Abstract
Background
Cellular immune responses are not well characterized during the initial days of acute symptomatic influenza infection.
Methods
We developed a prospective cohort of human subjects with confirmed influenza illness of varying severity who presented within a week after symptom onset. We characterized lymphocyte and monocyte populations as well as antigen-specific CD8+ T-cell and B-cell responses from peripheral blood mononuclear cells using flow cytometry and enzyme-linked immunospot assays.
Results
We recruited 68 influenza-infected individuals on average 3.5 days after the onset of symptoms. Three patients required mechanical ventilation. Influenza-specific CD8+ T-cell responses expanded before the appearance of plasmablast B cells. However, the influenza-specific CD8+ T-cell response was lower in infected subjects than responses seen in uninfected control subjects. Circulating populations of inflammatory monocytes were increased in most subjects compared with healthy controls. Inflammatory monocytes were significantly reduced in the 3 subjects requiring mechanical ventilation. Inflammatory monocytes were also reduced in a separate validation cohort of mechanically ventilated patients.
Conclusions
Antigen-specific CD8+ T cells respond early during acute influenza infection at magnitudes that are lower than responses seen in uninfected individuals. Circulating inflammatory monocytes increase during acute illness and low absolute numbers are associated with very severe disease.
Keywords: Influenza, cellular immunity, illness severity, CD8+ T cell, B cell, monocyte
We show that circulating antigen-specific CD8+ T cells expand early during acute influenza infection. Inflammatory monocytes in peripheral blood increase during acute infection and low absolute numbers of circulating inflammatory monocytes are associated with respiratory failure.
Influenza is a major human respiratory pathogen and leads to approximately half a million deaths worldwide each year [1]. Between 15% and 35% of the world population is infected with seasonal circulating influenza viruses each year [2]. The majority of these naturally acquired influenza infections are asymptomatic. Only 2%–10% of individuals with influenza infection will have symptoms that motivate them to seek medical care [2]. Understanding the limitations of the influenza-specific immune response that contribute to such severe disease in this group of individuals may help to illuminate avenues for the development of novel therapeutics and vaccines.
Robust influenza-specific CD8+ T-cell responses after the establishment of acute infection are important in preventing influenza-associated disease and death in animal models, especially in animals without preexisting anti-influenza antibodies [3–5]. High-magnitude influenza-specific CD8+ T-cell responses have been associated with decreased viral shedding in volunteers experimentally infected with influenza A virus [6]. The development of an early and high-magnitude influenza-specific CD8+ T-cell response is critical for protection against fatal disease in humans who have been infected with the avian H7N9 virus [7]. These studies suggest that human influenza-specific CD8+ T-cell responses play a role in diminishing the severity of illness after infection with influenza virus.
The role of influenza-specific CD8+ T-cell responses during the first several days of naturally acquired seasonal influenza infection, presumably when these cells would be exerting their protective effects, has not been well studied. Evaluation of the human influenza experimental challenge model suggests that high-magnitude influenza-specific CD8+ T-cell responses within the first week of infection are protective [8]; however, this model system is limited to the evaluation of low-severity and asymptomatic influenza cases. Most studies evaluating CD8+ T-cell responses during symptomatic, naturally acquired influenza A infection use samples with average collection a week or more after the start of symptoms [9, 10]. Studies that have been performed with samples from earlier time points have been extremely limited in sample size [11].
Monocytes possess and exert critical antiviral activity. Inflammatory monocytes seem to mediate lung injury in animal models of severe influenza [12]. Variations in human monocyte populations have been associated with influenza severity [10, 13]. Individuals with more severe naturally acquired influenza illness than a comparison group had increased circulating peripheral blood CD14++CD16+ “inflammatory” and CD14lowCD16+ “patrolling” monocytes approximately 25 days after the start of symptoms [10]. Increased patrolling CD14lowCD16+ monocytes in the nasal mucosa of individuals with less severe illness in a separate study were associated with a signature of anti-inflammatory effect on local cytokine expression [13].
We evaluated circulating monocyte and lymphocyte populations as well as influenza-specific B-cell and CD8+ T-cell populations in human subjects who presented to the emergency department of a large US hospital for medical care during the 2017–2018 (H3N2 virus predominant) and 2018–2019 (H1N1 virus predominant) influenza seasons. We find that the majority of patients with acute symptomatic influenza infection demonstrate very low magnitude influenza A peptide-responsive CD8+ T-cell populations but that these antigen-responsive CD8+ T cells expand very early during acute infection before the expansion of circulating antigen-specific B-cell populations. Finally, we show that CD14++CD16+ inflammatory monocytes are prominently elevated in peripheral blood during the first days of acute symptomatic infection, except in individuals with respiratory failure necessitating mechanical ventilation.
METHODS
EDFLU Study Design
The EDFLU study was approved by the Washington University in St Louis Institutional Review Board (approval nos. 201710220 and 201808115). EDFLU complied with the ethical standards of the Helsinki Declaration. We approached patients with influenza A diagnosed in the emergency department with a clinical influenza real-time reverse-transcription polymerase chain reaction test (Cepheid Xpert Flu/RSV) performed during the normal course of workup and evaluation. We obtained written informed consent from each subject or from a legally authorized representative. EDFLU study inclusion criteria required that subjects actively experience influenzalike illness (ILI) symptoms at some point in the 24 hours before enrollment.
We excluded potential participants if they were <18 years old, if they had not experienced ILI symptoms in the last 24 hours, or if we were unable to obtain written informed consent from the subject or their legally authorized representative. Peripheral blood samples were obtained from enrolled subjects into ethylenediaminetetraacetic acid–anticoagulated tubes (BD Biosciences) and prepared within 8 hours of phlebotomy into peripheral blood mononuclear cells (PBMCs) using Ficoll density gradient purification. Nasal swab samples were also obtained at enrollment for viral analysis.
We classified illness severity in our influenza-infected cohort based on measured peripheral blood oxygen saturation using pulse oximetry. This classification strategy has been used by others [14]. We classified as having “severe” illness any subject who presented with measured oxygen saturation ≤93% on room air, or who required supplemental oxygen. The category of “very severe” illness encompassed subjects who required tracheal intubation and mechanical ventilation owing to hypoxic respiratory failure. We established a “not severe” illness group to control for significant variation in clinical attributes of study subjects who did not meet criteria for severe or very severe illness. The subjects in the “not severe” group met all of the following criteria: (1) oxygen saturation ≥98% on room air, 2) age <65 years, (3) a chest radiograph obtained during normal clinical care and read by the attending radiologist as clear, (4) a medical history that did not include any of the Advisory Committee on Immunization Practices–specified risk factors for severe influenza illness [15], and (5) discharge from the hospital after a stay of <48 hours from the time of presentation to the hospital.
We enrolled control subjects without influenza infection in 2 additional ongoing studies. Control subjects had not experienced ILI symptoms at the time of sample collection or within the previous 90 days. These studies were independently approved by the Washington University Institutional Review Board (approval nos. 201707160 and 201808171). Control subjects received the inactivated seasonal influenza vaccine intramuscularly between 60 and 180 days before sample collection.
We analyzed a separate validation cohort of PBMC samples obtained from subjects hospitalized with severe pandemic 2009 H1N1 at the National Institute of Respiratory Diseases in Mexico City, Mexico. This study was approved by the National Institute of Respiratory Diseases Institutional Review Board in May of 2009 (approval no. B08-09).
Multiparameter Flow Cytometry
The PBMCs were analyzed using a panel of antibodies directed against the following antigens: CD45 fluorescein isothiocyanate (clone HI30), CD3 Alexa 700 (clone UCHT1), CD4 allophycocyanin–cyanine 7 (clone OKT4), CD8 BV421 (clone RPA-T8), CD38 phycoerythrin–cyanine 7 (clone HIT2), HLA-DR BV605 (clone L243), CD19 BV750 (clone HIB19), CD20 Pacific Blue (clone 2H7), CD14 APC (clone M5E2), CD16 BV570 (clone 3G8), CD71 phycoerythrin (clone CY1G4), and immunoglobulin (Ig) D SuperBright 702 (clone IA6-2). PBMCs (1–2 × 106) were stained with a master-mix containing appropriate pretitrated concentrations of the antibodies along with BD Brilliant Buffer (BD Biosciences) and Zombie NIR Fixable Viability Marker (BioLegend) to differentiate live and dead cells. Samples were run on a Cytek Aurora spectral flow cytometer using SpectroFlo software, version 2.1.0 (Cytek) and unmixed before final analysis using FlowJo software, version 10.6.2 (BD Biosciences).
Enzyme-Linked Immunospot Assay
We measured influenza A-specific CD8+ T-cell populations using Mabtech’s interferon (IFN) γ ELISpotPRO horseradish peroxidase–precoated plates according to the manufacturers recommendations. We obtained an array of 91 individual 9-mer peptides representing highly conserved Major histocompatibility complex class I epitopes from the influenza A matrix protein M1, polymerase protein PB1 and nucleoprotein (NP) from BEI resources (NR-2667). This panel of CD8+ T-cell epitopes restricted by a diverse range of HLA alleles has been previously used to evaluate representative influenza-specific CD8+ T-cell populations in cohorts of human subjects with varied HLA haplotypes [16]. We tested peptides at a final concentration of 5 μmol/L with 300 000 PBMCs per well. We read plates with an ImmunoSpot plate reader (Cellular Technology). Each individual subject’s reported composite CD8+ T-cell response represents a summation of the total response to each of the 91 peptides.
To perform circulating B-cell plasmablast enzyme-linked immunospot (ELISPOT) assays, we coated ELISPOT plates with either the 2017–2018 Flublok vaccine (Protein Sciences) during the 2017–2018 influenza season or the 2018–2019 Flucelvax vaccine (Seqirus) during the 2018–2019 influenza season. Plates were developed with anti-human IgG and IgA reagents and then counted on an ImmunoSpot plate reader to provide the entire IgG- and IgA-secreting plasmablast population directed against the annual vaccine antigens.
Analysis
We compared absolute numbers of cells between influenza-infected and uninfected individuals using Mann-Whitney tests. We also used Mann-Whitney tests to compare the absolute number of antigen-specific plasmablasts and CD8+ T cells at each individual day after infection. We used the Kruskal-Wallis test with Dunn multiple comparisons test to evaluate variation in inflammatory monocytes in individuals grouped by duration of symptoms. Finally, we evaluated the various differences between control subject cell populations and cell populations found in the 3 classified influenza severity groups, using Kruskal-Wallis with Dunn multiple comparisons tests. All significance tests were 2 tailed. Statistical analyses were performed using Prism software, version 8 (GraphPad Software).
RESULTS
EDFLU Cohort Characteristics
We enrolled a total of 68 subjects with acute influenza A infection diagnosed in the emergency department, 22 in the 2017–2018 H3N2-predominant season and 46 in the 2018–2019 H1N1-predominant season. Viral sequencing of nasal swab samples from selected subjects in each influenza season confirmed that the majority of subjects recruited in 2017–2018 were infected with H3N2 viruses (21 of 22 viral isolates sequenced), and a majority of those recruited in 2018–2019 were infected with 2009 H1N1 viruses (18 of 21 isolates sequenced). Cohort characteristics are listed in Table 1. Subject-level characteristics are reported in the Supplementary Material. In our total cohort of 68 subjects, illness was classified as not severe in 7 subjects, as severe in 15, and as very severe in 3. Forty-three of the subjects did not meet the strict criteria for each of the defined severity groups and were therefore not included in severity of illness analyses.
Table 1.
Characteristics of Participants in EDFLU Cohort by Outcome Group
| 2017–2019 EDFLU Cohort Characteristics | Subjects by Outcome, % With Characteristic (No./Total in Group)a | ||||
|---|---|---|---|---|---|
| Total Cohort (N = 68) | Not Severe Outcome (n = 7) | Mixed Outcome (n = 43) | Severe Outcome (n = 15) | Very Severe Outcome (n = 3) | |
| Total for 2017–2018 (H3N2 predominant), no. | 22 | 2 | 14 | 6 | 0 |
| Total for 2018–2019 (H1N1 predominant), no. | 46 | 5 | 29 | 9 | 3 |
| Age, mean (IQR), y | 47 (34–61) | 36 (25–45) | 46 (29–62) | 55 (47–67) | 48 (45–50) |
| Female sex | 60 (41/68) | 43 (3/7) | 65 (28/43) | 53 (8/15) | 67 (2/3) |
| Duration of symptoms, mean (IQR), d | 3.5 (2–4) | 2.9 (2–3) | 3.4 (2–4) | 3.7 (2–4) | 5.0 (4–7) |
| Vaccinated in current year | 38 (25/65) | 14 (1/7) | 39 (16/41) | 53 (8/15) | 0 (0/2) |
| Hospital admission | 56 (38/68) | 0 (0/7) | 53 (23/43) | 87 (13/15) | 100 (3/3) |
| Hospital admission for >48 h | 32 (22/68) | 0 (0/7) | 23 (10/43) | 60 (9/15) | 100 (3/3) |
| ICU admission | 8.8 (6/68) | 0 (0/7) | 0 (0/43) | 20 (3/15) | 100 (3/3) |
| Intubation and mechanical ventilation | 4.4 (3/68) | 0 (0/7) | 0 (0/43) | 0 (0/15) | 100 (3/3) |
| In-hospital death | 1.5 (1/68) | 0 (0/7) | 0 (0/43) | 0 (0/15) | 33 (1/3) |
| Immunocompromise | 21 (14/68) | 0 (0/7) | 26 (11/43) | 20 (3/15) | 0 (0/3) |
| Chronic lung disease | 38 (26/68) | 0 (0/7) | 28 (12/43) | 87 (13/15) | 33 (1/3) |
| Chronic heart failure | 13 (9/68) | 0 (0/7) | 12 (5/43) | 27 (4/15) | 0 (0/3) |
| Active cancer | 7 (5/68) | 0 (0/7) | 5 (2/43) | 20 (3/15) | 0 (0/3) |
| Diabetes mellitus | 24 (16/68) | 0 (0/7) | 23 (10/43) | 27 (4/15) | 67 (2/3) |
Abbreviations: EDFLU, ; ICU, intensive care unit; IQR, interquartile range.
aData represent % with characteristic (no./total in group) unless otherwise specified.
We validated some of our findings using samples from a separate cohort of 6 subjects who were hospitalized at the National Institute of Respiratory Diseases in Mexico City with acute pandemic H1N1 influenza in 2009. One of these subjects met criteria for severe illness. The other 5 subjects required intubation and mechanical ventilation and were classified as having very severe illness. None of these subjects were immunocompromised. All 6 of these patients ultimately recovered from their illness after hospitalization.
Reduction in Lymphocyte Populations During the Earliest Days of Medically Attended Influenza Infection
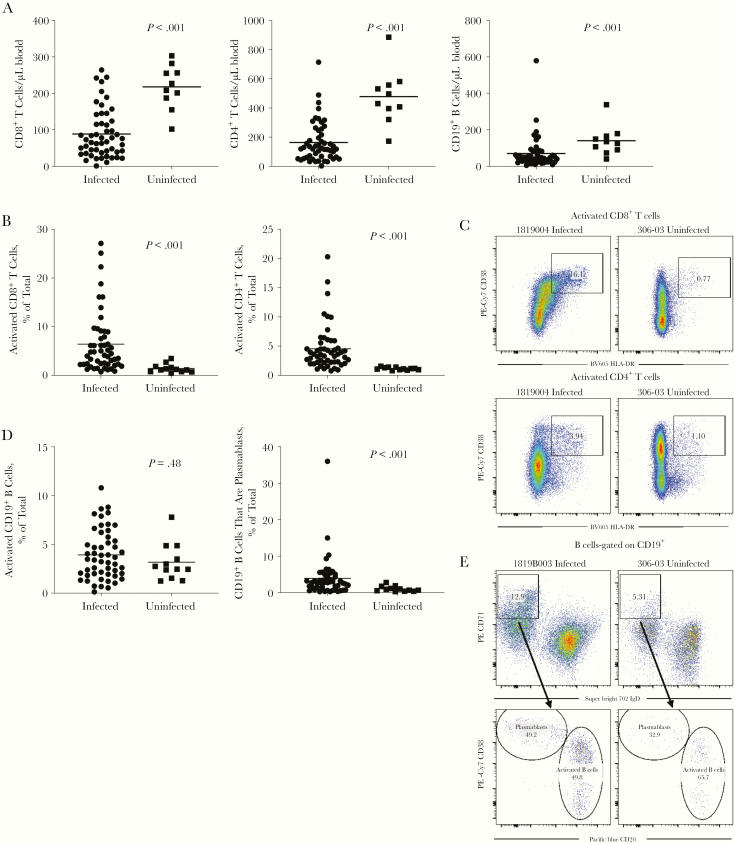
We measured B- and T-lymphocyte populations in both the infected and uninfected cohorts (Figure 1A). We found that CD8+ T-cell populations were decreased in the influenza-infected cohort compared with uninfected individuals. CD4+ T cells and CD19+ B cells were also decreased in infected subjects. We found significantly increased populations of activated (CD38+HLA-DR+) CD8+ T cells, activated CD4+ T cells and CD19+ plasmablasts (IgD−CD71highCD20lowCD38high) in the infected cohort (Figure 1B–1E), without a significant increase in numbers of activated CD19+ B cells (IgD−CD71highCD20highCD38low-intermediate) (Figure 1D and 1E).
Figure 1.
Peripheral blood lymphocyte populations during acute infection. Acutely infected subjects (n = 52) are compared with a cohort of uninfected control subjects (n = 12 total, n = 10 with absolute counts available). A, Total CD8+ T cells, total CD4+ T cells, and total CD19+ B-cell populations measured in peripheral blood. B, Quantified percentage of total CD8+ T cells and CD4+ T cells expressing both the HLA-DR and CD38 markers of T-cell activation. C, Representative fluorescence-activated cell sorting (FACS) plots of CD8+ and CD4+ T-cell activation staining gated on total live CD3+CD8+ or CD3+CD4+ cells, numbers are percentage of cells present in the gate that is drawn. Abbreviations: Cy7, cyanine 7; PE, phycoerythrin. D, The percentage of total CD19+ B cells with the activated B-cell surface phenotype—immunoglobulin (Ig) D−CD71hiCD20highCD38low-intermediate—or the plasmablast B-cell surface phenotype—IgD−CD71hiCD20lowCD38high. E, Representative FACS plots gated on live CD19+ B-cell populations, numbers are percentage of cells present in the gate that is drawn.
Selective Expansion of Inflammatory Monocyte Population During Acute Medically Attended Influenza Infection
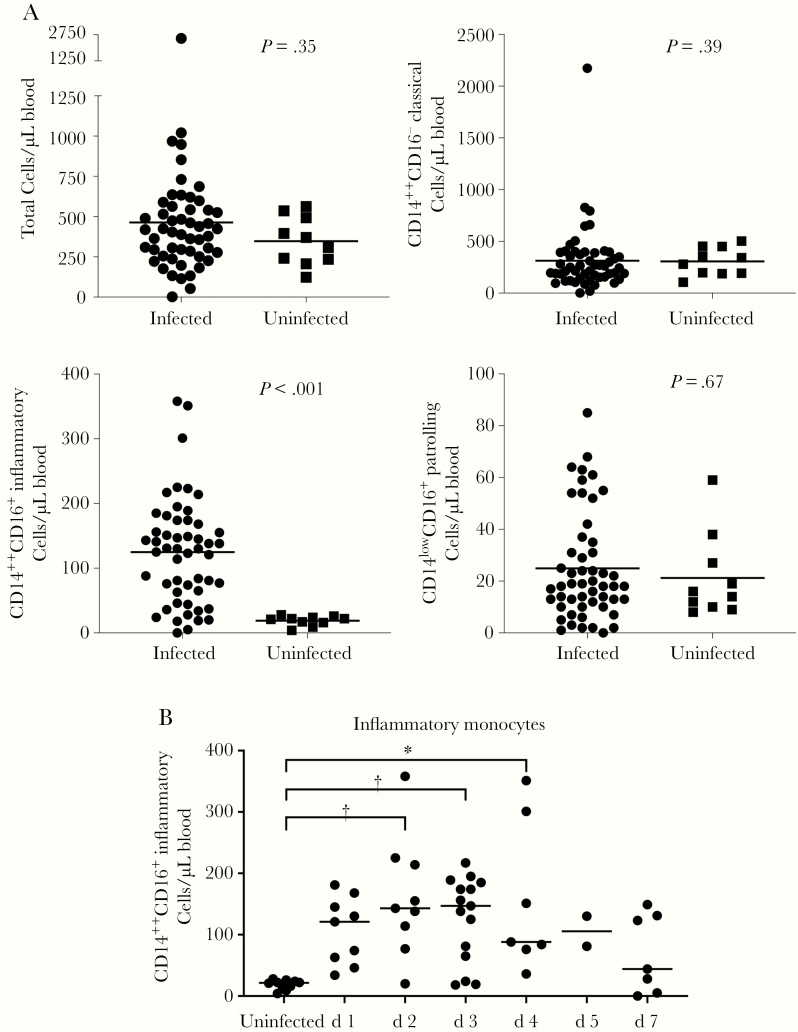
We evaluated specific monocyte populations in our cohort (Figure 2A) using cell surface markers that have been widely used by others [10, 14, 17, 18]. Absolute numbers of total circulating monocytes (CD14+), classical monocytes (CD14++CD16−) and patrolling monocytes (CD14lowCD16+) were similar between infected individuals and control subjects. We found that the absolute number of circulating inflammatory monocytes (CD14++CD16+) were elevated >6-fold in infected subjects compared with uninfected subjects.
Figure 2.
Circulating peripheral blood monocyte populations during acute infection in infected (n = 52) and uninfected (n = 10) subjects. A, Absolute numbers of live CD45+CD3−CD19− cells that are CD14+ “total,” CD14++CD16− “classical,” CD14++CD16+ “inflammatory,” and CD14lowCD16+ “patrolling” monocytes per microliter of peripheral blood. B, Inflammatory monocyte population size measured in subjects grouped by duration of symptomatic illness before sample collection. *P < .05; †P < .005.
We then grouped individual influenza-infected subjects by self-reported duration of symptoms before sample collection to determine when average numbers of inflammatory monocyte populations increase compared with controls during acute infection (Figure 2B). Inflammatory monocyte populations in individual patients were largest in those experiencing the first 4 days of symptomatic illness, compared with controls. Vaccination status of the infected subjects did not affect the time from symptom onset to presentation, nor the absolute number of circulating inflammatory monocytes (data not shown).
Early Influenza-Specific CD8+ T-Cell and B-Cell Recall Response Characterization
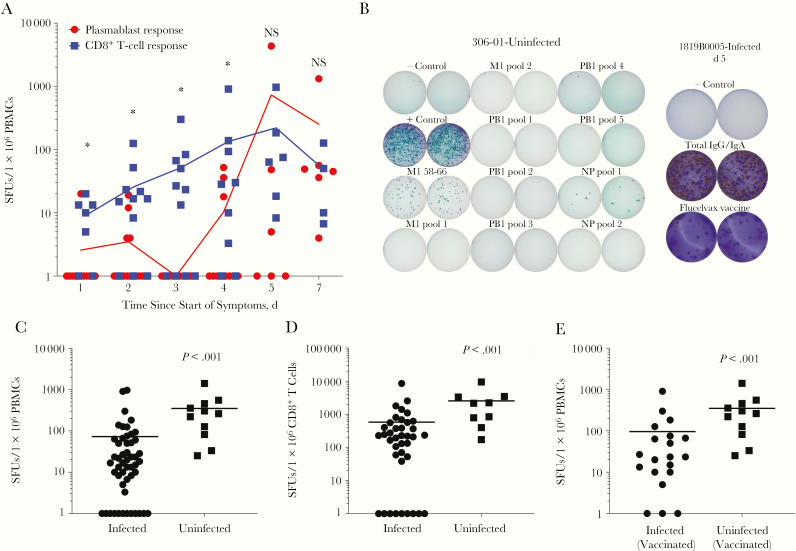
We evaluated the influenza-specific populations of CD8+ T cells and B cells to understand the dynamics of the adaptive immune response after establishment of infection. We found that the influenza antigen-responsive CD8+ T-cell population was higher in magnitude than the influenza antigen-specific B-cell plasmablast response in individuals presenting to the emergency department during the first 4 days of illness symptoms (Figure 3A). In individuals presenting after 5 or 7 days of symptoms, the B-cell plasmablast and CD8+ T-cell responses seemed to be equivalent and maximal.
Figure 3.
Influenza-responsive CD8+ T-cell populations expand early during acute infection, albeit at magnitudes lower than those observed in uninfected control subjects. A, The total magnitude of the interferon γ –producing CD8+ T-cell response directed against a panel of influenza CD8+ T-cell epitopes is plotted in blue (n = 48 subjects) along with the total immunoglobulin (Ig) G– and IgA-plasmablast response directed against the antigens found in the quadrivalent seasonal influenza vaccine in red (n = 57 subjects). Responses are grouped by self-reported number of days of symptomatic illness before sample collection. *P < .05; NS, not significant. B, Representative wells from CD8+ T-cell and plasmablast enzyme-linked immunospot assays, respectively, in 1 control and 1 infected subject. Abbreviations: IgG/IgA, immunoglobulin G/immunoglobulin A; NP, nucleoprotein. C, Decreased influenza-responsive CD8+ T-cell population in infected subjects (n = 51) compared with uninfected control subjects (n = 11). D, Influenza-specific CD8+ T-cell responses per 106 peripheral blood mononuclear cells (PBMCs) were normalized to the absolute number of CD8+ T cells in individual subjects to provide a value for spot-forming units (SFUs) per 106 circulating CD8+ T cells (n = 39 infected; n = 9 uninfected). E, Influenza-responsive CD8+ T-cell population in infected (n = 20) and uninfected (n = 11) subjects who confirmed receiving the seasonal influenza vaccine during the season in which they were enrolled in the study.
Circulating influenza-specific B-cell populations are a very rare population and difficult to measure in the peripheral blood of uninfected individuals. However, circulating influenza antigen-responsive CD8+ T-cell populations are readily detectable in uninfected human subjects. We next determined the magnitude of the influenza-responsive CD8+ T-cell population in our cohort of uninfected controls. We found that influenza-infected subjects had significantly reduced circulating frequencies of influenza-responsive CD8+ T cells when compared with uninfected individuals (Figure 3C). To control for the CD8+ T-cell lymphopenia we previously noted in infected subjects (Figure 1A), we normalized the measured influenza-responsive CD8+ T-cell population to the absolute count of CD8+ T cells circulating in blood for each individual subject. The significant reduction in circulating influenza-responsive CD8+ T cells in our cohort remained (Figure 3D).
It has been well established that the currently used seasonal influenza vaccines do not significantly expand influenza-responsive CD8+ T-cell populations [19]. Nevertheless, to control for the possibility that our uninfected cohort exhibited higher responses secondary to inactivated influenza vaccine administration, we analyzed influenza-responsive CD8+ T-cell population magnitude in only the infected individuals who reported receiving the seasonal influenza vaccine during the influenza season when they were enrolled in the EDFLU study. The significant reduction in magnitude of the antigen-responsive CD8+ T-cell population remained (Figure 3E).
Association Between Circulating Inflammatory Monocyte Population Magnitude and Illness Severity
We wanted to determine whether any components of the immunophenotype we describe in acute medically attended influenza infection are associated with illness severity. We measured a trend in decreasing absolute counts of CD8+ T cells, CD4+ T cells, and CD19+ B cells in increasingly severe illness, however, none of these met criteria for statistical significance (Supplementary Figure 1A). We did not detect an association in our cohort between CD4+ or CD8+ T-cell activation, magnitude of the influenza-responsive CD8+ T-cell population, or influenza-specific plasmablast response magnitude and illness severity (Supplementary Figure 1B–1D).
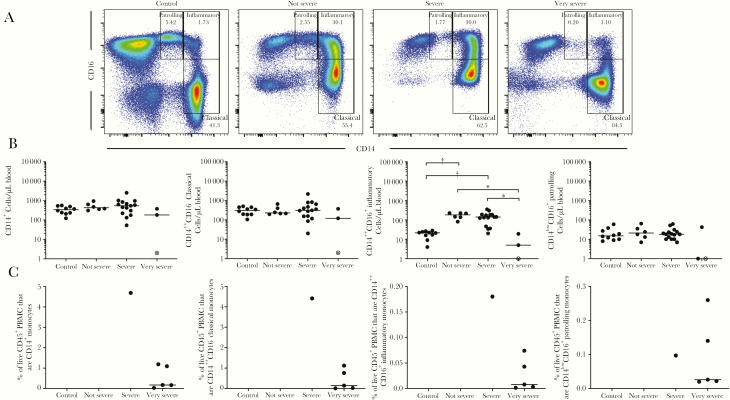
The 3 subjects with very severe illness requiring intubation exhibited a significant decrease in the inflammatory monocyte population (Figure 4). This is in contrast to the significant increase in inflammatory monocyte numbers in individuals with nonsevere illness and severe illness, when compared with uninfected subjects (Figure 4A and 4B).
Figure 4.
Evaluation of peripheral blood monocyte population magnitude in relation to severity of illness. A, Representative flow cytometry plots demonstrating increased inflammatory monocyte population (CD14++CD16+) in infected individuals and selective depletion of this population in infected individuals with very severe illness requiring mechanical ventilation. B, Absolute monocyte and monocyte subpopulation cell numbers in the EDFLU cohort (n = 10 uninfected, n = 6 not severe infected, n = 15 severe infected, n = 3 very severe infected). *P < .05; †P < .005. The open data point with the enclosed X denotes the single fatal case of severe influenza infection. C, The 2009 pandemic H1N1 subjects with severe and very severe illness admitted to the intensive care unit of the National Institute of Respiratory Diseases in Mexico City. Absolute cell count information for these samples was unavailable; therefore, frequency of live CD45+ peripheral blood mononuclear cells (PBMCs) are reported. Numbers are percentage of cells present in the gate that is drawn.
To validate our finding of decreased inflammatory monocytes in subjects requiring mechanical ventilation, we analyzed a collection of PBMC samples from patients with confirmed infection who were hospitalized during the pandemic 2009 H1N1 outbreak in Mexico City. Five of these subjects were mechanically ventilated and demonstrated lower total monocyte, classical monocyte, and inflammatory monocyte populations in their PBMCs, compared with a sixth subject with severe illness who did not require mechanical ventilation (Figure 4C).
DISCUSSION
The earliest immunologic events in naturally acquired, symptomatic human influenza infection are understudied. We present an analysis of peripheral blood cellular immune responses during the critical first week of illness, the time period during which influenza A viremia generally resolves [13, 20, 21] and most healthy patients recover from symptomatic illness. Individuals with medically attended symptomatic influenza A exhibit T-cell lymphopenia and a relative depletion in the frequency of influenza-responsive CD8+ T-cell populations found in the peripheral blood compared with uninfected human subjects. Recall circulating influenza-specific B-cell responses are not substantially present until≥ 5 days after symptoms have started, as others have noted [20, 22]. Finally, we report an association between inflammatory monocyte population magnitude in peripheral blood during the first week of illness and illness severity. Circulating inflammatory monocyte populations expand early and peak between the second and fourth day of symptomatic illness. Inflammatory monocyte populations in the blood are significantly decreased, however, in patients with severe illness requiring mechanical ventilation.
The immune mechanisms that control influenza viral replication in human subjects after infection are not well understood. Several lines of evidence in humans and animal models point to CD8+ T cells contributing to control of established infections [3, 4, 6, 7, 16]. Undoubtedly, innate mechanisms are also at play, given the preponderance of data from serum cytokine studies suggesting significant inflammatory signatures that include tumor necrosis factor α [10, 23], monocyte chemoattractant protein 1 [10], interleukin 8 [10], and interleukin 6 [23], that are independently associated with the degree of illness severity. Furthermore, neutrophil-related host factors [24], and monocyte populations [14] have also been implicated in viral control and symptom severity. Our current work provides evidence that inflammatory monocytes play a role by expanding very early during symptomatic illness. Furthermore, although influenza-responsive CD8+ T-cell populations expand during the first week of infection, the overall influenza-responsive CD8+ T-cell magnitude in our cohort of individuals with medically attended infection remains quite low compared with baseline responses in uninfected individuals.
Low circulating influenza-responsive CD8+ T-cell population magnitude in the majority of our subjects regardless of severity of illness can potentially be explained by several hypotheses that are not mutually exclusive. One possibility is that influenza-specific CD8+ T-cell populations circulating in blood during acute infection are not responsible for control of systemic infection. This leads to the prospect that influenza-specific CD8+ T-cell populations in blood reduce as other populations at the site of infection at or near the larger airways and mucosal surfaces of the upper airway expand owing to the recruitment of circulating CD8+ T lymphocytes. Alternatively, peripherally measured influenza-responsive CD8+ T-cell population size may be important in viral control, and our degree of illness severity is intrinsically biased to be so high, owing to our inclusion of only patients who seek medical care for illness, that we do not have sufficiently mild enough illness to serve as a comparator for correlation with illness severity.
Finally, influenza illness may decrease responsiveness of circulating influenza antigen-specific CD8+ T-cell populations to ex vivo stimulation with influenza A peptides in IFN- γ ELISPOT assays routinely used for measuring these populations resulting in a discrepancy between the responses measured and the actual magnitude of the circulating influenza-specific CD8+ T-cell population. Regardless, further work is required to delineate the role of influenza-specific CD8+ T cells in the control of human seasonal influenza A infection.
Earlier reports of pandemic 2009 H1N1 infection in small human cohorts have described decreased lymphocyte numbers [25] and antigen-specific T-cell responses [26]. Owing to limitations in patient numbers in these studies, responses grouped by time from onset of symptoms could not be assessed during the acute phase of infection. We did not find significant variation in T-cell lymphopenia during the first week of infection (data not shown). However, we do note that, despite the low overall magnitude of the influenza-responsive CD8+ T-cell population, the magnitude does change quickly over time with increasing responses throughout the first week. This suggests that the previous recognition of impaired antigen-specific T-cell numbers in peripheral blood was not an artifact of sample timing, because we have shown that our subjects do seem to be mounting a recall immune response. There is simply a global reduction in circulating antigen-responsive CD8+ T cells in symptomatic influenza-infected individuals.
A previous report evaluating samples from the MOSAIC cohort in the United Kingdom found specific elevation of inflammatory monocytes only in individuals with severe influenza without risk factors [14]. These subjects were sampled on average 13 days (range, 8–26 days) after the onset of symptoms. The study did not evaluate earlier time points to a large degree. Our work suggests that the significant burst of expansion in peripheral blood-located inflammatory monocytes occurs within the first few days of infection. Furthermore, this group’s work in the mouse model suggests a substantial contribution of lung-located monocytes to illness severity. Others have also noted associations between inflammatory monocyte recruitment to the lung and severe lung injury in mice [12], perhaps through this cell type’s unique ability to contribute to type I IFN production in the murine lung [12, 27]. We hypothesize that the reduction we see in peripheral blood inflammatory monocyte populations in individuals with very severe illness requiring mechanical ventilation may represent a relative depletion of this subset as more of these cells are recruited to the lung.
In conclusion, our work demonstrates that the influenza antigen-responsive CD8+ T-cell population during the first week of symptomatic influenza infection is low magnitude despite recall expansion. Circulating inflammatory monocyte populations expand early and are significantly decreased in individuals with severe illness requiring intubation and mechanical ventilation. The reason for this depletion in the peripheral blood is not presently known, but circulating inflammatory monocyte numbers during acute infection may serve as an easily measured biomarker for severe influenza infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Financial support. This work was supported by the Emergency Medicine Foundation (grant to P. A. M.) and the National Center for Advancing Translational Sciences, National Institutes of Health (grant UL1TR002345 to the Washington University Institute of Clinical and Translational Sciences).
Potential conflicts of interest. P. A. M. is listed as inventor on a provisional US patent entitled “Kits and Methods for Selecting Treatments for Respiratory Infections.” All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang QS, Bandaranayake D, Wood T, et al. ; Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Investigation Team Risk factors and attack rates of seasonal influenza infection: results of the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Seroepidemiologic Cohort Study. J Infect Dis 2019; 219:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinfurter JT, Brunner K, Capuano SV 3rd, et al. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog 2011; 7:e1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yap KL, Ada GL, McKenzie IF. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 1978; 273:238–9. [DOI] [PubMed] [Google Scholar]
- 5. Kreijtz JH, Bodewes R, van Amerongen G, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 2007; 25:612–20. [DOI] [PubMed] [Google Scholar]
- 6. McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med 1983; 309:13–7. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Wan Y, Qiu C, et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8⁺ T cells. Nat Commun 2015; 6:6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lillie PJ, Berthoud TK, Powell TJ, et al. Preliminary assessment of the efficacy of a T-cell–based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 2012; 55:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koutsakos M, Illing PT, Nguyen THO, et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat Immunol 2019; 20:613–25. [DOI] [PubMed] [Google Scholar]
- 10. Wong SS, Oshansky CM, Guo XJ, et al. SHIVERS Investigation Team Severe influenza is characterized by prolonged immune activation: results from the SHIVERS Cohort Study. J Infect Dis 2018; 217:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hillaire ML, van Trierum SE, Bodewes R, et al. Characterization of the human CD8+ T cell response following infection with 2009 pandemic influenza H1N1 virus. J Virol 2011; 85:12057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coates BM, Staricha KL, Koch CM, et al. Inflammatory monocytes drive influenza A virus-mediated lung injury in juvenile mice. J Immunol 2018; 200:2391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oshansky CM, Gartland AJ, Wong SS, et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med 2014; 189:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cole SL, Dunning J, Kok WL, et al. MOSAIC investigators M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight 2017; 2:e91868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. MMWR Recomm Rep 2018; 67:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305–12. [DOI] [PubMed] [Google Scholar]
- 17. Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abeles RD, McPhail MJ, Sowter D, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14hi/CD16neg monocytes: expansion of CD14hi/CD16pos and contraction of CD14lo/CD16pos monocytes in acute liver failure. Cytometry A 2012; 81:823–34. [DOI] [PubMed] [Google Scholar]
- 19. He XS, Holmes TH, Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 2006; 80: 11756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang KY, Li CK, Clutterbuck E, et al. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J Infect Dis 2014; 209:1354–61. [DOI] [PubMed] [Google Scholar]
- 21. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009; 200:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He XS, Holmes TH, Sanyal M, et al. Distinct patterns of B-cell activation and priming by natural influenza virus infection versus inactivated influenza vaccination. J Infect Dis 2015; 211:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest 1998; 101:643–9.9449698 [Google Scholar]
- 24. Tang BM, Shojaei M, Teoh S, et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat Commun 2019; 10:3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox A, Le NM, Horby P, et al. Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLoS One 2012; 7:e31535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohn KG, Cox RJ, Tunheim G, et al. Immune responses in acute and convalescent patients with mild, moderate and severe disease during the 2009 influenza pandemic in Norway. PLoS One 2015; 10:e0143281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uccellini MB, Garcia-Sastre A. ISRE-reporter mouse reveals high basal and induced type I IFN responses in inflammatory monocytes. Cell Rep 2018; 25:2784–96 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.