Abstract
Objective:
Few studies have investigated outcomes after truncal endovenous ablation in patients with combined deep and superficial reflux and no studies have evaluated patient-reported outcomes.
Methods:
We investigated the short- and long-term clinical and patient-reported outcomes among patients with and without deep venous reflux undergoing truncal endovenous ablation from 2015 to 2019 in the Vascular Quality Initiative. Preprocedural and postprocedural comparisons were performed using the t-test, χ2, or their nonparametric counterpart when appropriate. Multivariable logistic regression models were used to assess for confounding.
Results:
A total of 4881 patients were included, of which 2254 (46.2%) had combined deep and superficial reflux. The median follow-up was 336.5 days. Patients with deep reflux were less likely to be female (65.9% vs 69.9%; P = .003), more likely to be Caucasian (90.2% vs 86.5%; P = .003) and had no difference in BMI (30.6 ± 7.5 vs 30.6 6 7.2; P = .904). Additionally, no difference was seen in rates of prior varicose vein treatments, number of pregnancies, or history of deep venous thrombosis; however, patients without deep reflux were more likely to be on anticoagulation at the time of the procedure (10.9% vs 8.1%; P < .001). Patients without deep reflux had slightly higher median preprocedural Venous Clinical Severity Score (VCSS) scores (8 [interquartile range (IQR), 6–10]) vs 7 [IQR, 6–10]; P = .005) as well as postprocedural VCSS scores (5 [IQR, 3–7] vs 4 [IQR, 2–6]; P < .001). The median change in VCSS from before to after the procedure was lower for patients without deep reflux (3 [IQR, 1.0–5.5] vs 3.5 [IQR, 1–6]; P = .006). Total symptom score was higher for patients without deep reflux both before (median, 14 [IQR, 10–19] vs median, 13.5 [IQR, 9.5–18]; P = .005) and postprocedurally (median, 4 [IQR, 1–9] vs median, 3.25 [IQR, 1–7]; P < .001), but no difference was seen in change in symptom score (median, 8 [IQR, 4–13] vs median, 9 [IQR, 4–13]; P = .172). Patients with deep reflux had substantially higher rates of complications (10.4% vs 3.0%; P < .001), with a particular increase in proximal thrombus extension (3.1% vs 1.1%; P < .001). After controlling for confounding, this estimate of effect size for any complication increased (odds ratio, 5.72; 95% confidence interval, 2.21–14.81; P < .001).
Conclusions:
No significant difference is seen in total symptom improvement when patients undergo truncal endovenous ablation with concomitant deep venous reflux, although a greater improvement was seen in VCSS score in these patients. Patients with deep venous reflux had a significantly increased rate of complications, independent of confounding variables, and should be counseled appropriately before the decision for treatment.
Keywords: Chronic venous insufficiency, Deep venous reflux, Endovenous ablation
Disease of the superficial venous system of the lower extremities is common, with current estimates of prevalence as high as 30%.1 Superficial venous insufficiency has multiple important long-term health effects, including venous stasis ulceration, which effects up to 1% of the population, as well as pain, skin changes, and swelling, which can have important effects on patient’s quality of life.2 In addition, lower extremity superficial venous insufficiency represents an important economic entity, leading to direct medical expenditures on the order of $1 billion annually in the United States alone.3,4
Concomitant reflux of the deep and superficial venous system is common in this population, with recent estimates as high as 48% in a subgroup of patients with venous ulceration.5 Prior studies have suggested that superficial venous reflux may lead to deep venous reflux and that patients with deep venous reflux tend to have worse superficial venous disease.6–8 Additionally, ablation of the superficial venous system has been shown to improve reflux in the deep venous system.6,9 However, the effect of this combination of venous incompetence on patient symptoms, their response to endovenous truncal ablation, as well as postoperative complications has not been well-studied, with prior work being limited to small patient series and short-term assessment of outcomes.10
Our hypothesis was that patients undergoing superficial endovenous ablation of the truncal veins in the Vascular Quality Initiative (VQI) Varicose Vein Registry (VVR) who had concomitant deep vein reflux would have worse patient reported outcomes compared with those with isolated superficial venous reflux. Additionally, we hypothesized that patients with concomitant deep and superficial venous reflux would suffer from complications at higher rates compared to those undergoing truncal endovenous ablation for isolated superficial venous reflux.
METHODS
Study design.
The VQI consists of 12 major vascular procedural registries, one of which is the VVR, which is prospectively collected and was retrospectively reviewed for this study.11–13 The VQI VVR tracks data on superficial venous procedures. Inclusion criteria include patients who underwent procedures to ablate truncal veins using radiofrequency or laser in the lower extremity between 2015 and 2019. Patients who underwent procedures from 2014 were excluded because these data were entered into the registry retrospectively. Patients who underwent concomitant sclerotherapy or phlebectomy or who underwent treatment of nontruncal veins were also excluded from this analysis to minimize confounding. However, as a sensitivity analysis, we repeated the analysis without excluding patients who underwent concurrent sclerotherapy and/or phlebectomy. These results are shown in the Supplemental Figures. Cases are entered, and follow-up includes one early follow-up (0–3 months) and one late follow-up (>3 months after the procedure). At initial reporting, Clinical, Etiologic, Anatomic, and Physiologic (CEAP) class and Venous Clinical Severity Score (VCSS) are recorded for both legs.14,15 Follow-up reporting includes the treated leg’s CEAP class and VCSS along with outcomes. Data regarding the occurrence of specific postoperative complications are included in the VQI VVR data entry process. The presence of deep venous reflux is collected and separately recorded for each leg. Deep reflux for each leg in the VVR is defined as reflux for more than 1.0 seconds in the deep veins of that lower extremity. Superficial venous reflux is defined in the VVR as more than 0.5 seconds.
Patients also complete a quality-of-life survey before the procedure and then at follow-up. This survey defines seven different parameters, including leg heaviness, achiness, swelling, throbbing, itching, appearance, and work impact, and asks the participant to grade each parameter on an ordinal scale (from 0 [none of the time] to 5 [all of the time]).
Each patient-reported outcome was rated on a scale of 0 to 4 or 5, depending on the specific outcome. For heaviness, achiness, swelling, throbbing, and itching, the scale was as follows: 0, none of the time; 1, a little of the time; 2, some of the time; 3, a good bit of the time; 4, most of the time; and 5, all of the time. For appearance, the scale was as follows: 0, not at all noticeable; 1, slightly noticeable; 2, moderately noticeable; 3, very noticeable; and 4, extremely noticeable. For impact on work/activity, the scale was as follows: 0, none; 1, symptoms but full work/activity; 2, mildly reduced work/activity; 3, moderately reduced work/activity; 4, severely reduced work/activity; and 5, unable to do work/activity. The total possible scores range from 0 to 34.
This patient-reported outcomes assessment instrument resembles the HASTI instrument, which has been validated previously, with the addition of the impact on work/activity questions.16 Prior studies detail the usefulness of this instrument in the assessment of disease-specific quality of life for patients with chronic venous insufficiency.11–13
Patient demographic, diagnostic, preoperative, intraoperative, and postoperative data were collected prospectively by trained support staff at each center. Patients were included who underwent procedures from 2015 to 2019. There are webinars to train data managers, online support staff to field questions about all the variables, and strong data definitions. Entry of consecutive procedures by each participating center is ensured by annual audit against hospital claims data submitted by each center. VQI data forms contain error tracking software to prevent erroneous entry. Data that are entered are periodically checked for statistical aberration. Data that seem to be in error are then audited within centers to ensure accuracy and completeness. The University of Michigan Institutional Review Board waived the need for review, and informed consent of the patient was waived for this exempt analysis.
Statistical analysis.
Patient demographics as well as procedural and clinical characteristics are described using summary statistics. Categorical variables are presented as frequency counts with percentages and continuous variables are presented as mean ± standard deviation or median with interquartile range. Student’s t-tests and χ2 tests, or their nonparametric counterparts when the underlying distribution was non-normal as determined by Q-Q plot assessment, were used to compare continuous and categorical variables, respectively. For the multivariable models, covariates significant at a P value of less than .30 in the univariate analysis were included in a multivariable logistic regression model. Statistical significance was established at an alpha level of 0.05 throughout with Bonferroni adjusted P values reported for the univariate tests to adjust for multiple comparisons. Stata version 16 (StataCorp LP, College Station, Tex) was used for all analyses.
RESULTS
Characteristics of patients undergoing endovenous ablation.
A total of 4881 patients were included in the analysis, of which 2254 (46.2%) were identified as having concomitant deep venous reflux. Follow-up was longer in the group without deep reflux, with a median of 319 days, compared with the group with deep reflux, with a median of 182 days (P = .018) Patients with deep reflux were less likely to be female (65.9% vs 69.9%; P = .003), more likely to be white (90.2% vs 86.5%; P < .001), and less likely to be receiving anticoagulation (8.1% vs 10.9%; P < .001). Additionally, patients with deep reflux were less likely to routinely use compression therapy (P < .001) (Table I) and had a flatter distribution of CEAP classification (P < .001) (Fig 1). No significant differences were seen among patients included with respect to age, body mass index, history of varicose vein treatment, history of deep venous thrombosis, or number of prior pregnancies. Similar results were found during a sensitivity analysis including patients who also underwent sclerotherapy and/or (Supplementary Table I, online only).
Table I.
Patient and procedure characteristics for patients undergoing superficial venous ablation with and without deep venous reflux
| Characteristics | No deep reflux (n = 2627) | Deep reflux (n = 2254) | P value |
|---|---|---|---|
| Patient characteristic | |||
| Age, years | 56.9 (13.9) | 56.2 (13.6) | .650 |
| Female sex | 1835 (69.9) | 1486 (65.9) | .030 |
| Race (white) | 1984 (86.5) | 1544 (90.2) | <.01 |
| Length of follow-up, days | 319 (41–864) | 182 (96–631) | .018 |
| BMI | 30.6 (7.5) | 30.6 (7.2) | 1.000 |
| Prior VV treatment | 552 (21.1) | 477(21.2) | 1.000 |
| History of DVT | 161 (6.2) | 154 (6.8) | 1.000 |
| Receiving anticoagulation | 285 (10.9) | 183 (8.1) | .010 |
| No. of pregnancies, among females | 2(1–3) | 2 (1–3) | 1.000 |
| Compression compliance | <.01 | ||
| Never | 439 (21.6) | 487 (23.6) | |
| Intermittent | 330 (16.3) | 433 (21.01) | |
| Most days | 379 (18.7) | 372 (18.0) | |
| Every day | 881 (43.4) | 770 (37.3) |
BMI, Body mass index; DVT, deep venous thrombosis; VV, varicose vein.
Values are mean ± standard deviation, median (interquartile range), or number (%).
Fig 1.
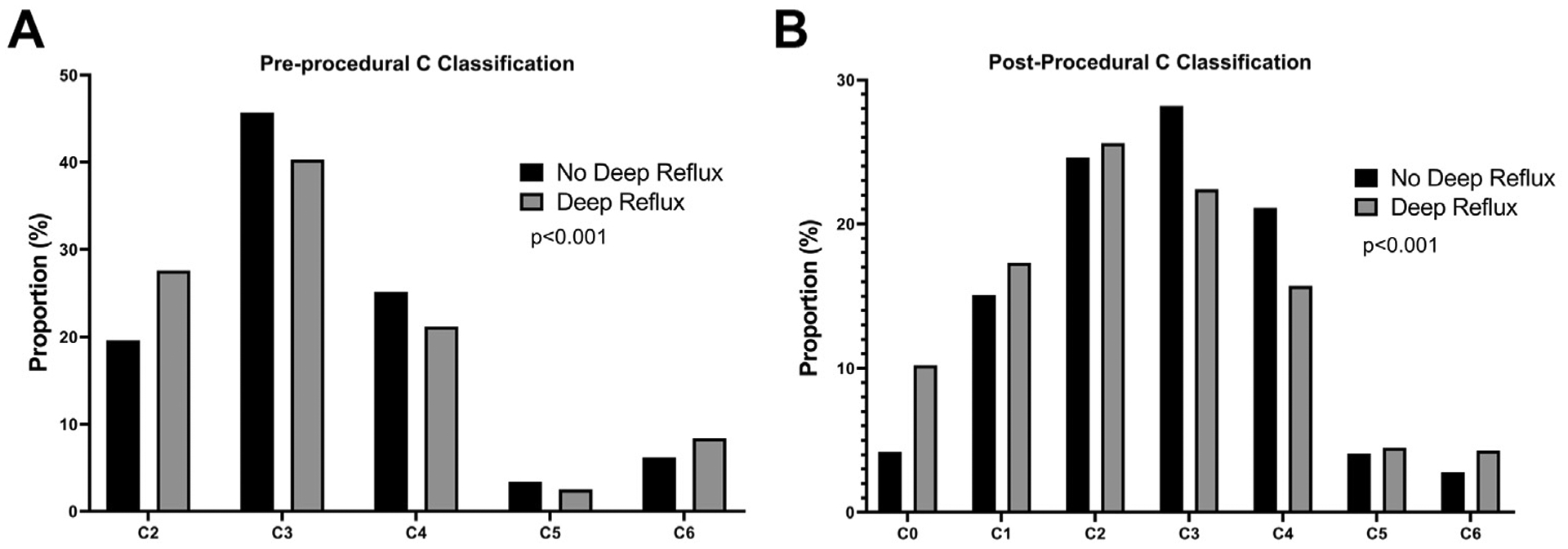
Comparison of C classification preprocedurally and postprocedurally among patients with and without deep reflux. A, Preprocedural C classification shown as proportion of the entire cohort as a percentage. B, Postprocedural C classification shown as proportion of the entire cohort as a percentage. P values of less than .05 are noted with an asterisk.
Procedural and patient-reported outcomes.
The comparison of distribution of CEAP postprocedurally followed a similar pattern to the preprocedural distribution, whereby patients with deep venous reflux were more likely to be at the extremes of the classification (shown specifically in Fig 1; P < .001). The inclusion of patients who underwent sclerotherapy and/or phlebectomy increased the relative proportion of patients in the C2 and C3 categories seen in Supplementary Fig 2 (online only). Preprocedural VCSS was lower among patients with concomitant deep venous reflux (median, 7 [IQR, 6–10] vs median, 8 [IQR, 6–10]; P = .005) as was the postprocedural VCSS (median, 4 [IQR, 2–6] vs median, 5 [IQR, 3–7]; P < .001). This was accompanied by a significantly greater improvement in VCSS score among patients with deep venous reflux (median, 3.5 [IQR, 1–6] vs median, 3 [IQR, 1.0–5.5]; P = .006).
The total symptom score was significantly lower among patients with deep venous reflux (median, 13.5; IQR, 9.5–18) compared with patients without deep venous reflux (median, 14; IQR, 10–19; P = .005). Significant improvement was seen for both groups after treatment, with postprocedural total symptom scores significantly lower in the deep reflux group compared with the group without deep venous reflux (median, 3.25 [IQR, 1–7] vs median, 4 [IQR, 1–9]; P < .001). When investigating improvement in total symptoms, no difference was seen between groups (median, 9 [IQR, 4–13] for patients with deep venous reflux vs median, 8 [IQR, 4–13] for patients without deep venous reflux; P = .172). These data are shown graphically in Fig 2.
Fig 2.
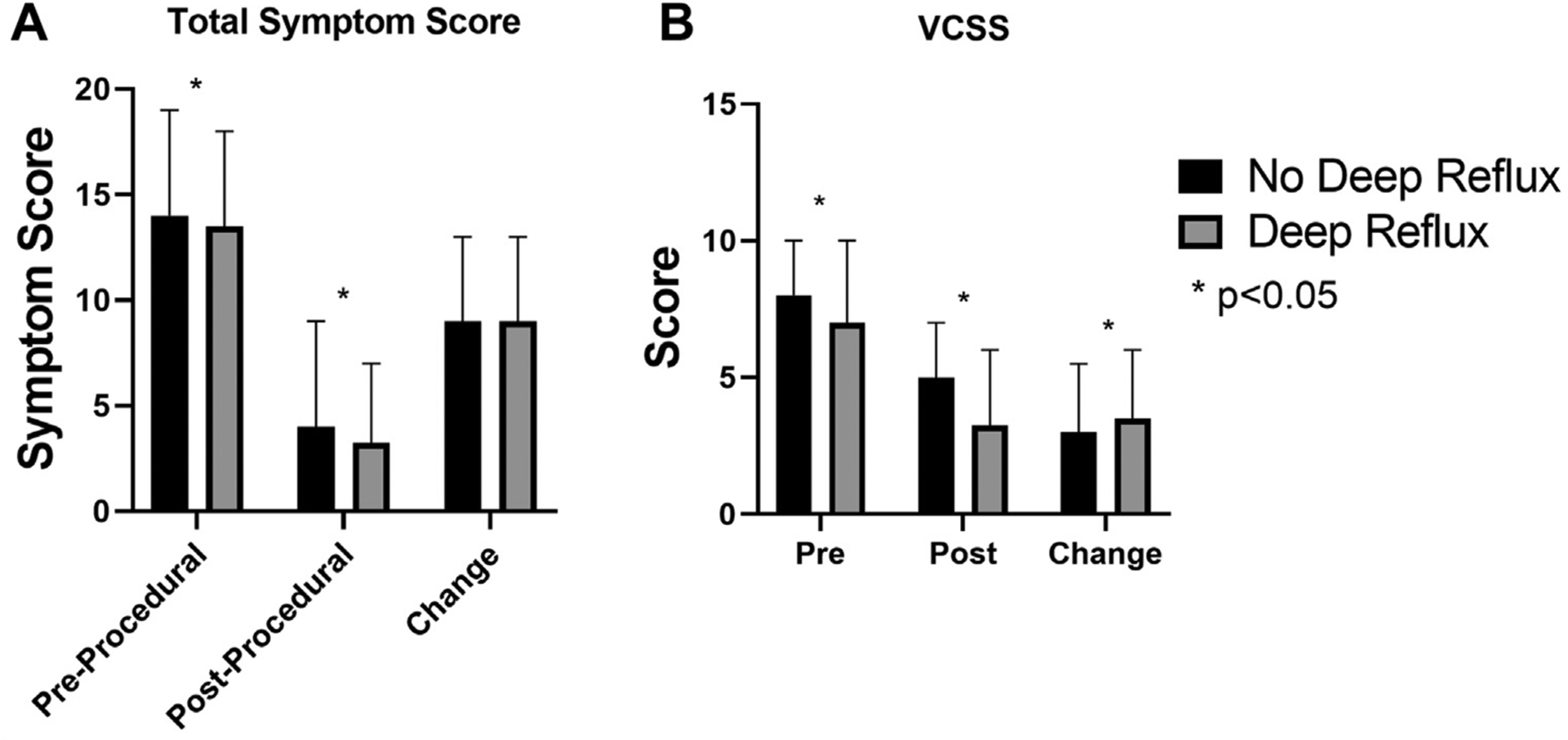
Comparison of total symptom score as well as Venous Clinical Severity Score (VCSS) among patients with and without deep reflux. A, Total symptom score and (B) VCSS in the preprocedural and postprocedural periods as well as the difference of the two, displayed here as change. P values of less than .05 are noted with an asterisk.
A multivariable logistic regression model was built to investigate potential for confounding of the relationship between deep reflux and improvement in VCSS. Table II includes a summary of the results from this model. Patients who underwent procedures in 2019 were significantly less likely to report improvement in VCSS (odds ratio [OR], 0.21; 95% confidence interval [CI], 0.068–0.28; P < .001), as were patients with more severe disease by CEAP classification (OR, 0.51; 95% CI, 1.29–80.69, for patients with C6 disease compared with the reference category C2 disease; P < .001). Supplementary Fig 1 (online only) shows the predicted probabilities for any complication stratified by presence or absence of deep reflux across the different C classifications. As is shown, patients with deep reflux had a significantly higher predicted probability of complication across all levels of C classification. Interestingly, the odds of improvement in VCSS were less likely for increasing compliance with compression therapy (intermittent OR, 0.29 [95% CI, 0.15–0.59; P = .001]; most days OR, 0.09 [95% CI, 0.04–0.18; P < .001]; and every day OR, 0.05 [95% CI, 0.03–0.10; P < .001]). After controlling for these confounding variables, the presence of concomitant deep venous reflux was not associated with improvement in VCSS (OR, 1.09; 95% CI, 0.79–1.49; P = .602). Supplementary Table II (online only) shows the results of the multivariable analysis including patients who underwent phlebectomy and/or sclerotherapy with ablation. The results were similar to the previous analysis.
Table II.
Multivariable analysis for predictors of improvement in Venous Clinical Severity Score (VCSS) among patients with and without deep reflux
| Characteristics | OR | 95% Cl | P value |
|---|---|---|---|
| Deep vein reflux | 1.09 | 0.79–1.49 | .602 |
| Year of surgery | |||
| 2016 | 0.68 | 0.47–1.16 | .121 |
| 2017 | 0.97 | 0.57–1.39 | .899 |
| 2018 | 0.67 | 0.38–0.95 | .099 |
| 2019 | 0.21 | 0.068–0.28 | <.001 |
| Age (per year) | 0.99 | 0.99–1.01 | .310 |
| BMI (per point) | 0.97 | 0.96–1.00 | .010 |
| No. of pregnancies | |||
| 0 | REF | REF | REF |
| 1 | 1.40 | 0.68–1.93 | .257 |
| 2 | 1.12 | 0.64–1.53 | .641 |
| 3 | 0.97 | 0.48–1.22 | .903 |
| 4 | 0.71 | 0.32–1.01 | .284 |
| 5 | 0.77 | 0.36–1.75 | 54 |
| 6 | 2.22 | 0.60–3.56 | .112 |
| Non-white race | 1.32 | 0.59–1.45 | .256 |
| Prior varicose vein procedure | 0.71 | 0.49–1.01 | .058 |
| History of DVT | 1.12 | 0.55–2.31 | .754 |
| Preoperative VCSS (per point) | 1.83 | 1.44–1.70 | <.001 |
| Preoperative CEAP | |||
| 2 | REF | REF | |
| 3 | 0.6 | 3.42–41.24 | .012 |
| 4 | 0.26 | 2.22–23.08 | <.001 |
| 5 | 0.75 | 1.34–13.77 | .789 |
| 6 | 0.51 | 1.29–80.69 | <.001 |
| Compression compliance | |||
| Never | REF | REF | |
| Intermittent | 0.29 | 0.15–0.59 | .001 |
| Most days | 0.09 | 0.04–0.18 | <.001 |
| Every day | 0.05 | 0.03–0.10 | <.001 |
BMI, Body mass index; CI, confidence interval; CEAP, Clinical, Etiologic, Anatomic, and Physiologic; DVT, deep venous thrombosis; OR, odds ratio.
Systemic and procedure-specific complications.
Major complications such as allergic reactions, pulmonary embolism, systemic infections, cerebrovascular accidents, migraines, visual disturbances, couch/chest tightness, transient ischemic attacks, and death were extremely uncommon (n = 6 for the entire cohort), and as such no statistical comparisons could be made for this outcome. On univariate analysis, patients with concomitant deep venous reflux had a significantly higher rate of procedure-specific complications (10.4%) compared with patients without deep venous reflux (3.0%; P < .001). When investigated individually, no difference was seen in the rates of deep venous thrombosis, bleeding requiring intervention, skin blistering, or hematoma. However, patients with deep venous reflux were more likely to suffer from paresthesias (2.5% vs 0.7%; P < .001), skin pigmentation (1.2% vs 0.4%; P = .023), superficial phlebitis (2.0% vs 0.9%; P = .018), wound infection (0.8% vs 0.2%; P = .040), and proximal thrombus extension (3.1% vs 1.1%; P < .001) (Fig 3).
Fig 3.
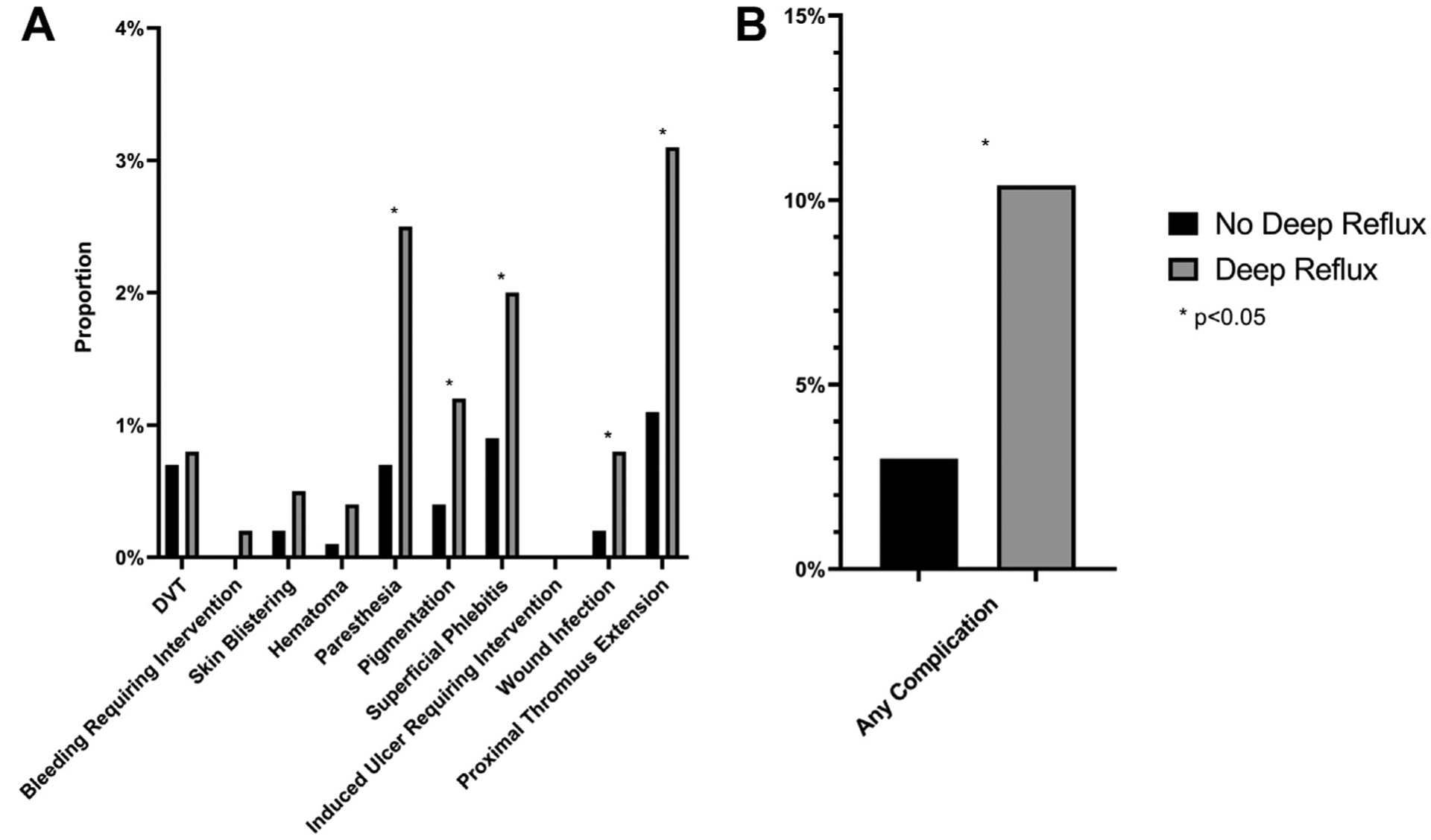
Comparison of complication rates among patients with and without deep reflux. A, Individual procedure specific complication rates and (B) overall procedure-specific complication rates. P values of less than .05 are noted with an asterisk. DVT, Deep vein thrombosis.
Results from a multivariable logistic regression model to assess the effect of deep reflux on the rate of total complications after controlling for confounding factors is shown in Table III. An increasing number of pregnancies was associated with a higher OR for complications, although patients with a history of 4 four pregnancies were the only group to reach statistical significance compared with the reference range of 0 (OR, 10.26; 95% CI, 1.07–98.50; P = .044). All other factors investigated were not predictive of complications, except for the presence of deep venous reflux, which had an OR of 5.72 (95% CI, 2.21–14.81; P < .001).
Table III.
Multivariable analysis for predictors of any complication among patients with and without deep reflux
| Characteristics | OR | 95% Cl | P value |
|---|---|---|---|
| Deep vein reflux | 5.72 | 2.21–14.81 | <.001 |
| Year of surgery | |||
| 2016 | 0.35 | 0.08–1.48 | .154 |
| 2017 | 0.83 | 0.28–2.49 | .743 |
| 2018 | 0.34 | 0.11–1.09 | .069 |
| 2019 | 0.64 | 0.15–2.72 | .545 |
| Age (per year) | 0.98 | 0.94–1.01 | .144 |
| BMI (per point) | 1.06 | 1.00–1.12 | .040 |
| No. of pregnancies | |||
| 0 | REF | REF | REF |
| 1 | 3.95 | 0.38–41.01 | .250 |
| 2 | 4.29 | 0.51–36.17 | .180 |
| 3 | 6.09 | 0.70–53.37 | .103 |
| 4 | 10.26 | 1.07–98.50 | .044 |
| 5 | 5.26 | 0.27–101.53 | .272 |
| 6 | 13.64 | 0.99–187.49 | .051 |
| Non-white race | 1.31 | 0.46–3.73 | .609 |
| Prior varicose vein procedure | 1.27 | 0.49–3.28 | .617 |
| History of DVT | 0.93 | 0.17–5.22 | .934 |
| Anticoagulation | 0.78 | 0.09–6.99 | .822 |
| Preoperative VCSS (per point) | 0.92 | 0.74–1.13 | .422 |
| Preoperative CEAP | |||
| 2 | REF | REF | REF |
| 3 | 0.43 | 0.16–1.13 | .087 |
| 4 | 0.67 | 0.15–3.04 | .599 |
| 5 | – | – | – |
| 6 | 1.15 | 0.07–20.02 | .923 |
| Compression compliance | |||
| Never | REF | REF | |
| Intermittent | 0.41 | 0.05–2.82 | .363 |
| Most days | 2.49 | 0.52–11.92 | .252 |
| Every day | 1.17 | 0.30–4.55 | .822 |
BMI, Body mass index; CEAP, Clinical, Etiologic, Anatomic, and Physiologic; CI, confidence interval; DVT, deep venous thrombosis; OR, odds ratio; VCSS, Venous Clinical Severity Score.
DISCUSSION
The effect of concomitant reflux of the deep venous system in patients with lower extremity superficial venous insufficiency has not been studied well previously, but the results from our study show a significantly increased rate of complications among these patients relative to patients without reflux of the deep venous system. Importantly, this association remained even after controlling for potentially confounding variables such as age, number of pregnancies, and compliance with compression therapy, among others. Specifically, increased rates of paresthesia, superficial phlebitis, wound infections, and proximal thrombus extension were seen among patients with concomitant deep venous reflux. One seemingly likely explanation is that venous stasis and increased swelling of the lower extremity leads to simultaneous lymphatic insufficiency, commonly referred to as phlebolymphedema, predisposing patients to superficial phlebitis as well as superficial wound infections.17,18 Additionally, stasis of the deep venous system likely predisposes these patients to proximal thrombus extension after ablation of the truncal veins. Increased risk of thrombus extension represents a potentially preventable outcome and has substantial implications for competing risk assessment in these patients. Given the substantially higher rate of thrombus extension, we suggest having a careful discussion regarding these risks with individual patients and use these data to weight alternative nonablative procedures for the treatment of superficial venous ablation in patients with concomitant deep venous reflux. We found a high proportion of concomitant deep venous reflux among patients included in this study, which may be representative of the highly specialized centers that participate in the VVR and not representative of the wider population of patients suffering from superficial venous reflux. Although this factor may result in an over-estimate of prevalence, it allows this cohort to serve as an enriched population to detect the effect of concomitant deep venous reflux on outcomes after truncal ablation.
Compliance with compression therapy was associated with a decreased odds of improvement in the VCSS. Although significant overlap exists in the CI for increasing time of compression therapy, there does seem to be a dose-response relationship. Overlap may exist in the potential treatment effect of compression stockings and ablation, such that those who would benefit from ablation would also benefit from compression therapy, but with exclusive mechanisms of improvement. In this way, ablation may serve as an alternative therapy for superficial reflux in patients who are noncompliant with compression therapy but add little effect to those who routinely use compression therapy. The results of our multivariable model suggest that this effect is consistent across groups with or without concomitant deep venous reflux.
Contrary to our hypothesis, no difference was seen in the magnitude of improvement in patient-reported outcomes between those with and without deep venous reflux. We found differences in preprocedural scores between the two groups, but the absolute magnitude of the differences were below a single point on the 34-point scale used in the VVR and likely represent statistically but not clinically significant differences between the two groups. The explanation for the lack of effect of concomitant reflux on patient-reported outcomes requires further characterization of the relative contribution of severity of reflux in the deep and superficial venous systems to development of the various symptoms tested in this study, but it may be that reflux of the deep venous system contributes to the complications, as described elsewhere in this article, while simultaneously having little impact on the symptoms that develop superficially.
Limitations to this study include those inherent to the VQI registry. The VVR is an extremely detailed database that captures a significant breadth of clinically important factors, but unmeasured variables may still exist that explain the associations seen here. We also were unable to study the effect of tributaries and their treatment on the outcomes in this study as these are not captured in the VVR.
The VVR collects information regarding the presence or absence of reflux within the deep venous system, but unfortunately does not contain information regarding the location of reflux if it is present. In this way, we cannot assess the effect that the presence of reflux at different anatomic locations may have on the outcomes included in this study. In the event that the presence of deep reflux at only specific locations confers the effects seen in this study, our results would only be biased toward the null rather than over-estimating the effect size.
Prior results suggest that ablation of the superficial venous system can result in resolution of reflux in the deep venous system in a substantial portion of patients.6,9 The VVR does not capture postoperative ultrasound data, and we are therefore unable to calculate rates of deep venous reflux resolution in this cohort. Without these data, we are unable to estimate the effect of deep venous reflux resolution on the outcomes assessed in this study, including its relationship with development of proximal thrombus extension, patient-reported outcomes, as well as the other complications captured in this registry. In the event that the resolution of reflux in the deep venous system results in no difference in outcomes, our estimates would be biased toward the null rather than inflated in their magnitude. We conclude that concomitant deep venous reflux results in at least a five-fold increased odds of complication compared with patients without deep reflux, but that this effect may in actuality be greater depending on the rate and effect of deep reflux resolution on complication rates.
Additionally, because the VVR is a procedural database, there may be a selection bias against patients who were not offered surgery for the treatment of their lower extremity venous disease. Finally, we excluded patients who underwent sclerotherapy or phlebectomy, as well as those who underwent only treatment of nontruncal veins, which may limit the generalizability of these results. We performed a sensitivity analysis to determine the effect of including patients with sclerotherapy and phlebectomy in the cohort as seen in the Supplementary Figures (online only), and we found significant differences in the results of the univariate proportions or point estimates for the multivariable model of improvement in VCSS, including the effect of disease severity, despite the fact that including these patients increased the proportion of the overall cohort with C2 disease. This finding suggests that the effects seen in this analysis are present for those patients independent of preoperative C classification.
Our results underscore the importance of identifying concomitant deep venous reflux among patients undergoing truncal endovenous ablation, primarily to inform the patient of increased risks and to attempt to mitigate them. Given the significant increase in postoperative complications identified in this study, further research should focus on elucidating the mechanisms involved and working toward strategies of mitigation. Additional work is required to understand the contribution of deep venous reflux to complications, the impact of anatomic location of deep reflux on outcomes, the effect of deep venous reflux resolution after ablation, and whether patients in this group should undergo treatment of the deep or superficial venous system or whether different options may better serve this group at clearly significant risk of postoperative complication.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective analysis of prospectively collected registry data (Vascular Quality Initiative)
Key Findings: Concomitant deep and superficial venous reflux is common, present in 46% of all patients undergoing truncal endovenous ablation. Patients with concomitant reflux have worse patient-reported outcomes, but similar improvements after treatment. Complications were significantly more common among those with concomitant reflux compared with those with superficial venous reflux alone (10.4% vs 3.0%).
Take Home Message: Patients with combined reflux in the deep and superficial venous systems have substantial improvement after truncal endovenous ablation, but suffer significantly higher rates of complications.
Footnotes
Author conflict of interest: A.O. reports serving as the principal investigator for a device contract with Medtronic related to deep vein thrombosis. All other authors report no competing interests.
Presented virtually in the plenary session at the 2020 Vascular Annual Meeting of the Society for Vascular Surgery, June 23, 2020.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Callam MJ. Epidemiology of varicose veins. Br J Surg 1994;81: 167–73. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology 2001;52(Suppl 1):S5–15. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg 2003;37:1047–53. [DOI] [PubMed] [Google Scholar]
- 4.Korn P, Patel ST, Heller JA, Deitch JS, Krishnasastry KV, Bush HL, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg 2002;35:950–7. [DOI] [PubMed] [Google Scholar]
- 5.Kanth AM, Khan SU, Gasparis A, Labropoulos N. The distribution and extent of reflux and obstruction in patients with active venous ulceration. Phlebology 2015;30:350–6. [DOI] [PubMed] [Google Scholar]
- 6.Sales CM, Bilof ML, Petrillo KA, Luka NL. Correction of lower extremity deep venous incompetence by ablation of superficial venous reflux. Ann Vasc Surg 1996;10:186–9. [DOI] [PubMed] [Google Scholar]
- 7.Lim KH, Hill G, Tarr G, van Rij A. Deep venous reflux definitions and associated clinical and physiological significance. J Vasc Surg Venous Lymphat Disord 2013;1:325–32. [DOI] [PubMed] [Google Scholar]
- 8.Meissner MH, Caps MT, Zierler BK, Bergelin RO, Manzo RA, Strandness DE. Deep venous thrombosis and superficial venous reflux. J Vasc Surg 2000;32:48–56. [DOI] [PubMed] [Google Scholar]
- 9.Puggioni A, Lurie F, Kistner RL, Eklof B. How often is deep venous reflux eliminated after saphenous vein ablation? J Vasc Surg 2003;38:517–21. [DOI] [PubMed] [Google Scholar]
- 10.Maleti O, Lugli M, Perrin M. After superficial ablation for superficial reflux associated with primary deep axial reflux, can variable outcomes be caused by deep venous valve anomalies? Eur J Vasc Endovasc Surg 2017;53:229–36. [DOI] [PubMed] [Google Scholar]
- 11.Sutzko DC, Obi AT, Kimball AS, Smith ME, Wakefield TW, Osborne NH. Clinical outcomes after varicose vein procedures in octogenarians within the Vascular Quality Initiative Varicose Vein Registry. J Vasc Surg Venous Lymphat Disord 2018;6:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutzko DC, Andraska EA, Obi AT, Sadek M, Kabnick LS, Wakefield TW, et al. Age is not a barrier to good outcomes after varicose vein procedures. J Vasc Surg Venous Lymphat Disord 2017;5:647–57.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obi AT, Sutzko DC, Almeida JI, Kabnick L, Cronenwett JL, Osborne NH, et al. First 10-month results of the Vascular Quality Initiative Varicose Vein Registry. J Vasc Surg Venous Lymphat Disord 2017;5:312–20.e2. [DOI] [PubMed] [Google Scholar]
- 14.Passman MA, McLafferty RB, Lentz MF, Nagre SB, Iafrati MD, Bohannon WT, et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. J Vasc Surg 2011;54(6 Suppl):2S–9S. [DOI] [PubMed] [Google Scholar]
- 15.Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248–52. [DOI] [PubMed] [Google Scholar]
- 16.Paty J, Turner-Bowker DM, Elash CA, Wright D. The VVSymQÒ instrument: use of a new patient-reported outcome measure for assessment of varicose vein symptoms. Phlebology 2016;31:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piller N Phlebolymphoedema/chronic venous lymphatic insufficiency: an introduction to strategies for detection, differentiation and treatment. Phlebol J Venous Dis 2009;24: 51–5. [DOI] [PubMed] [Google Scholar]
- 18.Farrow W Phlebolymphedema-a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec 2010;2:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


