Abstract
Background & Aims
Systemic retinol (vitamin A) homeostasis is controlled by the liver, involving close collaboration between hepatocytes and hepatic stellate cells (HSCs). Genetic variants in retinol metabolism (PNPLA3 and HSD17B13) are associated with non-alcoholic fatty liver disease (NAFLD) and disease progression. Still, little mechanistic details are known about hepatic vitamin A metabolism in NAFLD, which may affect carbohydrate and lipid metabolism, inflammation, oxidative stress and the development of fibrosis and cancer, e.g. all risk factors of NAFLD.
Methods
Here, we analyzed vitamin A metabolism in 2 mouse models of NAFLD; mice fed a high-fat, high-cholesterol (HFC) diet and Leptinob mutant (ob/ob) mice.
Results
Hepatic retinol and retinol binding protein 4 (RBP4) levels were significantly reduced in both mouse models of NAFLD. In contrast, hepatic retinyl palmitate levels (the vitamin A storage form) were significantly elevated in these mice. Transcriptome analysis revealed a hyperdynamic state of hepatic vitamin A metabolism, with enhanced retinol storage and metabolism (upregulated Lrat, Dgat1, Pnpla3, Raldh’s and RAR/RXR-target genes) in fatty livers, in conjunction with induced hepatic inflammation (upregulated Cd68, Tnfα, Nos2, Il1β, Il-6) and fibrosis (upregulated Col1a1, Acta2, Tgfβ, Timp1). Autofluorescence analyses revealed prominent vitamin A accumulation in hepatocytes rather than HSC in HFC-fed mice. Palmitic acid exposure increased Lrat mRNA levels in primary rat hepatocytes and promoted retinyl palmitate accumulation when co-treated with retinol, which was not detected for similarly-treated primary rat HSCs.
Conclusion
NAFLD leads to cell type-specific rearrangements in retinol metabolism leading to vitamin A accumulation in hepatocytes. This may promote disease progression and/or affect therapeutic approaches targeting nuclear receptors.
Keywords: Fatty Liver Disease, Vitamin A, Autofluorescence
Abbreviations used in this paper: HFC, high-fat, high-cholesterol; HFD, high-fat diet; HSC, hepatic stellate cell; mRNA, messenger RNA; NAFLD, nonalcoholic fatty liver disease; qHSC, quiescent hepatic stellate cell; RAR, retinoic acid receptor; RBP4, retinol binding protein 4; RXR, retinoid X receptor
Graphical abstract
Summary.
Disturbed vitamin A metabolism in the liver of nonalcoholic fatty liver disease mice leads to cell type-specific redistribution of vitamin A, as a result of which hepatic retinol levels reduce but retinyl esters strongly accumulate in hepatocytes, rather than in hepatic stellate cells.
See editorial on page 291.
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide. The prevalence of NAFLD is estimated at 20%–30% in the general population in Western countries. Starting with benign steatosis, patients are at risk to develop nonalcoholic steatohepatitis, and progress to cirrhosis and hepatocellular carcinoma.1 NALFD prevalence is particularly high in obese individuals (80%–90%), diabetes (30%–50%), or hyperlipidemia (90%).1,2 Hepatic fat accumulation is a combined result of a high fat– and high carbohydrate–containing diet and insufficient catabolism of these energy sources, in part owing to low physical activity.3 A tipping point NAFLD pathology is when simple steatosis is being accompanied by hepatic inflammation and fibrosis. Inflammation leads to the activation of hepatic stellate cells (HSCs) that transdifferentiate to proliferative and migratory myofibroblasts (eg, activated HSCs) that produce excessive amounts of extracellular matrix proteins, like collagens and fibronectins, the typical feature of fibrosis.4
HSCs are considered “quiescent” in the healthy liver, though they play a key role in controlling vitamin A metabolism.5 Vitamin A is important for many physiological processes, including reproduction, embryogenesis, glucose and lipid metabolism and vision, most of which are controlled by the retinoic acid–activated transcription factors retinoid X receptor (RXR) and retinoic acid receptor (RAR). Quiescent HSCs (qHSCs) contain the main body reserve of vitamin A, which is stored as retinyl esters in large cytoplasmic lipid droplets.6,7 Controlled conversion to retinol in qHSCs is believed to maintain stable circulating serum retinol levels around 2.0 μmol/L in humans (1.0–1.5 μmol/L in rodents). The specific (control) mechanisms involved are, however, largely unknown. Dietary vitamin A follows a complex route for final storage in HSCs.7 In the small intestine, retinyl esters are incorporated in chylomicrons and after transport through the circulation taken up by hepatocytes. In hepatocytes, they are converted to retinol and subsequent binding to retinol binding protein 4 (RBP4) stimulates the release of the retinol-RBP4 complex back to the circulation. Via an unknown mechanism, retinol is transferred to HSCs, re-esterified, and stored in lipid droplets. Liver injury–induced activation of HSCs leads to the rapid loss of vitamin A–containing lipid droplets and, as a consequence, chronic liver diseases are associated with vitamin A deficiency, including viral hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, biliary atresia, alcoholic hepatitis, and also NAFLD.8 Vitamin A deficiency is defined as serum retinol levels below 0.7 mmol/L.9 Not only serum, but also hepatic retinol levels were found to be reduced in class III (body mass index ≥40 kg/m2) obese patients and negatively correlate with histological severity of hepatic steatosis.10,11 These analyses, however, do not take retinyl esters into account, which form the largest pool of vitamin A. It thus remains unclear whether the low serum and hepatic retinol levels truly reflect complete depletion of vitamin A stores or, alternatively, point to aberrant vitamin A metabolism in the fatty liver. Notably, individuals carrying the NAFLD risk variant of PNPLA3 (encoding adiponutrin) show low serum retinol levels, while hepatic retinyl ester levels are increased, pointing to impaired hepatic retinyl ester-to-retinol conversion.12 The role of disturbed vitamin A metabolism in the development of NAFLD has recently been further emphasized by the association of gene variants in HSD17B13, a retinol dehydrogenase, with hepatic steatosis.13 In addition, transcriptome analyses revealed a hyperdynamic state of hepatic retinol metabolism in NAFLD patients (eg, enhanced expression of genes involved in vitamin A storage, hydrolysis, and transport), though with unknown effects on the various vitamin A pools.14
Thus, in this study we analyzed serum and hepatic retinol, retinyl ester, and RBP4 levels in 2 NAFLD mouse models, as well as expression of genes and proteins involved in vitamin A metabolism. Our data show that NAFLD in mice does not lead to systemic vitamin A deficiency, but rather enhances vitamin A storage in the liver while retinol levels are reduced. Importantly, bulk of the vitamin A accumulates in hepatocytes, rather than in HSCs.
Results
High Fat, High Cholesterol Diet Increases Body Weight and Liver Fat Content
In order to study the effect of NAFLD on vitamin A metabolism, we fed mice a high fat, high cholesterol (HFC)–containing diet for 12 or 20 weeks and first confirmed the development of fatty liver disease. The HFC diet induced significant body and liver weight gain after 20 weeks (Figure 1A and B). Hepatic total and free cholesterol, as well as total triglycerides, were significantly increased after 12 weeks of HFC feeding and remained enhanced after 20 weeks (Figure 1C–E). Similarly, plasma total and free cholesterol, as well as insulin levels were significantly elevated HFC-fed mice (Figure 1F–H). hematoxylin and eosin and Oil Red O staining confirmed excessive fat accumulation in the livers of HFC-fed mice and was associated with accumulation of CD68-positive inflammatory cells (Figure 1I).
Figure 1.
Hepatic fat accumulation and steatohepatitis in HFC-fed mice. Mice fed chow or HFC diet for 12 or 20 weeks were analyzed for (A) body weight, (B) liver weight, (C) liver total cholesterol levels, (D) liver triglyceride (TG) levels, (E) liver free cholesterol levels, F) plasma total cholesterol levels, (G) plasma free cholesterol levels, (H) plasma insulin levels, and (I) hematoxylin and eosin (H&E) staining, Oil Red O staining, and CD68 immunohistochemistry. Quantification of (immuno)histochemistry is described in the Methods and Materials. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
HFC Diet Induces Markers of Hepatic Lipid Uptake and Synthesis, Inflammation, and Fibrosis in Mice
Hepatic expression of genes involved in lipid uptake (Srb1, Cd36) and synthesis (Scd1, Fasn, Acc1, Srebp1c) were strongly induced in HFC-fed mice (Figure 2A). Moreover, the HFC diet leads to an early progression to steatohepatitis, with enhanced expression of inflammatory markers, including Cd68, Tnf-α, Nos2, Ccl2, Il-1β, and Il-6 (Figure 2B) as well as the progressive induction of markers of fibrosis (Coll1a1, Acta2, Tgf-β, and Timp1) (Figure 2C).
Figure 2.
Expression of hepatic genes involved in lipid uptake, lipid synthesis, inflammation, and fibrosis in HFC-fed mice. Mice fed chow or HFC diet for 12 or 20 weeks were analyzed by Q-PCR for hepatic expression of genes involved in (A) hepatic lipid uptake (Srb1, Cd36) and synthesis (Scd1, Fasn, Acc1), (B) hepatic inflammation (Cd68, Tnf-α, Nos2, Ccl2, Il-1β, Il-6), and (C) liver fibrosis (Coll1a1, Acta2, Tgf-β, and Timp1). Hepatic lipid uptake and synthesis, hepatic inflammation, and hepatic fibrosis were strongly increased in HFC-fed mice. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
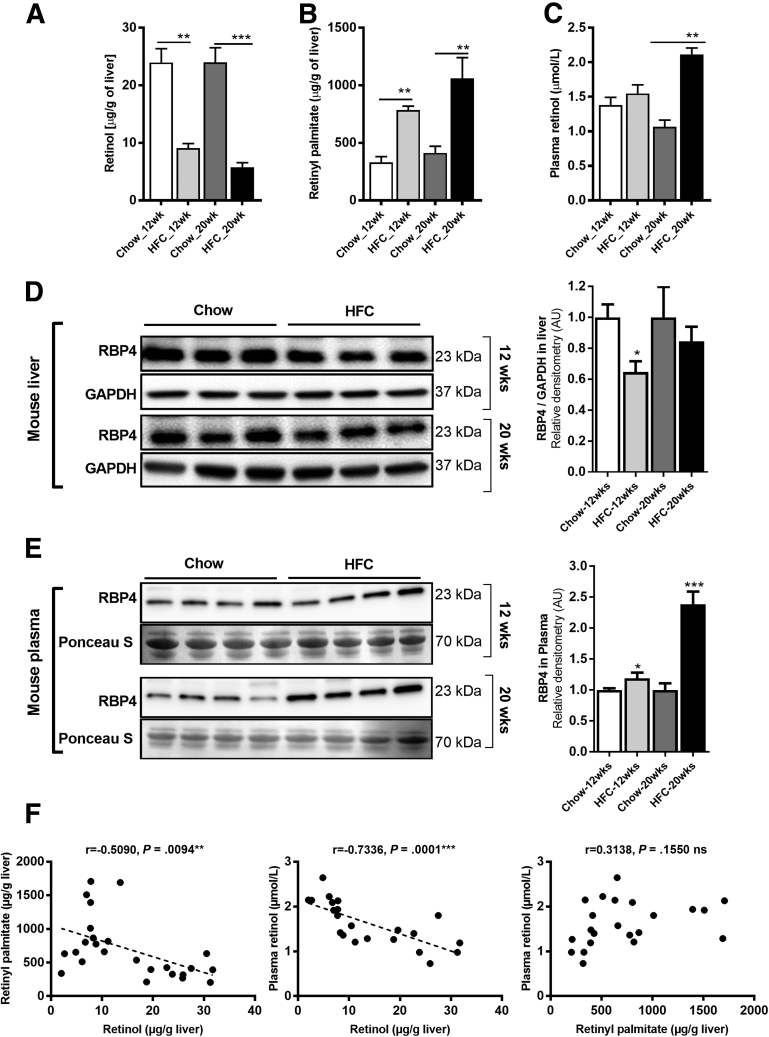
Reduced Retinol But Elevated Retinyl Palmitate in Livers of HFC-Fed Mice
Next, we determined the effect of the HFC diet on the levels of retinol in liver and plasma, as well as hepatic retinyl palmitate, the major storage form of vitamin A. On the one hand, hepatic retinol levels were comparable in 12- and 20-week chow-fed mice (24.0 ± 2.4 and 24.0 ± 2.5 μg/g liver, respectively) (Figure 3A). In contrast, hepatic retinol levels were progressively decreased in HFC-fed mice (9.1 ± 0.8 and 5.7 ± 0.8 μg/g liver after 12 and 20 weeks, respectively) (Figure 3A). On the other hand, hepatic retinyl palmitate levels progressively increased in HFC-fed mice (785 ± 34 and 1,060 ± 182 μg/g liver after 12 and 20 weeks, respectively) and were significantly higher than in chow-fed mice (331 ± 50 and 413 ± 58 μg/g liver after 12 and 20 weeks, respectively) (Figure 3B). The disturbed hepatic retinol/retinyl palmitate balance was not accompanied by changes in plasma levels of retinol after a 12-week HFC diet (1.4 ± 0.1 and 1.6 ± 0.1 μmol/L for chow and HFC diet, respectively), while it was increased in HFC-fed mice after 20 weeks (1.1 ± 0.1 and 2.1 ± 0.1 μmol/L for chow and HFC diet, respectively) (Figure 3C). Hepatic RBP4 protein levels were decreased in HFC-fed mice compared with chow-fed mice (Figure 3D), while hepatic messenger RNA (mRNA) expression of Rbp4 was comparable in all animal groups (Figure 4A). In contrast, serum RBP4 levels were elevated in HFC-fed mice, which was particularly evident after 20 weeks (Figure 3E). These results show that a drastic reduction in hepatic retinol levels in HFC-fed mice is accompanied by a significant increase in hepatic retinyl esters. Further, Pearson correlation analysis of tissue retinol levels showed a significant inverse correlation with tissue retinyl palmitate and plasma retinol levels, while tissue retinyl palmitate was not significantly correlated with plasma retinol (Figure 3F). Moreover, hepatic retinol levels in particular correlated inversely with biochemical and gene expression markers of NAFLD progression, including hepatic cholesterol and triglyceride levels, and hepatic expression of inflammatory (Tnfa, Nos2, Ccl2, and Il1b) and fibrosis (Col1a1, Acta2, Tgfb, and Timp1) markers (Supplementary Table 1).
Figure 3.
An HFC diet leads to impaired hepatic vitamin A metabolism in mice. Mice fed chow or HFC diet for 12 or 20 weeks were analyzed for hepatic levels of (A) retinol, (B) retinyl palmitate, and (C) plasma levels of retinol. Hepatic retinol levels were strongly reduced in HFC-fed mice, while retinyl palmitate levels were significantly increased compared with control mice. Twelve-week HFC-feeding did not alter plasma retinol levels, which were slightly elevated after 20 weeks. (D) Hepatic RBP4 protein levels were decreased in 12 week HFC-fed mice, while this decrease was no longer significant after 20 week HFC feeding. (E) Plasma RBP4 progressively increased in HFC-fed mice. (F) Correlation analysis of hepatic vitamin A (retinol, retinyl palmitate) with circulatory vitamin A (plasma retinol). ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
Figure 4.
Hepatic expression of genes involved in vitamin A homeostasis is strongly affected in HFC-fed mice. Mice fed chow or HFC diet for 12 or 20 weeks were analyzed by Q-PCR for hepatic expression of genes and transcription factors involved in (A) vitamin A storage (Lrat, Dgat1, Dgat2) and transport (Rbp4), (B) retinyl ester hydrolysis (Atgl/Pnpla2, Pnpla3, Lipe) and retinol-to-retinoic acid conversion (Raldh1, Raldh2, Raldh3, Raldh4), and (C) retinoic acid target genes (Rar-β, Cyp26a1, Hsd17b13, Ucp2, Cpt1a, Fgf21). Hepatic expression of vitamin A storage and hydrolysis were increased in HFC-fed mice, in conjunction with enhanced expression of retinoic acid responsive genes. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
Hepatic Expression of Genes Involved in Vitamin A Storage, Transport, and Hydrolysis as Well as Retinoic Acid Targets Are Elevated in HFC-Fed Mice
Hepatic mRNA levels of Lrat, coding for the main enzyme producing retinyl esters in the liver, increased with time in HFC-fed mice (Figure 4A). No or minor changes were detected in mRNA levels for alternative retinol-esterifying enzymes, like Dgat1 and Dgat2. Of the various retinyl ester hydrolases that mobilize retinol from hepatic retinyl ester stores, only Adpn/Pnpla3 mRNA levels were strongly induced in HFC-fed mice, while Atgl/Pnpla2 and Hsl/Lipe mRNA levels were not altered compared with chow-fed mice. Raldh2 mRNA levels were significantly enhanced in livers of HFC-fed mice, while levels of other members of the retinol to retinoic acid–converting enzymes15, 16, 17 were not changed (Raldh1, Raldh3, and Raldh4) (Figure 4B). Hepatic expression of retinoic acid-responsive genes (RAR-β, Cyp26a1, Hsd17b13, Ucp2, Cpt1a, Fgf21) was enhanced in the mice fed the HFC diet (Figure 4C). Thus, an HFC diet leads to a hyperdynamic state of retinol metabolism in mice, confirming earlier observations in NAFLD patients.14
Reduced Retinol and Enhanced Retinyl Palmitate in Livers of ob/ob Mice
To validate our findings, we next analyzed hepatic vitamin A metabolism in another model of fatty liver disease (eg, the leptin-deficient ob/ob mouse). In line with the observations described previously for HFC-fed mice, hepatic retinol levels were significantly lower in ob/ob mice compared with age-matched wild-type littermates (2.8 ± 0.1 vs 16.9 ± 5.1 μg/g liver, respectively) (Figure 5A), in conjunction with strongly enhanced levels of retinyl palmitate (359 ± 28 vs 160 ± 26 μg/g liver, respectively) (Figure 5B). Moreover, plasma retinol and RBP4 levels were significantly higher in ob/ob mice as compared with their wild-type littermates (2.5 ± 0.2 vs 0.9 ± 0.2 μM retinol, respectively; (Figure 5C). Finally, also hepatic RBP4 protein levels were reduced in ob/ob mice independently of (unchanged) Rbp4 mRNA levels as compared with wild-type mice (Figure 5D and 6A), all features being comparable between HFC-fed wild type mice and chow-fed ob/ob mice. Similar to HFC, plasma RBP4 levels were elevated in ob/ob mice as compared with wild-type mice (Figure 5E). Pearson correlation analysis of tissue retinol levels showed similarly a significant inverse correlation with tissue retinyl palmitate and plasma retinol levels, while tissue retinyl palmitate was not significantly correlated with plasma retinol (Figure 5F).
Figure 5.
Ob/ob mice show disturbed hepatic vitamin A metabolism.Ob/ob mice and age-matched wild-type littermates were sacrificed and analyzed for (A) hepatic retinol, (B) hepatic retinyl palmitate, (C) plasma retinol, and (D) hepatic and (E) plasma RBP4 levels. Hepatic retinol levels were strongly reduced, while retinyl palmitate levels were significantly increased in ob/ob mice. Plasma retinol and RBP4 protein levels were significantly elevated in ob/ob mice. In contrast, hepatic RBP4 protein levels were reduced. Note that hepatic RBP4 mRNA levels were not changed in ob/ob mice (see Figure 6A). (F) Correlation analysis of hepatic vitamin A (retinol, retinyl palmitate) with circulatory vitamin A (plasma retinol). ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001.
Hepatic Expression of Genes Involved in Vitamin A Storage, Transport, Hydrolysis, and Retinoic Acid-Responsive Targets Are Elevated in ob/ob Mice
Similar to HFC-fed mice, hepatic expression of Lrat and Dgat1 was significantly enhanced in ob/ob mice as compared with controls, while expression of Dgat2 and Rbp4 was not different between both animal groups (Figure 6A). Pnpla3 was strongly enhanced in ob/ob mice compared with control mice, while Pnpla2 and Lipe levels were unchanged. In contrast to HFC-fed mice, Raldh2 mRNA levels were reduced in ob/ob mice, while Raldh1 and Raldh3 levels were significantly elevated (Figure 6B). Finally, hepatic expression of various retinoic acid-responsive genes was also enhanced in ob/ob mice livers as compared with controls (Figure 6C). Taken together, both NAFLD mouse models present comparable impairments in hepatic vitamin A homeostasis.
Figure 6.
Hepatic expression of genes involved in vitamin A homeostasis is strongly affected in ob/ob mice.Ob/ob mice and age-matched wild-type (WT) littermates were sacrificed and analyzed by Q-PCR for hepatic expression of genes and transcription factors involved in (A) vitamin A storage (Lrat, Dgat1, Dgat2), transport (Rbp4), (B) vitamin A hydrolysis (Atgl/Pnpla2, Pnpla3, Lipe) and retinol-to-retinoic acid conversion (Raldh1, Raldh2, Raldh3, Raldh4), and (C) retinoic acid target genes (Rar-β, Cyp26a1, Hsd17b13, Ucp2, Cpt1a, Fgf21). Hepatic expression of vitamin A storage and hydrolysis was increased in ob/ob, in conjunction with enhanced expression of retinoic acid responsive genes. ∗P ≤ .05, ∗∗P ≤ .01.
Palmitate Increases Lrat Expression and Vitamin A Accumulation in Primary Rat Hepatocytes
Dietary vitamin A is absorbed by hepatocytes through retinyl ester-carrying chylomicron remnants, after which it is redistributed to HSCs for storage (see Introduction). Thus, both hepatocytes and HSCs are possible sites for retinyl ester accumulation, and earlier work has suggested vitamin A accumulation in hepatocytes of human and rodent fatty livers.18,19 Microscopical analysis of liver tissue for vitamin A–specific autofluorescence revealed few bright (auto)fluorescent dots in the hepatic parenchyme of chow-fed mice (Figure 7A, middle top panel) reminiscent of the distribution of quiescent HSCs and the pattern observed by others in healthy rat and human livers.18,19 The autofluorescence signal strongly increased in livers of HFC-fed mice, showing a completely altered staining pattern, which now appeared in large vesicular structures (“lipid droplets”) that localize predominantly in hepatocytes (Figure 7A, middle bottom panel; the Oil Red O staining for lipids is shown in the left panels for comparison). Immunohistochemical analysis of cell type–specific expression of hepatic LRAT revealed that it was predominantly present in (quiescent) HSCs in chow-fed mice (Figure 7A, top right panel) and remained undetectable in hepatocytes. In contrast, LRAT-specific staining was readily detected in hepatocytes in HFC-fed mice (Figure 7A, bottom right panel).
Figure 7.
Accumulation of vitamin A in lipid-loaded hepatocytes in vivo and in vitro. (A) Oil Red O staining (left panels) and vitamin A–specific autofluorescence (middle panels) immunohistochemistry of LRAT (right panels) of liver sections of chow-fed (top panels) and HFC-fed (bottom panels) mice. Vitamin A–specific autofluorescence was strongly increased in livers of HFC-fed mice and was located predominantly in hepatocytes compared with its location in sparsely present HSCs in livers of chow-fed mice. (B) Freshly isolated and 4-hour attached primary rat hepatocytes and quiescent primary HSCs were and immediately treated with and without palmitate (PA) for 24 hours. Cells were harvested and analyzed by Q-PCR analysis for Lrat and Pnpla3 expression. The gene expression is presented in 2-ΔΔCT and normalized to 18S. (C) Freshly isolated and 4-hour attached primary rat hepatocytes were treated for 48 hours with and without PA and retinol (ROH), followed by analysis of cellular retinyl PA and ROH levels. ∗P ≤ .05, ∗∗P ≤ .01.
In order to study cell type–specific effects of vitamin A storage in an in vitro model of fatty liver disease, we exposed primary rat hepatocytes, as well as quiescent and activated primary rat HSCs, to palmitate and found that it enhanced Lrat mRNA levels only in hepatocytes and not in quiescent HSCs (Figure 7B). Conversely, Pnpla3 mRNA levels were elevated by palmitate exposure specifically in HSCs. Moreover, cellular retinyl palmitate levels significantly increased only in primary rat hepatocytes exposed to palmitate together with retinol for 48 hours (Figure 7C).
These results suggest that NAFLD promotes vitamin A loss in HSCs while vitamin A storage is induced in hepatocytes.
Discussion
In this study, we show that steatohepatitis in mice heavily affects hepatic vitamin A metabolism, leading to reduced retinol and enhanced retinyl ester levels in the liver. Notably, retinyl esters accumulate in hepatocytes rather than in HSCs, cells that store vitamin A esters in a healthy liver. Following retinol levels, hepatic RBP4 levels are also reduced, while increased in circulation. Thus, steatohepatitis does not lead to true vitamin A deficiency as suggested by earlier studies, but rather leads to hepatic retinol deficiency due to metabolic changes. Vitamin A supplementation in the form of retinyl esters may therefore be counterproductive in NAFLD, as it will likely accumulate in the already overloaded hepatocytes. Also, aberrant vitamin A metabolism may directly affect the activity of nuclear receptors, such as RAR, PPARs, LXR, and FXR, as they require RXR for most of their actions.
Chronic liver diseases, including NAFLD, are associated with low serum and hepatic retinol levels, which is generally considered to be a sign of systemic vitamin A deficiency.10,11,20, 21, 22 Moreover, serum and hepatic retinol levels are negatively correlated with liver disease progression.10,20,23,24 However, systemic retinol levels are only a very small fraction of the total pool of vitamin A, which mostly consists of retinyl esters stored in liver (≥80%) and adipose tissue (10%–20%).25,26 Thus, systemic retinol levels alone are not a reliable marker for hypovitaminosis A per se.
Available information about the hepatic levels of the various retinoids (retinyl esters, retinol and retinoic acids) in NAFLD patients and animal models of NAFLD is highly controversial. Recent studies suggested that levels of all retinoids are reduced in mouse and human NAFLD livers,11,27 while earlier studies reported that NAFLD is associated with hepatic vitamin A accumulation in humans and rodents.18,19 Detailed knowledge about this is, however, crucial for patient care, as it will indicate whether vitamin A supplementation or therapy with specific vitamin A metabolites may have therapeutic value.
Our study largely confirms the reduction in hepatic retinol concentrations in fatty livers, as reported by others10,20,27,28 but reports the opposite effect for hepatic retinyl palmitate levels. Others reported that also retinyl palmitate levels in the livers of high-fat diet (HFD)–fed mice and ob/ob mice as wells as human NAFLD were decreased.11,27 Several reasons may account for this discrepancy. First was HFD vs HFC diet: A high-fat (only) diet may affect vitamin A metabolism differently than the HFC diets used in this study. However, we did not detect a reduction in hepatic retinyl palmitate levels in mice fed an HFD for 12 weeks, while hepatic retinol levels were reduced in both HFD- and HFC-fed mice (Figure 3A and 8). Second was the amount of vitamin A in the diets: in our study, both the control chow and HFC diet contained equal amounts of vitamin A (eg, 20 IU/g). The earlier study11 used an HFD that contained significantly less vitamin A compared with the control chow (3.8 vs 15 IU/g plus additional β-carotene, respectively). Dietary intake of vitamin A, even above daily recommendations, correlates directly with hepatic levels of retinyl palmitate and retinol.29, 30, 31, 32 Thus, for establishing an effect of a (high-fat) diet on hepatic vitamin A metabolism, it is crucial to standardize the dietary vitamin A intake for control and experimental diet. This is the case in the ob/ob mouse studies and cannot explain the opposing results between our and the earlier study. Third, retinol/retinyl ester extraction procedure: extraction of retinol and retinyl esters from serum or tissue is typically performed with either n-hexane25,33 or acetonitrile,11,27 though this is not always specified in methods sections. We compared both methods and found that acetonitrile very inefficiently extracts retinyl palmitate specifically from fatty liver tissue, while retinol extraction was similar with these solvents (Figure 9). This suggests that the extraction of retinyl esters by acetonitrile is compromised when a lot of fat is present in the (liver) tissue. Especially this latter procedural finding may explain the controversial findings on retinyl ester levels in fatty livers.
Figure 8.
An HFD leads to impaired hepatic vitamin A metabolism in mice. Mice were fed a chow diet or HFD diet for 12 weeks and analyzed for hepatic levels of retinol hepatic levels of retinyl palmitate. Hepatic retinol levels were strongly reduced in HFD-fed mice as compared with control mice. However, hepatic retinyl palmitate levels did change in both groups. ∗∗P ≤ .01.
Figure 9.
Comparison n-hexane and acetonitrile extraction in the mice liver. Effect of 2 different methods of vitamin A extractions was analyzed: (1) n-hexane (n-hex) (used in this study) or (2) acetonitrile method (ACN) as previously described11 from same mice livers fed a chow diet or HFC diet for 20 weeks (n = 3). Remarkably, both methods extracted a similar amount of retinol, but a significant less fraction of retinyl esters was extracted with ACN method. ∗P ≤ .05, ∗∗P ≤ .01.
We show that hepatic fat accumulation in mice is associated with a strong reduction in hepatic retinol levels, but not in circulation. This is in line with earlier observations10,11,20, 21, 22 and indicates that the mouse NAFLD models used do show the hepatic phenotype but do not replicate the reduced circulatory retinol levels observed in NAFLD patients.10,20,27,28 NAFLD mouse models likely reflect only the initiating phase of NAFLD, and serum retinol levels are also hardly affected at that stage in patients. Alternatively, the observed difference may be species related, but this requires analyses of vitamin A metabolism in more severe mouse NAFLD models. We did observe, however, that serum RBP4 levels are elevated in NAFLD mice, which is also found in obese individuals with or without established NAFLD.34, 35, 36, 37, 38 In contrast, hepatic RBP4 levels are reduced in NAFLD mice. This implies that, even though hepatic retinol levels are low, sufficient retinol is produced to promote—and even enhance—RBP4 secretion from the liver. Efficient RBP4 secretion from the liver strongly depends on retinol availability. Vitamin A deficiency leads to pronounced hepatic accumulation of retinol-free apo-RBP4 under unchanged Rbp4 mRNA levels and is rapidly released into the circulation upon retinol or retinoic acid treatment.39, 40, 41 As hepatic retinol levels were low, enhanced RBP4 secretion may also result from enhanced production of retinoic acids. Indeed, mRNA levels of Raldh1, the main enzyme responsible for RA production in the liver, were significantly elevated in ob/ob mice, with similar trends observed for 12- and 20-week HFC-fed mice. A similar increase in RALDH1 (ALDH1A1) expression in human nonalcoholic steatohepatitis liver was recently reported,27 though retinoic acid levels were reduced, when compared with control livers. We observed a more general hepatic induction of RA-responsive genes in NAFLD mice, including RAR-β, Cyp26a1, Hsd17b13, Ucp2, Cpt1a, and Fgf21, which is in line with the earlier reported hypermetabolic state of hepatic vitamin A metabolism and degradation in NAFLD patients.14 It may be important now to establish whether the extraction efficiency of retinoic acids are also different using acetonitrile, used by Zhong et al27 and many others, or n-hexane, as we observed for retinyl palmitate. Hepatic levels of Lrat and Dgat1, genes that encode enzymes that esterify retinol, were enhanced in NAFLD mice, observations also made in NAFLD patients.14 This coincides with a strong increase in hepatic retinyl palmitate levels in NAFLD mice, which is the main retinyl ester in both human and rodents.42,43 Particularly relevant is the apparent redistribution of vitamin A from HSCs in control livers to lipid-loaded hepatocytes in fatty livers. Retinyl esters are rapidly converted to retinol in the healthy liver. Accumulation of retinyl esters in lipid-loaded hepatocytes may therefore result from decreased hydrolysis or increased esterification. On the one hand, expression of Atgl/Pnpla2 (hydrolysis) was not changed, while both Lrat and Dgat1 (esterification) were enhanced in mouse fatty livers, indicating a shift to vitamin A storage in retinyl esters. On the other hand, PNPLA3/ADPN is also able to hydrolyze retinyl esters44 and its expression is strongly increased in mouse fatty liver, like in human NAFLD patients.45 Still, PNPLA3’s main substrates are triglycerides that also strongly accumulate in mouse fatty liver. This implies that its activity cannot compensate for the build-up of triglycerides in hepatocytes, as observed for retinyl palmitate. Notably, Lrat expression was induced in primary rat hepatocytes exposed to palmitate and they accumulate retinyl palmitate when co-exposed to retinol. These conditions did not regulate Lrat expression in qHSCs, but rather enhance retinyl ester hydrolysis by increasing PNPLA3 expression. These findings are in line with the shift in the main location of vitamin A to hepatocytes in fatty liver in mice (this study), rats,19 and humans.18 The redistribution of retinyl esters from HSCs to hepatocytes may also promote NAFLD-associated fibrosis, as differentiation of qHSCs to myofibroblasts is characterized by the loss of vitamin A-containing lipid droplets. In NAFLD, this apparently happens in conjunction with pronounced accumulation of retinyl esters in the liver, but then primarily in hepatocytes.
The mechanism(s) underlying the differential palmitate-mediated regulation of Lrat and Pnpla3 in hepatocytes and HSCs remain(s) to be determined. Saturated fatty acids, such as palmitate, regulate various transcriptional pathways that can directly or indirectly influence (cell type–specific) hepatic vitamin A metabolism.46 Hepatic de novo lipogenesis is under the primary transcriptional control of HFD and saturated fatty acids, such as palmitate, affect a multitude of transcription factors, including the sterol response element-binding protein-1c (SREBP-1c), carbohydrate response element-binding protein (ChREBP), CAAT/enhancer-binding protein- α or β (C/EBP α or β), hepatic nuclear factor-4α (HNF-4α), peroxisome proliferator-activated receptor α and γ (PPARα and γ), chicken ovalbumin upstream promoter transcription factor (COUP-TF), forkhead box O (FOXO), cAMP regulatory element-binding protein (CREBP), and nuclear factor κB.47, 48, 49 Indeed, PNPLA3 expression is under the direct control of SREBP-1c and ChREBP,50 and thus palmitate-induced SREBP-1c and ChREBP may enhance hepatic PNPLA3 and thereby promote retinyl ester loss. It will be therefore interesting now to analyze the possible contribution of the various transcription factors to Lrat and Pnpla3 regulation in hepatocytes and HSCs. Additionally, palmitate may induce general cellular responses, such as cellular endoplasmic reticulum stress, protein turnover, autophagy, mitochondrial dysfunction, and cell death49 that may indirectly affect vitamin A metabolism.
Taken together, our study shows that fatty liver disease is associated with hepatic accumulation of retinyl esters and disturbed vitamin A metabolism. The latter condition likely modulates disease progression, as it was recently shown that even moderate changes in hepatic RA production significantly enhance hepatic lipid accumulation.51 Moreover, lipid metabolism is tightly controlled by various nuclear receptors, like PPARs, FXR, LXR, and RAR, ligands of which are in advanced clinical trial stages for the treatment of NAFLD.46 All these factors require RXR as obligate partner and aberrant production of RXR-activating retinoids will affect nuclear receptor/RXR signaling. Further studies are needed to determine the impact of changed vitamin A metabolism in NAFLD on the therapeutic efficacy of these drug targets.
Materials and Methods
Animals
Animal experiments were approved by the Institutional Animal Care and Use Committee, University of Groningen, the Netherlands. Briefly, age- and sex-matched (8–10 weeks old) C57BL/6J mice were fed either regular chow or an HFC diet (containing 21% fat, with 45% calories from butterfat and 0.2% cholesterol; SAFE [Scientific Animal Food and Engineering], Villemoisson-sur-Orge, France) for a period of 12 or 20 weeks (n = 8), as reported previously.52,53 Both diets contained 20 IU of retinyl acetate/g as a source of vitamin A. Leptinob mutant (JAX ob/ob) mice (10–12 weeks old; Charles River Laboratories, Wilmington, MA) and appropriate controls (C57BL/6J) were also studied and received chow diet. Animals were kept in a pathogen-free environment with alternating dark-light cycles of 12 hours, and controlled temperature (20–24°C) and relative humidity (55 ± 15%) receiving food and water ad libitum. Animals were fasted 4 hours before sacrifice. Tissues were snap-frozen in liquid nitrogen or fixed in paraformaldehyde. Blood was collected by heart puncture.
Isolation and Culture of Primary Rat Hepatocytes and HSCs
Primary rat hepatocytes and HSCs were isolated from Wistar rats (Charles River Laboratories) and cultured as previously described.54 Briefly, primary hepatocytes were isolated after 2-step collagenase perfusion (Sigma-Aldrich, St. Louis, MO).55,56 Cell viability >80% was determined by Trypan blue (Sigma-Aldrich). Primary hepatocytes were used after 4 hours of attachment on collagen-coated plates in William’s E (Gibco by Life Technologies; Thermo Fisher Scientific, Bleiswijk, the Netherlands) medium with 10% fetal calf serum, 50-μg/mL gentamycin, and 100-U/mL penicillin/streptomycin/amphotericin B. Primary rat HSCs were isolated after pronase (Merck, Amsterdam, the Netherlands) and collagenase-P (Roche, Almere, the Netherlands) perfusion and followed by Nycodenz (Axis-ShieldPOC; Oslo, Norway) density gradient centrifugation.55 Primary hepatocytes and HSCs were exposed to bovine serum albumin–conjugated palmitic acid (0.25 or 0.5 mmol/L; Sigma-Aldrich), with or without 5-μM retinol (Sigma-Aldrich) for 24–48 hours as previously described.57
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Quantitative real-time reverse transcription polymerase chain reaction was performed as previously described.58 Briefly, total RNA was isolated from tissue samples using TRIzol reagent according to supplier’s instructions (Thermo Fisher Scientific). RNA quality and quantity were determined using a Nanodrop 2000c UV-vis spectrophotometer (Thermo Fisher Scientific). Complementary DNA was synthesized from 2.5 μg of RNA by using random nonamers and M-MLV reverse transcriptase (Thermo Fisher Scientific). TaqMan primers and probes were designed using Primer Express 3.0.1 (Thermo Fisher Scientific) and are shown in Supplementary Table 2. All target genes were amplified using the Q-PCR core kit master mix (Eurogentec, Maastricht, the Netherlands) on a 7900HT Fast Real-Time PCR system (Thermo Fisher Scientific). SDSV2.4.1 (Thermo Fisher Scientific) was used to analyze the data. Expression of genes is presented in 2-ΔCT and normalized to 36B4.
Western Blot Analysis
Protein samples were prepared for Western blot analysis as described previously.59 Protein concentrations were quantified using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as standard. Equal amounts of protein were separated on Mini-PROTEAN TGX precast 4%–5% gradient gels (Bio-Rad) and transferred to nitrocellulose membranes using the Trans-Blot turbo transfer system (Bio-Rad). Primary antibodies (anti-RBP4, 1:2,000; #ab109193 [Abcam, Cambridge, United Kingdom] and anti-GAPDH, 1:40,000 #CB1001 [Calbiochem; Merck-Millipore, Amsterdam-Zuidoost, the Netherlands]) and horseradish peroxidase–conjugated goat anti-rabbit secondary antibodies (1:2000; Agilent DAKO, Amstelveen, the Netherlands) were used for detection. Proteins were detected using the Pierce ECL Western blotting kit (Thermo Fisher Scientific). Images were captured using the chemidoc XRS system and Image Lab version 3.0 (Bio-Rad). The intensity of bands was quantified using ImageJ version 1.51 (National Institutes of Health, Bethesda, MD).
Histology and Pathological Scoring
Hematoxylin and eosin (hematoxylin and eosin) staining on liver sections (4 μm) was performed. Snap-frozen liver sections (5 μm) were stained using Oil Red O.60,61 A pathological score was calculated as previously described62 to determine the level of steatosis, as well as the grade of lobular inflammation.
Immunohistochemistry
Immunohistochemistry for CD68 (1:300; rabbit anti-mouse CD68; #137002; Biolegio, Nijmegen, the Netherlands) and LRAT (1:200 rabbit anti-mouse LRAT; #28075; Takara Immuno-Biological Laboratories, Gunma, Japan) was performed on snap-frozen liver sections as previously described.63 Antigen retrieval was performed by microwave irradiation in citrate buffer, pH 6.0, and blocking of endogenous peroxidase with 0.3% H2O2 for 30 minutes. Horseradish peroxidase–conjugated goat anti-rabbit antibodies (1:50; #170-6515; Bio-Rad) was used as secondary antibody. Slides were stained with DAB for 10 minutes and hematoxylin was used as a counter nuclear stain (2 minutes at room temperature). Finally, slides were dehydrated and mounted with Eukitt (Sigma-Aldrich). Slides were scanned using a nanozoomer 2.0 HT digital slide scanner (C9600-12; Hamamatsu Photonics, Hamamatsu, Japan) and analyzed with Aperio ImageScope (version 11.1; Leica Microsystems, Amsterdam, the Netherlands). CD68-positive cells were counted to assess the expansion of macrophages in the liver.
Lipid Analysis
Lipids were extracted from 15% (w/v) liver homogenate in phosphate-buffered saline by using the Bligh and Dyer method.64 A colorimetric assay was used to determine total cholesterol (11489232; Roche Molecular Biochemicals, Almere, the Netherlands) and free cholesterol (113609910930; DiaSys Diagnostic Systems GmbH, Holzheim, Germany). Cholesterol standards (DiaSys Diagnostic Systems GmbH) were used as a reference. Triglycerides were quantified using the Trig/GB kit (1187771; Roche Molecular Biochemicals), and Roche Precimat Glycerol standards (16658800) were used as a reference.
Serum and Hepatic Vitamin A Analysis
Serum and tissue vitamin A content was analyzed by reverse-phase high-performance liquid chromatography as previously described.33 Briefly, tissue (30–50 mg) was homogenized in phosphate-buffered saline to create a 15% (w/v) tissue homogenate. Then, tissue homogenate (66.70 μL equal to 10 mg of tissue) or serum (50 μL) were added in antioxidant mix (2.75 mL, containing 23.07-mmol/L pyrogallol, 1.32-mmol/L butylated hydroxytoluene, 5.83-mmol/L ethylenediaminetetraacetic acid, and 38.71-mmol/L ascorbic acid, dissolved in methanol:dH20 [2.67:1], pH 5.4) and vortexed thoroughly for 1 minutes.
Retinol and retinyl esters were extracted and deproteinized twice with n-hexane in the presence of retinol acetate (100 μL, concentration 4 μmol/L) as an internal standard to assess the level of recovery after the extraction procedure. Standard curves created from a range of concentrations of retinol and retinyl palmitate were used to determine absolute tissue and serum concentrations of these compounds. Additionally, 2 negative controls (only containing internal standard) and 2 positive controls (low and high concentrations of retinol plus internal standard) were included in each series of extractions. Samples were evaporated under N2 and diluted in 300-μL 100% ultrapure ethanol. Then, 50 μL was injected in high-performance liquid chromatography (HT Separations Module 2795; Waters Alliance, East Lyme, CT) for phase separation on a C18 column (Waters Symmetry C18, dimension 150 × 3.0 mm, particle size 5 μm; Waters Corporation, Milford, MA) and measurement (UV-VIS, dual wavelength, UV-4075 Jasco, Tokyo, Japan). Retinoids in samples were identified by exact retention time of known standards in ultraviolet absorption at 325 nm by high-performance liquid chromatography . Finally, retinol and retinyl palmitate concentrations were calculated and normalized to final volume or tissue weight.
Vitamin A Autofluorescence in Liver Tissue
Autofluorescence analysis was performed on unstained cryostatic liver sections using a Zeiss LSM 780 NLO 2-photon CLSM (Carl Zeiss, Jena, Germany) as previously described.18,65 Briefly, cryostat liver sections were illuminated with an excitation filter of 366-nm band-pass interference, and spectra were recorded in the range of 400–680 nm with spectrum acquisition from 0.2 to 3 seconds.
Statistical Analysis
Data is presented in group mean ± SEM and statistical analysis was performed using the GraphPad Prism 6 software package (GraphPad Software, San Diego, CA). Statistical significance was determined by Mann-Whitney test (2 groups), One-way analysis of variance or Kruskal-Wallis (more than 2 groups) followed by post hoc Dunn’s test (compare all pairs of columns). Pearson correlation was performed between tissue and circulatory vitamin A with various parameters of NAFLD progression.
Acknowledgments
The authors want to acknowledge Tim Van Zutphen and Dicky Struik for providing samples of ob/ob mice.
CRediT Authorship Contributions
Ali Saeed (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Software: Lead; Validation: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Paulina Bartuzi (Data curation: Supporting; Validation: Supporting)
Janette Heegsma, MSc (Data curation: Supporting; Formal analysis: Supporting; Methodology: Equal; Validation: Supporting)
Daphne Dekker (Data curation: Supporting; Formal analysis: Supporting)
Niels Kloosterhuis (Data curation: Supporting; Formal analysis: Supporting)
Alain de Bruin (Formal analysis: Supporting; Investigation: Supporting; Methodology: Supporting; Validation: Supporting; Visualization: Supporting)
Johan W Jonker (Resources: Supporting; Validation: Supporting; Visualization: Supporting; Writing – review & editing: Supporting)
Bart van de Sluis (Data curation: Equal; Formal analysis: Equal; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Resources: Equal; Supervision: Supporting; Validation: Equal; Visualization: Equal; Writing – review & editing: Equal)
Klaas Nico Faber (Conceptualization: Lead; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Equal; Methodology: Equal; Project administration: Lead; Resources: Lead; Supervision: Lead; Validation: Lead; Visualization: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of Interest The authors disclose no conflicts.
Funding This work was supported by the Dutch Digestive Disease Foundation, grant numbers MWO 03-38 (to Klaas Nico Faber) and MWO 08-70 (to Klaas Nico Faber).
Contributor Information
Ali Saeed, Email: a.saeed@umcg.nl.
Klaas Nico Faber, Email: k.n.faber@umcg.nl.
Supplementary Material
Supplementary Table 1.
Pearson Correlation Analysis of Vitamin A With Various Parameters of NAFLD in Mice Fed High-Fat, High-Cholesterol Diet
| Retinol |
Retinyl palmitate |
Plasma retinol |
Total cholesterol |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Retinol | 1.000 | |||||||
| Retinyl palmitate | –.5090a | .0094a | 1.000 | |||||
| Plasma retinol | –.8336a | .0001a | .3138 | .155 | 1.000 | |||
| Total cholesterol | –.6705a | .0002a | .4537b | .0227b | .4132b | .0447b | 1.000 | |
| Free cholesterol | –.6163a | .0023a | .2172 | .3316 | .3996 | .0654 | .8713b | <.0001b |
| Triglycerides | –.7264a | <.0001a | .4399b | .0357b | .3746 | .0858 | .8338b | <.0001b |
| Plasma total cholesterol | –.8558a | <.0001a | .3956 | .0557 | .7992b | <.0001b | .7002b | <.0001b |
| Plasma free cholesterol | –.6987a | .0001a | .3970 | .0547 | .7933b | <.0001b | .6201b | .0007b |
| Plasma insulin | –.4819a | .0199a | .1850 | .3981 | .3288 | .1352 | .4454b | .0257b |
| Ldlr | –.3034 | .1404 | –.0385 | .8552 | .3247 | .1216 | .0712 | .724 |
| Srb1 | –.4420a | .0269a | .3330 | .1038 | .2512 | .2364 | .6968b | <.0001b |
| Cd36 | –.7852a | <.0001a | .4761b | .0161b | .8182b | <.0001 | .5167b | .0058b |
| Scd1 | –.8337a | <.0001a | .4571b | .0216b | .7339b | <.0001b | .6488b | .0003b |
| Fasn | –.6557a | .0004a | .2310 | .2666 | .5699b | .0036b | .3909b | .0438b |
| Acc1 | –.6317a | .0007a | .2304 | .2679 | .5549b | .0049b | .4235b | .0277b |
| Tnfa | –.6956a | .0001a | .2370 | .2541 | .8304b | .0001b | .5063b | .0071b |
| Nos2 | –.5468a | .0057a | .3073 | .1441 | .6059b | .0022b | .3951b | .0457b |
| Ccl2 | –.6086a | .0012a | .2781 | .1784 | .7633b | <.0001b | .4329b | .0241b |
| Il1b | –.6533a | .0004a | .4088b | .0425b | .3854 | .0629 | .6062b | .0008b |
| Il6 | –.3023 | .1511 | .3158 | .1327 | .0613 | .7863 | .4628b | .0198b |
| Co1a1 | –.6398a | .0006a | .1263 | .5473 | .7626b | <.0001b | .4320b | .0244b |
| Acta2 | –.6355a | .0006a | .1773 | .3965 | .6210b | .0012b | .5299b | .0045b |
| Tgfb | –.7158a | <.0001a | .2572 | .2145 | .7649b | <.0001b | .6454b | .0003b |
| Timp1 | –.5585a | .0037a | .0657 | .7552 | .7044b | .0001b | .3541 | .07 |
Pearson correlation analysis of hepatic vitamin A (retinol, retinyl palmitate) with circulatory vitamin A (plasma retinol) with various parameters of diet-induced NAFLD progression. A correlation was also calculated between tissue and circulatory vitamin A with hepatic gene expression of markers for the progression of NAFLD. D P ≤ .05 considered significant.
NAFLD, nonalcoholic fatty liver disease.
Significant inverse correlation.
Positive correlation.
Supplementary Table 2.
Primers and Probes Used in Study for Analysis of Target Genes
| Gene/ID | TaqMan primers and probe |
|---|---|
|
36B4 NM_022402 |
Fwd: 5′-GCTTCATTGTGGGAGCAGACA-3′ Rev: 5′-CATGGTGTTCTTGCCCATCAG-3′ Probe: 5′-TCCAAGCAGATGCAGCAGATCCGC-3′ |
|
Acaca/Acc1 NM_133360.1/NM_022193.1 |
Fwd: 5′-GCCATTGGTATTGGGGCTTAC-3′ Rev: 5′-CCCGACCAAGGACTTTGTTG-3′ Probe: 5′-CTCAACCTGGATGGTTCTTTGTCCCAGC-3′ |
|
Act2/α-Sma NM_007392 |
Fwd: 5′-TTCGTGTGGCCCCTGAAG-3′ Rev: 5′-GGACAGCACAGCCTGAATAGC-3′ Probe: 5′-TTGAGACCTTCAATGTCCCCGCCA-3′ |
|
Cd36 BC010262/NM_031561 |
Fwd: 5′-GATCGGAACTGTGGGCTCAT-3′ Rev: 5′-GGTTCCTTCTTCAAGGACAACTTC-3′ Probe: 5′-AGAATGCCTCCAAACACAGCCAGGAC-3′ |
|
Cd68 NM_009853 |
Fwd: 5′-CACTTCGGGCCATGTTTCTC-3′ Rev: 5′-AGGACCAGGCCAATGATGAG-3′ Probe: 5′-CAACCGTGACCAGTCCCTCTTGCTG-3′ |
|
Ccl2 NM_031530.1 |
Fwd: 5′-TGTCTCAGCCAGATGCAGTTAAT-3′ Rev: 5′-CCGACTCATTGGGATCATCTT-3′ Probe: 5′-CCCCACTCACCTGCTGCTACTCATTCA-3′ |
|
Col1a1 NM_007742 |
Fwd: 5′-TGGTGAACGTGGTGTACAAGGT-3′ Rev: 5′-CAGTATCACCCTTGGCACCAT-3′ Probe: 5′-TCCTGCTGGTCCCCGAGGAAACA-3′ |
|
Cpt1a NM_013495.1 |
Fwd: 5′-CTCAGTGGGAGCGACTCTTCA-3′ Rev: 5′-GGCCTCTGTGGTACACGACAA-3′ Probe: 5′-CCTGGGGAGGAGACAGACACCATCCAAC-3′ |
|
Mlxipl/Chrebp NM_021455.3/NM_133552.1 |
Fwd: 5′-GATGGTGCGAACAGCTCTTCT-3′ Rev: 5′-CTGGGCTGTGTCATGGTGAA-3′ Probe: 5′-CCAGGCTCCTCCTCGGAGCCC-3′ |
|
Cyp26a1 NM_007811.1 |
Fwd: 5′-GGAGACCCTGCGATTGAATC-3′ Rev: 5′-GATCTGGTATCCATTCAGCTCAAA-3′ Probe: 5′-TCTTCAGAGCAACCCGAAACCCTCC-3′ |
|
Dgat1 NM_010046.2/NM_053437.1 |
Fwd: 5′-GGTGCCCTGACAGAGCAGAT-3′ Rev: 5′-CAGTAAGGCCACAGCTGCTG-3′ Probe: 5′-CTGCTGCTACATGTGGTTAACCTGGCCA-3′ |
|
Dgat2 NM_026384.2/NM_001012345.1 |
Fwd: 5′-GGGTCCAGAAGAAGTTCCAGAAG-3′ Rev: 5′-CCCAGGTGTCAGAGGAGAAGAG-3′ Probe: 5′-CCCCTGCATCTTCCATGGCCG-3′ |
|
Fasn NM_007988/NM_017332 |
Fwd: 5′-GGCATCATTGGGCACTCCTT-3′ Rev: 5′-GCTGCAAGCACAGCCTCTCT-3′ Probe: 5′-CCATCTGCATAGCCACAGGCAACCTC-3′ |
|
Fgf21 NM_020013.4/NM_130752.1 |
Fwd: 5′-CCGCAGTCCAGAAAGTCTCC-3′ Rev: 5′-TGACACCCAGGATTTGAATGAC-3′ Probe: 5′-CCTGGCTTCAAGGCTTTGAGCTCC A-3′ |
|
Hsd17b13 NM_198030.2/NM_001163486.1 |
Fwd: 5′- AAAGCAGAAAAGCAGACTGGTTCT-3′ Rev: 5′- CCCCAGTTTCCTGCATTTGT-3′ Probe: 5′-CGGTTTCCTCAACACCACGCTTATTGA-3′ |
|
Lipe/HSL NM_010719/X51415 |
Fwd: 5′-GAGGCCTTTGAGATGCCACT-3′ Rev: 5′-AGATGAGCCTGGCTAGCACAG-3′ Probe: 5′-CCATCTCACCTCCCTTGGCACACAC-3′ |
|
IL-1β NM_008361 |
Fwd: 5′-ACCCTGCAGCTGGAGAGTGT-3′ Rev: 5′-TTGACTTCTATCTTGTTGAAGACAAACC-3′ Probe: 5′-CCCAAGCAATACCCAAAGAAGAAGATGGAA -3′ |
|
IL-6 NM_031168 |
Fwd: 5′-CCGGAGAGGAGACTTCACAGA-3′ Rev: 5′-AGAATTGCCATTGCACAACTCTT-3′ Probe: 5′-ACCACTTCACAAGTCGGAGGCTTAATTACA-3′ |
|
iNos/Nos2 AF049656/NM_010927/NM_012611 |
Fwd: 5′- CTATCTCCATTCTACTACTACCAGATCGA-3′ Rev: 5′- CCTGGGCCTCAGCTTCTCAT-3′ Probe: 5′- CCCTGGAAGACCCACATCTGGCAG-3′ |
|
Ldlr NM_010700/NM_175762 |
Fwd: 5′-GCATCAGCTTGGACAAGGTGT-3′ Rev: 5′-GGGAACAGCCACCATTGTTG-3′ Probe: 5′-CACTCCTTGATGGGCTCATCCGACC-3′ |
|
Lpl NM_008509/NM_012598 |
Fwd: 5′-AAGGTCAGAGCCAAGAGAAGCA-3′ Rev: 5′-CCAGAAAAGTGAATCTTGACTTGGT-3′ Probe: 5′-CCTGAAGACTCGCTCTCAGATGCCCTACA-3′ |
|
Lrat NM_023624 |
Fwd: 5′-TCCATACAGCCTACTGTGGAACA-3′ Rev: 5′-CTTCACGGTGTCATAGAACTTCTCA-3′ Probe: 5′-ACTGCAGATATGGCTCTCGGATCAGTCC-3′ |
|
LRAT NM_004744 |
Fwd: 5′-TGCGAGCACTTCGTGACCTA-3′ Rev: 5′-TTATCTTCACAGTCTCACAAAACTTGTC-3′ Probe: 5′-TGCAGATATGGCACCCCGATCAGTC-3′ |
|
Pck1 NM_011044/NM_198780 |
Fwd: 5′-GTGTCATCCGCAAGCTGAAG-3′ Rev: 5′-CTTTCGATCCTGGCCACATC-3′ Probe: 5′-CAACTGTTGGCTGGCTCTCACTGACCC-3′ |
|
Ppargc1 α/Pgc1α NM_008904/NM_031347 |
Fwd: 5′-GACCCCAGAGTCACCAAATGA-3′ Rev: 5′-GGCCTGCAGTTCCAGAGAGT-3′ Probe: 5′-CCCCATTTGAGAACAAGACTATTGAGCGAACC-3′ |
|
Pnpla2/Atgl NM_025802/XM_347183 |
Fwd: 5′-AGCATCTGCCAGTATCTGGTGAT-3′ Rev: 5′-CACCTGCTCAGACAGTCTGGAA-3′ Probe: 5′-ATGGTCACCCAATTTCCTCTTGGCCC-3′ |
|
Pnpla3 NM_054088 |
Fwd: 5′-ATCATGCTGCCCTGCAGTCT-3′ Rev: 5′-GCCACTGGATATCATCCTGGAT-3′ Probe: 5′-CACCAGCCTGTGGACTGCAGCG-3′ |
|
PNPLA3 NM_025225 |
Fwd: 5′-CTGTACCCTGCCTGTGGAATCT-3′ Rev: 5′-AGGACATCGTCGGGCATATCT-3′ Probe: 5′-CCATGTCACCAGTCTCTGGACAATCGC-3′ |
| RAR-β | Assay on demand, Mm01319677_m1 (ThermoFisher) |
| Raldh1 | Assay on demand, Mm00657317_m1 (ThermoFisher) |
| Raldh2 | Assay on demand, Mm00501306_m1 (ThermoFisher) |
| Raldh3 | Assay on demand, Mm00474049_m1 (ThermoFisher) |
|
Raldh4 NM_178713.4 |
Fwd: 5′-TGGAGCAGTCTCTGGAGGAGTT-3′ Rev: 5′- GAAGTTCAGAACAGACCGAGGAA-3′ Probe: 5′- AATCTAAAGACCAAGGGAAAACCCTCACGC-3′ |
|
Rbp4 NM_011255.2/XM_215285.3 |
Fwd: 5′-GGTGGGCACTTTCACAGACA-3′ Rev: 5′-GATCCAGTGGTCATCGTTTCCT-3′ Probe: 5′-CCCCAGTACTTCATCTTGAACTTGGCAGG-3′ |
|
Scd1 NM_009127.2 |
Fwd: 5′-ATGCTCCAAGAGATCTCCAGTTCT-3′ Rev: 5′-CTTCACCTTCTCTCGTTCATTTCC-3′ Probe: 5′-CCACCACCACCATCACTGCACCTC-3′ |
|
TGF-β1 NM_021578.1 |
Fwd: 5′-GGGCTACCATGCCAACTTCTG-3′ Rev: 5′-GAGGGCAAGGACCTTGCTGTA-3′ Probe: 5′-CCTGCCCCTACATTTGGAGCCTGGA-3′ |
|
TGF-β1 NM_021578.1 |
Fwd: 5′-GGGCTACCATGCCAACTTCTG-3′ Rev: 5′-GAGGGCAAGGACCTTGCTGTA-3′ Probe: 5′-CCTGCCCCTACATTTGGAGCCTGGA-3′ |
|
Timp1 NM_001044384.1/NM_011593.2 |
Fwd: 5′-TCTGAGCCCTGCTCAGCAA-3′ Rev: 5′-AACAGGGAAACACTGTGCACAC-3′ Probe: 5′-CCACAGCCAGCACTATAGGTCTTTGAGAAAGC-3′ |
|
TNF-α NM_013693/NM_012675 |
Fwd: 5′- GTAGCCCACGTCGTAGCAAAC-3′ Rev: 5′- AGTTGGTTGTCTTTGAGATCCATG-3′ Probe: 5′- CGCTGGCTCAGCCACTCCAGC-3′ |
|
Ucp2 NM_011671.2 |
Fwd: 5′-CGAAGCCTACAAGACCATTGC-3′ Rev: 5′-ACCAGCTCAGCACAGTTGACA-3′ Probe: 5′-CAGAGGCCCCGGATCCCTTCC-3′ |
References
- 1.Albhaisi S., Sanyal A. Recent advances in understanding and managing non-alcoholic fatty liver disease. F1000Res. 2018 doi: 10.12688/f1000research.14421.1. 7F1000 Faculty Rev-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellentani S., Scaglioni F., Marino M., Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 3.Katsagoni C.N., Georgoulis M., Papatheodoridis G.V., Panagiotakos D.B., Kontogianni M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Iredale J.P., Thompson A., Henderson N.C. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832:876–883. doi: 10.1016/j.bbadis.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber R., Taschler U., Preiss-Landl K., Wongsiriroj N., Zimmermann R., Lass A. Retinyl ester hydrolases and their roles in vitamin A homeostasis. Biochim Biophys Acta. 2012;1821:113–123. doi: 10.1016/j.bbalip.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaner W.S., O’Byrne S.M., Wongsiriroj N., Kluwe J., D’Ambrosio D.M., Jiang H., Schwabe R.F., Hillman E.M.C., Piantedosi R., Libien J. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senoo H., Mezaki Y., Fujiwara M. The stellate cell system (vitamin A-storing cell system) Anat Sci Int. 2017;92:387–455. doi: 10.1007/s12565-017-0395-9. [DOI] [PubMed] [Google Scholar]
- 8.Saeed A., Hoekstra M., Hoeke M.O., Heegsma J., Faber K.N. The interrelationship between bile acid and vitamin A homeostasis. Biochim Biophys Acta. 2017;1862:496–512. doi: 10.1016/j.bbalip.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Brown E., Akré J., editors. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. World Health Organization; Geneva, Switzerland: 1996. [Google Scholar]
- 10.Chaves G.V., Pereira S.E., Saboya C.J., Spitz D., Rodrigues C.S., Ramalho A. Association between liver vitamin A reserves and severity of nonalcoholic fatty liver disease in the class III obese following bariatric surgery. Obes Surg. 2014;24:219–224. doi: 10.1007/s11695-013-1087-8. [DOI] [PubMed] [Google Scholar]
- 11.Trasino S.E., Tang X.-H., Jessurun J., Gudas L.J. Obesity leads to tissue, but not serum vitamin A deficiency. Sci Rep. 2015;5:15893. doi: 10.1038/srep15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovarova M., Königsrainer I., Königsrainer A., Machicao F., Häring H.-U., Schleicher E., Peter A. The genetic variant I148M in PNPLA3 is associated with increased hepatic retinyl-palmitate storage in humans. J Clin Endocrinol Metab. 2015;100:E1568–E1574. doi: 10.1210/jc.2015-2978. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y., Belyaeva O.V., Brown P.M., Fujita K., Valles K., Karki S., de Boer Y.S., Koh C., Chen Y., Du X., Handelman S.K., Chen V., Speliotes E.K., Nestlerode C., Thomas E., Kleiner D.E., Zmuda J.M., Sanyal A.J., (for the Nonalcoholic Steatohepatitis Clinical Research Network), Kedishvili N.Y., Liang T.J., Rotman Y. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashla A.A., Hoshikawa Y., Tsuchiya H., Hashiguchi K., Enjoji M., Nakamuta M., Taketomi A., Maehara Y., Shomori K., Kurimasa A., Hisatome I., Ito H., Shiota G. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res. 2010;40:594–604. doi: 10.1111/j.1872-034X.2010.00646.x. [DOI] [PubMed] [Google Scholar]
- 15.Makia N.L., Bojang P., Falkner K.C., Conklin D.J., Prough R.A. Murine hepatic aldehyde dehydrogenase 1a1 is a major contributor to oxidation of aldehydes formed by lipid peroxidation. Chem Biol Interact. 2011;191:278–287. doi: 10.1016/j.cbi.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kathmann E.C., Naylor S., Lipsky J.J. Rat liver constitutive and phenobarbital-inducible cytosolic aldehyde dehydrogenases are highly homologous proteins that function as distinct isozymes. Biochemistry. 2000;39:11170–11176. doi: 10.1021/bi001120m. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt D.K., Gaedigk A., Pearce R.E., Leeder J.S., Prasad B. Age-dependent protein abundance of cytosolic alcohol and aldehyde dehydrogenases in human liver. Drug Metab Dispos. 2017;45:1044–1048. doi: 10.1124/dmd.117.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croce A.C., De Simone U., Freitas I., Boncompagni E., Neri D., Cillo U., Bottiroli G. Human liver autofluorescence: an intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg Med. 2010;42:371–378. doi: 10.1002/lsm.20923. [DOI] [PubMed] [Google Scholar]
- 19.Croce A.C., Ferrigno A., Piccolini V.M., Tarantola E., Boncompagni E., Bertone V., Milanesi G., Freitas I., Vairetti M., Bottiroli G. Integrated autofluorescence characterization of a modified-diet liver model with accumulation of lipids and oxidative stress. Biomed Res Int. 2014;2014:803491. doi: 10.1155/2014/803491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botella-Carretero J.I., Balsa J.A., Vázquez C., Peromingo R., Díaz-Enriquez M., Escobar-Morreale H.F. Retinol and alpha-tocopherol in morbid obesity and nonalcoholic fatty liver disease. Obes Surg. 2010;20:69–76. doi: 10.1007/s11695-008-9686-5. [DOI] [PubMed] [Google Scholar]
- 21.Havaldar P.V., Patel V.D., Siddibhavi B.M. Recurrent vitamin A deficiency and fatty liver. J Trop Pediatr. 1991;37:87–88. doi: 10.1093/tropej/37.2.87. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Chen H., Wang J., Zhou W., Sun R., Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr. 2015;102:130–137. doi: 10.3945/ajcn.114.105155. [DOI] [PubMed] [Google Scholar]
- 23.de Souza Valente da Silva L., Valeria da Veiga G., Ramalho R.A. Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition. 2007;23:392–397. doi: 10.1016/j.nut.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Neuhouser M.L., Rock C.L., Eldridge A.L., Kristal A.R., Patterson R.E., Cooper D.A., Neumark-Sztainer D., Cheskin L.J., Thornquist M.D. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr. 2001;131:2184–2191. doi: 10.1093/jn/131.8.2184. [DOI] [PubMed] [Google Scholar]
- 25.Kane M.A., Folias A.E., Napoli J.L. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaner W.S., Li Y., Brun P.-J., Yuen J.J., Lee S.-A., Clugston R.D. Vitamin A absorption, storage and mobilization. Subcell Biochem. 2016;81:95–125. doi: 10.1007/978-94-024-0945-1_4. [DOI] [PubMed] [Google Scholar]
- 27.Zhong G., Kirkwood J., Won K.-J., Tjota N., Jeong H., Isoherranen N. Characterization of vitamin A metabolome in human livers with and without nonalcoholic fatty liver disease. J Pharmacol Exp Ther. 2019;370:92–103. doi: 10.1124/jpet.119.258517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suano de Souza F.I., Silverio Amancio O.M., Saccardo Sarni R.O., Sacchi Pitta T., Fernandes A.P., Affonso Fonseca F.L., Hix S., Ramalho R.A. Non-alcoholic fatty liver disease in overweight children and its relationship with retinol serum levels. Int J Vitam Nutr Res. 2008;78:27–32. doi: 10.1024/0300-9831.78.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Sundaresan P.R., Kaup S.M., Wiesenfeld P.W., Chirtel S.J., Hight S.C., Rader J.I. Interactions in indices of vitamin A, zinc and copper status when these nutrients are fed to rats at adequate and increased levels. Br J Nutr. 1996;75:915–928. doi: 10.1079/bjn19960197. [DOI] [PubMed] [Google Scholar]
- 30.Johansson I., Hallmans G., Wikman A., Biessy C., Riboli E., Kaaks R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002;5:487–496. doi: 10.1079/phn2001315. [DOI] [PubMed] [Google Scholar]
- 31.Cifelli C.J., Ross A.C. Chronic vitamin A status and acute repletion with retinyl palmitate are determinants of the distribution and catabolism of all-trans-retinoic acid in rats. J Nutr. 2007;137:63–70. doi: 10.1093/jn/137.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olivares A., Daza A., Rey A.I., López-Bote C.J. Dietary vitamin A concentration alters fatty acid composition in pigs. Meat Sci. 2009;81:295–299. doi: 10.1016/j.meatsci.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y.-K., Quadro L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol Biol. 2010;652:263–275. doi: 10.1007/978-1-60327-325-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Q., Graham T.E., Mody N., Preitner F., Peroni O.D., Zabolotny J.M., Kotani K., Quadro L., Kahn B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 35.Haider D.G., Schindler K., Prager G., Bohdjalian A., Luger A., Wolzt M., Ludvik B. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92:1168–1171. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 36.Reinehr T., Stoffel-Wagner B., Roth C.L. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:2287–2293. doi: 10.1210/jc.2007-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tajtáková M., Semanová Z., Ivancová G., Petrovicová J., Donicová V., Zemberová E. [Serum level of retinol-binding protein 4 in obese patients with insulin resistance and in patients with type 2 diabetes treated with metformin] Vnitr Lek. 2007;53:960–963. [PubMed] [Google Scholar]
- 38.Ulgen F., Herder C., Kühn M.C., Willenberg H.S., Schott M., Scherbaum W.A., Schinner S. Association of serum levels of retinol-binding protein 4 with male sex but not with insulin resistance in obese patients. Arch Physiol Biochem. 2010;116:57–62. doi: 10.3109/13813451003631421. [DOI] [PubMed] [Google Scholar]
- 39.Ronne H., Ocklind C., Wiman K., Rask L., Obrink B., Peterson P.A. Ligand-dependent regulation of intracellular protein transport: effect of vitamin a on the secretion of the retinol-binding protein. J Cell Biol. 1983;96:907–910. doi: 10.1083/jcb.96.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon J.L., Goodman D.S. Studies on the metabolism of retinol-binding protein by primary hepatocytes from retinol-deficient rats. J Cell Physiol. 1987;130:14–20. doi: 10.1002/jcp.1041300104. [DOI] [PubMed] [Google Scholar]
- 41.Bellovino D., Lanyau Y., Garaguso I., Amicone L., Cavallari C., Tripodi M., Gaetani S. MMH cells: An in vitro model for the study of retinol-binding protein secretion regulated by retinol. J Cell Physiol. 1999;181:24–32. doi: 10.1002/(SICI)1097-4652(199910)181:1<24::AID-JCP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Futterman S., Andrews J.S. The composition of liver vitamin A ester and the synthesis of vitamin A ester by liver microsomes. J Biol Chem. 1964;239:4077–4080. [PubMed] [Google Scholar]
- 43.Schäffer M.W., Roy S.S., Mukherjee S., Nohr D., Wolter M., Biesalski H.K., Ong D.E., Das S.K. Qualitative and quantitative analysis of retinol, retinyl esters, tocopherols and selected carotenoids out of various internal organs form different species by HPLC. Anal Methods. 2010;2:1320–1332. doi: 10.1039/c0ay00288g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirazzi C., Valenti L., Motta B.M., Pingitore P., Hedfalk K., Mancina R.M., Burza M.A., Indiveri C., Ferro Y., Montalcini T., Maglio C., Dongiovanni P., Fargion S., Rametta R., Pujia A., Andersson L., Ghosal S., Levin M., Wiklund O., Iacovino M., Borén J., Romeo S. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. 2014;23:4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aragonès G., Auguet T., Armengol S., Berlanga A., Guiu-Jurado E., Aguilar C., Martínez S., Sabench F., Porras J.A., Ruiz M.D., Hernández M., Sirvent J.J., Del Castillo D., Richart C. PNPLA3 expression is related to liver steatosis in morbidly obese women with non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:630. doi: 10.3390/ijms17050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saeed A., Dullaart R.P.F., Schreuder T.C.M.A., Blokzijl H., Faber K.N. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD) Nutrients. 2018;10:29. doi: 10.3390/nu10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C., Chakravarty K., Kong X., Tuy T.T., Arinze I.J., Bone F., Massillon D. Several transcription factors are recruited to the glucose-6-phosphatase gene promoter in response to palmitate in rat hepatocytes and H4IIE cells. J Nutr. 2007;137:554–559. doi: 10.1093/jn/137.3.554. [DOI] [PubMed] [Google Scholar]
- 48.Zhao N., Li X., Feng Y., Han J., Feng Z., Li X., Wen Y. The nuclear orphan receptor Nur77 alleviates palmitate-induced fat accumulation by down-regulating G0S2 in HepG2 cells. Sci Rep. 2018;8:4809. doi: 10.1038/s41598-018-23141-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Piccolis M., Bond L.M., Kampmann M., Pulimeno P., Chitraju C., Jayson C.B.K., Vaites L.P., Boland S., Lai Z.W., Gabriel K.R., Elliott S.D., Paulo J.A., Harper J.W., Weissman J.S., Walther T.C., Farese R.V. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Mol Cell. 2019;74:32–44.e8. doi: 10.1016/j.molcel.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubuquoy C., Robichon C., Lasnier F., Langlois C., Dugail I., Foufelle F., Girard J., Burnol A.-F., Postic C., Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J Hepatol. 2011;55:145–153. doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Yang D., Vuckovic M.G., Smullin C.P., Kim M., Pui-See Lo C., Devericks E., Yoo H.S., Tintcheva M., Deng Y., Napoli J.L. Modest decreases in endogenous all-trans-retinoic acid produced by a mouse Rdh10Heterozygote provoke major abnormalities in adipogenesis and lipid metabolism. Diabetes. 2018;67:662–673. doi: 10.2337/db17-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartuzi P., Wijshake T., Dekker D.C., Fedoseienko A., Kloosterhuis N.J., Youssef S.A., Li H., Shiri-Sverdlov R., Kuivenhoven J.-A., de Bruin A., Burstein E., Hofker M.H., van de Sluis B. A cell-type-specific role for murine Commd1 in liver inflammation. Biochim Biophys Acta. 2014;1842:2257–2265. doi: 10.1016/j.bbadis.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartuzi P., Billadeau D.D., Favier R., Rong S., Dekker D., Fedoseienko A., Fieten H., Wijers M., Levels J.H., Huijkman N., Kloosterhuis N., van der Molen H., Brufau G., Groen A.K., Elliott A.M., Kuivenhoven J.A., Plecko B., Grangl G., McGaughran J., Horton J.D., Burstein E., Hofker M.H., van de Sluis B. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat Commun. 2016;7:10961. doi: 10.1038/ncomms10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shajari S., Laliena A., Heegsma J., Tuñón M.J., Moshage H., Faber K.N. Melatonin suppresses activation of hepatic stellate cells through RORα-mediated inhibition of 5-lipoxygenase. J Pineal Res. 2015;59:391–401. doi: 10.1111/jpi.12271. [DOI] [PubMed] [Google Scholar]
- 55.Shajari S., Saeed A., Smith-Cortinez N.F., Heegsma J., Sydor S., Faber K.N. Hormone-sensitive lipase is a retinyl ester hydrolase in human and rat quiescent hepatic stellate cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1258–1267. doi: 10.1016/j.bbalip.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Woudenberg-Vrenken T.E., Buist-Homan M., Conde de la Rosa L., Faber K.N., Moshage H. Anti-oxidants do not prevent bile acid-induced cell death in rat hepatocytes. Liver Int. 2010;30:1511–1521. doi: 10.1111/j.1478-3231.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 57.Song Y.M., Song S.-O., Jung Y.-K., Kang E.-S., Cha B.S., Lee H.C., Lee B.-W. Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction. Autophagy. 2012;8:1085–1097. doi: 10.4161/auto.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blokzijl H., Vander Borght S., Bok L.I.H., Libbrecht L., Geuken M., van den Heuvel F.A.J., Dijkstra G., Roskams T.A.D., Moshage H., Jansen P.L.M., Faber K.N. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis. 2007;13:710–720. doi: 10.1002/ibd.20088. [DOI] [PubMed] [Google Scholar]
- 59.Pellicoro A., van den Heuvel F.A.J., Geuken M., Moshage H., Jansen P.L.M., Faber K.N. Human and rat bile acid-CoA:amino acid N-acyltransferase are liver-specific peroxisomal enzymes: implications for intracellular bile salt transport. Hepatology. 2007;45:340–348. doi: 10.1002/hep.21528. [DOI] [PubMed] [Google Scholar]
- 60.Fischer A.H., Jacobson K.A., Rose J., Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 61.Mehlem A., Hagberg C.E., Muhl L., Eriksson U., Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 62.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.-C., Torbenson M.S., Unalp-Arida A., Yeh M., McCullough A.J., Sanyal A.J., Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 63.Aparicio-Vergara M., Hommelberg P.P.H., Schreurs M., Gruben N., Stienstra R., Shiri-Sverdlov R., Kloosterhuis N.J., de Bruin A., van de Sluis B., Koonen D.P.Y., Hofker M.H. Tumor necrosis factor receptor 1 gain-of-function mutation aggravates nonalcoholic fatty liver disease but does not cause insulin resistance in a murine model. Hepatology. 2013;57:566–576. doi: 10.1002/hep.26046. [DOI] [PubMed] [Google Scholar]
- 64.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 65.Croce A.C., De Simone U., Vairetti M., Ferrigno A., Boncompagni E., Freitas I., Bottiroli G. Liver autofluorescence properties in animal model under altered nutritional conditions. Photochem Photobiol Sci. 2008;7:1046–1053. doi: 10.1039/b804836c. [DOI] [PubMed] [Google Scholar]