Abstract
Sites along the Elizabeth River are contaminated with polycyclic aromatic hydrocarbons (PAHs) from historical creosote production and other industrial processes. Previous studies have demonstrated that Atlantic killifish collected from sites throughout the Elizabeth River display resistance to the teratogenic effects of PAH-exposure in a manner commensurate with sediment PAH concentrations. The current study characterized various chemical pollutants in sediment and investigated the effects of aqueous sediment extracts from sites along the Elizabeth River to the cardiac development of Atlantic killifish embryos from fish collected from an uncontaminated reference site. Embryonic cardiac deformities were more prevalent after exposure to extracts from sites with high PAH loads. However, activation of cytochrome P4501A, a gene up-regulated by PAH-induction of the aryl hydrocarbon receptor and measured using an in ovo EROD assay, did not consistently increase with PAH concentrations. This work further characterizes sediments in the Elizabeth River, as well as provides insight into the evolutionary pressures at each ER site.
Keywords: Polycyclic aromatic hydrocarbons, Teratogenesis, CYP P4501A, Aryl hydrocarbon receptor
Introduction
The Elizabeth River (ER) is an estuarine system south of the Chesapeake Bay in Virginia, USA. During the nineteenth and twentieth centuries, several wood treatment facilities along the Southern Branch of the ER processed creosote, a complex mixture of hydrocarbons that include polycyclic aromatic hydrocarbons (PAHs), and polluted the river system with remnants of the preservative (Di Giulio and Clark 2015). Sites with pronounced creosote pollution include the location of the former Republic Creosoting Company, the former Eppinger & Russell (site currently owned by the Hess Corporation; hereafter called, Money Point), and the Atlantic Wood Industries site (which ceased operation and was added to EPA’s National Priorities List in 1990) (Di Giulio and Clark 2015). Other locations in the ER exhibit varying PAH concentrations in surficial sediments (Clark et al. 2013).
PAHs are ubiquitous environmental contaminants released primarily from incomplete combustion of organic material from natural and anthropogenic sources (Cerniglia 1992). They are structurally comprised of two or more fused benzene rings, a molecular structure that lends to hydrophobicity and recalcitrance in sediments (Cerniglia 1992; Kanaly and Harayama 2000). PAHs include known carcinogenic, teratogenic and mutagenic compounds and have been found to elicit a number of toxic effects across taxa (Nebert et al. 2004; Wills et al. 2010; Jung et al. 2011). In aquatic organisms, PAHs have been reported to cause hepatic neoplasms (Vogelbein et al. 1990), pericardial edema (Billiard et al. 2008), and other cardiac abnormalities (Clark et al. 2010). Some PAHs are potent aryl hydrocarbon receptor (AHR) pathway agonists and produce similar toxicological effects to those caused by dioxin-like compounds (Billiard et al. 2006; Clark et al. 2010; Incardona et al. 2005).
Several subpopulations of ER Fundulus heteroclitus (also known as mummichog, or Atlantic killifish; hereafter referred to as killifish) inhabit sites with relatively high PAH contamination and have evolved varying levels of resistance to the developmental teratogenicity of these complex PAH mixtures (Clark et al. 2013; Ownby et al. 2002). There exists an association between genetic variation amongst killifish subpopulations and PAH concentrations (Mulvey et al. 2002) and it has recently been determined that PAH-tolerant killifish subpopulations have independently and more recently diverged from the Elizabeth River killifish gene pool (Reid et al. 2016). A number of studies have described the molecular and cellular underpinnings of this adaptive response, which includes the downregulation of AHR pathways, and ER killifish have become a useful model for understanding mechanisms of PAH toxicity (Billiard et al. 2006; Jung et al. 2011; Meyer et al. 2005; Wassenberg and Di Giulio 2004).
Consequences to this adaptive resistance have been demonstrated in recent studies which measured altered swimming performances, reduced thermal plasticity, and decreased aerobic scope in killifish with PAH-tolerance (Brown et al. 2017; Jayasundara et al. 2017). These fitness costs may impair killifish ability to escape predators and find optimum environments in the face of habitat destruction or climate change. This work also suggests that there are other compromised biological processes (i.e., reproduction or stress responses) that may be impacted by the energy allocated to surviving in a chemically polluted environment (Jayasundara et al. 2017).
This species is beneficial for use in environmental research due to its non-migratory behavior, high fecundity, transparent embryonic development, and the availability of extensive physiological and genetic information (Armstrong and Child 1965; Burnett et al. 2007). In the context of this work, the limited migratory range of ER killifish, which effectively produces subpopulations of killifish within the estuary, enhances the study of the effects of chemical and other localized stressors (Lotrich 1975). Additionally, the distribution of killifish within the ER watershed, coupled with each study site’s location relative to historical contamination, provides a natural experiment to examine intra-population responses to anthropogenic stressors. Indeed, Clark et al. (2013) evaluated ER subpopulation-specific adaptive resistance to PAHs by exposing killifish embryos from differentially contaminated sites in the ER to sediment extracts derived from the highly PAH contaminated Atlantic Wood Industries Superfund site. Clark and colleagues demonstrated that ER killifish, in addition to PAH-resistance, are resistant to the effects of a number of other developmental and environmental toxicants including polychlorinated biphenyls (PCBs) and pesticides (Clark et al. 2013). Furthermore, Clark et al. (2013) inferred that multiple, independently evolved mechanisms might be underlying resistance to these chemicals in ER subpopulations. Among several explanations for this hypothesis, local adaption to a unique contaminant mixture at each site remains probable.
The goal of this study was to quantify the effects of contaminated sediment exposure for developmental toxicity in killifish embryos that have not been previously exposed (and therefore have not adapted) to environmental conditions impacted by PAHs and other industrial activities. Embryos of killifish obtained from an uncontaminated reference site (King’s Creek, VA) were exposed to sediment extracts collected from the same ER sites as Clark et al. (2013) and were monitored for sublethal developmental effects (e.g., cardiovascular abnormalities, such as edema and gross malformations). The test sediments were also characterized for total PAH content (the expected driver of toxicity for this selection of samples) as well as other potential contaminants of concern (i.e., PCBs, trace metals) that might contribute to exposures of mixtures. Overall, these experiments were designed to describe site-specific chemical profiles that may be serving as selection pressures that are influencing adaptive responses at various locations along the Elizabeth River.
Materials and methods
Fish collection and embryo extraction
Atlantic killifish were collected with minnow traps from the KC site (Fig. 1) during the summers of 2013 and 2014 and transported to Duke University (Durham, NC, USA). The fish were prophylactically treated for parasites (PraziPro Parasite Treatment, Hikari, Hayward, CA) and allowed to acclimate to the lab setting for 1 month before being used for embryo production. Fish were maintained in a recirculating artificial seawater system at ~15 ppt salinity and 28 °C on a 14:10 light:dark cycle with daily feeding of Purina® AquaMax® Fingerling Starter 300 (Land O’Lakes, Minneapolis, MN, USA) food. Spawning activity was monitored and during peak fertility fish were manually spawned to fertilize eggs (Armstrong and Child 1965). All animal collection, handling, and experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee (Protocol number: A184-13-07).
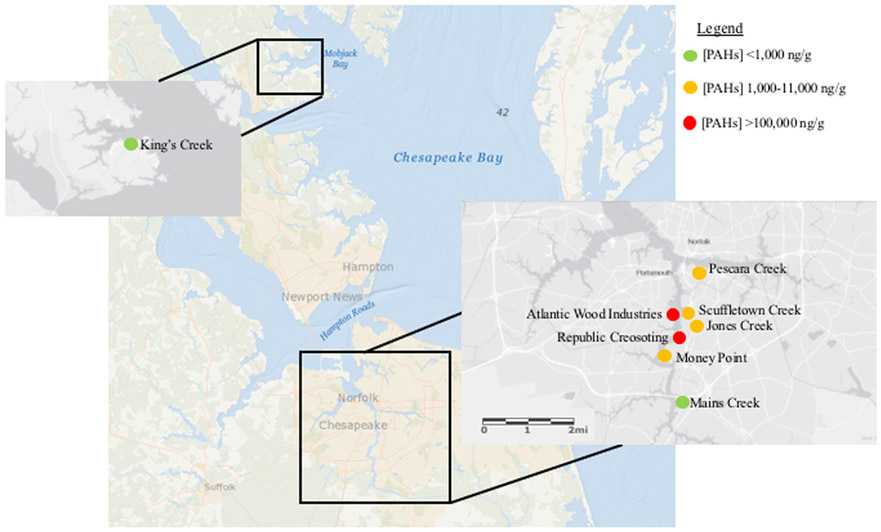
Fig. 1.
Map of sampling sites in Elizabeth River and King’s Creek (reference) sampling site in the Severn River, Virginia. Red dots indicate sites with measured high concentrations of PAHs, orange dots indicate moderate PAH concentrations, and green dots indicate low PAH concentrations
Sediment collection and storage
Sediment was collected in June 2014 from a reference site located at King’s Creek on the Severn River, VA, and seven sites in the Elizabeth River, VA (Fig. 1): Mains Creek (MC), Scuffletown Creek (SC), Pescara Creek (PC), Jones Creek (JC), Money Point, Republic Creosoting, and Atlantic Wood Industries (AW) (Clark et al. 2013; Di Giulio and Clark 2015). Sediment samples were collected at low tide by researchers accessing the site by foot. Sampling points were determined based on previously reported coordinates, reasonable access points, and proximity to perceived killifish egg-laying locations (Clark et al. 2013). Sediment samples were collected using a stainless steel hand shovel at six random pre-determined points along three 30-m transects running parallel to the shore (18 samples per site). Each transect was separated by 1 m. Approximately 1500 g of sediment was collected from each sample point. The 18 samples collected were combined and mixed within a 7.5 L plastic bucket to form a composite sediment sample from each site. Composite samples were stored in a walk-in cold room kept at 4 °C.
A second sample collection at King’s Creek, used for the metals analysis, was conducted in August 2014 applying the same collection, processing, and storage methods as the other site sediments. The sediments from the Republic Creosoting site were collected in August 2014 using a stainless steel Ponar grab, as the site was only accessible by boat. Five Ponar grabs in various locations throughout the killifish habitat in the river enclave, resulted in a site composite sample with an approximate mass of 750 grams. Sediment with visible creosote sheen was specifically collected at the Republic site, as opposed to the random sediment collection method employed at the other sites. This strategy, of obtaining a highly polluted representative sample or worst-case scenario, was employed to investigate the PAH profile at this site, which had not been previously accessible by our researchers.
Sediment extract preparation
Composite sediment extracts for each site were created based on the methods described by Clark et al. (2013). Sediments were processed into extracts within 1 week of collection to avoid excessive PAH-to-plastic adsorption. Each composite sediment sample was thoroughly mixed using a stainless steel paint-mixing electric drill attachment. Aliquots of 25 mL of sediment and 25 mL of deionized water were added to a 50 mL centrifuge tube (VWR International, Radnor, Pennsylvania). All tubes were placed horizontally on a rotary shaker for 24 h in the dark at 150–200 revolutions per minute (RPM) at 20 °C. Tubes were then centrifuged at 1000 relative centrifugal force (RCF) for 25 min. The supernatant was decanted from each tube to a single glass flask to produce a composite extract sample for each site (n = ~120 replicates per composite). Composite extract samples (~3000 mL/site) were aliquoted into amber glass jars with PTFE-lined lids and stored at −20 °C until thawed and used in embryo assays.
Embryo dosing, erod activity, and cardiac deformity assessment
At 24-h post fertilization (hpf), fertilized killifish embryos from the KC site were placed in clear glass scintillation vials with 10 mL dosing solution. Dosing solutions were prepared in glass beakers and contained 25 v/v % sediment extract from each Elizabeth River site, 21 μg/L ethoxyr-esorufin (a substrate used in the EROD assay), artificial seawater (Instant Ocean, Foster & Smith, Rhinelander, WI, USA) added as necessary to attain 15 ppt salinity, and deionized water. Control dosing solutions excluded sediment extracts. Fifty killifish embryos were dosed with the control solution and 30 embryos were dosed with the solutions containing sediment extracts from each site. Embryos that did not survive dosing exposures were removed from analyses.
The ethoxyresorufin-O-deethylase (EROD) assay serves as a sensitive biomarker of exposure to PAHs and other planar halogenated or aromatic xenobiotic chemicals, by indicating cytochrome P4501A (CYP1A) induction and AHR pathway upregulation (Whyte et al. 2000). EROD activity was quantified as previously described (Nacci et al. 2010; Timme-Laragy et al. 2007). At 96-hpf, embryos were observed under a fluorescence microscope (50x magnification, Zeiss Axioskop, Thornwood, NY, USA) and the difference in fluorescence intensity between the cardiac and yolk regions was measured.
Embryos at 144-hpf were blindly analyzed for cardiac deformities and general abnormalities utilizing light microscopy (40x magnification). Cardiac abnormalities were assessed on a 0–2 scale with a “0”-scored heart appearing normal, “1”-scored hearts appearing moderately deformed, and a “2”-scored heart resembling an elongated or “stringy-heart” as previously described (Brown et al. 2016; Clark et al. 2013, 2010; Matson et al. 2008).
Determination of PAHs in sediment
For PAH extraction, wet sediment (0.05 g) was ground with 0.25 g Na2SO4, spiked with 100 μL deuterated PAH surrogate mix (D10-2-methylnaphthalene, D10-fluorene, D10-fluoranthene, D12-chrysene, D12-perylene, and D12-indeno (1,2,3-c,d)pyrene), and extracted by ultrasonication for 5 min in 4 mL 1:1 hexane:acetone. Samples were centrifuged for 3 min at 3000 × g, and the pellets were extracted twice more, combining the supernatants. Samples were concentrated, treated to remove sulfur as previously described (Clark et al. 2013), and cleaned by silica solid phase extraction. All extractions were conducted in triplicate. Blanks (n = 3) and a standard reference material (SRM 1944, National Institute of Standards and Technology) were included with extractions. As previously described (Clark et al. 2013), PAHs were analyzed by gas chromatography-mass spectrometry (Agilent GC 6890N, MS 5975, Newark, DE) in electron impact mode. Sediment moisture content was measured gravimetrically and used to correct PAH concentrations to dry weight (Clark et al. 2013).
Determination of PCBs in sediment
To extract PCBs, 1 g of freeze-dried sediment was mixed with diatomaceous earth in an 11 cm3 Dionex ASE-200® cell (Sunnyvale, CA, USA), fortified with 100 ng PCB198 internal standard (Ultra Scientific, Rhode Island, USA) and extracted in a Dionex (ASE-200) automated solvent extraction unit using methylene chloride. Extracts were evaporated by high purity nitrogen and solvent exchanged to hexane. The extract volume was reduced and final volume adjusted to 1.0 mL with hexane. The extract was treated with sulfuric acid to remove interferences and allowed to react with acid for at least 24 h. Activated copper was then added to the clear extract to remove elemental sulfur.
Quantitative analysis for PCB congeners was performed on an Agilent 6890® Gas Chromatograph equipped with an electron capture detector. Contaminants measured were 18 PCB Congeners (IUPAC numbers 8, 18, 28/31, 31, 52, 44, 66/95, 101,118, 153, 105/132, 138, 187, 128, 180, 170, 195, 206 and 209) from National Oceanic and Atmospheric Administration (NOAA) National Status and Trends (NS&T) Program. Total PCBs (ng/g dry) were reported as the sum of the eighteen PCB congeners. To achieve the separation, a J&W DB-5 60 m fused silica capillary column (0.25 μm film thickness, 0.25 mm i.d.) was used. Ultra-high purity helium was the carrier gas (flow rate = 1.3 mL/min) and 95%:5% Argon/Methane (P5) was used as the auxiliary gas. The initial column temperature was held at 90 °C for 1 min, increased to 170 °C at 25 °C/min and then to 215 °C at 1.5 °C/min. After 3 min at 215 °C, the temperature was increased to 250 °C at 1.5 °C/min. Final column temperature was taken to 300 °C at 20 °C/min and held for 5 min. The injection port temperature was 250 °C and the detector temperature was 300 °C. Integration and calculations were accomplished by Agilent ChemStation® software.
Quality assurance (QA) samples included procedural blanks and standard reference material (NIST SRM 1944, a freeze-dried marine sediment with certified concentrations for PCBs). Procedural blanks were free of any contaminants. Recoveries of the measured PCB values versus the certified values were within the acceptance criteria (60–113%). Our method detection limit (MDL) was 1 ng/g dry for sediment samples.
Determination of extractable trace metals in sediment
Triplicate sediment samples from each site were dried at 100 °C for 24 h. The wet-to-dry mass ratio was noted for each replicate. Sediment (0.4–0.6 g) was mixed with 1 mL of concentrated HCl and 9 mL of concentrated HNO3, and processed by microwave assisted digestion (CEM SPD), according to procedures outlined in EPA Method 3051 (EPA 2007). The supernatant of the digested material was diluted in doubly deionized water (Millipore Milli-Q) and analyzed for trace element concentrations (Cr, Ni, Cu, Zn, As, Cd, and Pb) by inductively coupled plasma mass spectrometry (Agilent Technologies 7900 operated in helium reaction gas mode). The six metals were selected based on extraction results of a soil standard reference material (NIST 2709a San Joaquin soil SRM) that was digested and analyzed in the same batch as the ER sediment samples. The measured acid-extractable contents for Cr, Ni, Cu, Zn, Cd, and Pb for the SRM were within the reported range of values (NIST 2010). The measured extractable As content for the SRM was less than the reported value but was 80% of the reported total As content in the SRM. All acid-extractable trace element contents are reported on a dry mass basis and as the average (±1 standard deviation) of triplicates per site.
A subset of the sediment samples was also analyzed for total Hg content by direct combustion, gold amalgamation, atomic absorption spectroscopy (Milestone DMA-80). The Hg values are considered a total element content and analysis methods were verified by analysis of the NIST 2709 SRM (total Hg recoveries were 95.5% of the certified value for 6 replicate measurements).
Determination of loss on ignition
The loss on ignition test is an indicator of the organic matter comprised within each sediment sample (Heiri et al. 2000). The extended drying and subsequent high-temperature baking periods remove organic matter, such as estuarine detritus. Three grams of wet sediment from each sample site was added to separate, pre-weighed aluminum weigh boats. The sediment was placed in a drying oven at 100 °C for 24 h. The weight of each sample was recorded before and after the drying period and then placed in a muffle furnace at 375 °C for 4 h. Samples were weighed after removal and cooling, and compared to their dry weight prior to being heated. Sediment from each site was analyzed in triplicate, with the average difference in sample weight recorded as a percentage of the dry weight.
Data analysis
Analyses were conducted using R, an open source statistical software (version 3.2.2, Vienna, Austria). Cardiac deformities and EROD response data were rank-transformed and analyzed using a two-way analysis of variance (ANOVA) test (Brown et al. 2016; Clark et al. 2013; Oziolor et al. 2016). A Tukey post-hoc comparison was conducted to determine significant differences among the cardiac deformities in KC embroys exposed to each site sediment extract. A p-value ≤ 0.05 was used to define statistical significance for all tests.
Results
Population responses to exposures
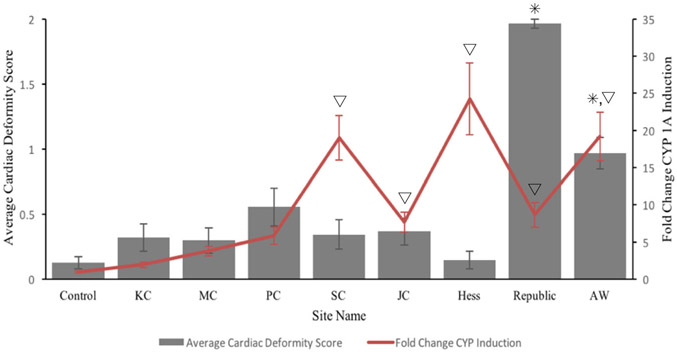
The developmental responses of KC killifish embryos to the different sediment extracts is presented in Fig. 2. Killifish exposed to KC and MC site sediment extracts, all with low PAH concentrations, did not exhibit significantly different cardiac deformities from the control. Similarly, there was not a significant increase in cardiac deformities in killifish exposed to sediment extracts from JC, PC, or SC—sites with moderate PAH concentrations. Republic and AW site sediment extracts induced significantly more cardiac deformities, with AW inducing more abnormal effects (heart score = 1) and Republic inducing either death or malformed hearts (heart score = 2). Cardiac deformity occurrences were determined to be more frequent and severe following exposures to extracts from sites with relatively high PAH concentrations.
Fig. 2.
King’s Creek embryonic cardiac deformities and induction of cytochrome P4501A (CYP1A) with exposure to sediment extracts from seven sites along the Elizabeth River and one reference site in the Severn River—King’s Creek (KC; n = 28). Elizabeth river sites are Mains Creek (MC; n = 30), Pescara Creek (PC; n = 27), Scuffletown Creek (SC; n = 29), Jones Creek (JC; n = 30), Hess/Eppinger & Russell/Money Point (Hess; n = 27), Republic Creosoting (Republic; n = 29), and the Atlantic Wood Industries (AW; n = 30). The Control site reflects an exposure that contained no sediment extract (n = 47). Gray bars represent average phenotypic cardiac deformity score, as calculated based on a 0–2 point weighted scale (left y-axis). The red line indicates the induction of CYP1A based on the EROD assay and is represented as fold changes from control values. The visual representation of a line does not indicate connectedness between site values. Triangles denote statistically significant (p ≤ 0.05) differences in responses to CYP1A induction from the control. Asterisks denote statistically significant differences in average cardiac deformities from the control
Embryos exposed to the Republic site extract were significantly more deformed than those exposed to extracts from any other site (p < 0.001). Embryos exposed to the Atlantic Wood site extract showed a significant increase in cardiac deformities from those exposed to Mains or Jones Creek site extracts. When compared to a control exposure (comprised of ASW, ethoxyresorufin, and DI water), the only site extracts that induced significantly elevated cardiac deformity scores were from Republic (p < 0.001) and Atlantic Wood (p = 0.0037). No other site extracts resulted in significant cardiac deformities when compared to the control exposure group. All of the sediment extract exposures significantly induced EROD except those from KC, MC, and PC sediments. Interestingly, the Money Point site, which began remediation in 2009 and included the dredging and disposal of contaminated sediment, displayed the most pronounced induction of EROD activity (~24 fold) when compared to the control group. However, the Money Point/Hess site did not exhibit significantly increased cardiac deformities.
Sediment characterization
Total selected PAHs detected in each site’s sediments are shown in Table 1. The concentrations of individual PAHs are included in the Supporting Material section. As expected, PAH measurements were highest at the AW and Republic sites and lowest at the KC and MC sites.
Table 1.
Concentrations of total selected PAHs at eight sites along the Elizabeth River, VA and a reference site, King’s Creek, Severn River, VA
| Site name | Total selected PAHs (ng/g dry sediment) |
|---|---|
| Mains Creek | 212.0 |
| King’s Creek | 953.3 |
| Money point (Eppinger & Russell; Hess) | 2729.7 |
| Jones Creek | 5136.4 |
| Pescara Creek | 7648.6 |
| Scuffletown Creek | 10,772.4 |
| Republic Creosoting | 295,902.9 |
| Atlantic Wood Industries | 786,947.9 |
Sediment PCB concentrations ranged from 106–122 ng/g in samples from AW, SC and PC (Table 2). Sediment PCB concentrations for Republic and JC were measured at 26.1 and 8.89 ng/g, respectively. Concentrations of PCBs were below the detection limits for KC, MC, and Money Point.
Table 2.
Concentrations of total PCBs (18 congeners) at seven sites along the Elizabeth River, VA and one reference site at King’s Creek, Severn River, VA
| Site name | Total PCBs (ng/g dry weight) |
|---|---|
| King’s Creek (reference site) | ND |
| Mains Creek | ND |
| Pescara Creek | 122.0 |
| Scuffletown Creek | 107.0 |
| Jones Creek | 8.9 |
| Hess (Eppinger & Russell) | ND |
| Republic Creosoting | 26.1 |
| Atlantic Wood Industries | 106.0 |
“ND” (not detected) describes a sample with values below the detection limit. The Atlantic Wood Industries reported value is an average of two replicate samples
Although the wide range of PAH contents were expected to be the major driver of the different toxicity outcomes between the sediment samples, analysis of the sediments for other potential toxicants varied widely between the samples. Among the acid-extractable trace elements quantified in the sediment samples (Cr, Ni, Cu, Zn, As, Cd, and Pb), PC sediment demonstrated higher trends of trace element values compared to the other sites (chromium was the exception). Moreover, the extractable zinc and lead contents in the sediments at PC exceeded the Effects Range Medium (ERM) sediment quality guidelines established by the National Oceanic and Atmospheric Administration (NOAA) (Table 3) (Buchman 1999). Total Hg contents were also generally within values expected of industrial and urbanization influences, with the reference sites containing the lowest Hg content and PC containing the highest total Hg. Every site, except for Money Point, had at least one trace element at concentrations greater than the NOAA Effects Range Low (ERL) guideline.
Table 3.
Average acid-extractable contents of selected trace elements in soil (shown in μg/g dry weight basis, n = 3 samples) at seven sites along the Elizabeth River Estuary, VA and one reference site at King’s Creek, Severn River, VA
| Site name | Lead | Chromium | Nickel | Copper | Zinc | Arsenic | Cadmium | Mercury |
|---|---|---|---|---|---|---|---|---|
| King’s Creek (reference site) | 18.9 ± 3.6 | 21.5 ± 3.5 | 10.2 ± 1.7 | 12.7 ± 2.3 | 62.4 ± 12.3 | 3.6 ± 0.6 | 0.3 ± 0.1 | 0.09 ± 0.01 |
| Mains Creek | 17.2 ± 0.8 | 2.2 ± 0.1 | 1.4 ± 0.03 | 4.4 ± 1.9 | 9.6 ± 0.1 | 0.5 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Pescara Creek | 334 ± 36.7 | 21.1 ± 2.8 | 22.7 ± 3.4 | 258.1 ± 33.3 | 426 ± 6.0 | 10.4 ± 1.2 | 0.99 ± 0.1 | 0.6 ± 0.03 |
| Scuffletown Creek | 37.6 ± 5.1 | 18.1 ± 1.7 | 10.5 ± 0.4 | 41.8 ± 1.5 | 208.3 ± 105.4 | 5.02 ± 0.1 | 0.4 ± 0.03 | 0.2 ± 0.01 |
| Jones Creek | 32.4 ± 2.4 | 13.9 ± 1.0 | 7.2 ± 0.5 | 34.2 ± 0.7 | 124.6 ± 8.3 | 4.1 ± 0.2 | 0.5 ± 0.04 | 0.19 ± 0.01 |
| Money Point | 1.7 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.9 | 1.6 ± 0.4 | 5.7 ± 0.8 | 0.7 ± 0.1 | 0.1 ± 0.01 | 0.02 ± 0.00 |
| Republic Creosoting | 48.2 ± 22.9 | 22.8 ± 6.8 | 13.1 ± 2.2 | 41.3 ± 2.8 | 185.7 ± 28.4 | 6.5 ± 0.5 | 0.5 ± 0.03 | 0.24 ± 0.01 |
| Atlantic Wood Industries | 72.6 ± 7.8 | 17.2 ± 1.9 | 13.6 ± 0.2 | 96.9 ± 6.0 | 208 ± 8.6 | 9.7 ± 0.4 | 0.9 ± 0.2 | 0.46 ± 0.1 |
The italicized values represent values greater than the Effects Range Low (ERL) and the bold values represent values greater than the Effects Range Medium (ERM)
Selected metals concentrations were positively correlated with the amount of organic matter (as measured by LOI) in sediment samples, such that site sediments with low organic matter had lower metals concentrations (Supplementary Material) (Di Giulio and Scanlon 1985). This trend was significant for all metals except for zinc and copper (p < 0.05). The PC site exceeded the NOAA ERL for all metals except for cadmium and had the highest concentrations of each metal type compared to every other sampling site. This is likely due to its proximity to an industrial shipyard.
The %LOI was relatively similar among site sediments (Table 4). However, sediments from the MC and Money Point sites exhibited an order of magnitude lower %LOI values compared to the other sites. The differences in organic matter contents at these site sediments may be due to the sediment composition of the site. Differences may also be biased by the specific point of sediment collection (i.e., proximity to shore, erosional or depositional zone).
Table 4.
Loss on ignition values, measuring the percent dry weight of organic matter in sediment samples from seven sites along the Elizabeth River, VA and one reference site at King’s Creek, Severn River, VA
| Site name | Loss on ignition values (% of sample dry weight) |
|---|---|
| King’s Creek (reference site) | 9.00 |
| Mains Creek | 0.53 |
| Pescara Creek | 11.37 |
| Scuffletown Creek | 6.28 |
| Jones Creek | 6.35 |
| Hess (Eppinger & Russell) | 0.39 |
| Republic Creosoting | 10.44 |
| Atlantic Wood Industries | 8.78 |
Discussion
This research was motivated by a gap in knowledge about the response of a reference population of killifish to sediment extracts from multiple sites with varying chemical contamination along the ER. A previous study by Clark et al. (2013) determined how embryos from multiple ER subpopulations responded to a highly polluted sediment extract from the AW site. In this study, killifish embryos from the reference site (King’s Creek) were exposed to sediment extracts from seven sites in the Southern Branch of the Elizabeth River, as well as their native site. Results show that embryonic exposure to site sediment extracts with relatively high PAH contamination induced developmental cardiac deformities, such as gross malformation and pericardial edema.
The EROD assay indicated higher CYP1A induction in embryos exposed to AW, Republic, SC, JC, and Money Point sediments. These results are inconsistent with the expectation that the most highly contaminated sediment extracts, producing the greatest phenotypic cardiac deformities, would generate greater CYP1A induction than extracts from other sites. These results may be due to the presence of PAHs within those site sediments that have the ability to activate AHR, but do not exist in concentrations significant enough to cause cardiac deformities (Clark et al. 2013). In support of this hypothesis, it has been shown that PAH-related toxicity can occur by AHR-independent mechanisms (Incardona et al. 2005; Reid et al. 2016). Further, EROD induction may be a more sensitive response in killifish than gross cardiac malformations.
One potential confounding factor in our study is that cytochrome P450s are induced by only certain PAHs and therefore EROD assay could be underestimating PAH toxicity in a given sediment mixture. While this is certainly a possibility, EROD assay has been utilized in determining PAH toxicity in a number of studies (Barron et al. 2004; Brunström et al. 1991; Fouchécourt et al. 1999; Wessel et al. 2010). Given that sediment chemical analyses show that each mixture contains at least some types of PAHs that can induce CYP activity, the EROD assay serves as a useful marker of PAH toxicity.
It is also important to note that specific PAH mixtures have been known to include AHR agonists and CYP1 inhibitors which can result in synergistic toxicity in teleost species (Timme-Laragy et al. 2007). However, only a few PAHs have been tested in mixtures and therefore, it is difficult to determine which PAHs or combinations of PAHs in the sediment extracts in this work are contributing to the toxic effects observed. Further, given that many PAHs inhibit CYP1A, the low EROD activity measured in embryos exposed to Republic and AW extracts may be an artifact of CYP1A inhibition by the PAHs present.
The PAH measurements for each site represented the same trend and magnitude reported by Clark et al. (2013), with the exception of the Money Point site. Previously reported values for PAH concentrations at the Money Point site, obtained by Vogelbein et al. (1990) and included in Clark et al. (2013), reflect sediment concentrations prior to site remediation, which likely account for the differences in trends reported in our work. This remediation effort, involving industry, government, and community partnerships, began in 2009 and involved sediment dredging and the installment of a sand containment cap (Di Giulio and Clark 2015). Remediation also likely explains the relatively low measurement of organic matter in Money Point sediments.
Dosing solutions prepared in this experiment were sediment extracts representing porewater concentrations of PAHs and therefore, based on partitioning properties of PAHs, are likely orders of magnitude lower in PAH concentrations than whole sediment measurements reported in this work. However, cardiac deformities and EROD induction was still observed and previously reported chemical analyses of PAHs detected the presence of potent AHR agonists in ER porewater (Fang et al. 2014).
This study is the first to report trace element concentrations at these sites in the ER. While our toxicity testing did not evaluate biological endpoints associated with exposure to metals, these data serve to enrich our knowledge of the complexity of environmental contamination at these sites and the potential for synergistic or alternative effects of environmental chemical pollution. This study also did not conduct PCB exposures, however Clark et al. (2013) reported effects in killifish embryos collected from fish from the KC, AW, SC, JC, PC, and MC sites after exposure to the PCB congener, PCB-126. Embryos from the MC site demonstrated sensitivity, and embryos from the KC site exhibited significantly higher cardiac deformities after PCB-126 exposure than the other sites (Clark et al. 2013). Our quantification of PCBs in the sediments indicate that fish at the KC and MC sites have not been exposed to elevated PCB concentrations within their habitats and therefore suggests that fish from these sites should be more sensitive to its teratogenicity compared to the other sites. Interestingly, Clark et al. (2013) demonstrated little embryonic sensitivity in SC, JC, and PC killifish embryos; sites where we identified PCB contamination. This may suggest that the presence of PCBs in their respective native habitats may have contributed to their lack of sensitivity to these compounds. However, the concentrations detected at these sites are relatively low and may not be responsible for adverse biological outcomes (Long et al. 1995).
While the LOI analysis can provide us with information about the natural organic matter within these sediments, further characterization of the sediments at each site is necessary to get a better understanding of PAH-to-sediment sorption potential. Since bioavailability of PAHs to both macro- and microbiota depends not only the chemical characteristics, but also the distribution of the chemical compounds in the sediment, an enhanced understanding of the physicochemical properties of the sediments and PAHs at each site would more adequately inform the hypotheses for the demonstrated differential resistance to PAH toxicity by ER fish populations.
Overall, we found that various sites in the Elizabeth River are differentially contaminated with complex mixtures of aromatic hydrocarbon compounds and potentially toxic trace metals and metalloids. The bioavailability of compounds in these mixtures may depend on the total organic matter in the sediment and may play an important role in determining the overall biological toxicity for organisms inhabiting this river. Indeed, this may lead to different selection pressures driving subpopulation level adaptive and acclamatory changes in killifish and other organisms. This hypothesis is supported by our findings on differential toxicity of sediment extracts from various sites coupled with the findings of Clark et al. (2013) on gradation of tolerance to PAH mixtures in fish from these sites. Our findings also highlight the importance of extensive characterization (i.e., chemical pollutants as well physiochemical properties) of sediment samples when determining biological effects of polluted watersheds.
Supplementary Material
Acknowledgements
We thank Dr. Michael Unger and George Vadas of Virginia Institute of Marine Sciences (VIMS) for their support in collecting sediment from the Republic field site. We authors also thank Marc Gutterman (USACE) for allowing us access to the Atlantic Wood Industries site and for assisting with sediment collection.
Funding This work was funded by the NIEHS-sponsored Duke University Superfund Research Center (P42ES010356).
Footnotes
Supplementary information The online version of this article (https://doi.org/10.1007/s10646-019-02116-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval Mention of trade names or commercial products does not constitute endorsement or recommendation for use. All applicable institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Armstrong PB, Child JS (1965) Stages in the normal development of fundulus heteroclitus. Biol Bull 128:143–168 [Google Scholar]
- Barron MG, Heintz R, Rice SD (2004) Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res 58:95–100. 10.1016/j.marenvres.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT (2008) Nonadditive effects of PAHs on early vertebrate development: Mechanisms and implications for risk assessment. Toxicol Sci 105:5–23. 10.1093/toxsci/kfm303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT (2006) The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol Sci 92:526–536. 10.1093/toxsci/kfl011 [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT (2016) Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol 53:55–63. 10.1016/j.ntt.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Thompson J, Chernick M, Hinton DE, Di Giulio RT (2017). Later life swimming performance and persistent heart damage following subteratogenic PAH mixture exposure in the atlantic killifish (Fundulus heteroclitus). Environ Toxicol Chem. 10.1002/etc.3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstrom B, Broman D, Näf C (1991) Toxicity and EROD-inducing potency of 24 polycyclic aromatic hydrocarbons (PAHs) in chick embryos. Arch Toxicol 65:485–489. 10.1007/BF01977361 [DOI] [PubMed] [Google Scholar]
- Buchman MF (1999). NOAA screening quick reference tables. [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gómez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL (2007) Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp Biochem Physiol—Part D Genomics Proteom 2:257–286. 10.1016/j.cbd.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351–368. 10.1007/BF00129093 [DOI] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT (2013) Compound and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus Subpopulations throughout the Elizabeth River Estuary (Virginia, USA). Environ Sci Technol 47:10556–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Matson CW, Jung D, Di Giulio RT (2010) AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus). Aquat Toxicol 99:232–240. 10.1016/j.aquatox.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio RT, Clark BW (2015) The Elizabeth River Story: a case study in evolutionary toxicology. J Toxicol Environ Heal Part B 18:259–298. 10.1080/15320383.2015.1074841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio RT, Scanlon PF (1985) Heavy metals in aquatic plants, clams, and sediments from the chesapeake Bay, U.S.A.: implications for waterfowl. Sci Total Environ 41:259–274 [Google Scholar]
- Fang M, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, Ferguson PL, Stapleton HM (2014) Effect-directed analysis of Elizabeth River porewater: Developmental toxicity in zebrafish (Danio rerio). Environ Toxicol Chem 33:2767–2774. 10.1002/etc.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchécourt MO, Arnold M, Berny P, Videmann B, Rether B, Rivière JL (1999) Assessment of the bioavailability of PAHs in rats exposed to a polluted soil by natural routes: Induction of EROD activity and DNA adducts and PAH burden in both liver and lung. Environ Res 80:330–339. 10.1006/enrs.1998.3932 [DOI] [PubMed] [Google Scholar]
- Heiri O, Lotter AF, Lemcke G (2000) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25(1):101–110 [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL (2005) Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect 113:1755–1762. 10.1289/ehp.8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasundara N, Fernando PW, Osterberg JS, Cammen KM, Schultz TF, Di Giulio RT (2017) Cost of tolerance: physiological consequences of evolved resistance to inhabit a polluted environment in teleost fish Fundulus heteroclitus. Environ Sci Technol acs. est.7b01913. 10.1021/acs.est.7b01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Matson CW, Collins LB, Laban G, Stapleton HM, Bickham JW, Swenberg JA, Di Giulio RT (2011) Genotoxicity in Atlantic killifish (Fundulus heteroclitus) from a PAH-contaminated Superfund site on the Elizabeth River, Virginia. Ecotoxicology 20:1890–1899. 10.1007/s10646-011-0727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra Kanaly, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria MINIRE-VIEW biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067. 10.1128/JB.182.8.2059-2067.2000.Updated [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E, MacDonald D, Smith S (1995) Incidence of adverse bilogical effects within ranges of chemical concentrations in marine and estuarine sediments. Env Manag 19:81 [Google Scholar]
- Lotrich VA (1975) Summer home range and movements of fundulus heteroclitus (Pisces: Cyprinodontidae) in a Tidal Creek. Ecology 56:191–198 [Google Scholar]
- Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT (2008) Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol 87:289–295. 10.1016/j.aquatox.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Volz DC, Freedman JH, Di Giulio RT (2005) Differential display of hepatic mRNA from killifish (Fundulus heteroclitus) inhabiting a Superfund estuary. Aquat Toxicol 73:327–341. 10.1016/j.aquatox.2005.03.022 [DOI] [PubMed] [Google Scholar]
- Mulvey M, Newman MC, Vogelbein W, Unger MA (2002) Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol 61:195–209. 10.1016/S0166-445X(02)00055-3 [DOI] [PubMed] [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S (2010) Adaptation of the estuarine fish fundulus heteroclitus (Atlantic Killifish) to polychlorinated biphenyls (PCBs). Estuaries Coasts 33:853–864. 10.1007/s12237-009-9257-6 [DOI] [Google Scholar]
- National Institute of Standards and Technology Special Publication (2010), 260–172, 39 pages [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850. 10.1074/jbc.R400004200 [DOI] [PubMed] [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF (2002) Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem 21:1897–1902. 10.1002/etc.5620210917 [DOI] [PubMed] [Google Scholar]
- Oziolor EM, Dubansky B, Burggren WW, Matson CW (2016) Cross-resistance in Gulf killifish (Fundulus grandis) populations resistant to dioxin-like compounds. Aquat Toxicol 175:222–231. 10.1016/j.aquatox.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF, Crawford DL, Whitehead A (2016) The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science 354:1305–1308. 10.1126/science.aah4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT (2007) Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat Toxicol 85:241–250. 10.1016/j.aquatox.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (2007) Method 3051A (SW-846): Microwave assisted acid digestion of sediments, sludges, and oils, Revision 1 [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ (1990) Hepatic neoplasms in the Mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res 50:5978–5986. 10.1177/019262339402200302 [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT (2004) Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res 58:163–168. 10.1016/j.marenvres.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Wessel N, Santos R, Menard D, Le Menach K, Buchet V, Lebayon N, Loizeau V, Burgeot T, Budzinski H, Akcha F (2010) Relationship between PAH biotransformation as measured by biliary metabolites and EROD activity, and genotoxicity in juveniles of sole (Solea solea). Mar Environ Res 69:S71–S73. 10.1016/j.marenvres.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Whyte J, Jung R, Schmitt C, Tillitt DE (2000) Ethoxyresorufin- O-deethylase (EROD) Activity in Fish as a Biomarker of Chemical Exposure. Crit Rev Toxicol 30:347–570. 10.1080/10408440091159239 [DOI] [PubMed] [Google Scholar]
- Wills LP, Jung D, Koehrn K, Zhu S, Willett KL, Hinton DE, di Giulio RT (2010) Comparative chronic liver toxicity of benzo[a]pyrene in two populations of the atlantic killifish (Fundulus heteroclitus) with different exposure histories. Environ Health Perspect 118:1376–1381. 10.1289/ehp.0901799 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.