Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease at a global scale and is strongly associated with the obesity and metabolic syndrome epidemic.1 NAFLD embodies a spectrum of liver conditions that may progress from an apparently benign accumulation of lipids (steatosis) to inflammatory stages accompanied by hepatocellular damage known as nonalcoholic steatohepatitis (NASH). In a significant number of patients NASH progression can lead to cirrhosis and hepatocellular carcinoma development.2 In fact, NASH will soon surpass alcoholic liver disease as the leading transplant indication for all patients.2 The clinical and socioeconomic burden of NAFLD has spurred extraordinary research activity on this disease.3 The mechanisms of NAFLD development appear complex and multifarious.4 A systemic dysregulation of energy metabolism characterized by excess dietary intake of fat and carbohydrates, insulin resistance, enhanced de novo hepatic lipogenesis, and increased hepatocyte uptake of fatty acids results in the hepatic accumulation of toxic lipids, hepatocellular death, and parenchymal inflammation.5 In addition, compromised expression and activity of transcription factors such as liver X receptor, farnesoid X receptor, peroxisome proliferator-activated receptors α and γ, sterol response element binding protein-1c, and carbohydrate response element binding protein, key regulators of lipid and glucose metabolism, are also involved in NAFLD progression.6 Chronic parenchymal injury and inflammation trigger a potent tissue repair reaction dominated by the transdifferentiation of quiescent hepatic stellate cells (HSCs) into extracellular matrix-producing myofibroblasts, which drive liver fibrosis.4 Inflammation and fibrosis are key risk factors for liver disease progression in NASH, and together with the transcription factors mentioned above, they encompass major potential targets for NASH therapy.3,4
Chronic liver diseases (CLD), including NAFLD, are commonly associated with nutrient and vitamin deficiencies such as those of vitamins D and A.7,8 In the broad context of NAFLD pathogenesis, vitamin A sits at a critical crossroad between metabolic regulation and HSC activation and thus may be regarded as a pivotal factor.9 Indeed, HSCs represent the principal systemic reserve of retinol. After passing through the hepatocytes, dietary retinol is mostly taken up and stored as retinyl-palmitate esters (RE) in lipid droplets characteristic of quiescent HSCs.10 In response to liver injury HSCs become activated and rapidly loss their vitamin A contents in a process that has been mechanistically linked to CLD progression and the onset of vitamin A deficiency (VAD).10 On the other hand, on conversion into retinoic acids (RA) vitamin A exerts potent systemic regulatory effects on many physiological processes including lipid and carbohydrate metabolism. This is achieved through RA binding to retinoid X receptor (RXR) and retinoid acid receptor (RAR) transcription factors, which operate in direct or indirect coordination with the metabolic transcription factors previously mentioned.8,9 Experimental and clinical findings indicate that normal RA signaling is important to prevent NAFLD,10 and that patients carrying the NAFLD risk variant of patatin-like phospholipase domain-containing protein 3 (PNPLA3-I148M) have low serum retinol concentrations.11 Moreover, low serum and hepatic retinol levels, taken as indicators of VAD, have been observed in NAFLD patients in association with disease progression.8 These findings strongly suggest the presence of a pathogenic VAD in NAFLD. However, the ultimate mechanisms determining the nature of this vitamin A inadequacy are not fully understood. In this issue of Cellular and Molecular Gastroenterology and Hepatology Saeed et al12 provide clarifying experimental insights into this matter that may be relevant for patients’ management.
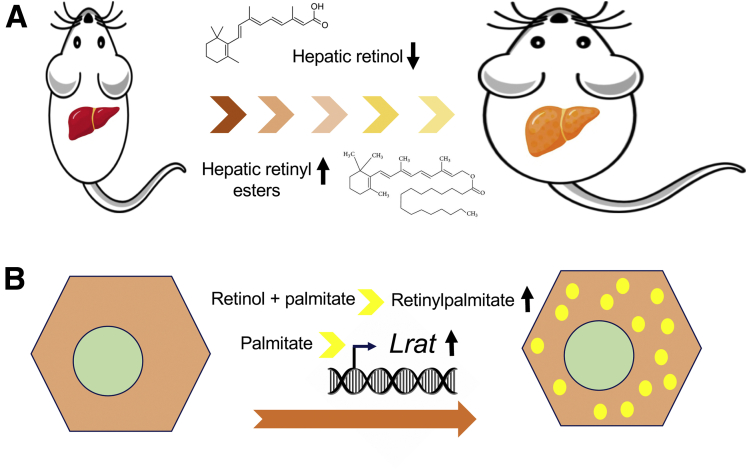
The authors implement 2 complementary models of NASH, mice fed with a high-fat and high-cholesterol–containing diet and leptin-deficient ob/ob mouse. In both models a reduction in hepatic retinol levels was confirmed in correlation with NASH progression, but most importantly, a significant increase in hepatic retinyl palmitate was found (Figure 1A). This is a relevant observation, because RE are the most abundant form of vitamin A in the body, and their hepatic accumulation would suggest that in spite of reduced serum retinol levels a true VAD does not occur in NASH. This finding seemed at variance with previous reports that showed a reduction in hepatic RE in NAFLD models and patients.13,14 However, as the authors demonstrate, different experimental procedures for RE extraction from lipid-laden liver tissue may account for such discrepancy. This observation raises a general note of caution for future studies addressing vitamin A metabolism in fatty livers. Another important finding of this work was the realization that hepatic accumulation of retinyl palmitate in NASH took place in hepatocytes, not in HSCs, which are their natural cellular depot. This situation was attributed to the overexpression in hepatocytes of lecithin-retinol acyltransferase (Lrat), coding for the main enzyme in RE synthesis. Increased hepatic LRAT expression has been previously reported in NAFLD patients.15 However, Saeed et al12 provide an interesting observation by showing that in primary hepatocytes (Figure 1B), but not in primary HSCs, Lrat expression can be induced by palmitate, and that retinyl-palmitate accumulated when cells were co-treated with retinol. Conversely, in HSCs, palmitate enhanced the expression of Pnpla3, which is endowed with RE hydrolase activity. The specific mechanisms underlying the differential effects of palmitate on Lrat and Pnpla3 expression in hepatocytes and HSCs still need to be worked out. Yet, this work contributes to explain the accumulation of vitamin A in hepatocytes previously found in human fatty liver.16 From a broader perspective, the study of Saeed et al would also identify novel pathogenic mechanisms for palmitate in NAFLD. The ectopic accumulation of retinyl esters in hepatocytes and the overall dysregulation of vitamin A metabolism, likely impacting on RAR/RXR signaling networks, should now be added to the list of toxic stimuli mediated by this fatty acid.5,17 Although many aspects of vitamin A metabolism in NAFLD remain to be clarified, this experimental report provides insightful information that may help guide future NAFLD treatment strategies.
Figure 1.
Nonalcoholic fatty liver disease results in disturbed hepatic vitamin A metabolism. (A) Feeding a high-fat and high-cholesterol (HFC) diet, or leptin deficiency (ob/ob mice), leads to reduced hepatic retinol levels and to the accumulation of retinyl esters in mice. (B) Incubation of primary rat hepatocytes with palmitate results in up-regulation of expression of lecithin-retinol acyltransferase (Lrat), the main enzyme in retinyl ester synthesis normally expressed in hepatic stellate cells. When primary rat hepatocytes are incubated with retinol and palmitate, retinyl palmitate is readily synthetized and accumulated. These responses contribute to understand the in vivo findings showing increased LRAT staining and vitamin A–laden autofluorescent vesicular structures found in parenchymal liver cells from HFC-fed mice.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by grants PI02019-104878RB-100 and PI02019-104265RB-100 from FEDER/Ministerio de Ciencia, Innovación y Universidades-Agencia Estatal de Investigación, and HEPACARE Project from Fundación La Caixa.
References
- 1.Muthiah M.D., Sanyal A.J. Burden of disease due to nonalcoholic fatty liver disease. Gastroenterol Clin North Am. 2020;49:1–23. doi: 10.1016/j.gtc.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Sheka A.C., Adeyi O., Thompson J. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 3.Byrne C.D., Targher G. What’s new in NAFLD pathogenesis, biomarkers and treatment? Nat Rev Gastroenterol Hepatol. 2020;17:70–71. doi: 10.1038/s41575-019-0239-2. [DOI] [PubMed] [Google Scholar]
- 4.Friedman S.L., Neuschwander-Tetri B.A., Rinella M. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parthasarathy G., Revelo X., Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4:478–492. doi: 10.1002/hep4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steensels S., Qiao J., Ersoy B.A. Transcriptional regulation in non-alcoholic fatty liver disease. Metabolites. 2020;10:283. doi: 10.3390/metabo10070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacifico L., Osborn J.F., Bonci E. Association between vitamin D levels and nonalcoholic fatty liver disease: potential confounding variables. Mini-Reviews Med Chem. 2018;19:310–332. doi: 10.2174/1389557518666181025153712. [DOI] [PubMed] [Google Scholar]
- 8.Saeed A., Dullaart R.P.F., Schreuder T.C.M.A. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD) Nutrients. 2017;10:29. doi: 10.3390/nu10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaner W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153–178. doi: 10.1016/j.pharmthera.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haaker M.W., Vaandrager A.B., Helms J.B. Retinoids in health and disease: a role for hepatic stellate cells in affecting retinoid levels. Biochim Biophys Acta - Mol Cell Biol Lipids. 2020:1865. doi: 10.1016/j.bbalip.2020.158674. [DOI] [PubMed] [Google Scholar]
- 11.Kovarova M., Königsrainer I., Königsrainer A. The genetic variant I148M in PNPLA3 is associated with increased hepatic retinyl-palmitate storage in humans. J Clin Endocrinol Metab. 2015;100:E1568–E1574. doi: 10.1210/jc.2015-2978. [DOI] [PubMed] [Google Scholar]
- 12.Saeed A., Bartuzi P., Heegsma J. Impaired hepatic vitamin A metabolism in NAFLD mice leading to vitamin A accumulation in hepatocytes. Cell Mol Gastroenterol Hepatol. 2021;11:309–325. doi: 10.1016/j.jcmgh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trasino S.E., Tang X.H., Jessurun J. Obesity leads to tissue, but not serum vitamin A deficiency. Sci Rep. 2015;5 doi: 10.1038/srep15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong G., Kirkwood J., Won K.J. Characterization of vitamin A metabolome in human livers with and without nonalcoholic fatty liver disease. J Pharmacol Exp Ther. 2019;370:92–103. doi: 10.1124/jpet.119.258517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashla A.A., Hoshikawa Y., Tsuchiya H. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res. 2010;40:594–604. doi: 10.1111/j.1872-034X.2010.00646.x. [DOI] [PubMed] [Google Scholar]
- 16.Croce A.C., Simone U De, Freitas I. Human liver autofluorescence: an intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg Med. 2010;42:371–378. doi: 10.1002/lsm.20923. [DOI] [PubMed] [Google Scholar]
- 17.Hirsova P., Ibrabim S.H., Gores G.J. Thematic review series: lipotoxicity—many roads to cell dysfunction and cell death lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]