Abstract
BACKGROUND:
Aging is accompanied by alterations in immune functions. How these changes translate into levels of circulating inflammatory mediators and network expression after severe trauma is not well characterized. To address this, we compared time-dependent changes in the levels of an extensive biomarker panel in cohorts of severely injured young and aged adults.
STUDY DESIGN:
Cohorts of young (18 to 30 years old, n = 115) and aged (65 to 90 years old, n = 101) blunt trauma patients admitted to the ICU with plasma sampled 3 times within the first 24 hours and daily from day 1 to day 7 were assayed for 30 inflammatory biomarkers using Luminex analyzer. Stringently matched groups controlling for sex ratio and Injury Severity Score (n = 56 young vs n = 56 aged) were generated. Data were analyzed using 2-way ANOVA, area under the curve analysis, Dynamic Bayesian Network inference, and Dynamic Network Analysis.
RESULTS:
In the overall cohorts, the young group had a significantly higher Injury Severity Score, which was associated with higher circulating levels of 18 inflammatory mediators from admission to day 7. The aged group had higher levels of C-X-C motif chemokine ligand 10/interferon gamma-induced protein 10 and C-X-C motif chemokine ligand 9/monokine induced by gamma interferon. In groups that were matched for Injury Severity Score, the significantly higher levels of interferon gamma-induced protein 10 and monokine induced by gamma interferon persisted in the aged. Dynamic Bayesian Network revealed interferon gamma-induced protein 10 and monokine induced by gamma interferon as key mediators in the aged, and Dynamic Network Analysis revealed higher network complexity in the aged.
CONCLUSIONS:
These findings indicate that differences in the early inflammatory networks between young and aged trauma patients are not simply a suppression of pro-inflammatory responses in the aged, but are characterized by a major shift in the mediator profile patterns with high levels of CXC chemokines in the aged.
Traumatic injury is known to result in the simultaneous release of both pro- and anti-inflammatory mediators.1 These post-traumatic inflammatory responses can be altered in the elderly as a consequence of the biology of aging. This age-related dysfunction of the immune system, known as immunosenescence, involves changes in both innate and adaptive immunity,2 including the phenotype and function of a variety of immune cells.3 Aging is also associated with a chronic subclinical level of systemic inflammation termed inflammaging,2 which is characterized by increased pro-inflammatory cytokines.4,5
Earlier studies have shown that aging correlates with differential outcomes, including but not limited to, organ failure, longer ICU and hospital lengths of stay (LOS), increased likelihood of discharge to nursing or rehabilitation facility, and increased mortality.6–9 The literature is varied, however, on whether the age-related changes of the immune system and inflammatory response are wholly or partly responsible for the adverse clinical outcomes observed in the elderly population.10 Vanzant and colleagues,8 using data obtained from the Inflammation and the Host Response to Injury Collaborative Program Trauma Glue Grant of young (younger than 55 years) vs aged (55 years and older) patients, examined levels of 7 pro-inflammatory mediators and found significantly lower levels of 6 biomarkers in the patients 55 years and older. They concluded that inflammaging in and of itself does not lead to an exaggerated inflammatory response post trauma, but that the inflammatory response takes longer to recover in older compared with younger trauma patients.
How immunosenescence and inflammaging affect levels of inflammatory mediators after traumatic injury has not been well characterized. To address this question, we sought to analyze the dynamic changes in an extensive panel of inflammatory mediators in stringently matched trauma patients that differed in age. This panel included mediators that represent all the major pathways of the immune response. Our results revealed that the inflammatory response of the aged patients (65 years and older) was represented by a unique pattern of circulating cytokine and chemokine levels relative to the young patients (18 to 30 years old). The aged cohort had elevated levels of C-X-C motif chemokine ligand 10/interferon gamma-induced protein 10 (IP-10) and C-X-C motif chemokine ligand 9/monokine induced by gamma interferon (MIG) compared with the young cohort, and IP-10 and MIG were of particular importance in the dynamic interactions among systemic biomarkers in the aged. The systemic inflammatory response to aging is not simply a matter of suppressed inflammation, and is better characterized by a shift in the pathways and mediators that manifest after severe injury.
MATERIALS AND METHODS
Subjects and blood sampling
Blunt trauma patients were enrolled in this study after University of Pittsburgh IRB approval and informed consent. Inclusion criteria were age 18 years or older, admission to the ICU, and expectation to survive beyond initial 24 hours after injury. Exclusion criteria were isolated head injury, brain death criteria, and pregnancy. Plasma was sampled 3 times within the first 24 hours, starting with the initial blood draw on arrival, and then daily from day (D)1 to D7 after injury.
Data collection
Demographic and clinical data including mechanism of injury, ICU LOS, hospital LOS, days on mechanical ventilation, Abbreviated Injury Scale (AIS), Injury Severity Score (ISS), Marshall Multiple Organ Dysfunction Score, WBC, rates of nosocomial infection (NI), comorbidities, and disposition were collected from the inpatient electronic medical records and trauma registry database.
Study design
This was a retrospective study of a prospectively maintained clinical and Biobank database of 472 blunt trauma survivors at a Level I trauma center. Patients were stratified into 2 groups: young (18 to 30 years old, n = 115) and aged (65 to 90 years old, n = 101). Although trauma studies vary widely in their definition of aged,11 we chose 65 years old as a cutoff based on the US Census Bureau definition of age 65 years and older as the “older population.”12 Given the heterogeneity in the trauma patient population, and to avoid the potential impact of sex and ISS when comparing aged patients with young patients, stringently matched groups were generated based on these variables. To do so, a 1:1 matching was performed using IBM SPSS Statistics software case-control matching controlling for sex ratio and ISS calculated on hospital arrival.
Multiplex biomarker assay
Blood samples were centrifuged and plasma was stored at −80°C for subsequent analysis of inflammatory mediators. A Luminex 100 IS analyzer (Luminex) and Human Cytokine/Chemokine MILLIPLEX Panel kit (Millipore Corporation) were used to measure plasma levels of C-X-C motif chemokine ligand (CXCL) 10/IP-10, CXCL9/MIG, eotaxin/C-C motif chemokine ligand (CCL) 11, interleukin (IL)-1β, IL-1 receptor antagonist, IL-2, soluble IL-2 receptor-α, IL-4, IL-5, IL-6, IL-7, IL-8/CXCL8, IL-10, IL-13, IL-15, IL-17A, interferon-α, interferon-γ, CCL3/macrophage inflammatory protein (MIP)-1α, CCL4/MIP-1β, CCL2/monocyte chemoattractant protein (MCP)-1, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-α. Human T helper 17 MILLIPLEX Panel kit was used to measure IL-9, IL-21, IL-22, IL-23, IL-17E/25, and IL-33. Soluble ST2 was measured by a sandwich ELISA assay (R&D Systems).
Statistical analyses
All data were analyzed using SigmaPlot 11 software (Systat Software) and GraphPad Prism (GraphPad). Statistical differences between groups were determined by either Manne–Whitney U test or chi-square as appropriate. Group by time interaction of plasma inflammatory mediators’ levels was determined by 2-way ANOVA. To quantify the overall production of the statistically significant mediators, we calculated the area under the curve (AUC) using the mean values for each time point in a given time frame and then calculated the fold-change difference between the 2 groups. Spearman’s correlation was performed to measure the strength of association between admission levels of inflammatory mediators and clinical outcomes. A p value < 0.05 was considered statistically significant for all analyses.
Data-driven modeling
Dynamic Bayesian Network (DyBN) inference was carried out to define the most likely single-network structure that best characterizes the dynamic interactions among systemic inflammatory mediators across all time points, in the process suggesting likely feedback structures that define central nodes. The networks might also suggest possible mechanisms by which progression of the inflammatory response differs within a given experimental group. In this analysis, time courses of unprocessed inflammatory mediator measurements from each patient were used as input for a DyBN inference algorithm, implemented in Matlab (MathWorks) essentially as described previously for gene array data13 and modified by our group for the study of systemic acute inflammation.14–16
Dynamic Network Analysis (DyNA) was carried out to define, in a granular fashion, the central inflammatory network nodes as a function of both time and age. The main difference between DyNA and DyBN is that DyNA allows for granular temporal resolution of networks over distinct time intervals, and DyBN helps suggest feedback structures. Using inflammatory mediator measurements, networks were created during 3 consecutive time periods (0 to 8 hours, 8 to 16 hours, and 16 to 24 hours) using Matlab.17,18 Connections (network edges) were created if the Pearson correlation coefficient between any 2 inflammatory mediators (network nodes) at the same time interval was greater or equal to a threshold of 0.7. Connections represent trajectories of inflammatory mediators that move in parallel, either in the same or opposite direction. The network complexity for each time interval was calculated using the following formula: sum (N1 + N2 +…+ Nn) / (n − 1), where N represents the number of connections for each mediator and n is the total number of mediators analyzed. The total number of connections represents the sum of the number of connections across all time intervals for all patients in a given group. Dynamic robustness index was also performed to quantify how network complexity changes as a function of correlation stringency. As done in our previous work,19 this was calculated for each time period and patient group using the lowest (0.7) and highest (0.95) correlation stringency and network complexity (C) using the formula (C0.7 – C0.95) / (0.95 – 0.7).
RESULTS
Demographics and clinical outcomes
The overall study cohort was 472 blunt trauma survivors admitted to the ICU of a single Level I trauma center, described previously.17 To compare the circulating inflammatory profiles between young and aged trauma patients, patients between 18 to 30 years (young, n = 115) and 65 to 90 years (aged, n = 101) were identified for analysis (Table 1). Overall, males were predominant in both groups, but comprised a significantly greater proportion in the young (p = 0.01). In terms of mechanism of injury, motor vehicle crash was the most common mechanism in both groups, although there were significantly more falls in the aged (p < 0.001). The young patients had a higher mean ISS (p < 0.001), although, on average, the ISS of both groups are considered of moderate injury (ISS 16 to 24). An analysis of the AIS revealed a significantly higher degree of injury to the head/neck, face, and abdomen in the young patients (eFig. 1A). There were no differences between the groups with regard to other body regions, mean Multiple Organ Dysfunction Score from D1 to D7, and ICU LOS or hospital LOS, although the young patients trended toward having longer hospital LOS, possibly as a consequence of their higher ISS. Patients in the young group had significantly more days on mechanical ventilation (p = 0.007) compared with the aged. Patients in the aged group had a greater proportion of disposition to a rehabilitation or other facility compared with a home discharge in the young patients (p < 0.001).
Table 1.
Demographics, Clinical Outcomes, Mechanism of Injury, Comorbidities, and Disposition of the Overall Cohorts
| Variable | Young* (n = 115) | Aged† (n = 101) | p Value |
|---|---|---|---|
| Demographic | |||
| Age, y, mean ± SEM | 23.8 ± 0.3 | 75.1 ± 0.7 | <0.001 |
| Sex, male/female, n | 87/28 | 60/41 | 0.01 |
| Injury Severity Score, mean ± SEM | 22.9 ± 1.1 | 16.8 ± 0.8 | <0.001 |
| Multiple Organ Dysfunction score day 1 to 7, mean ± SEM | 1.7 ± 0.2 | 1.4 ± 0.2 | 0.34 |
| Glasgow Coma Scale, mean ± SEM | 13.1 ± 0.4 | 14.6 ± 0.2 | <0.001 |
| WBC, mean ± SEM | 17.6 ± 0.7 | 13.1 ± 0.5 | <0.001 |
| Neutrophil to lymphocyte ratio, mean ± SEM | 7.8 ± 0.7 | 8.1 ± 0.6 | 0.19 |
| Clinical outcomes | |||
| ICU LOS, d, mean ± SEM | 8.2 ± 0.8 | 6.9 ± 0.8 | 0.22 |
| Hospital LOS, d, mean ± SEM | 13.8 ± 1 | 11.1 ± 0.8 | 0.18 |
| Mechanical ventilation, d, mean ± SEM | 4.7 ± 0.7 | 3.4 ± 0.7 | 0.007 |
| Nosocomial infection, n (%) | 36 (31) | 30 (30) | 0.80 |
| Mechanism of injury, n (%) | |||
| Motor vehicle crash | 99 (86) | 50 (50) | <0.001 |
| Fall | 10 (9) | 43 (43) | <0.001 |
| Other | 6 (5) | 8 (8) | 0.4 |
| Comorbidity, n (%) | |||
| Cardiovascular disease | 8 (7) | 71 (70) | <0.001 |
| Diabetes mellitus | 3 (3) | 19 (19) | <0.001 |
| Asthma/COPD | 8 (7) | 14 (14) | 0.09 |
| Disposition, n (%) | <0.001 | ||
| Home | 67 (58) | 33 (33) | — |
| Rehabilitation/other facility | 48 (42) | 68 (67) | — |
Mann-Whitney U test or chi-square test were used as appropriate with statistical significance set at p < 0.05.
Ages 18 to 30 years.
Ages 65 to 90 years.
LOS, length of stay.
A number of confounders are likely to influence the magnitude and nature of the systemic inflammatory response after injury, including the individual’s sex and severity of the traumatic injury. To address this, we performed a stringent matching strategy to compare aged patients with a highly matched group of young patients based on sex ratio and ISS. The groups matched for ISS identified 56 young patients and 56 aged patients both with a mean ISS of 18 ± 1.2 (Table 2). There was no difference in the mean Multiple Organ Dysfunction Score from D1 to D7. However, the aged experienced a more complicated course with significantly longer total LOS (p = 0.04) and trend toward higher NI rates and longer mean ICU LOS, but these did not reach statistically significant differences. They were also less likely to be discharged to home (p = 0.002), despite a similar level of injury magnitude. Of note, AIS revealed a significantly higher degree of chest injury in the aged vs higher abdominal injury in the young (eFig. 1B). Aged patients that experienced a similar level of injury severity were more likely to have a complicated course develop compared with young patients.
Table 2.
Demographics, Clinical Outcomes, Mechanism of Injury, Comorbidities, and Disposition of the Groups Matched for Injury Severity Score
| Variable | Young* (n = 56) | Aged† (n = 56) | p Value |
|---|---|---|---|
| Demographic | |||
| Age, y, mean ± SEM | 23.8 ± 0.5 | 74.4 ± 0.9 | <0.001 |
| Sex, male/female, n | 41/15 | 41/15 | 1.0 |
| Injury Severity Score, mean ± SEM | 18.3 ± 1.2 | 18.3 ± 1.2 | 1.0 |
| Multiple Organ Dysfunction score day 1 to 7, mean ± SEM | 1.4 ± 0.3 | 1.7 ± 0.3 | 0.21 |
| Glasgow Coma Scale, mean ± SEM | 13.9 ± 0.5 | 14.3 ± 0.3 | 0.4 |
| WBC, mean ± SEM | 18.8 ± 1 | 13.8 ± 0.6 | <0.001 |
| Neutrophil to lymphocyte ratio, mean ± SEM | 8.9 ± 1 | 8.4 ± 0.8 | 0.78 |
| Clinical outcomes | |||
| ICU LOS, d, mean ± SEM | 5.6 ± 0.8 | 8.8 ± 1.2 | 0.06 |
| Hospital LOS, d, mean ± SEM | 10.6 ± 1.1 | 12.8 ± 1.2 | 0.04 |
| Mechanical ventilation, d, mean ± SEM | 2.7 ± 0.7 | 5.1 ± 1.1 | 0.45 |
| Nosocomial infection, n (%) | 10 (18) | 19 (34) | 0.05 |
| Mechanism of injury, n (%) | |||
| Motor vehicle crash | 47 (84) | 31 (55) | 0.001 |
| Fall | 5 (9) | 18 (32) | 0.002 |
| Other | 4 (7) | 7 (13) | 0.3 |
| Comorbidity, n (%) | |||
| Cardiovascular disease | 4 (7) | 32 (57) | <0.001 |
| Diabetes mellitus | 0 | 11 (20) | |
| Asthma/COPD | 6 (11) | 7 (13) | 0.77 |
| Disposition, n (%) | 0.002 | ||
| Home | 40 (71) | 24 (43) | — |
| Rehabilitation/other facility | 16 (29) | 32 (57) | — |
Mann-Whitney U test or chi-square test were used as appropriate with statistical significance set at p < 0.05.
Ages 18 to 30 years.
Ages 65 to 90 years.
LOS, length of stay.
Circulating levels of inflammatory mediators
The levels of 30 circulating inflammatory mediators were measured at 3 time points in the first 24 hours and then daily for 7 days. Differences in the patterns of the mediators were assessed in 3 ways. First, we plotted the temporal patterns in average absolute levels of circulating biomarkers from time of admission and during the 7-day course after injury between the young and aged groups, and determined the significant differences between these groups by 2-way ANOVA (eFigs. 2 and 3). Second, to assess differences between young and aged patients at early vs late time points, we separated the time course into early (0 to 24 hours) and late (D1 to D7) time periods and assessed difference by 2-way ANOVA. We also measured the AUC for average levels of the mediators for the 2 time periods and identified mediators that were significantly different.
Table 3 shows the mediators that were significantly higher in either the young or aged patient groups for the overall comparison and the patients matched for ISS. The mediators identified as significantly different (p < 0.05) are listed from most to least significant. Two-way ANOVA identified 18 of 30 mediators between admission and D7 as higher in the young patients in the overall comparison. When matched for ISS, the number dropped to a single mediator, MIP-1β, which was elevated in the young. For aged patients, 2-way ANOVA revealed that 3 mediators were higher for both the overall and matched group comparisons. Interestingly, the mediators varied in these 2 group comparisons, and only MIG was found to be significantly higher in the aged patients across both comparisons. However, IL-5 and IL-7 were also identified as significantly higher in the aged patients matched for ISS.
Table 3.
Statistically Significant Different Inflammatory Mediators among the Overall Cohorts and the Groups Matched for Injury Severity Score for 3 Time Periods
| Elevated in young* |
Elevated in aged† |
||||||
|---|---|---|---|---|---|---|---|
| Overall cohorts |
Matched-ISS groups |
Overall cohorts |
Matched-ISS groups |
||||
| Mediator | p Value | Mediator | p Value | Mediator | p Value | Mediator | p Value |
| Admission to day 7 | |||||||
| IL-lβ | <0.001 | MIP-1β | 0.026 | MIG | <0.001 | MIG | <0.001 |
| IL-5 | <0.001 | — | — | IP-10 | 0.001 | IL-5 | 0.006 |
| IFN-α | <0.001 | — | — | IL-23 | 0.006 | IL-7 | 0.012 |
| IL-1RA | <0.001 | — | — | — | — | — | — |
| IL-6 | <0.001 | — | — | — | — | — | — |
| IL-13 | <0.001 | — | — | — | — | — | — |
| IL-2 | <0.001 | — | — | — | — | — | — |
| IL-15 | <0.001 | — | — | — | — | — | — |
| sIL-2Rα | <0.001 | — | — | — | — | — | — |
| IL-17A | <0.001 | — | — | — | — | — | — |
| IL-4 | <0.001 | — | — | — | — | — | — |
| TNF-α | <0.001 | — | — | — | — | — | — |
| MIP-1β | <0.001 | — | — | — | — | — | — |
| MCP-1 | 0.004 | — | — | — | — | — | — |
| GM-CSF | 0.011 | — | — | — | — | — | — |
| MIP-1α | 0.02 | — | — | — | — | — | — |
| IL-10 | 0.023 | — | — | — | — | — | — |
| IL-8 | 0.042 | — | — | — | — | — | — |
| 0 to 24 h | |||||||
| IL-6 | <0.001 | IL-6 | 0.024 | MIG | <0.001 | MIG | <0.001 |
| IL-13 | <0.001 | — | — | IP-10 | <0.001 | IL-7 | 0.017 |
| MCP-1 | <0.001 | — | — | — | — | IP-10 | 0.032 |
| IL-8 | 0.001 | — | — | — | — | — | — |
| IL-10 | 0.005 | — | — | — | — | — | — |
| IL-1RA | 0.017 | — | — | — | — | — | — |
| IL-21 | 0.021 | — | — | — | — | — | — |
| MIP-1β | 0.023 | — | — | — | — | — | — |
| Days 1 to 7 | |||||||
| IL-1β | <0.001 | — | — | MIG | <0.001 | MIG | 0.023 |
| IFN-α | <0.001 | — | — | Eotaxin | 0.004 | IL-5 | 0.024 |
| IL-1RA | <0.001 | — | — | IL-23 | 0.021 | — | — |
| IL-13 | <0.001 | — | — | IP-10 | 0.042 | — | — |
| IL-2 | <0.001 | — | — | — | — | — | — |
| IL-15 | <0.001 | — | — | — | — | — | — |
| sIL-2Rα | <0.001 | — | — | — | — | — | — |
| IL-17A | <0.001 | — | — | — | — | — | — |
| IL-4 | <0.001 | — | — | — | — | — | — |
| IL-5 | 0.001 | — | — | — | — | — | — |
| TNF-α | 0.001 | — | — | — | — | — | — |
| MIP-1α | 0.001 | — | — | — | — | — | — |
| IL-6 | 0.002 | — | — | — | — | — | — |
| GM-CSF | 0.014 | — | — | — | — | — | — |
Statistical significance set at p < 0.05 by 2-way ANOVA.
Ages 18 to 30 years.
Ages 65 to 90 years.
GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IL-1RA, IL-1 receptor antagonist; IP-10, interferon gamma-induced protein 10; ISS, Injury Severity Score; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; sIL-2Rα, soluble IL-2 receptor-α; TNF, tumor necrosis factor.
To determine which mediators demonstrated the greatest difference early vs late, we carried out a similar analysis of mediator levels divided in separate analysis of levels from 0 to 24 hours and from D1 to D7. The mediators that were significantly different by 2-way ANOVA are shown in Table 3 and the fold difference in the significantly different mediators are shown in the AUC analysis in Tables 4 and 5. Within the first 24 hours, the overall comparison revealed that 8 mediators were higher in the young patients compared with the aged patients, although only IL-6 was higher in the patients matched for ISS. For aged patients, 3 mediators were significantly higher vs young patients, with MIG and IP-10 identified for both group comparisons. For D1 to D7, the levels of 14 mediators were higher in the young for the overall comparison, and none for the comparison for the ISS-matched groups. Across this same period, 4 mediators in the overall comparison and 2 mediators in the ISS-matched groups were higher in aged patients. Here again, MIG was found to be elevated across all comparisons in the aged. Interleukin-7 was higher early and IL-5 late in the aged patients matched for ISS.
Table 4.
Area Under the Curve Analysis in the First 24 Hours Post-Injury and Day 1 to Day 7 for Statistically Significantly Different Inflammatory Mediators (by 2-Way ANOVA) among the Overall Young vs Aged Cohorts
| AUC, pg/h/mL |
||||
|---|---|---|---|---|
| Mediator | Young* | Aged† | Fold-change (aged/young) | p Value |
| 0 to 24 h | ||||
| MIG | 13,049.1 | 23,334.8 | 1.8 | <0.001 |
| IP-10 | 5,346 | 8,458.2 | 1.6 | <0.001 |
| IL-21 | 765 | 1,030 | 1.3 | 0.02 |
| MIP-1β | 2,749.5 | 1,692.8 | 0.6 | 0.02 |
| IL-10 | 1,644.1 | 973.8 | 0.6 | 0.005 |
| MCP-1 | 19,810.8 | 11,263 | 0.6 | <0.001 |
| IL-13 | 404.5 | 223.3 | 0.6 | <0.001 |
| IL-1RA | 15,783.4 | 7,420.6 | 0.5 | 0.02 |
| IL-8 | 1,834 | 781 | 0.4 | 0.001 |
| IL-6 | 7,768.1 | 2,211.9 | 0.3 | <0.001 |
| Days 1 to 7 | ||||
| MIG | 4,118.2 | 6,578 | 1.6 | <0.001 |
| IL-23 | 13,300.3 | 18,119.8 | 1.4 | 0.02 |
| Eotaxin | 245.8 | 297.9 | 1.2 | 0.004 |
| IP-10 | 3,108.4 | 3,709.6 | 1.2 | 0.04 |
| TNF-α | 85.1 | 61.4 | 0.7 | 0.001 |
| IFN-α | 415.0 | 281.9 | 0.7 | <0.001 |
| sIL-2Rα | 2,309.5 | 1500.7 | 0.6 | <0.001 |
| IL-4 | 267.5 | 167.0 | 0.6 | <0.001 |
| GM-CSF | 269.3 | 146.6 | 0.5 | 0.01 |
| IL-6 | 711.7 | 377.8 | 0.5 | 0.002 |
| IL-17A | 356.6 | 185.3 | 0.5 | <0.001 |
| MIP-1β | 876.6 | 434.8 | 0.5 | 0.001 |
| IL-1RA | 3,350.2 | 1,634.8 | 0.5 | <0.001 |
| IL-13 | 132.2 | 65.3 | 0.5 | <0.001 |
| IL-2 | 73.5 | 36.0 | 0.5 | <0.001 |
| IL-1 β | 160.8 | 77.5 | 0.5 | <0.001 |
| IL-15 | 229.7 | 109 | 0.5 | <0.001 |
| IL-5 | 167.1 | 77.5 | 0.5 | 0.001 |
Ages 18 to 30 years.
Ages 65 to 90 years.
AUC, area under the curve; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IL-1RA, IL-1 receptor antagonist; IP-10, interferon gamma-induced protein 10; ISS, Injury Severity Score; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; sIL-2Rα, soluble IL-2 receptor-α.
Table 5.
Area Under the Curve Analysis in the First 24 Hours Post-Injury and Day 1 to Day 7 for Statistically Significantly Different Inflammatory Mediators (by 2-Way ANOVA) among the Groups Matched for Injury Severity Score
| AUC, pg/h/mL |
||||
|---|---|---|---|---|
| Mediator | Young* | Aged† | Fold-change (aged/young) | p Value |
| 0 to 24 h | ||||
| IL-7 | 178.1 | 588.7 | 3.3 | 0.02 |
| MIG | 2,724.2 | 5,757.8 | 2.1 | <0.001 |
| IP-10 | 1,358.4 | 1,798.4 | 1.3 | 0.03 |
| IL-6 | 2,054.4 | 700.2 | 0.3 | 0.02 |
| Days 1 to 7 | ||||
| IL-5 | 47.8 | 90.9 | 1.9 | 0.02 |
| MIG | 4,252.9 | 6,598.1 | 1.6 | 0.02 |
Ages 18 to 30 years.
Ages 65 to 90 years.
AUC, area under the curve; IL, interleukin; IP-10, interferon gamma-induced protein 10; MIG, monokine induced by gamma interferon.
We correlated the AUCs from 0 to 24 hours and D1 to D7 for the 6 mediators found to be different between the matched groups with ISS for all of the patients in the young and aged cohorts and found that the differences held for patients with moderate to severe injury (eFig. 4). The most significant differences in circulating mediators between young and aged patients experiencing a similar insult is greater IL-6 elevations in the young early after injury and higher CXC chemokines early and over time in the aged.
Additionally, we wanted to examine the biomarker trajectories of these 6 mediators as a function of advancing age. To do so, we clustered the overall aged cohort into 2 subgroups based on 10-year intervals of 65 to 75 years (n = 55) and 80 to 90 years (n = 30) and analyzed the biomarker levels using 2-way ANOVA. This analysis showed that the patients in the oldest age range of 80 to 90 years old had statistically significantly higher levels of the CXC chemokines, IP-10 and MIG, from admission to D7 compared with those 65 to 75 years old. However, there were no statistically significant differences in mean plasma levels of the 4 remaining key biomarkers (IL-5, IL-6, IL-7, and MIP-1β) between the 2 subgroups (eFig. 5).
We next aimed to determine whether the major differences between the inflammatory profiles of young and aged patients were potentially impacted by major comorbidities. Because the aged patients had more comorbidities than the young, we excluded the young in this analysis. We derived a subgroup of aged patients with cardiovascular disease (n = 71) and compared their mediator levels with a subgroup of aged patients without significant comorbidities (n = 21). Analysis of the key biomarkers from admission to D7 showed that the aged without comorbidities had significantly elevated levels of IL-5, IL-6, and IL-7 compared with the aged with cardiovascular disease (eFig. 6). The aged with cardiovascular disease did not have significantly elevated levels of any of the key mediators. When comparing aged patients with diabetes mellitus (n = 19) with those without comorbidities, no significant differences in the levels of key mediators were found. These results support the premise that the contribution of major comorbidities to the levels of inflammation biomarkers after trauma is minimal compared with other factors, such as age and injury severity. However, the presence of cardiovascular disease might be a factor that impairs the release of some regulatory cytokines.
In addition to comorbidities, we sought to analyze how the levels of inflammatory mediators correlated with outcomes. To do so, we derived subgroups of young and aged patients based on 2 clinical outcomes, NI and ICU LOS. We first segregated the overall young vs aged cohorts into those with NI and those without NI. When examining the key mediators from admission to D7, all 6 (IL-5, IL-6, IL-7, MIP-1β, IP-10, and MIG) were found to be significantly different between the young without NI (n = 79) compared with the aged without NI (n = 71) (eFig. 7A). The young had significantly higher levels of IL-5, IL-6, IL-7, and MIP-1β, and IP-10 and MIG were higher in the aged. However, when examining those with NI, only 2 of the key mediators were different between the subgroups (eFig. 7B). The young with NI (n = 36) had significantly higher levels of IL-6, and the aged with NI (n = 30) had significantly higher levels of IL-7. We next stratified the overall young vs aged cohorts into those with ICU LOS ≤5 days vs ICU LOS >5 days to better determine the influence of complicated clinical courses on biomarker trajectories. When comparing the young and aged subgroups with a shorter ICU LOS (n = 71 and n = 68, respectively), IP-10 and MIG were significantly higher in the aged, and MIP-1b was significantly higher in the young from admission to D7 (eFig. 8A). During this same time period, IL-5, IL-6, and MIP-1b were significantly higher in the young compared with the aged, with a longer ICU LOS (n = 44 and n = 33, respectively) (eFig. 8B). Taken together, these analyses show that the major differences between inflammatory mediator patterns of young and aged patients persist even when the patients have similar clinical outcomes (eg complicated or uncomplicated courses as determined here by NI and ICU LOS). We believe this suggests age is a primary factor in driving the differences.
To determine whether admission levels of mediators that correlated with specific adverse outcomes varied between the young and aged cohorts, Spearman’s correlations were performed between admission levels of mediators and 3 outcomes (rate of NI, ICU LOS, and rate of home disposition). NI was found to have a significantly positive correlation with admission levels of MCP-1 for both the young (r = 0.37, p = 0.001) and the aged (r = 0.29, p = 0.01). This end point also correlated positively with the admission levels of IL-6 (r = 0.38, p < 0.001), IL-8 (r = 0.28, p = 0.02), and IL-10 (r = 0.25, p = 0.03) in the young cohort. These 4 mediators also significantly positively correlated with ICU LOS in both the young and the aged cohorts. For the young, the strongest positive correlations were with MCP-1 (r = 0.55, p < 0.001) and IL-6 (r = 0.47, p < 0.001), followed by IL-8 (r = 0.45, p < 0.001) and IL-10 (r = 0.44, p < 0.001). For the aged, the strongest positive correlations were with MCP-1 (r = 0.48, p < 0.001) and IL-8 (r = 0.48, p < 0.001), followed by IL-6 (r = 0.41, p = 0.004) and IL-10 (r = 0.34, p < 0.001). Not surprisingly, the rate of home disposition negatively correlated with admission levels of MCP-1 (r = −0.42, p < 0.001), IL-6 (r = −0.38, p < 0.001), IL-8 (r = −0.26, p = 0.03), and IL-10 (r = −0.24, p = 0.03) in the young. In addition, IP-10 (r =−0.25, p = 0.03) and MIG (r =−0.23, p = 0.04) negatively correlated with home disposition in the young, but not the aged. These findings indicate that even though the levels of some mediators are dampened in the aged, they still correlated with adverse outcomes when measured at admission.
Dynamic systemic inflammatory profiles
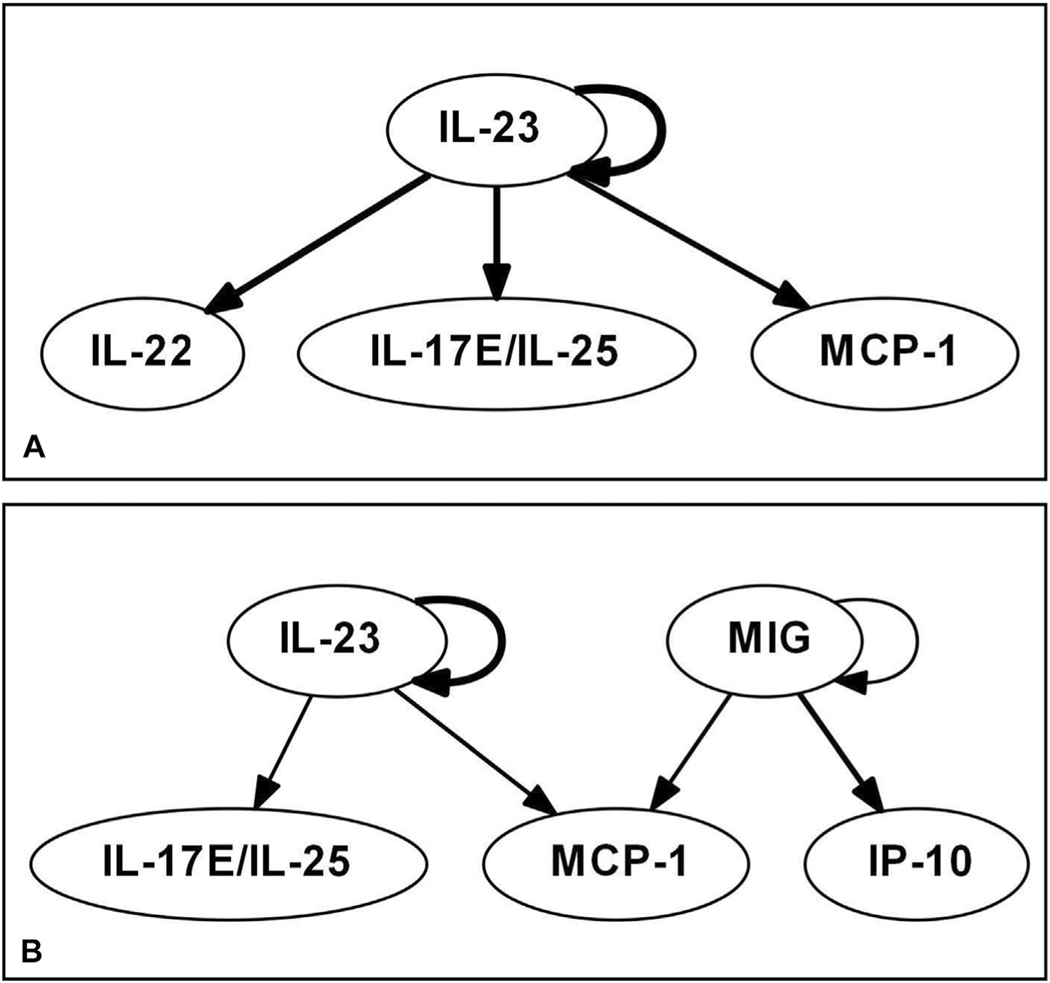
Dynamic Bayesian Network is a method used to demonstrate the potential regulatory relationships among multiple variables that change over time. Dynamic Bayesian Network inference performed for the matched ISS groups suggested key differences in inflammatory programs between the young and the aged. Dynamic Bayesian Network inference in the young patients matched for ISS showed that IL-23 is a central mediator that exhibits self-feedback (which we refer to as a central node), with downstream effects on IL-22, IL-17E/IL-25, and MCP-1 (Fig. 1A). In the aged patients matched for ISS, DyBN inference suggested that MIG is a central mediator along with IL-23, both of which display self-feedback on their own production and are inferred as central nodes (Fig. 1B). This analysis also suggested that MIG and IP-10 are key chemokines in the dynamic inflammatory network of the aged patients. Monokine induced by gamma interferon influenced IP-10 directly, and neither chemokine appears in the systemic inflammatory networks of the young patients.
Figure 1.
Dynamic Bayesian Network (DyBN) suggests differential expression of dynamic networks among the (A) young group matched for Injury Severity Score and (B) aged group matched for Injury Severity Score.
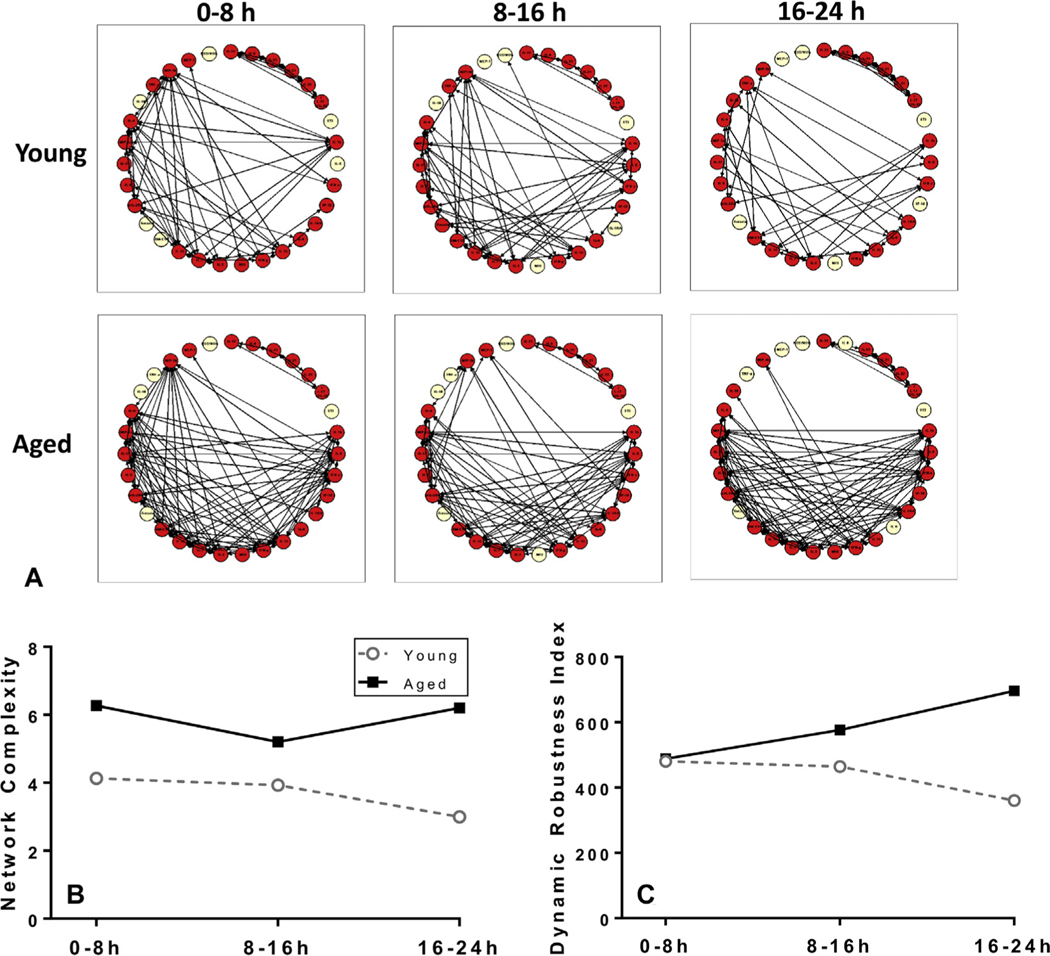
We next performed DyNA, which is used to visualize possible connectivity between measured inflammatory mediators and to estimate network complexity among mediators over multiple time points. This analysis was performed for the matched ISS groups during 3 different time periods after admission (0 to 8 hours, 8 to 16 hours, and 16 to 24 hours), and demonstrated different dynamic networks of systemic inflammation between the young and aged groups (Fig. 2A). By 24 hours after injury, the networks of both the young and aged patients became sparser. There was a higher network complexity in the aged patients (Fig. 2B). The network complexity of the aged was higher than the young throughout all 3 time periods, and the difference was greatest in the last time period (16 to 24 hours). Next, the dynamic robustness index was calculated to quantify how network complexity changes as a function of correlation stringency. This analysis showed that the aged had a higher network robustness at all time intervals compared with the young (Fig. 2C). Taken together, DyNA suggested that the aged patients exhibited a higher degree of correlation between inflammatory mediators within the first 24 hours after injury compared with the young patients.
Figure 2.
(A) Dynamic Network Analysis (DyNA) of inflammatory mediators among the groups matched for Injury Severity Score suggests a differential inflammation profile from 0 to 24 hours. (B) DyNA network complexity and (C) Dynamic Robustness Index differs between the young and aged patients.
DISCUSSION
Normal aging is associated with changes in immune function commonly viewed as a decline of adaptive immune responses and an increase in baseline inflammation.3 Regardless of the definition used to categorize patients as aged, advanced age has been shown to be an independent predictor of adverse outcomes after severe traumatic injury.6,20 By comparing young and aged adult patients, we were able to identify differences in pattern-specific mediator levels between young and aged trauma victims. Matching patients by injury severity narrowed the differences to just a few mediators out of the 30 measured in the circulation. Compared with the young, aged patients show minimal IL-6 elevations early but exaggerated and sustained increases in CXC chemokines, including MIG and IP-10. This pattern was associated with longer ICU stays and a higher incidence of NI after moderate injury compared with the young. These findings support the conclusion that aged patients have a different immune response to injury compared with young adults. This should be taken into consideration when testing immune-based interventions in trauma populations and in the identification of inflammation biomarkers for predicting outcomes.
The demographics of aged trauma patients differ from those of the young. For example, we show that aged patients experience the same injury severity with mechanisms that involve lower energy (more falls in the elderly vs more motor vehicle crashes in the young) and at a given level of injury severity are more likely to have complications develop. The study using data gathered through the Glue Grant Consortium reported that patients 55 years and older who go on to have a complicated clinical course had lower circulating levels of IL-6, IL-8, IL-10, MCP-1, IL-1β, and tumor necrosis factor-a (of 7 cytokines and chemokines measured) acutely after injury compared with patients younger than 55 years.8 That study included a cohort of 17 highly matched patients older than 55 years who had complicated courses after severe injury. By looking at a larger number of mediators that are more representative of the different components of the immune response, we also found that not only are IL-6 levels suppressed in the aged patients, levels of other mediators were significantly higher in the aged patients. Therefore, our results indicate that it is not simply a matter of suppressed responses in aged patients; instead, young and aged patients appeared to differ in the nature of the immune response, as reflected in circulating mediator levels. This notion was further supported by our comparative computational modeling work using DyBN and DyNA that revealed major differences in the correlations and inferred regulatory relationships among key inflammatory mediators in the young vs aged patients.
Among the circulating inflammatory mediators notably present at higher levels in aged trauma patients were MIG and IP-10, pro-inflammatory mediators in the CXC family of chemokines.21 Both chemokines were also identified as potential key regulators of inflammation in the aged patients by DyBN inference. Monokine induced by gamma interferon and IP-10 bind to a common receptor, CXCR3, and act as chemoattractants for T helper 1 lymphocytes that secrete interferongamma.21,22 Monokine induced by gamma interferon and IP-10 are important regulators of type 1 immune responses by contributing to the recruitment of proinflammatory cells to sites of inflammation.23 How elevations in systemic levels of these chemokines relate to inflammation in tissues is not clear, but we speculate that elevated circulating levels of chemokines could contribute to a dysfunctional immune response by failing to provide immune cells with appropriate direction based on chemokine gradients. Alternatively, the excessive release of these chemokines could be compensatory to an inadequate response for dysfunctional immune cells in aged patients and an attempt to activate or mobilize immune cells from the bone marrow. The same might be true for IL-7, which we also found to be elevated early in the aged in our evaluation of ISS-matched groups.
As people age, they can have a propensity to produce more CXC chemokines. A 2007 study of healthy volunteers found a gradual increase of serum MIG and IP-10 levels with aging.24 Monokine induced by gamma interferon showed a slight trend upward to a median serum level of approximately 130 pg/mL at 66 to 70 years. Interferon gamma-induced protein 10 levels also trended upward with age to a median serum level of approximately 30 pg/mL at 76 to 90 years. Although elevated compared with younger healthy volunteers, these concentrations are well below the exacerbated levels of CXC chemokines seen in our aged trauma patients across all study groups. In the aged group matched for ISS, mean levels of MIG were between 1,000 and 1,500 pg/mL across time points and mean levels of IP-10 peaked at approximately 650 pg/mL by day 4. The mechanisms and reasons for the gradual elevations in CXC chemokines as people age or their exaggerated release after severe injury in aged patients are not clear. Also unclear is whether MIG or IP-10 could be used selectively as biomarkers or therapeutic targets in aged patients after injury.
Interleukin-23 was identified as an important proximal regulator of the inflammatory cytokine and chemokine networks of both the young and the aged. It is a pro-inflammatory cytokine that plays a role in the differentiation of T helper 17 cells in type 3 immunity.23 It has been identified as a key mediator in inflammatory and autoimmune diseases, including inflammatory bowel disease, inflammatory joint disease, and Graves disease.25–27 In mouse studies of hemorrhagic shock, IL-23 has been shown to be a driver of lung polymorphonuclear leukocyte infiltration and IL-17A.28 The specific role of IL-23 in aging is not known, although it has been suggested that decreased production of IL-23 is associated with frailty in the elderly.29 Recent work from our group and others has pointed to IL-17A and T helper 17 in adverse outcomes after trauma,30,31 and the dominant role for IL-23 aligns well with these observations. Our results suggest the activation of type 3 immune responses in severely injured trauma patients regardless of age. The higher levels of IL-5 identified late in the aged group in the ISS-matched analysis might indicate an enhanced type 2 response, although other markers of type 2 responses, such as IL-4 and IL-13, were not different between the groups.
Several studies have reported increased baseline levels of IL-6 in healthy aging as a consequence of inflammaging.4,32,33 Our analysis revealed a robust and early peak in IL-6 in the young patients after trauma, and no increase in IL-6 in the matched aged patients. Although we found differences in many other mediators in the unmatched young and aged patient groups, only IL-6 persisted as significantly different when the patients were matched for injury severity. The study mentioned also found a difference in IL-6 levels between young and aged trauma patients.8 Together, these studies suggest that certain components of the innate immune response are severely impaired in aged patients. Although experimental studies have established that high levels of IL-6 early after injury contribute to organ injury,34,35 the consequence of inadequate IL-6 levels cannot be determined from our studies.
A limitation of this study is the size of the matched patient groups. The results of the present study should be validated in a larger patient population and at other institutions. Another limitation is the age ranges we used to define “young” and “aged” in this study. We decided to focus on the patients at the age extremes of our study population to allow for the greatest discrimination between study groups. Sixty-five years old was chosen as “aged” based on the US Census Bureau definition of elderly. Frailty can be another determinant of how immune and inflammatory responses vary with aging. Unfortunately, this variable is not routinely recorded in the electronic medical records associated with trauma patients. As such, we do not have a measured frailty index or record of pre-injury health status in our patient population. Finally, our results are limited by the inflammatory mediators that were assayed based on the available multiplex assay kits. The inclusion of other biomarkers or measurement of other parameters of the immune response and phenotype would likely lead to the identification of other differences between young and aged trauma patients.
Supplementary Material
Acknowledgments
Support: NIH grant P50-GM-53789.
Presented at the 41st Annual Conference on Shock, Scottsdale, AZ, June 2018.
Presented at the American College of Surgeons 104th Annual Clinical Congress, Scientific Forum, Boston, MA, October 2018.
Abbreviations and Acronyms
- AIS
Abbreviated Injury Scale
- AUC
area under the curve
- CCL
C-C motif chemokine ligand
- CXCL
C-X-C motif chemokine ligand
- D
day
- DyBN
Dynamic Bayesian Network
- DyNA
Dynamic Network Analysis
- IL
interleukin
- IP-10
interferon gamma-induced protein 10
- ISS
Injury Severity Score
- MCP
monocyte chemoattractant protein
- MIG
monokine induced by gamma interferon
- NI
nosocomial infection
Footnotes
CME questions for this article available at http://jacscme.facs.org
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
REFERENCES
- 1.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Experiment Med 2011;208: 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazeldine J, Lord JM, Hampson P. Immunesenescence and inflammaging: a contributory factor in the poor outcome of the geriatric trauma patient. Age Res Rev 2015;24:349–357. [DOI] [PubMed] [Google Scholar]
- 3.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 2006;24:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin N Am 2003; 23:15–39. [DOI] [PubMed] [Google Scholar]
- 5.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opinion Hematol 2001;8: 131–136. [DOI] [PubMed] [Google Scholar]
- 6.Sauaia A, Moore FA, Moore EE, et al. Early predictors of postinjury multiple organ failure. Arch Surg 1994;129:39–45. [DOI] [PubMed] [Google Scholar]
- 7.Nacionales DC, Szpila B, Ungaro R, et al. A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol 2015;195:2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanzant EL, Hilton RE, Lopez CM, et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care 2015;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MD, Tracy JK, Meyer W, et al. Trauma in the elderly: intensive care unit resource use and outcome. J Trauma 2002; 53:407–414. [DOI] [PubMed] [Google Scholar]
- 10.Smith RM. Immunity, trauma and the elderly. Injury 2007; 38:1401–1404. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs DG, Plaisier BR, Barie PS, et al. Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma 2003;54:391–416. [DOI] [PubMed] [Google Scholar]
- 12.He W, Goodkind D, Kowal P. An Aging World: 2015 International Population Reports, P95/16–1. Washington, DC: US Census Bureau; 2016. [Google Scholar]
- 13.Grzegorczyk M, Husmeier D. Improvements in the reconstruction of time-varying gene regulatory networks: dynamic programming and regularization by information sharing among genes. Bioinformatics 2011;27:693–699. [DOI] [PubMed] [Google Scholar]
- 14.Azhar N, Ziraldo C, Barclay D, et al. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One 2013;8:e78202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaaqoq AM, Namas R, Almahmoud K, et al. Inducible protein-10, a potential driver of neurally controlled interleukin-10 and morbidity in human blunt trauma. Crit Care Med 2014;42:1487–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emr B, Sadowsky D, Azhar N, et al. Removal of inflammatory ascites is associated with dynamic modification of local and systemic inflammation along with prevention of acute lung injury: in vivo and in silico studies. Shock 2014;41:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namas RA, Vodovotz Y, Almahmoud K, et al. Temporal patterns of circulating inflammation biomarker networks differentiate susceptibility to nosocomial infection following blunt trauma in humans. Ann Surg 2016;263:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Malak O, Vodovotz Y, Zaaqoq A, et al. Elevated admission base deficitis associated with a complex dynamic network of systemic inflammation which drives clinical trajectories in blunt trauma patients. Mediators Inflammation 2016;2016:7950374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora R, Vodovotz Y, Mi Q, et al. Data-driven modeling for precision medicine in pediatric acute liver failure. Mol Med 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams SD, Cotton BA, McGuire MF, et al. Unique pattern of complications in elderly trauma patients at a Level I trauma center. J Trauma Acute Care Surg 2012;72:112e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasperini S, Marchi M, Calzetti F, et al. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunology 1999;162:4928–4937. [PubMed] [Google Scholar]
- 22.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukocyte Biol 1997;61:246–257. [PubMed] [Google Scholar]
- 23.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 2015;135:626–635. [DOI] [PubMed] [Google Scholar]
- 24.Shurin GV, Yurkovetsky ZR, Chatta GS, et al. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 2007;39:123–129. [DOI] [PubMed] [Google Scholar]
- 25.Duvallet E, Semerano L, Assier E, et al. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med 2011;43: 503–511. [DOI] [PubMed] [Google Scholar]
- 26.Jia H, Tao F, Liu C, et al. Both interleukin-23A polymorphism and serum interlukin-23 expression are associated with Graves’ disease risk. Cell Immunol 2015; 294:39–43. [DOI] [PubMed] [Google Scholar]
- 27.Maloy KJ. The interleukin-23/interleukin-17 axis in intestinal inflammation. J Int Med 2008;263:584–590. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Yuan Y, Li Y, et al. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunology 2009;182:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compte N, Zouaoui Boudjeltia K, Vanhaeverbeek M, et al. Frailty in old age is associated with decreased interleukin-12/ 23 production in response to Toll-like receptor ligation. PloS One 2013;8:e65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abboud A, Namas RA, Ramadan M, et al. Computational analysis supports an early, type 17 cell-associated divergence of blunt trauma survival and mortality. Crit Care Med 2016;44:e1074–e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri A, Brat GA, Yorkgitis BK, et al. Phenotyping the immune response to trauma: a multiparametric systems immunology approach. Crit Care Med 2017;45:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulop T, Larbi A, Douziech N, et al. Cytokine receptor signalling and aging. Mech Ageing Dev 2006;127:526–537. [DOI] [PubMed] [Google Scholar]
- 33.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Experimental Gerontol 2004;39: 687–699. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang J, Korff S, et al. Delayed neutralization of interleukin 6 reduces organ injury, selectively suppresses inflammatory mediator, and partially normalizes immune dysfunction following trauma and hemorrhagic shock. Shock 2014;42:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol 2001;280:C343–C351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.