Abstract
Introduction
With the threat of a worldwide pandemic of COVID-19, it is important to identify the prognostic factors for critical conditions among patients with non-critical COVID-19. Prognostic factors and models may assist front-line clinicians in rapid identification of high-risk patients, early management of modifiable factors, appropriate triaging and optimising the use of limited healthcare resources. We aim to systematically assess the clinical, laboratory and imaging predictors as well as prediction models for severe or critical illness and mortality in patients with COVID-19.
Methods and analysis
All peer-reviewed and preprint primary articles with a longitudinal design that focused on prognostic factors or models for critical illness and mortality related to COVID-19 will be eligible for inclusion. A systematic search of 11 databases including PubMed, EMBASE, Web of Science, Cochrane Library, CNKI, VIP, Wanfang Data, SinoMed, bioRxiv, Arxiv and MedRxiv will be conducted. Study selection will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data extraction will be performed using the modified version of the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies checklist and quality will be evaluated using the Newcastle-Ottawa Scale and the Quality In Prognosis Studies tool. The association between prognostic factors and outcomes of interest will be synthesised and a meta-analysis will be conducted with three or more studies reporting a particular factor in a consistent manner.
Ethics and dissemination
Ethical approval was not required for this systematic review. We will disseminate our findings through publication in a peer-reviewed journal.
PROSPERO registration number
CRD 42020178798.
Keywords: infectious diseases, public health, COVID-19, adult intensive & critical care
Strengths and limitations of this study.
The evidence synthesis on prognostic factors and models of COVID-19-related critical conditions will play a pivotal role in assisting front-line clinical decision-making.
The quality of included studies will be evaluated using a validated tool (Quality In Prognosis Studies) specifically developed to assess the risk of bias of prognosis studies.
Given that primary studies can be conducted in different region, population or setting, prognostic factors or models can be assessed using different tools, heterogeneity in the pooled data may be a limitation of this review; however, subgroup analyses will help overcome this limitation.
Introduction
Description of the condition
COVID-19, a newly emerged respiratory disease caused by SARS-CoV-2, was first reported in December 2019.1 2 The infection has recently spread to at least 188 countries and regions, with more than 25 million confirmed cases and 850 000 deaths worldwide as of 1 September 2020.3 The number of people infected is probably much higher due to the shortage of tests for COVID-19. Despite a variety of rapid public health responses aimed at containing the disease, many countries have been confronted with enormous challenges to the healthcare systems posed by the overwhelming number of patients requiring hospital admission, especially by those with progression to severe or critical illness according to the criteria in the WHO recommendations or the local guidelines.4–8
Why is it important to do this review?
A report of 72 314 cases from the Chinese Center for Disease Control and Prevention showed that most of the patients with COVID-19 are asymptomatic or exhibit mild or moderate symptoms.9 The vast majority of patients with mild and moderate symptoms are recommended to stay at home or are admitted in shelter/field hospitals.10–14 However, patients with mild symptoms may develop rapidly worsening respiratory failure that requires intubation.7 Approximately 5%–29% of the patients progressed to a severe or critical condition such as acute respiratory distress syndrome or septic shock and/or multiple organ failure that required admission to the intensive care unit.9 15–18 Patients who exhibited severe or critical symptoms or patients at high risk to develop severe conditions were the main reason behind the overwhelming number of patients who required admission or even intensive care. Hence, it is crucial to determine the prognostic factors associated with the risk of a subsequent critical outcome among patients with non-critical COVID-19. Prognostic factors and prediction models for severe or critical COVID-19 have many potential uses in various settings including informing individuals about the future course of their illness, aiding triage and referral, early management of modifiable factors, treatment and other factors related to clinical decision-making.
Status of the current literature
Evidence is rapidly accumulating about prognostic factors and models for critical conditions or mortality related to COVID-19. Recently, two systematic reviews focusing on specific perspectives of COVID-19 have been published.19 20 Henry and colleagues published a systematic review that included only the laboratory biomarkers and excluded the clinical and imaging predictors associated with severe illness and mortality in COVID-19.19 Another review by Wynants and colleagues focused on the prediction models for diagnosis and prognosis of COVID-19 infection.20 Eight studies regarding prognostic models for severe state or mortality were included. However, only the studies aimed at developing or validating a model or a scoring system were included, while those aimed at predictor findings were excluded from this systematic review.20 In addition, since China was the first epicentre of COVID-19, many studies on the prediction of COVID-19 may have been published in Chinese journals. According to our preliminary results, more than 15 studies regarding prognostic factors or models have been published in four Chinese databases (China National Knowledge Infrastructure (CNKI), Wanfang, CBM and VIP) that were not included in the aforementioned systematic review. Limited data are available on the overview of evidence that focuses on clinical, laboratory and imaging prognostic factors for critical illness or mortality associated with COVID-19. Moreover, a huge number of recent articles have emerged with the worldwide pandemic. Many valuable articles on prognostic factors or models of COVID-19 have not been included in these published reviews. Among these, some high-quality papers have been published in leading journals,21 22 which provided us with more evidence and insights into this topic. Therefore, there is a need for a systematic review to evaluate and synthesise the data from the current studies from a comprehensive perspective on clinical, laboratory and imaging prognostic factors and prediction models for critical illness and mortality associated with COVID-19.
Research aims
We aim to systematically assess the clinical, laboratory and imaging predictors as well as models for severe or critical illness and mortality in patients with COVID-19. Predictors and models for critical illness may be different from that of mortality, so it will be assessed according to different outcomes.
Methods
This systematic review protocol followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) recommendations23 and the Cochrane Handbook. The PRISMA-P checklist is presented in online supplemental appendix 1. This review protocol was started in early April and has been registered with the International Prospective Register of Systematic Reviews.24
bmjopen-2020-039813supp001.pdf (202.7KB, pdf)
Search strategy
A systematic search of 11 public domain databases including PubMed, EMBASE, Web of Science, Cochrane Library, CNKI, Chinese Science and Technology Periodical Database (VIP), Wanfang database (Wanfang Data), China Biology Medicine disc (SinoMed), bioRxiv, Arxiv and MedRxiv will be performed. We will use exploded Medical Subject Headings and the appropriate corresponding keywords related to the population, combined with exposure and outcomes such as: ‘COVID-19’ OR ‘SARS-CoV-2’ OR ‘2019-nCoV’ OR ‘novel coronavirus’ AND ‘critically’ OR ‘severe’ OR ‘mortality’ OR ‘deterioration’ AND ‘predictor’ OR ‘prediction’ OR ‘prognostic’ OR ‘factor’. Additionally, a publication list of the COVID-19 Living Systematic Review25 and other resources26 will be screened for additional relevant references. There will be no restrictions on language or publication status (preprint or peer-reviewed articles). The research will be restricted to articles concerning humans from December 2019 to the present. We will include additional papers from other sources including the references of review articles or studies identified during screening. A sample search strategy for PubMed is shown in online supplemental appendix 2.
bmjopen-2020-039813supp002.pdf (93.6KB, pdf)
Eligibility criteria
Participants
All patients with confirmed diagnosis of COVID-19, explicitly classified as mild, moderate, severe or critically ill according to accepted diagnostic criteria such as the WHO recommendations or the local guidelines, will be included. The criteria in the guidelines may be modified over time. Thus, the criteria in different periods or regions will be acceptable.
Exposures
Any data related to demographics, symptoms and signs, pulmonary functions, laboratory tests, radiological findings, comorbidities and interventions will be considered potential predictors for critical illness or mortality in patients with COVID-19. This information may include factors such as the age, fever, shortness of breath, underlying diseases, mechanical ventilation, and dexamethasone or other interventions.
Comparators
Based on the published studies, many factors including older age; underlying diseases such as hypertension, diabetes and cardiovascular diseases; and chest radiographic abnormalities were independent predictive factors for critical illness in hospitalised patients with COVID-19.21 22 These potential variables will be considered the comparators. Participants with and without specific clinical, laboratory and imaging information will be compared to clarify the significance of this information in predicting critical illness and mortality associated with COVID-19.
Outcomes
The outcomes will include deterioration, progression, severe critical illness or death related to COVID-19 according to accepted criteria.
Timing and setting
There will be no restriction on the time point when the prognostic factors were under review as well as on the period when the outcomes were predicted. No restriction will be imposed on the setting.
Types of study to be included
Both experimental and longitudinal observational studies including randomised controlled trials, cohort studies, case–control studies and registry studies will be included. Review articles, editorials, letters, comments, case reports, cross-sectional studies and studies that failed to investigate the prognostic factors or models for critical illness or mortality will be excluded.
Study selection
Two reviewers (JL, LF) will independently perform the initial search and examine the titles, abstracts and full texts (if necessary) to identify eligible studies according to the inclusion and exclusion criteria. Disagreements between the reviewers will be resolved by consensus and by adjudication of a third reviewer (QL) in case of persistent disagreement. The selection process is illustrated in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram (figure 1).27
Figure 1.
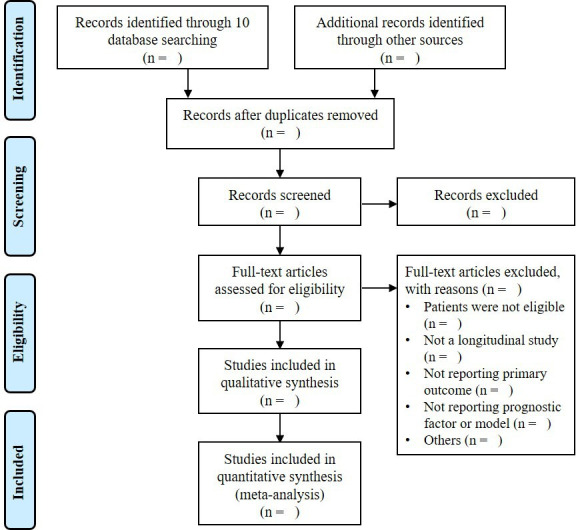
Flow diagram of the study selection process.
Data extraction
Data extraction will be independently conducted by two reviewers (TZ, PJ) using a standard data extraction form developed according to the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS) checklist for prediction model studies and its modified version (CHARMS-PF)28 29 as well as according to the Prediction Model Risk of Bias Assessment Tool.30 For each included trial, the following key information will be extracted based on availability: name of the first author, year of publication, study location, study design, study setting, participants, sample size, follow-up period, outcomes of interest, risk and prognostic factors, missing data, summary statistics, results, interpretation and discussion. The authors of the studies will be contacted through email or telephone in case of missing relevant data.
Assessment of the risk of bias
We will evaluate the risk of bias using the Newcastle-Ottawa Scale31 and the Quality In Prognosis Studies checklist, which has been recommended by the Cochrane Group to assess the risk of bias in studies related to prognostic factors.32 Quality assessment will be performed independently by two reviewers (TZ, LK) and discrepancies will be resolved through consensus.
Data synthesis
Essential data will be summarised in tables for evaluation. Estimates of risk difference in terms of critical illness and mortality will be calculated. For categorical variables, ORs, relative risk or HRs will be analysed to compare these variables between mild/moderate and severe/critical COVID-19 cases. Studies reporting adjusted or unadjusted results will be analysed separately. Only the unadjusted effect estimates for prognostic factors will be combined, while effect estimates from multivariate models will be described qualitatively. With three or more studies reporting a particular factor in a consistent manner, a meta-analysis will be conducted using the Review Manager software (RevMan V.5.3, the Cochrane Collaboration, London, UK) to synthesise the association of prognostic factors and critical illness or mortality in patients with COVID-19. For severe or critical illness and mortality, the data will be synthesised according to different outcomes. Heterogeneity among the included studies will be tested using the I2 statistic.33 Forest plots will be presented as significant predictors. In case of substantial heterogeneity, subgroup analyses will be conducted to examine or to explore the causes of heterogeneity. Subgroup analysis will be based on the categories defined by the following characteristics: study location/region, risk of bias and particular population such as children and elderly people.
Ethics and dissemination
Ethical approval was not required for this systematic review. We will disseminate our findings through publication in a peer-reviewed journal.
Patient and public involvement
There is no patient or public involvement in the whole process of conducting this systematic review.
Discussion
With an unprecedented threat of a worldwide COVID-19 pandemic, there has been an increasing need for early identification of patients at higher risk of progression to critical illness or even death. This systematic review will comprehensively summarise the existing evidence on clinical, laboratory and imaging factors and models for predicting critical conditions and mortality in patients with COVID-19. The findings of this review will provide front-line clinicians an early surrogate for disease severity before the onset of critical illness, which may play a key role in assisting the clinicians in early management of modifiable factors, appropriate triaging of patients and optimising the use of limited healthcare resources.
Supplementary Material
Acknowledgments
We gratefully acknowledge Wei Chen from the Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, for her great help on the improvement of methodology of this protocol. We thank Editage (www.editage.com) for English language editing.
Footnotes
Contributors: XL and YG conceived the research question. TZ, LF, PJ and LK developed the search strategy and performed the preliminary search, screening and data extraction. QL and YG contributed to the methodological development of the protocol. XL and JL drafted the manuscript. QL and YG revised the manuscript and all authors developed and approved the final manuscript before submission.
Funding: The study was funded by the Chinese Medicine Inheritance and Innovation Talent Project-National Leading Talent Support Program for Traditional Chinese Medicine (Grant [2018] No.12)
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Wang C, Horby PW, Hayden FG, et al. . A novel coronavirus outbreak of global health concern. Lancet 2020;395:470–3. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, et al. . A novel coronavirus emerging in china - key questions for impact assessment. N Engl J Med 2020;382:692–4. 10.1056/NEJMp2000929 [DOI] [PubMed] [Google Scholar]
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track covid-19 in real time. Lancet Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-cov-2 admitted to ICUs of the lombardy region, Italy. JAMA 2020;323:1574. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J, Tong Z, Guan X, et al. . Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med 2020;46:837–40. 10.1007/s00134-020-05979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the covid-19 outbreak in lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020;323:1545–6. 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 7.Goh KJ, Choong MC, Cheong EH, et al. . Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from covid-19 infection. Ann Acad Med Singap 2020;49:108–18. 10.47102/annals-acadmedsg.202057 [DOI] [PubMed] [Google Scholar]
- 8.Arabi YM, Murthy S, Webb S. Covid-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 10.Gostin LO, Wiley LF. Governmental public health powers during the covid-19 pandemic: stay-at-home orders, business closures, and travel restrictions. JAMA 2020;323:2137–8. 10.1001/jama.2020.5460 [DOI] [PubMed] [Google Scholar]
- 11.Park PG, Kim CH, Heo Y, et al. . Out-of-hospital cohort treatment of coronavirus disease 2019 patients with mild symptoms in Korea: an experience from a single community treatment center. J Korean Med Sci 2020;35:e140. 10.3346/jkms.2020.35.e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kmietowicz Z. Covid-19: highest risk patients are asked to stay at home for 12 weeks. BMJ 2020;368:m1170. 10.1136/bmj.m1170 [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, He S, Li F, et al. . Mobile field hospitals, an effective way of dealing with COVID-19 in China: sharing our experience. Biosci Trends 2020;14:212–4. 10.5582/bst.2020.01110 [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Zhang Z, Yang J, et al. . Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet 2020;395:1305–14. 10.1016/S0140-6736(20)30744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. . Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Liu X, Xiong L, et al. . Imaging and clinical features of patients with 2019 novel coronavirus SARS-cov-2: a systematic review and meta-analysis. J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry BM, de Oliveira MHS, Benoit S, et al. . Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020;58:1021–8. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 20.Wynants L, Van Calster B, Collins GS, et al. . Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 2020;369:m1328. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang W, Liang H, Ou L, et al. . Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020;180:1081–9. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, She Z-G, Cheng X, et al. . Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–77. 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2016;354:i4086. 10.1136/bmj.i4086 [DOI] [PubMed] [Google Scholar]
- 24.Centre for Reviews and Dissemination, University of York Prospero: International prospective register of systematic reviews. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020178798
- 25.Institute of Social and Preventive Medicine Living evidence on COVID-19, 2020. Available: https://ispmbern.github.io/covid-19/living-review/index.html
- 26.Shokraneh F. Keeping up with studies on covid-19: systematic search strategies and resources. BMJ 2020;369:m1601. 10.1136/bmj.m1601 [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley RD, Moons KGM, Snell KIE, et al. . A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019;364:k4597. 10.1136/bmj.k4597 [DOI] [PubMed] [Google Scholar]
- 29.Moons KGM, de Groot JAH, Bouwmeester W, et al. . Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the charms checklist. PLoS Med 2014;11:e1001744. 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moons KGM, Wolff RF, Riley RD, et al. . Probast: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 31.Wells GA, Shea B, O’Connell D, et al. . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, 2009. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 32.Hayden JA, van der Windt DA, Cartwright JL, et al. . Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039813supp001.pdf (202.7KB, pdf)
bmjopen-2020-039813supp002.pdf (93.6KB, pdf)


