjuvant chemotherapy, and endocrine therapy (ET).3,4 Adjuvant ET (aromatase inhibitors [AIs] and/or antiestrogens with or without ovarian suppression) is standard treatment of HR+, HER2− early breast cancer (EBC) and has been associated with a significant reduction in risk of recurrence and death.4 Although many patients with HR+, HER2− disease will not experience recurrence or have distant recurrence with standard therapies alone, up to 20% of patients may experience disease recurrence in the first 10 years, often with distant metastases, at which time the disease is incurable.5 For those patients with high-risk clinical and/or pathologic features, risk of recurrence is higher, especially during the first few years on adjuvant ET.6 It is therefore critical to optimize adjuvant therapy to prevent early recurrences and metastases for these patients.
CONTEXT
Key Objective
Does adding a CDK4/6 inhibitor to endocrine therapy (ET) in the adjuvant setting provide additional benefit for patients with HR+, HER2− early breast cancer (EBC)? monarchE is a global, randomized, phase III trial that evaluated the combination of the CDK4/6 inhibitor abemaciclib and standard ET among 5,637 randomly assigned patients with HR+, HER2−, node-positive EBC at high risk of early recurrence.
Knowledge Generated
The results showed that abemaciclib added to ET significantly improved invasive disease-free survival (IDFS) in patients with high-risk EBC in the adjuvant setting. There was a 25% reduction in the risk of developing an IDFS event relative to ET alone and a 3.5% absolute improvement in 2-year IDFS rates (92.2% v 88.7%). Safety was consistent with the known safety profile of abemaciclib.
Relevance
If approved, abemaciclib added to standard adjuvant ET could become a new standard of care for patients with HR+, HER2− high-risk EBC.
Abemaciclib is an oral, continuously dosed, cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor approved in combination with ET for the treatment of HR+, HER2− advanced breast cancer (ABC) on the basis of significant improvements in progression-free survival (PFS) and overall survival (OS) in combination with fulvestrant7,8 and in PFS in combination with nonsteroidal AIs.9,10 As such, exploration of the combination of abemaciclib with ET is warranted in the adjuvant setting.
monarchE is an open-label, global, randomized, phase III trial that investigated the addition of abemaciclib to standard adjuvant ET in patients with HR+, HER2−, node-positive, high-risk EBC. The design of monarchE was motivated by large clinical datasets in EBC, which all noted a subset of HR+, HER2− patients with high-risk clinical features and/or highly proliferative disease that were likely to experience recurrence quickly.6,11-13 The goal in monarchE was to treat the patients with primary endocrine-resistant disease who were likely to experience recurrence earlier in the course of their disease, especially in the first 5 years. This report describes the first results from monarchE following a preplanned interim analysis.
METHODS
Trial Oversight
This study was funded by the sponsor (Eli Lilly) and designed together with members of the study Executive Committee. The study was performed in compliance with the Declaration of Helsinki. The study protocol and all amendments received approval from ethical/institutional review boards before implementation, and all patients gave written informed consent. All authors contributed to the writing and review of the manuscript and were involved in the interpretation of the data. Executive and Global Steering Committees provided oversight of the conduct of the trial, and an independent data monitoring committee reviewed the safety data approximately every 6 months and efficacy data at prespecified interim analyses. Although this was an open-label study, the sponsor and all investigative sites remained blinded to treatment group assignments for aggregate data until the study was confirmed as positive. A complete list of all committee members and investigators is available in the Data Supplement (online only).
Patients
Female (any menopausal status) and male patients ≥ 18 years of age with HR+14 and HER2−15 disease were eligible. High risk was defined as patients with four or more positive pathologic axillary lymph nodes or one to three positive axillary lymph nodes and at least one of the following: tumor size ≥ 5 cm, histologic grade 3, or centrally assessed Ki-67 ≥ 20% (please refer to the Data Supplement, online only, for details on Ki-67 methodology).
Patients may have received up to 12 weeks of ET after the last non-ET before randomization and must have been randomly assigned within 16 months of definitive breast cancer surgery.
Radiotherapy and both adjuvant and neoadjuvant chemotherapy were allowed, but not required. Patients with occult breast cancer, metastatic disease, or node-negative breast cancer, and, after a protocol amendment, patients with inflammatory breast cancer, were excluded. Patients who had received treatment with ET for breast cancer prevention, raloxifene, and/or a CDK4/6 inhibitor, and those with a history of venous thromboembolic events (VTEs) were also excluded.
An interactive Web response system was used to randomly assign patients (1:1) to receive either abemaciclib (150 mg twice daily on a continuous dosing schedule) plus ET or ET alone. Stratification factors included previous chemotherapy (neoadjuvant, adjuvant, or none), menopausal status (as determined at the time of breast cancer diagnosis), and region (North America/Europe, Asia, or other). Patients were treated for 2 years (treatment period) or until meeting criteria for discontinuation. After the treatment period, all patients continued ET for 5 to 10 years, as clinically indicated (Data Supplement). The 2-year treatment duration was chosen based on historical studies indicating recurrence events first peaked at 2 years for patients with EBC.16 Post-discontinuation treatment was at the discretion of the investigator. Crossover was not permitted at any time.
Trial Procedures
Visits occurred every 2 weeks for the first 2 months, monthly from months 3-6, and then every 3 months until the end of year 2. Thereafter, visits were every 6 months until year 5 and then annually from years 6-10.
All randomly assigned patients were followed for local/regional and distant recurrence and OS. At each visit, patients were assessed by a medically qualified individual for adverse events (AEs) and any signs or symptoms of recurrence. At clinic visits, central chemistry and hematology laboratories were drawn, performance status was assessed, and physical examinations were conducted. Tests to confirm recurrence after detection of signs or symptoms were performed at the discretion of the treating medically qualified individual.
Statistical Analysis
The primary end point was invasive disease-free survival (IDFS) per the Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials (STEEP) criteria17 and was measured from the date of randomization to the date of first occurrence of ipsilateral invasive breast tumor recurrence, local/regional invasive breast cancer recurrence, distant recurrence, death attributable to any cause, contralateral invasive breast cancer, or second primary nonbreast invasive cancer. Confirmation by biopsy or imaging was required, when possible. All patients who experienced local recurrence continued to be followed for distant recurrence. Distant relapse–free survival (DRFS), a secondary end point, was defined as the time from randomization to distant recurrence or death from any cause, whichever occurred first. Patients for whom no event was observed were censored on the day of their last assessment for recurrence or date of randomization if no post-baseline assessment for recurrence occurred. Other secondary end points included OS, safety, pharmacokinetics (PK), and patient-reported outcomes (PROs). PK and PRO data will be reported separately.
The study was powered at approximately 85% to detect the superiority of abemaciclib plus ET versus ET in terms of IDFS, assuming a hazard ratio (HR) of 0.73 at a cumulative two-sided α level of .05, with a 5-year IDFS rate of 82.5% in the control arm for this high-risk population.6,12,13 This required approximately 390 IDFS events in the intent-to-treat (ITT) population at the time of the primary analysis. There were two planned efficacy interim analyses at approximately 50% and 75% of the total required events. The overall type I error for the preplanned interim analyses and primary analysis was maintained using the Lan-DeMets method with an O’Brien-Fleming stopping boundary.
Efficacy analyses were performed on the ITT population. The primary objective was to test the superiority of abemaciclib plus ET versus ET on IDFS using a log-rank test stratified by randomization factors. A stratified Cox proportional hazard model with treatment arm as a variable was used to estimate the HR and the corresponding 95% CI. The Kaplan-Meier method was used to estimate the 2-year IDFS rates in each treatment arm. Similar analyses were performed on DRFS, but there was no α control for statistical significance on this end point. All reported P values are two-sided.
Subgroup analyses of IDFS in the ITT population were performed for potential prognostic subgroup variables prespecified in the statistical analysis plan, including stratification factors and some clinicopathological features. HR estimates were reported within each subgroup, with P values for interaction tests across subgroups.
Safety was analyzed in all randomly assigned patients who received at least one dose of study treatment (defined as either abemaciclib or ET after randomization). Investigator-reported terms were mapped to MedDRA terms, and AEs were graded according to Common Terminology Criteria Adverse Events v4.0. Please refer to the protocol, included in the Data Supplement, for additional details on statistical methods and analyses.
RESULTS
Patients and Treatment
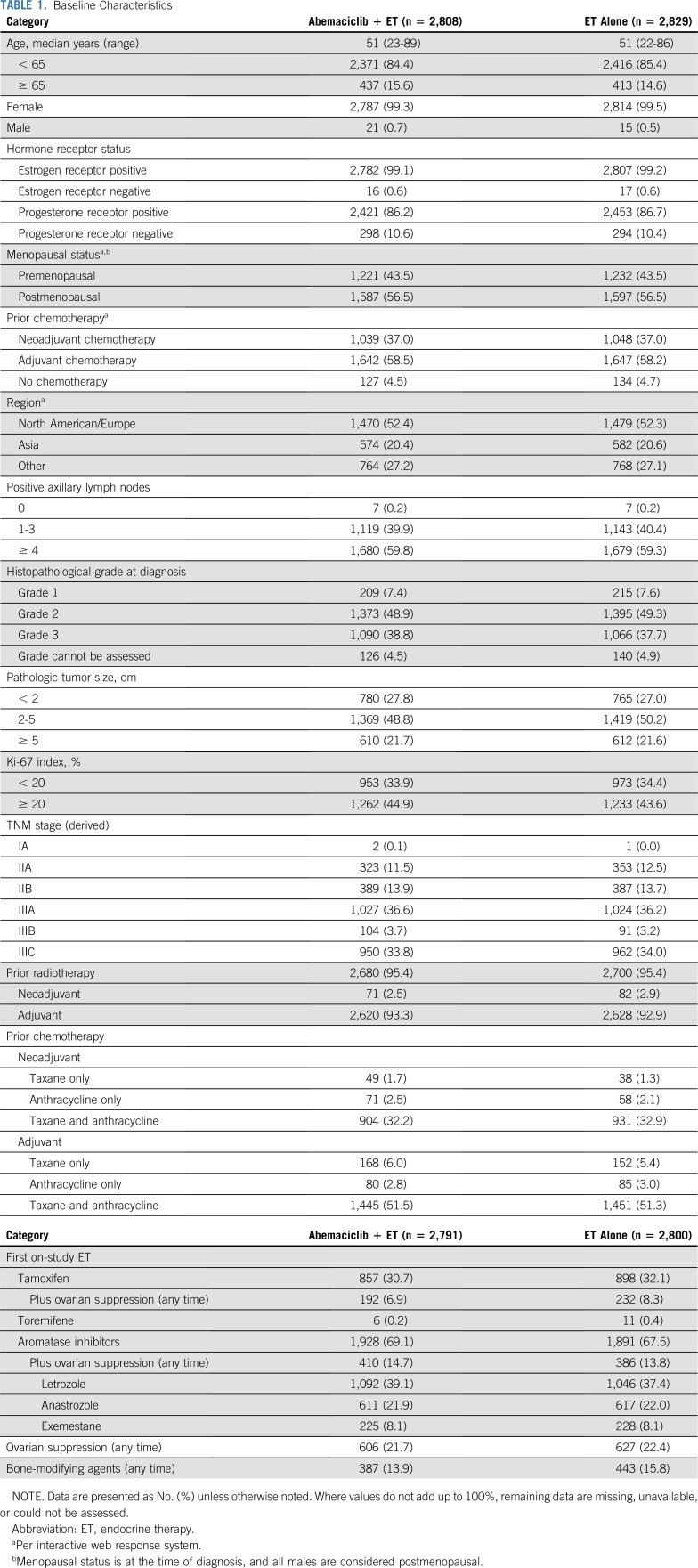
From July 2017 to August 2019, 5,637 patients from 603 sites in 38 countries were randomly assigned 1:1 to receive either abemaciclib plus ET or ET alone (Fig 1). Baseline characteristics were balanced between study arms (Table 1). The population had a median age of 51.0 years (12.6% patients < 40 years) and was predominantly female (99.4%), and postmenopausal (56.5%) at the time of diagnosis. Nearly 60% of patients were eligible on the basis of four or more nodes. Details on the specific high-risk clinical and/or pathologic feature(s) that made patients eligible are described in the Data Supplement. A total of 95.4% of patients had received radiotherapy, and 95.4% of patients had received prior chemotherapy (37.0% neoadjuvant, 58.3% adjuvant, 3.5% received both, and all patients who received both were counted in the neoadjuvant total). AIs were prescribed as the first ET on study treatment in 68.3% of patients (including 14.2% treated with AI plus ovarian function suppression), and tamoxifen in 31.4% (including 7.6% treated with tamoxifen plus ovarian function suppression; Table 1).
FIG 1.
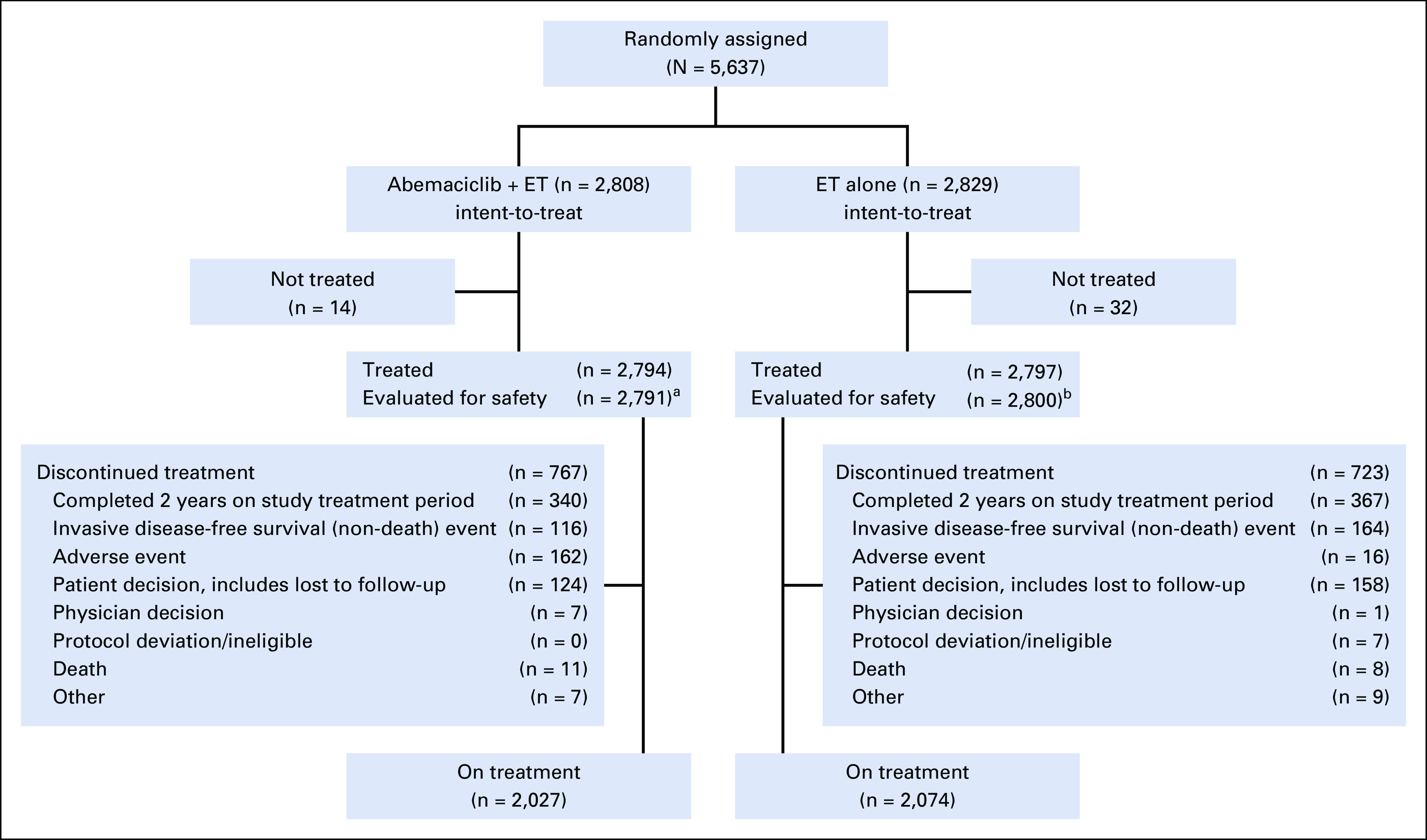
CONSORT diagram. (a) Four patients randomly assigned to the abemaciclib arm only received endocrine therapy (ET) and were evaluated for safety in the control arm. (b) One patient randomly assigned to the control arm received abemaciclib and was evaluated for safety in the abemaciclib arm.
TABLE 1.
Baseline Characteristics
At the time of the data cutoff (March 16, 2020), 707 (12.5%) patients had completed the 2-year treatment period, and 4,101 (72.8%) patients were still in the 2-year treatment period. Median follow-up time was approximately 15.5 months in both arms.
Efficacy
With a total of 323 IDFS events observed at the second efficacy interim analysis, the two-sided P value boundary for positive efficacy was .026. At the time of data cutoff, there were 136 (4.8%) IDFS events in the abemaciclib arm and 187 (6.6%) in the control arm. Abemaciclib plus ET demonstrated a statistically significant improvement in IDFS versus ET alone (P = .01; HR, 0.75; 95% CI, 0.60 to 0.93), with 2-year IDFS rates of 92.2% (abemaciclib arm) versus 88.7% (control arm; Fig 2). Most IDFS events were distant recurrences (87 in the abemaciclib arm and 138 in the control arm; Table 2). The addition of abemaciclib to ET also resulted in an improvement in DRFS compared with ET alone (nominal P = .01; HR, 0.72; 95% CI, 0.56 to 0.92), with 2-year DRFS rates of 93.6% (abemaciclib arm) and 90.3% (control arm; Fig 3A). Common sites of distant recurrence were bone, liver, and lung (Table 2). HRs within prespecified subgroups are presented in Figure 2B (IDFS) and Figure 3B (DRFS).
FIG 2.
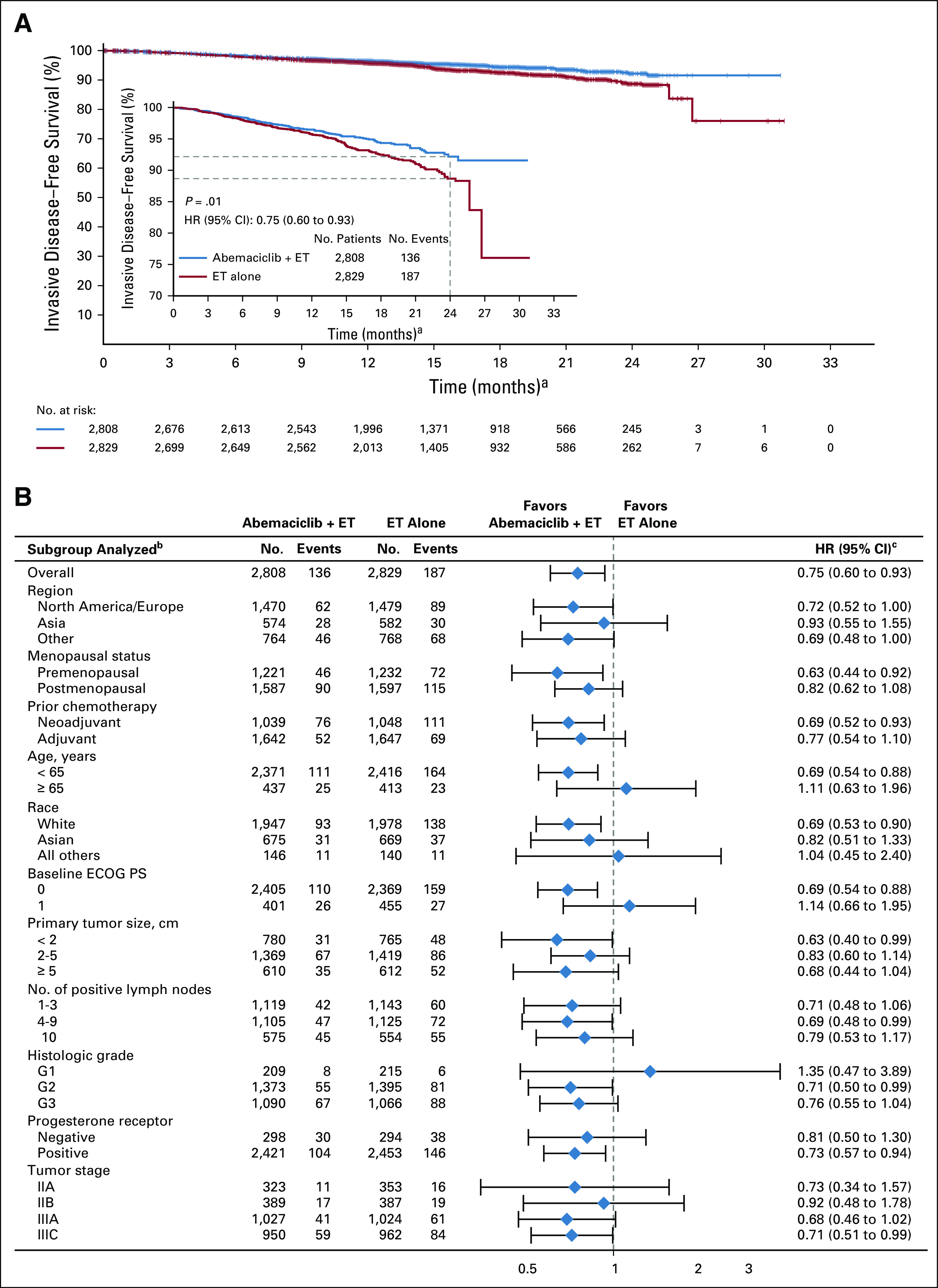
Invasive disease-free survival (IDFS). (A) Kaplan-Meier curves of IDFS and IDFS zoomed in to better visualize separation of the curves in the intent-to-treat population. (B) IDFS of patient subgroups. Hazard ratios (HRs) are stratified in overall population and unstratified in subgroups for abemaciclib plus endocrine therapy (ET) versus ET alone. HR estimates for IDFS are indicated by diamonds, and 95% CIs are indicated by the crossing horizontal lines. (a) Curves should not be interpreted beyond 24 months because of the limited follow-up. (b) If a subgroup consists of < 5% of randomly assigned patients, analysis within that subgroup was omitted. (c) The width of CIs in subgroups has not been adjusted for multiplicity; thus, the subgroup results are exploratory in nature. ECOG PS, Eastern Cooperative Oncology Group performance status.
TABLE 2.
Recurrence Events
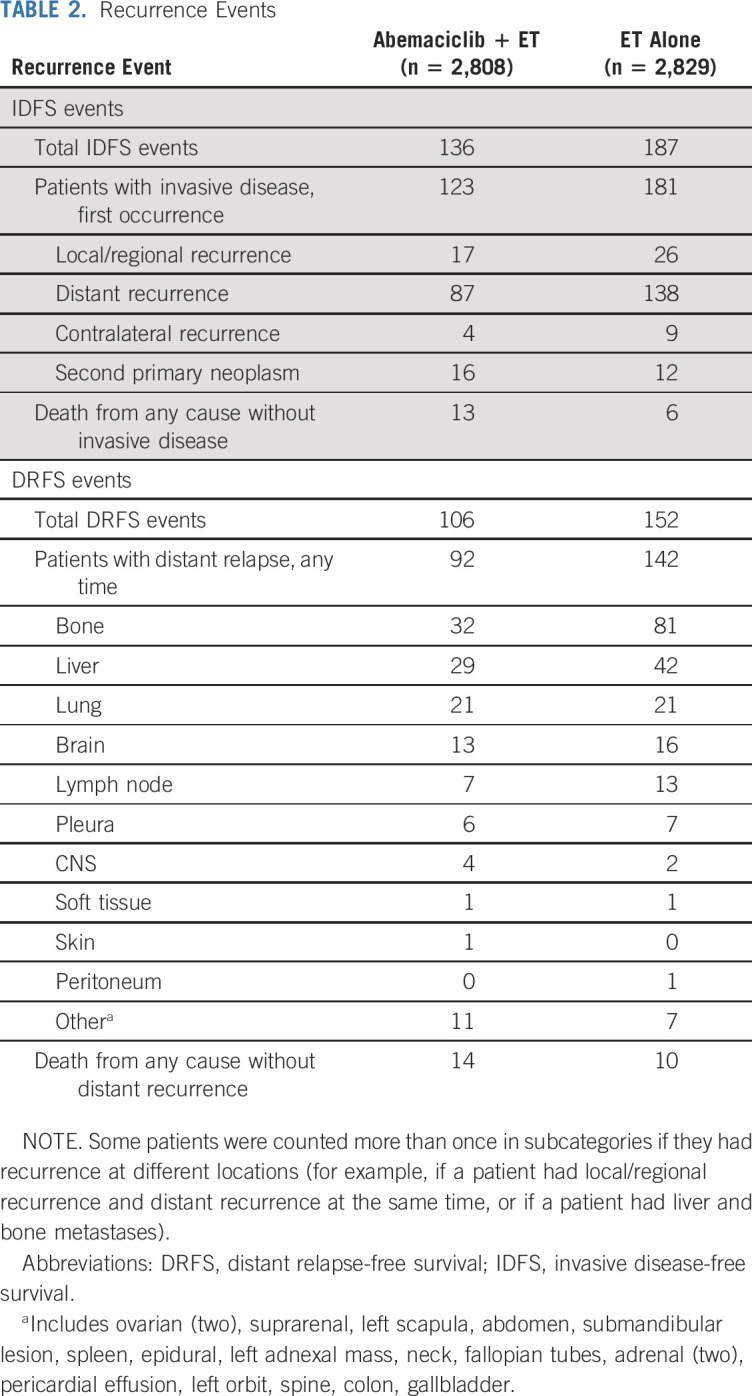
FIG 3.
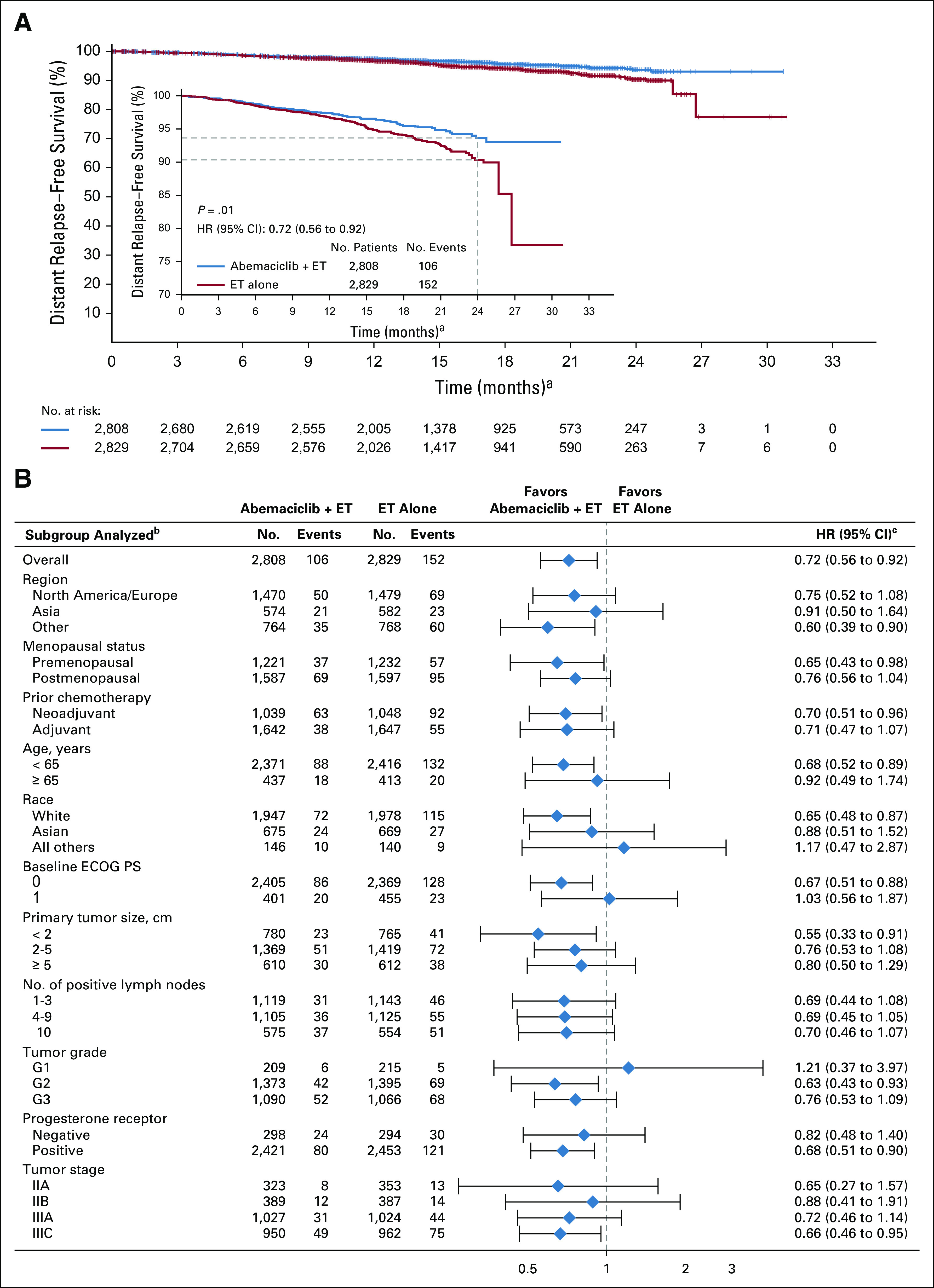
Distant relapse–free survival (DRFS). (A) Kaplan-Meier curves of DRFS and DRFS zoomed in to better visualize separation of the curves in the intent-to-treat population. (B) DRFS of patient subgroups. Hazard ratios (HRs) are stratified in overall population and unstratified in subgroups for abemaciclib plus endocrine therapy (ET) versus ET alone. HR estimates for DRFS are indicated by diamonds, and 95% CIs are indicated by the crossing horizontal lines. (a) Curves should not be interpreted beyond 24 months because of the limited follow-up. (b) If a subgroup consists of < 5% of randomly assigned patients, analysis within that subgroup was omitted. (c) The width of CIs in subgroups has not been adjusted for multiplicity; thus, the subgroup results are exploratory in nature. ECOG PS, Eastern Cooperative Oncology Group performance status.
OS data were immature, with 39 (1.4%) deaths observed in the abemaciclib arm and 37 (1.3%) observed in the control arm. The study will continue to a final analysis of OS.
Safety
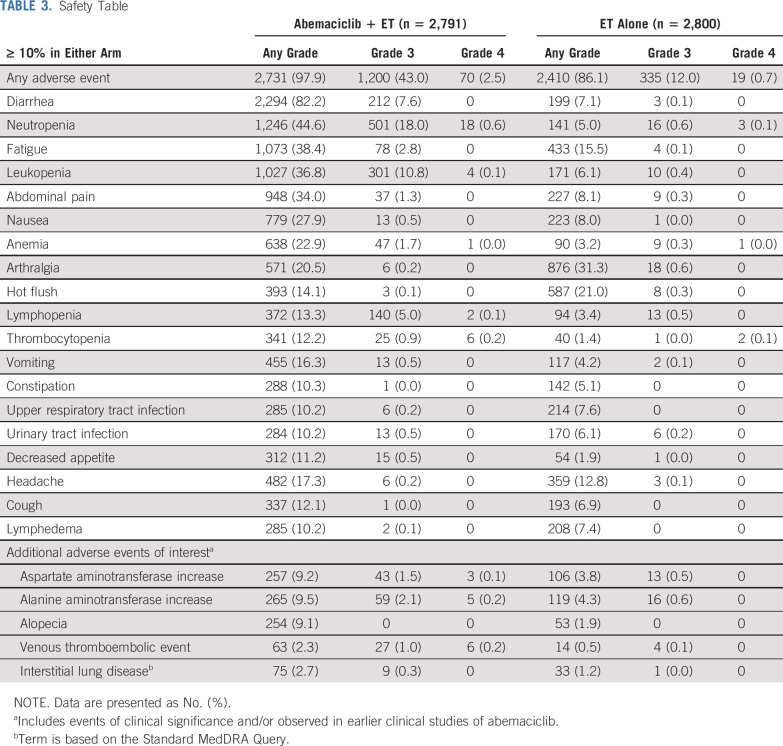
A total of 5,591 patients (2,791 in the abemaciclib arm and 2,800 in the control arm) were included in the safety analysis. The median duration of ET was approximately 15 months in each arm. The median duration of abemaciclib was 14 months. Abemaciclib dose adjustments due to AEs occurred in 1,901 patients (68.1%), with 56.9% having dose omissions and 41.2% having reductions. In the abemaciclib arm, 463 patients (16.6%) discontinued abemaciclib because of AEs, of whom 306 remained on ET when abemaciclib was discontinued. A total of 172 patients (6.2%) discontinued both treatments (157 at the same time) because of AEs. In the control arm, 21 patients (0.8%) discontinued ET because of AEs.
A total of 5,141 patients experienced at least one treatment-emergent AE (97.9% in the abemaciclib arm and 86.1% in control arm). The most frequent AEs were diarrhea, neutropenia, and fatigue in the abemaciclib arm and arthralgia, hot flush, and fatigue in the control arm (Table 3). VTEs were reported in 2.3% of patients in the abemaciclib arm and 0.5% in the control arm (pulmonary embolism, 0.9% v 0.1%). Details on VTEs are in the Data Supplement. Interstitial lung disease (ILD) occurred in 2.7% of patients in the abemaciclib arm (0.3% grade 3) and 1.2% of patients in the control arm (one patient with grade 3; Table 3).
TABLE 3.
Safety Table
Grade ≥ 3 AEs occurred in 45.9% of patients in the abemaciclib arm and 12.9% of patients in the control arm. Serious adverse events (SAEs) occurred in 12.3% of patients in the abemaciclib arm and 7.2% of patients in the control arm, with the most frequently reported SAE in both arms being pneumonia (0.8% and 0.5%, respectively). Although the number of grade ≥ 3 AEs was higher in the abemaciclib arm, this did not translate into proportional increases in SAEs.
Deaths on study treatment or within 30 days of discontinuation were balanced between the arms, with 14 (0.5%) in each arm. In the abemaciclib arm, 11 were due to AEs (two, diarrhea and pneumonitis, considered possibly related to study treatment by the investigator) versus seven as a result of AEs in the control arm.
DISCUSSION
In this global, open-label, randomized phase III trial in patients with HR+, HER2−, node-positive, high-risk EBC, the addition of abemaciclib to standard adjuvant ET resulted in a statistically significant improvement in IDFS. All patients had primary breast surgery, and despite > 95% of patients having received prior chemotherapy and radiotherapy, the estimated 2-year IDFS rate in the monarchE control arm indicates that 11.3% of patients with high-risk clinicopathological features will develop an invasive disease event within 2 years. The addition of abemaciclib resulted in a 25% reduction in the risk of developing an IDFS event relative to ET alone and an absolute improvement of 3.5% in 2-year IDFS rates. This is a clinically meaningful result, and the treatment effect was observed in all prespecified subgroups. Of note, among the 43.5% of patients who were premenopausal at diagnosis, there was a significant 37% reduction in the risk of recurrence relative to ET alone. This is relevant because there have been very few treatment advances in adjuvant therapy for HR+, HER2− EBC for nearly 20 years.
Early recurrence within the first 2 years of adjuvant ET in HR+ breast cancer is known to represent primary endocrine resistance.18 More than 75% of the early recurrences seen in the control arm were distant recurrence. Overcoming endocrine resistance and reducing the risk of distant metastasis is an essential objective for any novel therapy in HR+ EBC. Abemaciclib is an approved CDK4/6 inhibitor in HR+, HER2− ABC, with clinical efficacy both as monotherapy in pretreated ABC19 and in combination with ET.8,9 Significant improvement in OS was reported in patients with endocrine-resistant disease, including patients with visceral metastases.7 The effect now seen in the EBC adjuvant setting in monarchE indicates a similar impact on preventing early primary endocrine resistant recurrences and on reducing the risk of metastatic recurrence by a clinically meaningful 28% relative to ET alone, especially in bone and liver metastases (Table 2). Of note, the Kaplan-Meier curves for both IDFS and DRFS separate at 9-12 months (Figs 2 and 3), which may indicate the need for early treatment of very-high-risk patients who may have subclinical micrometastatic disease at diagnosis.
The ability to conclude the statistically significant benefit of adding abemaciclib to ET is dependent on the number of IDFS events observed. By enrolling patients whose cancer is at high risk of early recurrence, monarchE reached the target number of events for the efficacy assessment faster relative to a patient population at a lower risk of recurrence. At this preplanned interim analysis, 323 events were observed, corresponding to > 80% of the total planned number of events (390). Overall type I error was robustly controlled using an O’Brien-Fleming spending function, ensuring highly statistically significant and definitive results. Although the median follow-up of 15 months at this time is short for an EBC adjuvant study, the positive outcome demonstrated the effectiveness of abemaciclib in reducing the risk of invasive disease within 2 years—a critical outcome for this high-risk population. Additional follow-up in monarchE will determine the persistence of this benefit on later recurrences and overall survival in node-positive, high-risk HR+ HER2− EBC, which is unknown at present.
Understanding the subgroups of patients with HR+, HER2− breast cancer for whom the addition of a targeted therapy is most beneficial is an important goal. In monarchE, a population with increased risk of recurrence on the basis of disease characteristics such as axillary lymph node involvement, tumor size, and biology was selected, and a clear efficacy benefit for the addition of abemaciclib was demonstrated. Whether benefit of a CDK4/6 inhibitor can be demonstrated in patients at lower risk is unclear, especially given that many of these patients experience recurrence late (> 10 years after diagnosis), and extended adjuvant ET is increasingly used and may benefit these patients. There are additional ongoing clinical trials evaluating CDK4/6 inhibitors in EBC (ClinicalTrials.gov identifiers: NCT03701334 and NCT02513394). Results from these trials using different compounds in different populations will further determine the role of CDK4/6 inhibitors in EBC. The definitive results from monarchE have identified a specific high-risk patient population for whom the addition of abemaciclib prevents early disease recurrences and reduces the risk of distant metastases. In addition, understanding the tumor biology of patients who benefit most from adjuvant abemaciclib is important, and parallel translational research to investigate tissue and plasma samples from patients in monarchE is underway.
Adherence to therapy is a challenge in the adjuvant setting, and a manageable toxicity profile for any novel therapy is crucial. The safety profile of abemaciclib in this adjuvant study was consistent with the known adverse effect profile of abemaciclib previously reported in the ABC setting. Of interest, arthralgia and hot flush are known frequent and often troublesome AEs related to ET. Both were significantly reduced in those treated with abemaciclib plus ET compared with ET alone (arthralgia, 20.5% v 31.3%; hot flush, 14.1% v 21.0%). This intriguing observation has been reported previously in a randomized study of a CDK4/6 inhibitor plus ET.20
The most frequently reported AE in the abemaciclib arm was diarrhea. Diarrhea occurred early (median time to onset for any grade of 8 days), was short-lived (median duration for grades 2 and 3 of 5 to 6 days), and was managed with antidiarrheal medication and dose adjustments per protocol. The frequency of diarrhea grade ≥ 3 was 7.6%; however, a very low number of patients (4.8%) discontinued abemaciclib because of diarrhea. Neutropenia grade ≥ 3 occurred in < 20% of abemaciclib-treated patients (0.3% febrile neutropenia, any grade). VTE and ILD were reported more often in the abemaciclib arm, and although SAEs were uncommon, signs and symptoms should be closely monitored for patients on abemaciclib.
In conclusion, monarchE has demonstrated that abemaciclib added to standard adjuvant ET significantly improves IDFS in women and men with HR+, HER2−, node-positive EBC at high risk of early recurrence.
ACKNOWLEDGMENT
We thank the patients who participated in the trial, their families, and those who provided support to the participants. We also thank all the site personnel who helped enroll monarchE and who supported and continue to support monarchE, and to Sarah C. Nabinger for editorial assistance for this manuscript. We thank Mary Stuart, MD, for her involvement in the start-up and execution of the study. We would like to honor the memory of Ayse Gül Kurt, MD, a monarchE investigator at LMU Munich, who died of metastatic breast cancer at the age of 43 in May 2020. Please refer to the Data Supplement for a complete list of committee members and investigators involved in monarchE.
SUPPORT
Funded and sponsored by Eli Lilly and Company. Additional support provided by National Institute for Health Research funding to the Royal Marsden and Institute of Cancer Research Biomedical Research Center.
CLINICAL TRIAL INFORMATION
NCT03155997 (monarchE)
See accompanying editorial on page 3977
Processed as a Rapid Communication manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Stephen R. D. Johnston, Nadia Harbeck, Masakazu Toi, Miguel Martin, Ian C. Smith, Martin Frenzel, Desirée Headley, Maarten Hulstijn, Joyce O’Shaughnessy, Priya Rastogi
Provision of study material or patients: Stephen R. D. Johnston, Nadia Harbeck, Roberto Hegg, Masakazu Toi, Miguel Martin, Zhi Min Shao, Qing Yuan Zhang, Jorge Luis Martinez Rodriguez, Mario Campone, Erika Hamilton, Joohyuk Sohn, Valentina Guarneri, Morihito Okada, Frances Boyle, Patrick Neven, Javier Cortés, Jens Huober, Andrew Wardley, Sara M. Tolaney, Irfan Cicin, Joyce O’Shaughnessy, Priya Rastogi
Collection and assembly of data: Stephen R. D. Johnston, Nadia Harbeck, Roberto Hegg, Masakazu Toi, Miguel Martin, Zhi Min Shao, Qing Yuan Zhang, Erika Hamilton, Joohyuk Sohn,Valentina Guarneri, Morihito Okada, Frances Boyle, Patrick Neven, Sara M. Tolaney, Irfan Cicin, Ian C. Smith
Data analysis and interpretation: Stephen R. D. Johnston, Nadia Harbeck, Masakazu Toi, Miguel Martin, Jorge Luis Martinez Rodriguez, Mario Campone, Erika Hamilton, Valentina Guarneri, Patrick Neven, Javier Cortés, Jens Huober, Andrew Wardley, Sara M. Tolaney, Irfan Cicin, Ian C. Smith, Martin Frenzel, Desirée Headley, Ran Wei, Belen San Antonio, Maarten Hulstijn, Joanne Cox, Priya Rastogi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephen R. D. Johnston
Honoraria: Pfizer, Novartis, Eisai, AstraZeneca, Roche, Eli Lilly
Consulting or Advisory Role: Eli Lilly, Puma Biotechnology, Novartis, Pfizer
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst)
Nadia Harbeck
Stock and Other Ownership Interests: West German Study Group
Honoraria: Roche, Novartis, Amgen, Pfizer, Genomic Health, AstraZeneca, Zodiac Pharma, Pierre Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Celgene, Pfizer, Eli Lilly, Sandoz, Daiichi Sankyo, Agendia, AstraZeneca, Merck Sharp & Dohme, Odonate Therapeutics, Seattle Genetics, West German Study Group (I), Pierre Fabre
Research Funding: Roche/Genentech (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Merck Sharp & Dohme (Inst)
Masakazu Toi
Honoraria: Novartis, Takeda, AstraZeneca, Eisai, Genomic Health, Chugai Pharma, Taiho Pharmaceutical, Daiichi Sankyo, Yakult, Shimadzu, Pfizer, Konica Minolta, Eli Lilly, Kyowa Kirin, Devicore Medical Japan
Consulting or Advisory Role: Daiichi Sankyo, Kyowa Kirin, Konica Minolta, Bertis, Athenex Oncology, BMS
Speakers' Bureau: Pfizer, AstraZeneca, Eli Lilly
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Shimadzu, Astellas Pharma, AFI Technology, Japan Breast Cancer Research Group, Pfizer, Eisai, Daiichi Sankyo, AstraZeneca, Ono Pharmaceutical, Nippo Kayaku, Kyoto Breast Cancer Research Network
Patents, Royalties, Other Intellectual Property: JP 2017-143763WO2017/131162A1 (Inst), PCT/JP2016/004374 (Inst)
Travel, Accommodations, Expenses: Eisai, Takeda
Other Relationship: Japan Breast Cancer Research Group, Kyoto Breast Cancer Research Network, Organization for Oncology and Translational Research
Miguel Martin
Honoraria: Roche/Genentech, Eli Lilly, Pfizer (I), Novartis, Pierre-Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Eli Lilly, AstraZeneca, Taiho Pharmaceutical, PharmaMar
Speakers' Bureau: Eli Lilly/ImClone, Roche/Genentech, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), PUMA (Inst)
Mario Campone
Honoraria: Novartis, Eli Lilly, GT1
Consulting or Advisory Role: Novartis (Inst), SERVIER (Inst), Menarini, Sanofi (Inst), Eli Lilly (Inst), Pfizer, AstraZeneca/MedImmune (Inst), AbbVie (Inst), Pierre Fabre (Inst), ACCORD (Inst) Sandoz-Novartis (Inst)
Speakers' Bureau: Novartis, Amgen
Research Funding: Novartis (Inst)
Travel, Accommodations, Expenses: Novartis, Astra Zeneca, Pfizer
Other Relationship: Roche
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Eli Lilly (Inst), Puma Biotechnology (Inst), Daiichi Sankyo (Inst), Mersana (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Novartis (Inst), Silverback Therapeutics (Inst), Black Diamond (Inst), CytomX Therapeutics (Inst), Dantari (Inst), H3 Biomedicine (Inst), Merck (Inst), Novartis (Inst), Seattle Genetics (Inst), Eisai (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune (Inst), Eli Lilly (Inst), Medivation (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Takeda (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho Pharmaceutical (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros Pharmaceuticals (Inst), Clovis Oncology (Inst), CytomX Therapeutics (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro (Inst), Aravive (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Torque (Inst), Harpoon (Inst), Fochon (Inst), Black Diamond (Inst), Orinove (Inst), Molecular Templates (Inst), Silverback (Inst), Seattle Genetics (Inst), Puma Biotechnology (Inst), Compugen (Inst), G1 Therapeutics (Inst), Karyopharm Therapeutics (Inst), Torque (Inst), Dana Farber Cancer Hospital (Inst), Compugen (Inst), Infinity Pharmaceuticals (Inst), Onconova Therapeutics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Eli Lilly, Pfizer, Puma Biotechnology, Daiichi Sankyo
Joohyuk Sohn
Research Funding: MSD (Inst), Roche (Inst), Novartis (Inst), Eli Lilly (Inst), Pfizer (Inst), Bayer (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst)
Valentina Guarneri
Consulting or Advisory Role: Eli Lilly, Roche, Novartis
Speakers' Bureau: Novartis, Eli Lilly
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Tesaro, Celgene
Morihito Okada
Speakers' Bureau: Taiho Pharmaceutical, Johnson & Johnson, Covidien, Eli Lilly, Chugai Pharma, AstraZeneca, Ono Pharmaceutical, CSL Behring
Research Funding: Taiho Pharmaceutical (Inst), Nippon Kayaku (Inst), Chugai Pharma (Inst), Covidien (Inst), Johnson & Johnson (Inst), Daiichi Sankyo (Inst), Yakult Honsha (Inst), Eli Lilly Japan (Inst), Nihon Medi-Physics (Inst), Pfizer (Inst), Mochida Pharmaceutical (Inst), Shionogi (Inst), Ono Pharmaceutical (Inst), Kyowa Hakko Kirin (Inst)
Frances Boyle
Honoraria: Eli Lilly, Eisai, Roche
Consulting or Advisory Role: Roche, Eli Lilly, Novartis, Pfizer
Travel, Accommodations, Expenses: Novartis
Other Relationship: Paxman, Breast Cancer Network of Australia, Clinical Oncology Society of Australia, Pam McLean Communications Centre
Uncompensated Relationships: Paxman
Patrick Neven
Consulting or Advisory Role: Eli Lilly (Inst), Pfizer (Inst), Novartis (Inst), Roche (Inst), Radius Health (Inst)
Travel, Accommodations, Expenses: Eli Lilly (Inst), Pfizer (Inst), Roche (Inst)
Javier Cortés
Stock and Other Ownership Interests: MedSIR
Honoraria: Novartis, Eisai, Celgene, Pfizer, Roche, Samsung, Eli Lilly, Merck Sharp & Dohme, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Cellestia Biotech, AstraZeneca, Biothera, Merus, Roche, Seattle Genetics, Daiichi Sankyo, ERYTECH Pharma, Polyphor, Athenex, Eli Lilly, SERVIER, Merck Sharp & Dohme, GlaxoSmithKline, Leuko, Clovis Oncology, Bioasis, Boehringer Ingelheim
Research Funding: ARIAD (Inst), Astrazeneca (Inst), Baxalta GMBH/ Servier Affaires (Inst), Bayer (Inst), Eisai Farmaceutica (Inst), Guardant Health (Inst), Merck Sharp & Dohme (Inst), Pfizer (Inst), Puma (Inst), Queen Mary University of London (Inst), Roche (Inst), Piqur (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Eisai, Novartis, Daiichi Sankyo
Jens Huober
Honoraria: Novartis, Roche, Eli Lilly, Pfizer, Celgene, MSD Oncology, AstraZeneca, Celgene, AbbVie
Consulting or Advisory Role: Novartis, Roche, Pfizer, Eli Lilly, Celgene, AstraZeneca, AbbVie, Eisai, MSD Oncology, Hexal
Research Funding: Novartis (Inst), Celgene (Inst), Hexal
Travel, Accommodations, Expenses: Novartis, Roche, Pfizer, Celgene, Daiichi Sankyo
Andrew Wardley
Stock and Other Ownership Interests: Andrew Wardley Limited, Manchester Cancer Academy Limited, Outreach Research and Innovation Group Limited
Honoraria: Roche, Novartis, AstraZeneca, Eli Lilly, Pfizer
Consulting or Advisory Role: Roche, Eli Lilly, Novartis, AstraZeneca, MSD Oncology, Daiichi Sankyo, Athenex, Pfizer, AstraZeneca, Coleman Expert Network, Guidepoint Global, Gerson Lehrman Group, Athenex, Boehringer Ingelheim, Helios, Simo
Speakers' Bureau: Roche, AstraZeneca, Novartis, Eisai, Eli Lilly, Pfizer
Research Funding: Roche (Inst), Novartis (Inst), Pfizer (Inst), Most Cancer Pharma (Inst)
Travel, Accommodations, Expenses: Roche, Daiichi Sankyo
Other Relationship: MSD Oncology
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Eli Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Immunomedics, Sanofi, Celldex, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate, AbbVie, Silverback Therapeutics, G1 Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Eli Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Eli Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Eisai, NanoString Technologies, Puma Biotechnology, Celldex
Irfan Cicin
Consulting or Advisory Role: Pfizer (Inst), MSD Oncology (Inst), Roche (Inst), Novartis/Ipsen (Inst), Eli Lilly (Inst), Bristol Myers Squibb (Inst), SERVIER (Inst), Abdi Ibrahim (Inst), Nobelpharma (Inst), AbbVie (Inst), Teva, Janssen Oncology (Inst)
Speakers' Bureau: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Abdi Ibrahim (Inst)
Ian C. Smith
Employment: Eli Lilly, Artios Pharma
Honoraria: Roche, Eisai, AstraZeneca, Genomic Health, Pfizer
Consulting or Advisory Role: Roche, AstraZeneca, Eisai, Pfizer, Genomic Health
Travel, Accommodations, Expenses: Roche, Pfizer
Martin Frenzel
Employment: Eli Lilly
Stock and Other Ownership Interests: Eli Lilly
Desirée Headley
Employment: Eli Lilly
Stock and Other Ownership Interests: Eli Lilly, Evgen, Amgen, AstraZeneca, Bayer, Chugai Pharma, Johnson & Johnson, Merck, Novartis, Pfizer, Roche, Takeda, Varex Imaging, Utah Medical Products, Varian Medical Systems, Zimmer BioMet, Zoetis
Ran Wei
Employment: Eli Lilly and Company
Stock and Other Ownership Interests: Eli Lilly and Company
Belen San Antonio
Employment: Eli Lilly
Stock and Other Ownership Interests: Eli Lilly
Maarten Hulstijn
Employment: Eli Lilly, Biotest (I)
Joanne Cox
Employment: Eli Lilly, Alnylam (I)
Stock and Other Ownership Interests: Eli Lilly
Joyce O'Shaughnessy
Honoraria: AstraZeneca, Eli Lilly, AbbVie, Celgene, Eisai, Novartis, Pfizer, Agendia, Amgen, Bristol Myers Squibb, Genentech, Genomic Health, GRAIL, Immunomedics, HERON, Ipsen, Jounce Therapeutics, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Seattle Genetics, Syndax, Ondonate, Sanofi, Samsung, Daiichi Sankyo
Consulting or Advisory Role: Novartis, Pfizer, Eli Lilly, AbbVie, AstraZeneca, Celgene, Eisai, Agendia, Amgen, Bristol Myers Squibb, Genentech, Genomic Health, GRAIL, Immunomedics, HERON, Ipsen, Jounce Therapeutics, Merck, Myriad Pharmaceuticals, Puma Biotechnology, Roche, Seattle Genetics, Syndax, Ondondate, Sanofi, Samsung, Daiichi Sankyo
Speakers' Bureau: AstraZeneca, Novartis, Eli Lilly, Pfizer, Pfizer
Research Funding: Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Celgene, Eli Lilly, Novartis, Pfizer, AbbVie, Agendia, Amgen, Eisai, Genomic Health, GRAIL, Ipsen, Jounce Therapeutics, Myriad Pharmaceuticals, Puma Biotechnology, Seattle Genetics, AstraZeneca, Sanofi, Roche
Priya Rastogi
Travel, Accommodations, Expenses: Genentech/Roche, Eli Lilly, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1093/jnci/dju055. Howlader N, Altekruse SF, Li CI, et al: US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106:dju055, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso F Spence D Mertz S, et al. : Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast 39:131-138, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Senkus E Kyriakides S Ohno S, et al. : Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v8-v30, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network: Breast Cancer (version 4.2020). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) : Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 386:1341-1352, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Mamounas EP Tang G Paik S, et al. : 21-Gene Recurrence Score for prognosis and prediction of taxane benefit after adjuvant chemotherapy plus endocrine therapy: Results from NSABP B-28/NRG Oncology. Breast Cancer Res Treat 168:69-77, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.1001/jamaoncol.2019.4782. Sledge GW Jr, Toi M, Neven P, et al: The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol 6:116-124, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sledge GW Jr Toi M Neven P, et al. : MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875-2884, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP Toi M Campone M, et al. : MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638-3646, 2017 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1038/s41523-018-0097-z. Johnston S, Martin M, Di Leo A, et al: MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5, 2019, doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso F van’t Veer LJ Bogaerts J, et al. : 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717-729, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Gluz O Nitz UA Christgen M, et al. : West German Study Group phase III planB trial: First prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 34:2341-2349, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Marmé F Lederer B Blohmer JU, et al. : Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 53:65-74, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Hammond ME Hayes DF Wolff AC, et al. : American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 6:195-197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC Hammond MEH Allison KH, et al. : Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 36:2105-2122, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group, Forbes JF Cuzick J, et al. : Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45-53, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA Barlow WE Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol 25:2127-2132, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Cardoso F Senkus E Costa A, et al. : 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 29:1634-1657, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickler MN Tolaney SM Rugo HS, et al. : MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res 23:5218-5224, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston S Puhalla S Wheatley D, et al. : Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET Trial. J Clin Oncol 37:178-189, 2019 [DOI] [PubMed] [Google Scholar]