Abstract
Aims:
The objective of this review was to examine the relationship between osteoarthritis (OA) and mobility-related comorbidities, specifically diabetes mellitus (DM) and cardiovascular disease (CVD). It also investigated the relationship between OA and mortality.
Methods:
An overview of meta-analyses was conducted by performing two targeted searches from inception to June 2020. The association between OA and (i) DM or CVD (via PubMed and Embase); and (ii) mortality (via PubMed) was investigated. Meta-analyses were selected if they included studies that examined adults with OA at any site and reported associations between OA and DM, CVD, or mortality. Evidence was synthesized qualitatively.
Results:
Six meta-analyses met inclusion criteria. One meta-analysis of 20 studies demonstrated a statistically significant association between OA and DM, with pooled odds ratio of 1.41 (95% confidence interval: 1.21, 1.65; n = 1,040,175 patients). One meta-analysis of 15 studies demonstrated significantly increased risk of CVD among OA patients, with a pooled risk ratio of 1.24 (1.12, 1.37, n = 358,944 patients). Stratified by type of CVD, OA was shown to be associated with increased heart failure (HF) and ischemic heart disease (IHD) and reduced transient ischemic attack (TIA). There was no association reported for stroke or myocardial infarction (MI). Three meta-analyses did not find a significant association between OA (any site) and all-cause mortality. However, OA was found to be significantly associated with cardiovascular-related death across two meta-analyses.
Conclusion:
The identified meta-analyses reported significantly increased risk of both DM and CVD (particularly, HF and IHD) among OA patients. It was not possible to confirm consistent directional or causal relationships. OA was found to be associated with increased mortality, but mostly in relation to CVD-related mortality, suggesting that further study is warranted in this area.
Keywords: cardiovascular diseases, comorbidity, diabetes mellitus, osteoarthritis, review
Background
Osteoarthritis (OA) is the most common form of arthritis.1 The prevalence of OA in the general adult population by joint site is estimated to be 11%, 24%, and 43% for hip, knee, and hand OA, respectively.2 Among aged individuals in developed nations, OA is reported to be among the 10 most common disabilities.3 Current non-pharmacological management strategies for OA are based around nutrition, physical exercise, and pain management. The first two strategies intersect with treatment programs for cardiovascular disease (CVD) and diabetes mellitus (DM) — both of which are associated with loss of mobility.
Functional limitations exist when capacity for movement, such as walking or climbing stairs, is reduced in both ability and intensity.4 This outcome is prevalent among those who have experienced major CVD, including stroke, ischemic heart disease (IHD), atrial fibrillation, and heart failure (HF).5 Among DM patients, muscle weakness and peripheral neuropathy limit mobility on a daily basis.6 Key health risks associated with DM and CVD, including obesity, hypertension, and depression, are also associated with OA.7,8 Accordingly, OA is linked to increased rates of comorbidity for both DM and CVD.9
One currently unaddressed barrier to effective OA care is weight management, as it is tied to exercise and improved nutrition. However, as many as 30% of older adults report walking difficulties.10 Decreased mobility may consequently shift the burden of treatment to nutritional management, which is routinely challenging for many patients as it requires high self-management. Poor nutrition coupled with lack of exercise are two key factors in the development or progression of DM and CVD.
Currently, there is a lack of clarity on the magnitude of association between OA and the comorbidities of CVD and DM. Symptoms of OA may adversely affect the management of CVD and DM diseases. For example, arthritic pain may significantly limit mobility in OA patients. However, physical activity maintenance is vital to CVD management. While increased negative outcomes are shared across conditions, it is unknown if there is shared causality or association. Within DM and OA populations, the comorbidity of one is directly and significantly related to increased risk in the other, though evidence is conflicting when comparing across types of OA.11 Also poorly understood is the association between OA and mortality. Links between OA and all-cause mortality, and, more specifically, CVD death, are confounded by contrasting evidence.12
Therefore, the aim of this overview was to summarize evidence from systematic reviews and meta-analyses examining and quantifying the relationship between OA and mobility-related comorbidities, specifically DM and CVD, and mortality.
Methods
This was an overview of existing meta-analyses reporting associations between OA and DM or CVD, or OA and mortality, in adults (⩾18 years) with OA at any site.
Eligibility criteria
Eligible studies were any systematic review and meta-analysis examining associations between OA (any location) and DM or CVD. Only studies assessing associations quantitatively via odds ratio (OR), relative risk (RR), or hazard ratio (HR) were included.
Search methods
The Cochrane Handbook for Systematic Reviews of Interventions was used to guide the methods for conducting the overview.13 A Population, Intervention, Comparator, and Outcome (PICO) framework was used to implement the eligibility criteria. Search strategies included strings with Medical Subject Headings (MeSH) and keywords for “osteoarthritis”, “diabetes mellitus”, “cardiovascular disease”, and “mortality” (see Appendix for search strings). Only publications in English were considered. Hand-searching was also performed on the reference lists of identified publications.
Searches were conducted using PubMed and Embase from inception until April 2018. A subsequent search of PubMed from inception until September 2018 was used to identify associations between OA and mortality. We conducted an update to our searches from the time of the previous searches to the present (June 2020) to identify any eligible studies published within the last 2 years. We did not detect any additional meta-analysis that adhered to the eligibility criteria of this overview.
Study selection
Based on the PICO criteria, titles and abstracts were screened for eligibility in duplicate. Articles deemed possibly relevant were advanced to full-text screening and likewise examined in duplicate. Articles meeting inclusion criteria after full-text screening were included in the overview. Any discrepancies during title/abstract and full-text screening were resolved via consensus, with a third reviewer as needed. All screening and file storage were managed in DOC Library© (Doctor Evidence, LLC, Santa Monica, CA, USA).
Data extraction and synthesis
Information about the literature search strategy, population, rate of comorbidity or mortality, and quality of studies included in each meta-analysis was extracted and logged in a table of characteristics. Following this, pooled absolute and relative effects from each meta-analysis were extracted, with priority given to estimates based on multivariate models (OA versus non-OA) controlling for confounding variables. If two or more meta-analyses reported the exact same outcome, the more precise estimate was included in this overview. Pooled estimates from the meta-analyses were summarized and presented as forest plots.
Results
Literature search findings
Our search identified a total of 2874 records. After an initial title and abstract screening, 154 articles were retrieved for full-text screening, where they were assessed for eligibility. Of these, six meta-analyses met the inclusion criteria and were selected for the present overview (Figure 1).
Figure 1.
PRISMA flow diagram.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Of the six included publications, one examined the relationship between OA and diabetes,11 and one examined the relationship between OA and CVD.14 One publication examined the relationship between OA and CVD, as well as between OA and mortality.12 Three publications examined the relationship between OA and mortality.15–17
Description of the meta-analyses included in the overview
All included publications were published in 2015 or later. All but one used a standardized checklist or tool for quality assessment of included studies.15 Across the six publications, a total of 102 individual studies were included. Our own assessment of quality found that overall, included studies were of moderate-to-high quality and generally controlled for confounders (most commonly age and sex). A summary of the characteristics of the final six included meta-analyses are presented in Table 1.
Table 1.
Characteristics of included publications.
| Meta-analysis | Literature search strategy | Population | Rate of comorbidity or mortality | Quality of studies included in each review |
|---|---|---|---|---|
| Studies examining the association between OA and Diabetes | ||||
| Louati et al.11 | MEDLINE, Embase, Cochrane Library, major congresses; updated January 2015 | Any OA |
Diabetes (35 studies)
OA (n = 645, 089): 14.4 ± 0.1% |
STROBE quality score: median = 69% (range 33–91%) |
| Studies examining the association between OA and CVD | ||||
| Wang et al.12 | Included MEDLINE, Embase, Cochrane Library; grey literature; conducted November 2015 | Any OA (diagnosed with predefined criteria) |
CVD (15 studies)
OA (n = 80,911): 36.1% CV deaths (6 studies) OA (n = 5143): 57.9% |
NOS: range: medium to high quality |
| Hall et al.14 | Included MEDLINE, Embase, Cochrane Library, grey literature; conducted November 2014 | Any OAa |
CVD (4 studies)
OA (n = 6165): 38.4% (95% CI: 37.2–39.6%); Control (n = 9874): 9% (95% CI: 8–9%) |
3 of 4 studies satisfied all criteria CASP appraisal criteria |
| Studies examining the association between OA and Mortality | ||||
| OARSI15 | A literature review and expert advice identified cohort studies with appropriate individual patient data. | American & ROW cohorts with: • Symptomatic Knee ROA • Symptomatic Knee OA (no ROA) • Symptomatic hip OA (any ROA status)b |
All-cause mortality (7 studies)
OA (n = 36,884): range = 3.8–57.9% |
N/R |
| Veronese et al.16 | Included PubMed, Embase, CiNAHL; conducted November 2015 | Any OA (diagnosed with clinical and/or radiological assessment) |
All-cause mortality (7 studies)
OA (n = 10,018) Control (n = 18,541) HR between OA and mortality provided in Figure 3. |
NOS: median = 7 (IQR: 6–8) |
| Xing et al.17 | Included MEDLINE, Embase, Cochrane Library; grey literature; conducted November 2015 | Any SOA or ROA | All-cause mortality (10 studies) OA (n = 20,273) HR between OA and mortality provided in Figure 3. |
NOS: All, but 3 rated as high quality |
Diagnosed according to recognized criteria, self-report or self-reported physician-diagnosed OA.
A standardized definition of SROA was used, based on individual patient data.
CVD, cardiovascular disease; N/R, not reported; NOS, Newcastle–Ottawa Scale; OA, osteoarthritis; ROA, radiographic OA; ROW, Rest of world; SOA, symptomatic OA; SROA, symptomatic radiographic OA.
Associations between OA and DM
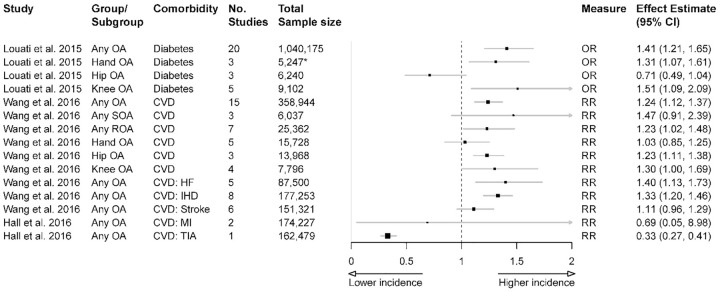
A summary of the estimated effects for OA and DM is presented in Figure 2. For patients with any OA, there was significantly increased risk of DM compared with the non-OA population, with OR of 1.41 [95% confidence interval (CI): 1.21, 1.65].11 There was also significantly increased risk of DM in knee OA (OR 1.51, 95% CI: 1.09, 2.09).11 Furthermore, by adding sample sizes from the three studies included in Louati et al.,11 there was significantly increased risk of DM in hand OA (OR 1.31, 95% CI: 1.07, 1.61).18–20 Finally, there was no significant association found between hip OA and DM.11
Figure 2.
Association between OA and DM or CVD across the included publications (based on separate meta-analyses).
*Calculated by adding sample sizes from the three studies18–20 included in Louati et al.,11 which contributed to the association measure for risk of diabetes in hand OA.
CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; HF, heart failure; IHD, ischaemic heart disease; MI, myocardial infarction; OA, osteoarthritis; TIA, transient ischaemic attack.
Associations between OA and CVD
A summary of the pooled effect estimates from two meta-analyses for association between OA and CVD is presented in Figure 2, plotted across 11 subgroup analyses. For patients with any OA, there was significantly increased risk of CVD, with RR of 1.24 (95% CI: 1.12, 1.37).12 The risk was similar for patients with hip OA (RR 1.23, 95% CI: 1.11, 1.38). For patients with any radiographic OA, the risk of CVD was also significant (RR 1.23, 95% CI: 1.02, 1.48).12 For patients with any symptomatic OA, hand OA, and knee OA, there was no significant risk for general CVD. However, the reported RR trended towards increased risk.
When analyses were stratified by type of CVD, any OA was associated with increased HF (RR 1.4, 95% CI: 1.13, 1.73) and IHD (RR 1.33, 95% CI: 1.2, 1.46).12 Any OA was also associated with reduced transient ischemic attack (TIA; RR 0.33, 95% CI: 0.27, 0.41).14 There was no association reported for stroke or myocardial infarction (MI).12,14
Associations between OA and mortality
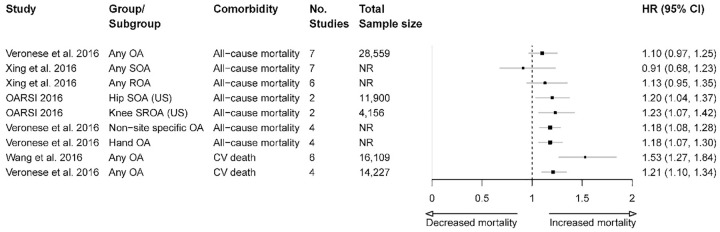
A summary of the estimated effects for OA and all-cause and cause-specific mortality from nine separate meta-analyses (from three systematic reviews) is presented in Figure 3.
Figure 3.
Association between OA and mortality across the included publications (based on separate meta-analyses).
CI, confidence interval; CV, cardiovascular; NR, not reported; OA, osteoarthritis; ROA, radiographic osteoarthritis; SOA, symptomatic osteoarthritis; SROA, symptomatic radiographic osteoarthritis; US, United States.
A significant increase in risk of all-cause mortality was found for those with symptomatic hip OA (HR 1.2, 95% CI: 1.04, 1.37); symptomatic and radiographic knee OA (HR 1.23, 95% CI: 1.07, 1.42);15 non-site specific OA (HR 1.18, 95% CI: 1.08, 1.28); and hand OA (HR 1.18, 95% CI: 1.07, 1.3).16 In contrast, three other meta-analyses found no significant relationship between all-cause mortality and any OA,16 any symptomatic OA,17 or any radiographic OA.17
When cause-specific mortality was examined, one meta-analysis (six unique studies, with ~16,000 patients) reported that people with OA are at increased risk of CVD-related death compared with those without OA (RR 1.53, 95% CI: 1.27, 1.84).12 Another meta-analysis (four unique studies, with ~14,000 patients) reported a similar increase in risk of CVD-related death in the OA patient population (HR 1.21, 95% CI: 1.1, 1.34).16
Discussion
The relationship between OA and mobility-related comorbidities such as DM and CVD, as well as links to mortality, have been the subject of much research. Our overview sought to examine these associations in further detail by gathering high-quality evidence from existing meta-analyses.
Findings from our overview provide additional support for a significant association between OA and DM. One meta-analysis (comprised of >1,000,000 participants in total) demonstrated increased risk of DM in those living with any type of OA.11 Intriguingly, the relationship between OA and DM was found to be a two-way association, as patients with DM were at higher risk of developing OA when compared with a non-DM population (OR 1.46, 95% CI: 1.08, 1.96).11 This association may be due to shared risk factors common to both conditions (e.g., age and obesity).11
When stratified according to joint site, findings for OA and DM were mixed. Hand OA and knee OA were significantly linked to DM, while no association was found between hip OA and DM. This observation may be explained in part by an effect of obesity: in a 10-year cohort study of 1675 participants, a high body mass index (BMI >30 kg/m2) was significantly associated with hand OA (OR 2.59, 95% CI: 1.08, 6.19) and knee OA (OR 2.81, 95% CI: 1.32, 5.96), but not with hip OA (OR 1.11, 95% CI: 0.41, 2.97).21 However, we caution against making conclusive remarks regarding obesity’s role as a confounder, as the studies included in the meta-analysis were not without their limitations. Many of the datasets were based on medical or prescription records. Therefore, there was a lack of control for other confounding variables (e.g., individual participant data). Moreover, the inclusion of cross-sectional studies combined with a lack of longitudinal studies were contributing factors to the mixed findings.
Findings from our overview also revealed that OA is associated with a higher risk of CVD (particularly, HF and IHD). Similar to findings for OA and DM, conclusive directional and causal claims could not be made due to the observational nature of the study designs. No association was reported for stroke or MI, and interestingly, a protective effect for TIA was reported. Authors attributed these null and protective effects to survival bias: people at greater CVD risk may die prematurely, consequently leaving the OA participant group comprised of mostly healthier individuals with better CVD risk profiles.14 Another limitation of the included meta-analyses was the high statistical heterogeneity observed (I2 > 85% across all pooled analyses).14
The mechanisms underlying the relationship between OA and CVD are still relatively unclear. While the lack of appropriate research design is an important contributor to the knowledge gap, there are also distinct disease-management factors in OA and CVD contributing to the complexity of this association. For example, commonly used medications for OA [e.g., nonsteroidal anti-inflammatory drugs (NSAIDs)] can increase CVD risk, and the commonly reduced physical activity levels of OA patients can be detrimental for CVD management.
Other mediators must also be considered, including low-grade systemic inflammation observed across all three conditions. In DM for example, hyperglycemia can promote joint inflammation, cartilage degradation,22 and atherosclerosis,23 which is also a key component of CVD. The clinical implications of these shared risk factors are critical to outcomes. Thus, treatment options for OA patients with adverse risk profiles for CVD or DM need to be considered in OA management. Physical activity needs further promotion in clinical practice, as exercise impacts OA as well as CVD and DM disease progression.
It was challenging to pool evidence on OA and mortality rates. Many mediators and confounders, such as systemic inflammation and frailty, influence this relationship. Overall, there was insufficient evidence from the meta-analyses to suggest a direct relationship between general OA and all-cause mortality. However, certain specific OA joint sites were associated with greater all-cause mortality (hip, knee, and hand).9,16 The strongest evidence was for OA and an increased risk of CVD mortality. This finding fits in with our previous CVD findings where OA populations had a higher risk of CVD. As well, the increased inflammation and low physical activity levels associated with the OA population all contribute to a context in which there is increased risk of CVD mortality. More research is warranted to better understand the relationship between OA and CVD-related mortality.
Limitations
Findings from the present overview come from observational evidence. Therefore, it is not possible to claim causality for any relationship reported. Among the included meta-analyses, longitudinal studies were lacking, and these studies are better suited for observing relationships between chronic conditions such as OA and comorbidities. Certain populations may have been underrepresented, as it was not possible to stratify meta-analysis results according to certain characteristics (e.g., sex). This is pertinent as females are at higher risk of OA.
Furthermore, analysis of site-specific OA was based on a limited number of studies, many of which were contrasting (e.g., some evidence suggests lower limb OA can increase risk of mortality, whereas hand OA appears to decrease risk). Another limitation is that other comorbidities may be associated with OA, whereas this review considered only the mobility-related comorbidities DM and CVD. Importantly, the present overview was not designed to capture elements governing the association between OA and related comorbidities. The underlying etiologies are likely complex, involving contributions from genetic, environmental, and treatment-related factors. Though beyond the scope of this overview, characterization of these determinants would help establish the direction of causality as well as potential preventative measures. Finally, it was often not possible to control for confounding factors such as age and obesity, both of which have clinical implications for OA, DM, and CVD.
Conclusion
Overall, the meta-analyses identified reported significant associations between OA and the mobility-related comorbidities of DM and CVD. OA populations were found to have a higher risk of both DM and CVD (particularly, HF and IHD). It was not possible to confirm consistent directional or causal relationships. OA was found to be associated with increased mortality, but mostly in relation to CVD mortality, suggesting that further study is warranted in this area.
Acknowledgments
Medical writing was provided by Mir-Masoud Pourrahmat from Evidinno Outcomes Research Inc., contracted by Doctor Evidence LLC, which was funded by Sanofi.
Appendix
Appendix Table 1.
Search strategy for Embase (DM and CVD).
| # | String |
|---|---|
| 1 | exp *cardiovascular disease/or (Cardiac or cardiovascular or heart or coronary or arter$ or STEMI$ or NSTEMI$ or ischaem$ or ischem$ or emboli$ or MACE or stroke$ or cerebral or brain or infarct$ or hemorrhag$ or haemorrhag$ or emboli$ or cerebrovascular or thromboemboli$ or thromb$ or vascular or peripheral arterial disease or atheroscleros$ or bypass or CABG or stent or stents).ti,ab. or (CAD or CHF or CHD or myocardial or MI or CVA or CVAs or CVE or CVEs or PAD or PCI).ti. |
| 2 | exp *insulin dependent diabetes mellitus/or exp *non insulin dependent diabetes mellitus/or (diabetes or diabetic or NIDDM or IDDM or T2DM or T1DM or T2D or T1D).ti,ab,kw. or DM.ti. |
| 3 | (gestational or insipidus).ti. |
| 4 | 2 not 3 |
| 5 | 1 and 4 |
| 6 | exp *osteoarthritis/or (osteoarthriti$ or osteo-arthriti$ or osteoarthro$ or osteo-arthro$ or degenerative arthr$ or degenerative joint or OA or gonarthriti$ or gonarthro$).ti,ab. |
| 7 | 5 and 6 |
| 8 | 7 not ((exp animal/or nonhuman/) not exp human/) |
| 9 | Clinical study/or clinical article/ |
| 10 | Case control study/ |
| 11 | Family study/ |
| 12 | Longitudinal study/ |
| 13 | Retrospective study/ |
| 14 | Prospective study/ |
| 15 | Randomized controlled trials/or randomized controlled trial/ |
| 16 | 14 not 15 |
| 17 | Cohort analysis/ |
| 18 | (Cohort adj (study or studies)).mp. |
| 19 | (Case control adj (study or studies)).tw. |
| 20 | (follow up adj (study or studies)).tw. |
| 21 | (observational adj (study or studies)).tw. |
| 22 | (epidemiologic$ adj (study or studies)).tw. |
| 23 | (cross sectional adj (study or studies)).tw. |
| 24 | 9 or 10 or 11 or 12 or 13 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 |
| 25 | 8 and 24 |
| 26 | limit 25 to (article or article in press or conference paper) |
| 27 | limit 26 to (yr=“2016–2018” and (conference abstract or “conference review”)) |
| 28 | 26 or 27 |
| 29 | remove duplicates from 28 |
CVD, cardiovascular disease; DM, diabetes mellitus.
Appendix Table 2.
Search strategy for MEDLINE (DM and CVD).
| # | String |
|---|---|
| 1 | Cardiovascular Diseases[Mesh] OR (Cardiac [tiab] OR cardiovascular [tiab] OR heart [tiab] OR coronary [tiab] OR arter* [tiab] OR CAD [ti] OR CHF [ti] OR CHD [ti] OR myocardial [tiab] OR MI [ti] OR STEMI* [tiab] OR NSTEMI* [tiab] OR ischaem* [tiab] OR ischem* [tiab] OR emboli* OR MACE [tiab] OR stroke* OR cerebral [tiab] or brain [tiab] OR infarct* [tiab] OR hemorrhag* [tiab] OR haemorrhag* [tiab] OR emboli* [tiab] OR cerebrovascular [tiab] OR CVA [ti] OR CVAs [ti] OR CVE [ti] OR CVEs [ti] OR thromboemboli* [tiab] OR thromb* [tiab] OR vascular [tiab] OR peripheral arterial disease [tiab] OR PAD [ti] OR atheroscleros* [tiab] OR PAD [ti] OR PCI [ti] OR bypass [tiab] OR CABG [tiab] OR stent [tiab] OR stents [tiab]) |
| 2 | (“Diabetes Mellitus, Type 2”[Mesh] OR “Diabetes Mellitus, Type 1”[Mesh] OR diabetes [tiab] OR diabetic [tiab] OR DM [ti] OR NIDDM [tiab] OR IDDM [tiab] OR T2DM [tiab] OR T1DM [tiab]) NOT (gestational [ti] OR insipidus [ti]) |
| 3 | 1 OR 2 |
| 4 | (“Osteoarthritis”[majr] OR osteoarthriti*[tiab] OR osteo-arthritis[tiab] OR degenerative arthritis[tiab] OR degenerative joint[tiab] OR OA[tiab]) |
| 5 | 3 AND 4 |
| 6 | 5 NOT ((animals[mh] NOT humans[mh])) |
| 7 | 6 AND systematic[sb] |
CVD, cardiovascular disease; DM, diabetes mellitus.
Appendix Table 3.
Search strategy for Embase (mortality).
| # | String |
|---|---|
| 1 | exp *morbidity/or (death or deaths or die or died or fatal$).ti. or (morbid$ or mortalit$).ti,ab. |
| 2 | exp *osteoarthritis/or (osteoarthriti$ or osteo-arthriti$ or osteoarthro$ or osteo-arthro$ or degenerative arthr$ or degenerative joint or OA or gonarthriti$ or gonarthro$).ti,ab. |
| 3 | 1 and 2 |
| 4 | 3 not ((exp animal/or nonhuman/) not exp human/) |
| 5 | Clinical study/or clinical article/ |
| 6 | Case control study/ |
| 7 | Family study/ |
| 8 | Longitudinal study/ |
| 9 | Retrospective study/ |
| 10 | Prospective study/ |
| 11 | Randomized controlled trials/or randomized controlled trial/ |
| 12 | 10 not 11 |
| 13 | Cohort analysis/ |
| 14 | (Cohort adj (study or studies)).mp. |
| 15 | (Case control adj (study or studies)).tw. |
| 16 | (follow up adj (study or studies)).tw. |
| 17 | (observational adj (study or studies)).tw. |
| 18 | (epidemiologic$ adj (study or studies)).tw. |
| 19 | (cross sectional adj (study or studies)).tw. |
| 20 | 5 or 6 or 7 or 8 or 9 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 |
| 21 | 4 and 20 |
| 22 | limit 21 to (article or article in press or conference paper) |
| 23 | limit 22 to (yr=“2016–2018” and (conference abstract or “conference review”)) |
| 24 | 22 or 23 |
| 25 | remove duplicates from 24 |
Appendix Table 4.
Search strategy for MEDLINE (mortality).
| # | String |
|---|---|
| 1 | “Morbidity”[Mesh] OR “Mortality”[Mesh] OR death[ti] OR deaths[ti] OR die[ti] OR died[ti] OR fatal*[ti] OR morbid*[tiab] OR mortalit*[tiab] |
| 2 | (“Osteoarthritis”[majr] OR osteoarthriti*[tiab] OR osteo-arthritis[tiab] OR degenerative arthritis[tiab] OR degenerative joint[tiab] OR OA[tiab]) |
| 3 | 1 AND 2 |
| 4 | 3 NOT ((animals[mh] NOT humans[mh])) |
| 5 | 4 AND systematic[sb] |
Footnotes
Conflict of interest statement: Gustavo Campos reports speaker/honoraria and consulting for Sanofi. Raman Mundi reports personal fees received for participation in advisory board meetings from Sanofi, Pendopharm, and KCI. Craig Whittington is currently employed by Sanofi, and is a former employee of Doctor Evidence, LLC, who were contracted by Sanofi to conduct this study. Marie-Josée Toutounji and Wilson Ngai are currently employed by Sanofi. Brendan Sheehan reports speaker/honoraria and consulting for Sanofi. All authors met the ICMJE authorship criteria.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Sanofi.
ORCID iD: Craig Whittington  https://orcid.org/0000-0002-1950-0334
https://orcid.org/0000-0002-1950-0334
Contributor Information
Gustavo Constantino de Campos, Department of Orthopedic Surgery, Faculty of Medical Sciences, Universidade Estadual de Campinas (UNICAMP), PO Box 6111, Campinas, São Paulo 13087-000, Brazil.
Raman Mundi, Sunnybrook Health Sciences Centre, ON, Canada.
Craig Whittington, Doctor Evidence, Santa Monica, CA, USA; Sanofi, Global Medical, Bridgewater, NJ, USA.
Marie-Josée Toutounji, Sanofi, Global Medical, Bridgewater, NJ, USA.
Wilson Ngai, Sanofi, Global Medical, Bridgewater, NJ, USA.
Brendan Sheehan, Department of Medicine, Dalhousie University, NS, Canada.
References
- 1. Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013; 72: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 2. Pereira D, Peleteiro B, Araújo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011; 19: 1270–1285. [DOI] [PubMed] [Google Scholar]
- 3. Palmer KT, Goodson N. Ageing, musculoskeletal health and work. Best Pract Res Clin Rheumatol 2015; 29: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grimmer M, Riener R, Walsh CJ, et al. Mobility related physical and functional losses due to aging and disease - a motivation for lower limb exoskeletons. J Neuroeng Rehabil 2019; 16: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phan HM, Alpert JS, Fain M. Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol 2008; 17: 101–107. [PubMed] [Google Scholar]
- 6. Hillson R. Muscles in diabetes. Pract Diabetes 2017; 34: 42–43. [Google Scholar]
- 7. Nüesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 2011; 342: d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hawker GA, Croxford R, Bierman AS, et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One 2014; 9: e91286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R 2012; 4(Suppl. 5): S10–S19. [DOI] [PubMed] [Google Scholar]
- 10. Shumway-Cook A, Ciol MA, Yorkston KM, et al. Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc 2005; 53: 1217–1221. [DOI] [PubMed] [Google Scholar]
- 11. Louati K, Vidal C, Berenbaum F, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015; 1: e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Bai J, He B, et al. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep 2016; 6: 39672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration, 2011. [Google Scholar]
- 14. Hall AJ, Stubbs B, Mamas MA, et al. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur J Prev Cardiol 2016; 23: 938–946. [DOI] [PubMed] [Google Scholar]
- 15. March L, Cross M, Lo C, et al. Osteoarthritis: a serious disease. U.S. Food and Drug Administration, https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf (2016, accessed November 2020).
- 16. Veronese N, Cereda E, Maggi S, et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum 2016; 46: 160–167. [DOI] [PubMed] [Google Scholar]
- 17. Xing D, Xu Y, Liu Q, et al. Osteoarthritis and all-cause mortality in worldwide populations: grading the evidence from a meta-analysis. Sci Rep 2016; 6: 24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahaghin S, Bierma-Zeinstra SMA, Koes BW, et al. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam study. Ann Rheum Dis 2007; 66: 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orellana C, Navarro N, Calvet J, et al. FRI0305 Higher frequency of metabolic syndrome in patients with hand osteoarthritis is more pronounced in OBESE patients. Ann Rheum Dis 2013; 71(Suppl. 3): 417. [Google Scholar]
- 20. Haugen IK, Ramachandran VS, Misra D, et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis 2015; 74: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 2008; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis 2011; 70: 1354–1356. [DOI] [PubMed] [Google Scholar]
- 23. Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol 2002; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]