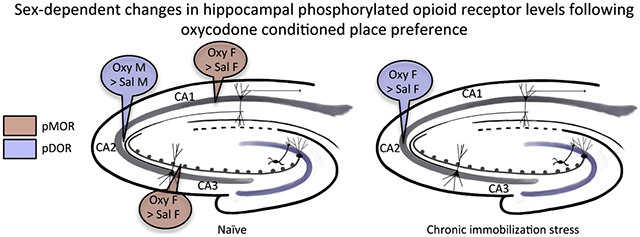
Abstract
Following oxycodone conditioned place preference (CPP) in naïve female and male Sprague Dawley rats, delta- and mu-opioid receptors (DORs and MORs) redistribute in hippocampal CA3 pyramidal cells and GABAergic interneurons in a manner that would promote opioid-associative learning processes, particularly in females. MORs and DORs similarly redistribute in CA3 and hilar neurons following chronic immobilization stress (CIS) in females, but not males, essentially “priming” the opioid system for oxycodone-associative learning. Following CIS, only females acquire oxycodone CPP. The present study determined whether sex and CIS differentially affect the levels of phosphorylated MORs and DORs (pMORs and pDORs) in the hippocampus following oxycodone CPP as phosphorylation is important for opioid receptor internationalization and trafficking. In naïve oxycodone-injected (Oxy) female rats, the density of pMOR-immunoreactivity (ir) was increased in CA1 stratum oriens and CA3a,b strata lucidum and radiatum compared to saline-injected (Sal)-females. Additionally, the density of pDOR-ir increased in the pyramidal cell layer and stratum radiatum of CA2/3a in Oxy-males compared to Sal-males. In CIS females that acquire CPP, pDOR-ir levels were increased in the CA2/3a. These findings indicate only rats that acquire oxycodone CPP have activated MORs and DORs in the hippocampus but that the subregion containing activated opioid receptors differs in females and males. These results are consistent with previously observed sex differences in the hippocampal opioid system following Oxy-CPP.
Keywords: opioid receptors, learning, Drug addiction, hippocampus
Graphical abstract
1. INTRODUCTION
With the rise of the opioid epidemic over the past twenty years [1], elucidating the mechanisms associated with addiction is of prime importance. In the rodent hippocampus, opioid signaling in the CA3 region is implicated in spatial memory and associative learning [2, 3], two factors that are involved in the addiction process. Following chronic stress, spatial learning and memory are impaired in male, but not female, rodents [4, 5]. Additionally, chronically stressed male rodents undergo morphological changes such as dendritic retraction in CA3 pyramidal cells and loss of parvalbumin (PARV)-containing interneurons [6], suggesting that the hippocampus adapts to chronic stress differently in females and males.
Previously, we found that both naive female and male rats acquired conditioned place preference (CPP) to the mu opioid receptor (MOR) agonist oxycodone [7]. Examination of anatomical changes in naive rats following oxycodone CPP revealed that delta opioid receptors (DORs) and MORs redistributed in CA3b pyramidal cells and γ-amino butyric acid (GABA)-ergic hilar interneurons [7] in a manner that would promote opioid-mediated long-term potentiation (LTP) [8]. Moreover, chronic immobilization stress (CIS) in female, but not male, rats redistributed DORs and MORs within CA3 pyramidal cells and GABAergic interneurons in a manner that “primes” the hippocampus for opioid-associated learning [9–11]. Importantly, following CIS, only female rats acquired oxycodone CPP [11]. Taken together, these findings show that sex and CIS can differentially interact to influence both the hippocampal opioid system as well as behavioral responses to oxycodone.
The present study determined whether sex and CIS differentially affect the levels of phosphorylated MORs and DORs (pMORs and pDORs) in the hippocampus following oxycodone CPP as phosphorylation is important for opioid receptor internationalization and trafficking. [12–14]. Determining the sex differences in pDOR and pMOR may provide insight into the differing mechanisms of addiction between females and males.
2. MATERIALS AND METHODS
2.1. Animals:
All animal procedures were in accordance with the 2011 Eighth edition of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This study used three cohorts of adult Sprague-Dawley rats (N=72; RGD Cat# 734476, RRID:RGD_734476): Cohort 1. Saline-injected (Sal) naïve males and females or oxycodone-injected (Oxy) naïve males and females that were subjected to CPP (N = 6/group; 24 total). Cohort 2. Naïve males and females or CIS males and females not subjected to oxycodone CPP (N = 6/group; 24 total). Cohort 3. CIS Sal- males and females or CIS Oxy- males and females that were subjected to CPP (N = 6/group; 24 total). On the day of euthanasia, all female rats were in the estrus phase of the estrous cycle as determined by vaginal smear cytology. Rats were single-housed (cohorts 1 and 3) or double-housed (cohort 2) with a 12-hour light/dark cycle with unrestricted access to food and water. Tissues from these cohorts of rats were used in our previous studies [7, 9, 11].
2.2. CIS:
In cohorts 2 and 3, rats were subjected to CIS for 10 consecutive days [9, 11]. For this, rats were placed in plastic cone shaped polyethylene bags with a small hole at the apex and a Kotex mini-pad for urine collection. The rats were placed with their noses at the hole of the bag, sealed in with tape and left undisturbed for 30 mins each day. Rats in cohort 2 were euthanized 1 day after the last stress session. Rats in cohort 3 started CPP training two days following the last stress session.
2.3. Oxycodone CPP:
The CPP paradigm is described in our previous studies [7, 11, 15]. Briefly, the apparatus is divided into three compartments: white, black and a central gray compartment, which are separated by removable doors. The 14-day CPP protocol has three phases: 1) Preconditioning (day 1): The rats explored the CPP apparatus freely for 30 mins. 2) CPP training (days 2 – 9): The removable doors were added to separate the black and white compartments and the rats underwent 4 training sessions. On the first day of each session, the rats were injected with oxycodone (3 mg/kg, i.p.) or saline and placed in one compartment (e.g., white) for 30 min. On the second day of each session, the rats were injected with saline and placed in the other compartment (e.g., black) for 30 min. Control rats were injected with saline on both days. 3) CPP test (day 14): The he doors were removed from the apparatus. Four days following the last injection, the rats were placed in the central gray section and allowed to explore the CPP apparatus freely for 30 mins. Infrared photo beam breaks were used to determine the amount of time each rat spent in each section. The difference in percent of time spent in the drug-paired compartment vs. the drug-unpaired compartment was determined. Our previous studies demonstrated that the naïve (unstressed) female and male rats used in this study (cohort 1) both acquired oxycodone CPP [7], while only the CIS female rats used in this study (cohort 3) acquired oxycodone CPP [11]. Rats in cohorts 1 and 3 were euthanized immediately after the CPP test session on day 14.
2.4. Antibody Characterization:
pMOR:
This study used a rabbit polyclonal antibody generated against a synthetic phosphopeptide corresponding to residues surrounding Ser377 of human (homologous to Ser375 of mouse) pMOR (#3451 Cell Signaling, Danvers, MA, USA). Specificity of this antibody has been shown using western blots and immunoprecipitation in both HEK293 cells and neuronal cells [16]. In the presence of the opioid receptor agonist DAMGO, or the antagonist naloxone, pMOR-immunoreactivity (ir) in HEK293 cells is increased and decreased, respectively [16]. Mutating the SER 375 residue of the MOR in HEK293 cells abolished morphine-induced phosphorylation as measured by immunoprecipitation [17]. Prior studies have shown that pMOR-ir is abolished when the antibody adsorbed with the peptide to which the antiserum was raised [18].
pDOR:
A rabbit polyclonal antibody generated against a synthetic phosphopeptide corresponding to residues surrounding Ser363 of human pDOR was used (#3641 Cell Signaling, Danvers, MA). On Western blots of hippocampal samples from wild-type mice 20 minutes after being treated with the DOR agonist SNC80 (10 mg/kg), this antibody demonstrated a 65kDa band; this same band was absent in DOR knockout mice with the same treatment [19].
2.5. Section preparation:
Procedures are described in detail in prior publications [7, 11]. Briefly, the rats were anesthetized either with Ketamine (100mg/kg) and xylazine (10mg/kg) I.P. (cohorts 1 and 3) or sodium pentobarbital (150mg/kg; cohort 2) and perfused through the ascending aorta sequentially with: 1) 10-15 ml 0.9% saline and 2% heparin; 2) 50 ml 3.75% acrolein and 2% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB); 3) 200 ml 2% PFA in PB. Coronal sections (40 μm thick) through the hippocampus were cut on a Vibratome and stored in cryoprotectant solution at −20°C until use. One rostral hippocampal section (between AP-3.5 and -4.00 from Bregma [20]) from each rat was selected for immunocytochemistry and sections from each cohort were processed separately. To ensure identical labeling conditions between groups in each cohort, hippocampal sections were coded with hole-punches in the cortex, and processed in a single container throughout the immunocytochemical procedures [21].
2.6. Immunocytochemistry:
Previously described immunoperoxidase labeling methods were used [21]. The tissue sections were incubated in 1% sodium borohydride in PB for 30 mins to reduce reactive aldehydes. Sections were rinsed in PB until gaseous NaBH4 bubbles disappeared, washed in tris-buffered saline (TS; pH 7.6), and blocked in 0.5% bovine serum albumin (BSA) in TS for 30 mins. The tissues then were incubated with 0.1% BSA and either rabbit anti-pMOR (1:800) or rabbit anti-pDOR (1:500) for 24 hours at room temperature and 48 hours at 4°C. The sections were incubated in donkey-anti-rabbit antibody (1:400; Jackson Immunoresearch Laboratories, Cat# 711-506-152, RRID:AB_2616595) for 30 minutes in 0.1% BSA in TS. The tissue sections incubated in avidin-biotin complex (ABC; Vectastain elite kit, Vector Laboratories, Burlingame, CA) in TS for 30 mins. All incubations had TS washes in between them and were carried out on a shaker at 145 rpm. The sections were placed in 3,3’-diaminobenzidine (Sigma-Aldrich, St. Louis, MO) and 3% H2O2 in TS for 3 mins (pMOR) or 7-9 mins (pDOR). Sections were mounted onto gelatin-coated slides, dehydrated, and cover slipped in DPX mounting media (Sigma-Aldrich).
2.7. Data Collection:
To ensure unbiased analysis of the data, an experimenter blinded to treatment performed all data collection and analyses. Images were captured at 2x (pMOR) or 10x (pDOR) on a Nikon Eclipse 80i microscope using a Dage MTI CCD-72 camera and IP Lab software (Scanalytics IPLab, RRID:SCR_002775). Average pixel density within regions of interest (ROI) was determined using ImageJ64 (RRID:SCR_003070) software. For pMOR, ROIs were the stratum lucidum (SLu) and stratum radiatum (SR) in CA3a, b, and c, and stratum oriens (SO) and SR in CA1. For pDOR, ROIs were the SR and pyramidal cell layer (PCF) in CA2/3a. The pixel density of a small region lacking labeling was subtracted from ROI measurements to control for variations in illumination and background staining between images. The accuracy of this technique has been corroborated by a strong linear correlation between average pixel density and neutral density values of gelatin filters with defined transmittances ranging from 1 to 80% (Eastman Kodak) [22].
2.8. Figure Preparation:
No feature within an image was obscured, moved, removed, introduced or enhanced. Adjustments to brightness, sharpness, and contrast were made in Microsoft PowerPoint 2010 and applied uniformly to all portions of the image. Graphs were generated using Graphpad Prism 8 software (RRID:SCR_002798).
2.9. Statistical Analysis:
Data are expressed as mean ± SEM. Significance was set to an alpha < 0.05. All statistical analyses were conducted on JMP Pro 12 software (RRID:SCR_014242). Optical density sample comparisons were performed between groups in each cohort using a one-way ANOVA or two-tailed t-test with a Welch correction for samples with unequal variances (as determined by Levene’s test).
3. RESULTS
3.1. pMOR levels are increased in naïve Oxy-females compared to Sal-females
Consistent with our previous study [18], dense diffuse pMOR-ir is found in SLu of CA3a, b and c and sparser diffuse pMOR-ir is seen in the central hilus of the DG and in SO and SR of CA1 and CA3 (Fig. 1). In naïve (unstressed) rats, the density of pMOR-ir in Oxy-females is increased compared to Sal-females in the CA1 SO (t(8.25)=−2.39; p=0.0381; Fig. 1B–D), the CA3a SLu (t(10)=−2.77; p=0.0197), CA3a SR (t(10)=−2.72; p=0.0215), the CA3b SLu (t(7.53)=−2.87; p=0.0166; Fig. 1E–G), CA3b SR (t(8.20)=−3.00; p=0.0134; Fig. 2D–F), and the CA3c SR (t(10)=−2.80; p=0.0189). No change, m pMOR-ir were observed between unstressed Sal- and Oxy-males in any lamina of either the CA1 or CA3 regions (Fig. 1D,G).
Fig 1: pMOR levels are increased in naïve Oxy-females compared to Sal-females in CA1 and CA3b.
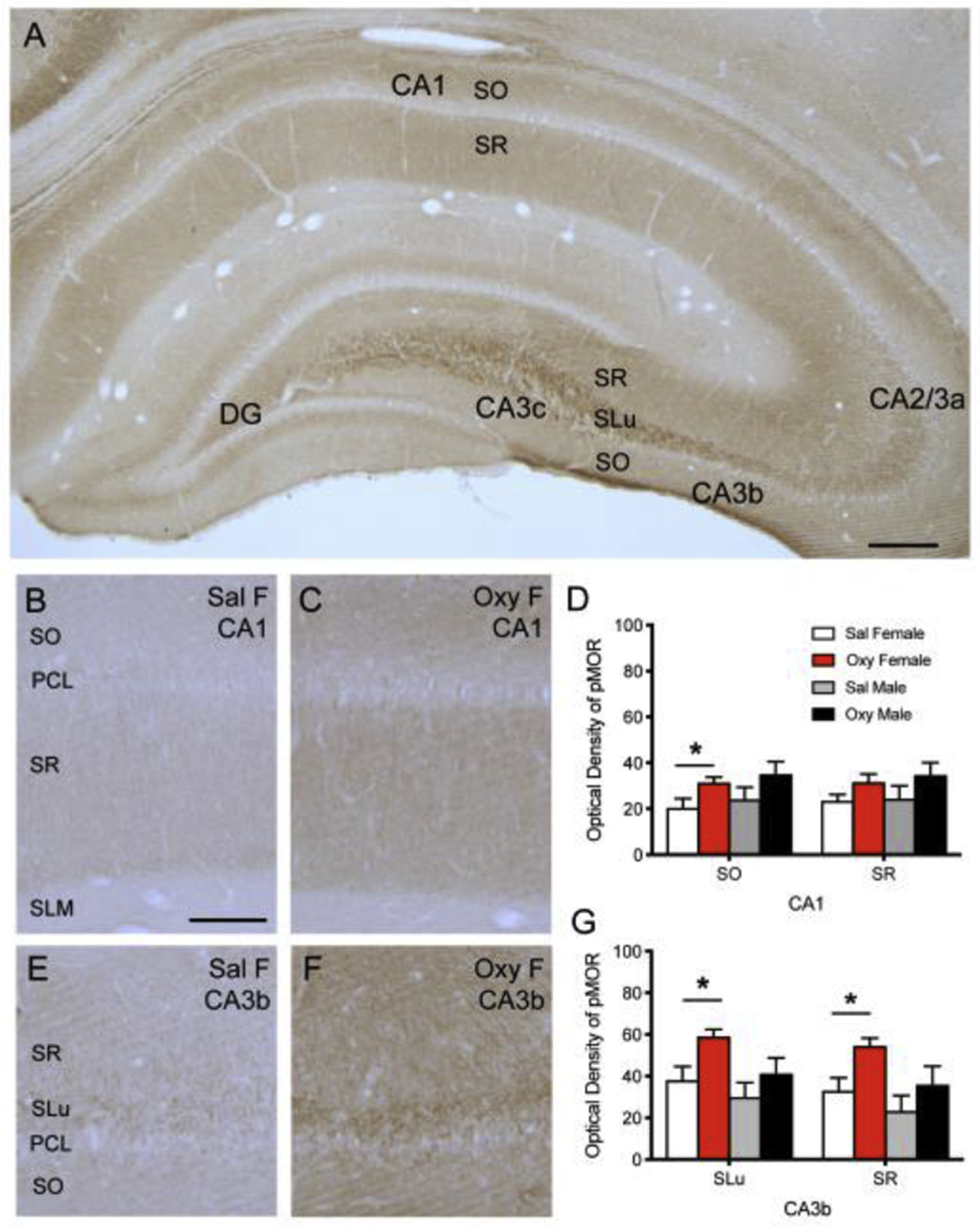
A. pMOR-ir is most dense in the SLu of CA3a, b and c and less dense in the SO and SR of the CA1 and CA3. Sparse diffuse pMOR-ir is seen in the central hilus of the dentate gyrus (DG). B,C. Representative images show pMOR-ir in the SO, PCL, SR, and SLM of CA1 in a Sal-female (B) and an Oxy-female (C) rat. D. The density of pMOR-ir increases in the SO of CA1 in Oxy-females compared to Sal-females. E,F. Representative images of pMOR-ir in the SR, SLu, PCL, and SO of CA3b in a Sal-female (E) and an Oxy-female (F) rat. G. The density of pMOR-ir increases in the SLu and SR of CA3b in Oxy-females compared to Sal-females. Scale bar A = 250 μm; B,C,E,F = 100 μm; *p < 0.05.
Fig 2: pDOR levels are increased in naïve Oxy-males compared to Sal-males and in CIS Oxy-females compared to CIS Sal-females in CA2/3a.
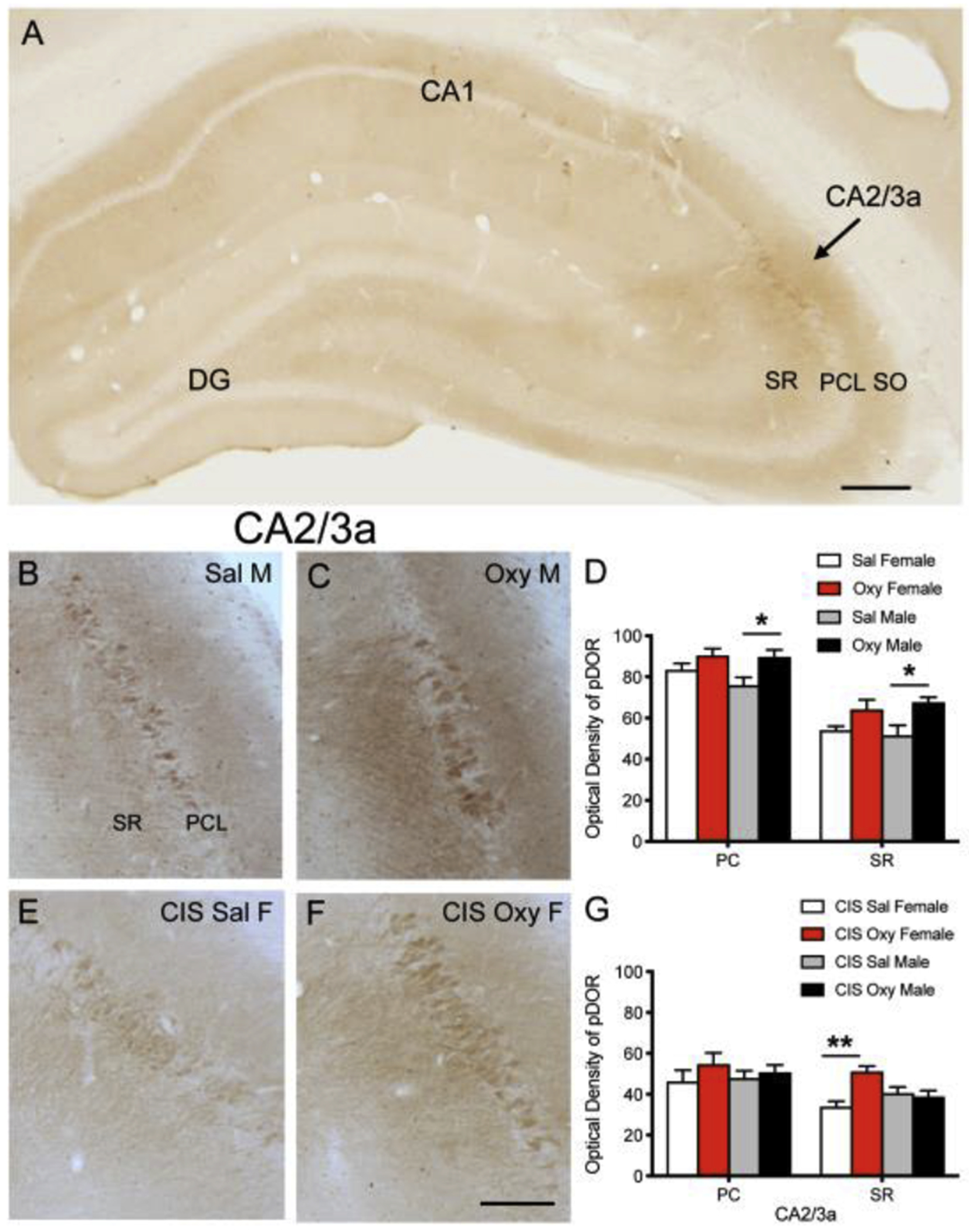
A. pDOR-ir is most dense in the pyramidal cell layer and SR of CA2/3a. B,C. Representative images show pDOR-ir in the SR, PCL, and SO of CA2/3a in a Sal-male (B) and an Oxy-male (C) rat. D. The density of pDOR-ir increases in the PCL and SO of CA2/3a in Oxy-males compared to Sal-males. E,F. Representative images of pDOR-ir in the SR, PCL, and SO of CA2/3a in a CIS Sal-female (E) and a CIS Oxy-female (F) rat. G. The density of pDOR-ir increases in the SR of CA2/3a in CIS Oxy-females compared to CIS Sal-females. Scale bar A = 250 μm; B,C,E,F = 100 μm; *p < 0.05; **p < 0.01.
3.2. pDOR levels are increased in naïve Oxy-males compared to Sal-males
Consistent with our previous study [23], pDOR-ir was found primarily in CA2/3a pyramidal cell bodies and dendrites in SR (Fig. 2A). In unstressed (naïve) rats, the optical density of pDOR-ir is increased in Oxy-males compared to Sal-males in the CA2/3a PCL (t(10)=−2.71; p=0.0220; Fig 2B–D) and CA2/3a SR (t(10)=−2.98; p=0.0138; Fig 2B–D). No changes in pDOR-ir were observed between unstressed (naïve) Sal- and Oxy-females in either subregion of CA2/3a (Fig. 2D).
3.3. CIS alone has little effect on pMOR and pDOR levels in females and males
The optical density of pMOR-ir increased in control males compared to control females in the CA3c SO (p=0.0135; not shown). No changes in pMOR-ir were observed in any other lamina in CA3 or in CA1 in CIS females and males.
3.4. pMOR-ir is similar in CIS Sal- and CIS Oxy-rats while pDOR is elevated in CIS Oxy-females
In contrast to naïve rats, there were no differences in the optical density of pMOR-ir in any subregion of CA1 or CA3 in CIS Oxy- and CIS Sal-females or males. However, the optical density of pDOR-ir is increased in the CA2/3a SR of CIS Oxy-female compared to CIS Sal-female rats (t(8)=−4.70; p=0.0015) with no change in pDOR-ir in CA2/3a in CIS Sal- and CIS Oxy-males (Fig. 2E–G).
4. Discussion
Here we find that oxycodone CPP increases the levels of pMOR in CA1 and CA3 neurons in naïve females and pDOR in CA2/3A neurons in naïve males. As phosphorylation is important for the internalization and trafficking of opioid receptors [12–14], the results suggest that oxycodone CPP redistributes MORs and DORs within select circuits in a sex-dependent manner. Moreover, we find that following CIS, in which only females acquire oxycodone CPP [11], the levels of pDOR are increased in CA2/3a pyramidal neurons in females but not males. These results have important implications for understanding the mechanisms of our previously reported sex differences in opioid receptor trafficking within hippocampal neurons following oxycodone-associative learning [7, 11, 15].
MOR agonists such as oxycodone can bind with various affinities to MOR splice variants and phosphorylation of MORs may result in differentiated actions of those variants [24]. MOR1D, a prominent splice variant in the rat hippocampus, is found in the mossy fiber pathway and throughout lamina containing CA1 and CA3 pyramidal cell dendrites as well as interneurons [25]. Thus, the pattern of pMOR-ir labeling reflects a combination of labeled MOR1A, which is in interneurons [26], MOR1D and other MOR splice variants [18]. pMOR-ir is abundant within mossy fiber axons [18], suggesting that MOR activation may influence the transduction of electrical signals to the terminal and/or protein transport [27, 28]. As mossy fibers also contain opioid peptides [26], activation of MORs could auto-regulate granule cell synaptic transmission.
pMOR-ir is also found in axon terminals forming asymmetric synapses with dendritic spines in CA3 [18], suggesting that MOR activation could affect excitatory transmission of other afferents to pyramidal cell neurons [29]. Thus, the elevation of pMOR in CA1 and CA3 in females following oxycodone CPP suggests that circuits in these regions have been altered in a manner that would enhance synaptic plasticity. Our prior studies showed that the threshold for MOR basal excitatory transmission in mossy fiber-CA3 pyramidal cell synapses is lower in proestrus (high estrogen) females compared to males [8]. However, the elevation of pMOR in mossy fiber axons in females following oxycodone CPP could provide an alternate mechanism that would promote excitation at these synapses. This would have important functional consequences as opioid signaling in the CA3 region is important in promoting associative learning processes [2, 3].
The density of pDOR-ir in CA2/3a pyramidal cells and dendrites was increased in naïve Oxy-males compared to Sal-males in a way that elevated them to the same levels as Sal- and Oxy-females. As our prior studies showed that baseline pDOR levels are higher in estrus females compared to males [23], the lack of change in the naïve Oxy-females compared to Sal-females may indicate that pDOR levels are already maximal. Within CA2/3a pyramidal cell soma and proximal dendrites, pDOR-ir is localized to endomembranes and endoplasmic reticula, organelles associated with protein synthesis and receptor trafficking [29]. Moreover, acute morphine (1 hour, 20mg/kg, I.P.) elevates pDOR-ir in CA2/3a pyramidal cell soma in naïve male rats [23]. As morphine and oxycodone are structurally similar in that they are both small opiate alkaloids with morphinan base structures [30], DORs in CA2/3a pyramidal cells likely are activated during oxycodone CPP training. In addition to soma, pDOR-ir is prominent within the spines of CA3 dendrites contacted by mossy fibers and other excitatory afferents [23]. Thus, the elevation of pDOR levels in CA2/3a pyramidal cell soma and dendrites in males compared to females could be important for plasticity processes through their downstream effects. In support, DORs are elevated in mossy fiber-CA3 synapses in naïve Oxy-males to levels known to promote opioid-mediated LTP in naïve proestrus females [7, 8].
The increase in pDOR-ir in CA3 SR in the naïve Oxy-males parallels the upregulation of the immediate early gene activity-regulated cytoskeleton-associated protein (Arc) mRNA CA3 in naïve Oxy-males compared to Sal-males [15]. Additionally, our prior study [15] demonstrated that naïve Oxy-males compared to naïve Oxy-females have elevated levels of brain derived neurotrophic factor (BDNF) expression in the dentate gyrus, which harbors the granule cells known to produce BDNF in the mossy fibers [31]. Both Arc and BDNF are important regulators of synaptic plasticity processes including synaptogenesis and LTP [32, 33]. Together these findings suggest that DORs as well as key synaptic molecules in CA2/3a pyramidal neurons and mossy fiber afferents are activated in males following oxycodone CPP. Moreover, although it is not know what signaling molecules contribute to the increase in pDOR-ir seen in CIS Oxy-females compared to CIS Sal-females, our prior studies in naïve Oxy-females suggest that ARC may be involved [15].
Unlike CIS Oxy-females, CIS Sal- and Oxy-males had unaltered pMOR and pDOR levels. This finding is in agreement with our prior findings examining the hippocampal opioid system in this same cohort of rats [11]. Specifically, the levels of MORs in GABAergic interneuron dendrites are similar in CIS Sal- and Oxy-males [11]. Furthermore, the number of DOR-labeled dendritic spines contacted by mossy fibers in CA3 was significantly reduced in CIS Sal- and Oxy-males [11]. Additionally, corticotropin releasing hormone receptor, which is colocalized with DORs in pyramidal cell neurons [6] and is important for synaptic efficacy and LTP [34], is increased in naïve Oxy-males but significantly decreased after CIS [35]. Together, these findings indicate that CIS Oxy-males compared to their unstressed counterparts did not activate MORs and DORs in a manner that allows them to develop the synaptic plasticity necessary for opioid associated learning processes such as CPP.
In conclusion, the present demonstration that oxycodone CPP activates MORs in CA1 and CA3 neurons in naïve females and in contrast DORs in CA2/3a neurons in naïve males suggests that facilitation of drug-associated learning may occur through differing mechanisms in the two sexes. Moreover, these findings suggest that the absence of activation of MORs and DORs in CIS Oxy-males may contribute to their inability to acquire CPP.
Highlights:
Oxycodone conditioned place preference alters opioid receptor phosphorylation
The hippocampal region containing activated opioid receptors varies with sex
Phosphorylated mu opioid receptor levels increase in CA1 and CA3a,b in females
Phosphorylated delta opioid receptors (pDOR) increase in CA3/CA2a in males
After chronic immobilization stress, pDOR levels increase CA3/CA2a in females
Acknowledgements
Supported by NIH grants DA08259 (T.A.M., M.J.K., B.S.M.), HL098351 (T.A.M.), HL 136520 (T.A.M.), MH041256 (B.S.M.) and MH102065 (J.D.G.), and Hope for Depression Research grant (B.S.M.). We thank NYC Dept. of Education Work Based Learning program for sponsoring J.R.B. We thank Mr. Konrad T. Ben, Mr. Joshua Kogan, Ms. June Chan and Dr. Diane Lane for technical assistance and Ms. Megan Johnson and Ms. Fangmin Yu for assistance with figure preparation.
ABBREVIATIONS
- ABC
avidin-biotin complex
- ARC
activity-regulated cytoskeleton-associated protein
- BSA
bovine serum albumin
- CIS
chronic immobilization stress
- CPP
conditioned place preference
- DG
dentate gyrus
- DOR
delta opioid receptor
- GABA
Gamma-amino butyric acid
- ir
immunoreactivity
- LTP
long-term potentiation
- MOR
mu opioid receptor
- Oxy
oxycodone
- PARV
parvalbumin
- PB
phosphate buffer
- PCL
pyramidal cell layer
- pDOR
phosphorylated DOR
- PFA
paraformaldehyde
- pMOR
phosphorylated MOR
- ROI
region of interest
- Sal
saline
- SLu
stratum lucidum
- SO
stratum oriens
- SR
stratum radiatum
- TS
tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References:
- [1].Prescription Painkiller Overdoses: A growing epidemic, especially among women. CDC Vital Signs, Centers for Disease Control and Prevention, 2013. [Google Scholar]
- [2].Kesner RP, Warthen DK, Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats, Hippocampus 20 (2010) 550–557. [DOI] [PubMed] [Google Scholar]
- [3].Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL Jr., Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory, J Neurosci 24 (2004) 2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ, Chronic stress and neural function: accounting for sex and age, J Neuroendocrinol 19 (2007) 743–751. [DOI] [PubMed] [Google Scholar]
- [5].McEwen BS, Milner TA, Hippocampal formation: shedding light on the influence of sex and stress on the brain, Brain Res Rev 55 (2007) 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McEwen BS, Milner TA, Understanding the broad influence of sex hormoner and sex differences in the brain, J Neurosci Res 95 (2017) 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ryan JD, Zhou Y, Contoreggi NH, Bshesh FK, Gray JD, Kogan JF, Ben KT, McEwen BS, Jeanne Kreek M, Milner TA, Sex differences in the rat hippocampal opioid system after oxycodone conditioned place preference, Neuroscience 393 (2018) 236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE, Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat, J Neurosci 35 (2015) 1723–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA, Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress, Neurobiol Stress 5 (2016) 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM, Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus, Synapse 67 (2013) 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reich B, Zhou Y, Goldstein E, Srivats SS, Contoreggi NH, Kogan JF, McEwen BS, Kreek MJ, Milner TA, Gray JD, Chronic immobilization stress “primes” the hippocampal opioid system for oxycodone-associated learning in female but not male rats, Synapse (2019) 73(5): e22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deng HB, Yu Y, Wang H, Guang W, Wang JB, Agonist-induced mu opioid receptor phosphorylation and functional desensitization in rat thalamus, Brain Res 898 (2001) 204–214. [DOI] [PubMed] [Google Scholar]
- [13].Law PY, K ouhen OM, Solberg J, Wang W, Erickson LJ, Loh HH, Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor, J Biol Chem 275 (2000) 32057–32065. [DOI] [PubMed] [Google Scholar]
- [14].Doll C, Konietzko J , Poll F, Koch T, Hollt V, Schulz S, Agonist-selective patterns of mu-opiod receptor phosphorylation revealed by phosphosite-specific antibodies, Br J Pharmacol 164 (2011) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Randesi M, Contoreggi NH, Zhou Y, Rubin BR, Bellamy JR, Yu F, Gray JD, McEwen BS, Milner TA, Kreek MJ, Sex Differences in neuroplasticity- and stress-related gene expression and protein levels in the rat hippocampus following oxycodone conditioned place preference, Neuroscience 410 (2019) 274–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V, Morphine induces terminal mu-opioid receptor desensitization by sustained phosphorylation of serine-375, Embo j 23 (2004) 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chu J, Zheng H, Loh HH, Law PY, Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins, Cell Signal 20 (2008) 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gonzales KL, Chapleau JD, Pierce JP, Kelter DT, Williams TJ, Torres-Reveron A, McEwen BS, Waters EM, Milner TA, The influences of reproductive status and acute stress on the levels of phosphorylated mu opioid receptor immunoreactivity in rat hippocampus, Front Endocrinol (Lausanne) 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL, In vivo delta opioid receptor internalization controls behavioral effects of agonists, PLoS One 4 (2009) e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Swanson LW, Brain Maps: Structure of the Rat Brain, Elsevier, Amsterdam, 1992. [Google Scholar]
- [21].Milner TA, Waters EM, Robinson DC, Pierce JP, Degenerating processes identified by electron microscopic immunocytochemical methods, Methods Mol Biol 793 (2011) 23–59. [DOI] [PubMed] [Google Scholar]
- [22].Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA, Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress, J Chem Neuroanat 55 (2014) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burstein SR, Williams TJ, Lane DA, Knudsen MG, Pickel VM, McEwen BS, Waters EM, Milner TA, The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus, Brain Res 1518 (2013) 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pasternak GW, Pan YX, Mu opioids and their receptors: evolution of a concept, Pharmacol Rev 65 (2013) 1257–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abbadie C, Pan Y, Drake CT, Pasternak GW, Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS, Neuroscience 100 (2000) 141–153. [DOI] [PubMed] [Google Scholar]
- [26].Drake CT, Chavkin C, Milner TA, Opioid systems in the dentate gyrus, Prog Brain Res 163 (2007) 245–263. [DOI] [PubMed] [Google Scholar]
- [27].Cheung DW, Synaptic transmission in the guinea-pig vas deferens: the role of nerve action potentials, Neuroscience 37 (1990) 127–134. [DOI] [PubMed] [Google Scholar]
- [28].Cunnane TC, Stjarne L, Frequency dependent intemittency and ionic basis of impulse conduction in postganglionic sympathetic fibres of guinea-pig vas deferens, Neuroscience 11 (1984) 211–229. [DOI] [PubMed] [Google Scholar]
- [29].Peters A, Palay SL, Webster H.d., The fine structure of the nervous system: neurons and their supporting cells, Oxford University Press, New York, 1991. [Google Scholar]
- [30].Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT, Differential activation and trafficking of mu-opioid receptors in brain slices, Mol Pharmacol 74 (2008) 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gray JD, Milner TA, McEwen BS, Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors, Neuroscience 239 (2013) 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K, The Arc of synaptic memory, Exp Brain Res 200 (2010) 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cunha C, Brambilla R, Thomas KL, A simple role for BDNF in learning and memory?, Front Mol Neurosci 3 (2010) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen Y, Andres A, Frotscher M, Baram TZ, Tuning synaptic transmission in the hippocampus by stress: the CRH system, Frontiers in Cellular Neuroscience 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, Waters EM, Milner TA, Sex differences in the subcellular distribution of corticotropin-releasing factor receptor 1 in the rat hippocampus following chronic immobilization stress, Neuroscience 383 (2018) 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]


