Abstract
Background
Neonatal herpes simplex virus (HSV) disease results in unacceptable morbidity and mortality. The primary humoral immune response to natural infection is neutralizing antibodies (Abs). However, Abs that activate Fc gama receptors (FcγRs) and mediate antibody-dependent cell-mediated cytotoxicity (ADCC) may play a dominant role in protection. In adult mice, a single-cycle HSV candidate vaccine deleted in glycoprotein-D (ΔgD-2) that induces ADCC provided complete protection against HSV disease and prevented the establishment of latency. Passive transfer studies showed that Abs were sufficient for protection. The current study tested the hypothesis that maternal immunization with ΔgD-2 would protect neonates.
Methods
C57BL/6 female mice were vaccinated 3 weeks apart with ΔgD-2, and pups were challenged at different times postnatally with lethal doses of HSV-1 or HSV-2. Concentration and functionality of Abs and immune cells were assessed.
Results
Maternal ΔgD-2 immunization provided significant protection and reduced viral dissemination after lethal challenge with HSV-1 or HSV-2. Protection correlated with Abs acquired transplacentally or from breastmilk that mediated ADCC. Protection was reduced when pups were challenged on Day 1 of life, and this was associated with decreased ability of newborn cells to mediate Ab-dependent cell killing.
Conclusions
Antibodies mediating ADCC provide significant protection against neonatal HSV.
Keywords: antibody-dependent cellular cytotoxicity, HSV vaccines, neonatal herpes
Transfer of placental and breastmilk antibodies mediating antibody-dependent cytolysis, which are elicited in response to vaccination of female mice with a single-cycle HSV-2 glycoprotein D-null candidate vaccine, provides significant protection to pups challenged with clinical isolates of HSV-1 or HSV-2.
Neonatal herpes simplex virus types 1 or 2 (HSV-1 and HSV-2) disease is associated with an estimated 60% fatality rate without treatment and substantial morbidity even with appropriate therapy [1]. Approximately two thirds of neonates who survive central nervous system (CNS) disease have long-term sequelae [1]. The incidence of neonatal HSV is unknown, but mathematical modeling suggests an annual worldwide rate of 14 000 cases (4000 for HSV-1 and 10 000 for HSV-2) [2]. Herpes simplex virus type 2 dominates in Africa, whereas HSV-1 is emerging as a significant serotype associated with neonatal disease in the United States [2, 3].
Primary maternal infection with either serotype near the time of delivery is associated with a greater risk of transmission and more severe neonatal disease reflecting exposure to higher viral loads and/or little to no maternal transfer of protective antibodies (Abs) [4]. The risk for neonatal transmission is 30%–50% with first-episode maternal infection compared with 1%–3% with recurrent genital herpes [4, 5]. The functionality of maternal Abs may also play a role in mitigating neonatal disease [6, 7]. In a study of 47 neonates with HSV disease (20 with skin, 11 with disseminated and 16 with CNS disease), higher levels of Abs that mediate antibody-dependent cell-mediated cytotoxicity (ADCC) were significantly associated with protection against disseminated disease after controlling for the neutralizing titer [8]. Thus, strategies designed to elicit or boost transfer of HSV-specific Abs, and specifically ADCC Abs, may prevent or reduce the severity of neonatal disease.
Efforts to develop a safe and effective vaccine against HSV have met with limited success and, until very recently, have focused primarily on induction of neutralizing Abs. Subunit vaccines comprising glycoprotein D (gD) alone or combined with other viral proteins and with different adjuvants have dominated the HSV vaccine field [9–15]. Two clinical trials with an adjuvanted HSV-2 gD subunit vaccine (gD2-AS04) in serodiscordant couples found that the vaccine provided some efficacy in women (but not men), and only in those who were seronegative for both HSV-1 and HSV-2 before vaccination [11]. However, a subsequent field trial conducted in doubly seronegative women was disappointing. The vaccine elicited gD-specific neutralizing Abs, but it provided only partial protection against HSV-1 and no protection against HSV-2 [14, 16]. The ADCC responses were not reported, but subsequent murine studies demonstrated little or no ADCC response to a similarly adjuvanted gD subunit vaccine [17]. Moreover, an earlier trial with a combination of gD and gB and the adjuvant MF59 also failed to protect despite eliciting neutralizing Abs; absence of ADCC was postulated to have contributed to the lack of efficacy [18].
Building on this framework, we hypothesized that a single-cycle viral strain deleted in gD (designated ΔgD-2) might elicit a different type of immune response than previously observed with adjuvanted subunit vaccines either by unmasking polyantigenic responses to less immunodominant viral proteins and/or because gD may have an immunomodulatory role. ΔgD-2 is grown on complementing cells that express HSV-1 gD to generate phenotypically gD positive but genetically gD-null viral particles [19]. In preclinical studies, the vaccine is safe and highly effective, providing complete protection against primary disease and preventing the establishment of latency in adult mice after intravaginal or skin (male and female) challenge with clinical isolates of HSV-1 or HSV-2 [17, 20, 21]. In contrast to subunit vaccines, the vaccine elicits high-titer Abs that are weakly neutralizing, but they activate Fc gamma receptors (FcγRs) to mediate ADCC and Ab-dependent phagocytosis (ADCP), collectively referred to as Ab-dependent cell killing (ADCK). Passive transfer of immune serum from vaccinated to naive wild-type, but not FcγR or neonatal Fc receptor (FcRn) knockout mice, completely protects against subsequent viral challenge, highlighting the key role of ADCK in protection [17, 20, 21]. The current studies were designed to test the role of ADCK Abs in preventing neonatal disease and to test the hypothesis that immunization of female mice would protect their pups from postnatal viral challenge.
METHODS
Mice and Ethics Statement
Murine studies were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine, Protocol number 2016-1205. C57BL/6 mice were purchased from Jackson Laboratory ([JAX] Bar Harbor, ME).
Cells and Viruses
Vero (African green monkey kidney) (CCL-82; ATCC), HaCAT (human keratinocytes) (CLS 300493; ATCC), and VD60 (Vero cells encoding gD-1 under its endogenous promoter [22]) were passaged in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum ([FBS] Gemini Bio-Products, West Sacramento, CA) and penicillin-streptomycin (Invitrogen). The clinical isolates HSV-2(4674) and HSV-1(B3x1.1) [21] and the laboratory strain HSV-2(333)ZAG, which expresses a green fluorescence protein (GFP) under control of the cytomegalovirus promoter inserted at an intergenic site within the virus, were propagated and titered (plaque assay) on Vero cells. ΔgD-2 was constructed by inserting GFP in place of the gene for gD as previously described and was propagated and titered on VD60 cells; no plaques were detected on Vero cells [19].
Immunizations and Viral Challenge Studies
Female adult mice were vaccinated subcutaneously at 4 (prime) and 7 (boost) weeks of age with 5 × 106 plaque-forming units (pfu) of ΔgD-2 (grown on VD60 cells) or an uninfected VD60 cell lysate as a control. Alternatively, adult mice were intranasally infected with a sublethal dose of HSV-2(4674). One week after boost or 2 weeks after primary sublethal infection, mice were mated. Pups were inoculated on Days 1, 7, or 14 of life with lethal doses of HSV-1(B3x1.1) or HSV-2(4674) delivered intranasally by micropipette (final volume, 5 μL/nares), monitored daily, and euthanized if signs of encephalitis (eg, paralysis, tremors, gait instability, hunched posture) developed. Surviving mice were euthanized on Day 14.
Detection of Herpes Simplex Virus Deoxyribonucleic Acid by Quantitative Polymerase Chain Reaction
At time of euthanasia, liver, lung, kidney, brain, and trigeminal ganglia were collected, and deoxyribonucleic acid (DNA) was extracted using the DNeasy Blood and Tissue DNA Extraction Kit (QIAGEN) and assayed for HSV DNA by real-time quantitative polymerase chain reaction (qPCR) (ABsolute qPCR ROX Mix; Thermo Fisher Scientific) using primers that target HSV polymerase (UL30) (10 ng of DNA per sample) [17]. Mouse β-actin was included as a loading control (Applied Biosystems, Foster City, CA). Samples with fewer than 4 copies were considered negative.
Quantification of Antibody Responses, Neutralizing Titers, and Fcγ Receptor Activation
Total or subtype-specific HSV immunoglobulin G (IgG) was quantified in serum or breastmilk by enzyme-linked immunosorbent assay (ELISA) [17, 20, 21]. Breastmilk was collected from nursing mice on days 8–12 postparturition by separating them from their offspring for at least 2 hours, administering 2 IU/kg of oxytocin via intraperitoneal injection 5 minutes before manually expressing milk, which was frozen at −20°C. The ELISA plates were coated with Vero cell lysates harvested 24 hours after infection with HSV-1(B3x1.1) or HSV-2(4674) at a multiplicity of infection (MOI) of 0.1 pfu/cell or uninfected control lysates. Serial dilutions of serum or breastmilk were incubated with the coated plates overnight at 4°C, and bound total IgG, IgG1, IgG2, or IgG3 was quantified using specific biotin-labeled secondary Abs (BD Biosciences, San Jose, CA). The anti-HSV IgG level was determined after subtracting optical densitometry values obtained for uninfected cell lysates. Neutralization was assessed in a plaque assay by incubating virus with heat-inactivated serum for 1 hour before inoculating Vero cells. Plaques were counted after 48 hours, and the percentage reduction in pfu relative to cells infected in the absence of mouse serum was calculated [17, 20, 21]. The ability of immune serum to activate the FcγRIV, a biomarker of ADCC, was determined using the mFcγRIV ADCC Reporter Bioassay (Promega, Madison, WI) with HSV-2(4674) or HSV-1(B3x1.1)-infected Vero cells as targets [17, 21].
Antibody-Dependent Cell-Mediated Killing
Antibody-dependent cell-mediated killing (ADCK) was assessed by measuring target cell death after incubating HSV-2(333)ZAG-infected HaCAT (MOI 1 pfu/cell, 4 hours at 37°C) or uninfected control cells with serum from vaccinated mice in the presence of pooled immune effector cells. Effectors were isolated from spleens and livers of 5–10 naive neonatal or 3–5 naive adult mice. Target cells were added to each well of a 96-well, U-bottom plate at a density of 2 × 105 cells in 100 μL and incubated with 100 µL heat-inactivated immune serum (1:5 dilution in DMEM) isolated from ΔgD-2 or VD60 control vaccinated adult mice for 15 minutes at room temperature. The effectors were then added at an effector-to-target cell ratio of 10:1 for 1 hour at 37°C, 5% CO2. The cells were stained with eBioscience Fixable Viability Dye eFluor 450 (FVD450) (Thermo Fisher Scientific) for 30 minutes at 4°C, fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), and resuspended in FACs buffer (2% FBS in PBS). The cells were analyzed by flow cytometry on an LSRII (Becton Dickinson), and the percentage of GFP+ (HSV-2 infected) and FVD450+ (dead cells) were quantified using FlowJo analysis software.
Fcγ Receptor Expression
Fcγ receptor expression was assessed by staining immune cells (isolated as for ADCK assays) with live/dead fixable viability marker (Live/Dead Fixable Red; BD Sciences, Carlsbad, CA) and fluorescein isothiocyanate-labeled anti-FcγRI, II, III, or IV (MCA5997F, MCA6000F, MCA5998F, MCA5999F; Bio-Rad, Hercules, CA) or anti-HVEM-APC (BD Biosciences). Cells were also stained with anti-CD11b-PE, anti-CD11c-PECy7, anti-Ly6G-APC, anti-Ly6C-AF700, anti-CD3-BV421 (all BD Biosciences) and anti-F4/80-PerCP (TONBO Biosciences, San Diego, CA). Neutrophils were defined as CD11bhiF4/80loLy6GhiLy6Cint; monocytes were defined as CD11bhiF4/80loLy6GintLy6Chi; and macrophages were defined as CD11bintF4/80hi. Unstained and fluorescence minus one (FMO) controls were included in the analysis.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 7.0 software (GraphPad Software Inc., San Diego, CA). A P value less than or equal to 0.05 was considered statistically significant. Survival curves were compared using Kaplan-Meier test; other results were compared using analysis of variance with multiple testing as indicated.
RESULTS
Maternal ΔgD-2 Protects 7- and 14-Day-Old Pups From Lethal Challenge With Herpes Simplex Virus HSV-1 or HSV-2
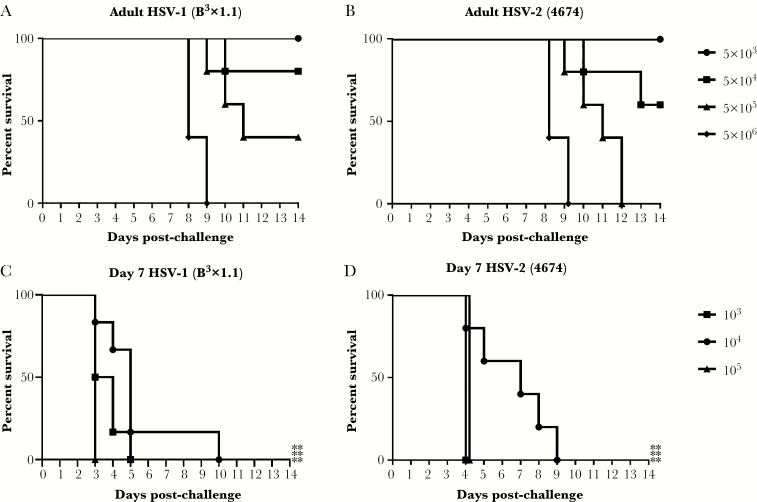
Seven-day-old pups, which are considered comparable to a human term infant with respect to immunity [23], or adult (7–8 weeks old) mice were challenged intranasally with escalating doses of HSV-1(B3x1.1) or HSV-2(4674) to determine susceptibility. Pups were significantly more susceptible than adults after intranasal challenge with either serotype (P < .01) and rapidly succumbed at doses as low as 103 pfu/mouse, whereas 100% lethality was observed in adults only at doses greater than 5 × 105 pfu/mouse for HSV-2 and 5 × 106 pfu/mouse for HSV-1 (Figure 1). The dominant signs of disease were neurologic with pups demonstrating gait disturbances, hunched posture, and paralysis. Because all of the doses were lethal in the pups, vaccine challenge studies were subsequently conducted with 103 pfu/pup for HSV-2 and, as an even more stringent challenge, 105 pfu/pup of HSV-1.
Figure 1.
Susceptibility of adult and 7-day-old pups to intranasal inoculation with herpes simplex virus (HSV)-1 and HSV-2. Seven-week-old adult female mice (A and B) or 7-day-old pups (C and D) were intranasally challenged with the indicated doses of HSV-1(B3x1.1) or HSV-2(4674) and monitored for 2 weeks for signs of disease. Survival curves are shown (n = 5–6 mice per dose). The asterisks indicate significant difference in survival comparing pups and adults (**, P < .01).
To determine whether vaccinated females transferred protective Abs to their offspring, pups born to ΔgD-2 or control (uninfected VD60 cell lysate) prime-boost immunized mice were challenged intranasally on Day 7 of life. In subsequent studies, the challenge was extended to 14 day-old (modeling postnatal exposure) and 1 day-old (modeling preterm infant) pups. Significant protection against HSV-1 or HSV-2 (Figure 2) was observed in 7- and 14-day-old pups born to ΔgD-2 immunized dams compared with control-vaccinated dams (P < .001). However, protection was reduced when pups were challenged on Day 1 of life with only modest protection against HSV-1 (P = .03) and no protection against HSV-2.
Figure 2.
Maternal immunization with ΔgD-2 protects 7- and 14-day-old pups from herpes simplex virus (HSV) disease. Female C57BL/6 mice were prime-boost subcutaneously immunized with 5 × 106 plaque-forming units (pfu) of ΔgD-2 or VD60 cell lysate control, mated 1-week postboost, and monitored for birth. Pups were then challenged intranasally at the indicated day of life with (A) 103 pfu of HSV-2(4674) (Day 1: ΔgD n = 5, VD60 n = 6; Day 7: ΔgD n = 16, VD60 n = 40; Day 14: ΔgD n = 22, VD60 n = 14) or (B) 105 pfu of HSV-1(B3x1.1) (Day 1: ΔgD n = 5, VD60 n = 17; Day 7: ΔgD n = 48, VD60 n = 45; Day 14: ΔgD n = 23, VD60 n = 14). Statistical significance was determined by Kaplan-Meier (Day 7, 14) and analysis of variance with Tukey’s multiple comparison test (Day 1). Significance is shown relative to VD60 lysate control (*, P < .05; ***, P < .001).
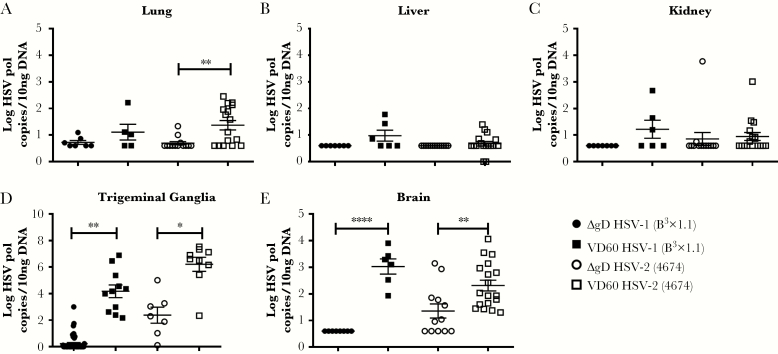
To determine whether the maternally derived Abs reduced viral dissemination, tissues were harvested at time of euthanasia (either for disease or in surviving mice at the conclusion of the 2-week observation period) and assayed for HSV DNA by qPCR (7-day-old pup results) (Figure 3). The highest levels of viral DNA were recovered in lung and neuronal tissue, which is consistent with the route of inoculation and neurological signs; maternally acquired Abs provided significant protection against viral dissemination to these sites in the pups.
Figure 3.
Maternal vaccination with ΔgD-2 decreases viral dissemination to lung, trigeminal ganglia, and brain. Neonatal mice born to either ΔgD-2 (blue) or VD60 (red) immunized mothers were challenged intranasally with 105 plaque-forming units (pfu) of herpes simplex virus HSV-1(B3x1.1) or 103 pfu of HSV-2(4674) on Day 7 of life. Lung (A), liver (B), kidney (C), trigeminal ganglia (D), and brain tissue (E) were collected at time of sacrifice or Day 14 postchallenge, and HSV viral load was determined by quantitative polymerase chain reaction of homogenized tissue. Statistical significance was determined by Student’s t test compared with VD60 control for each serotype (*, P < .05; **, P < .01; ****, P < .0001).
Optimal Protection Requires Antibodies Acquired From Breastmilk
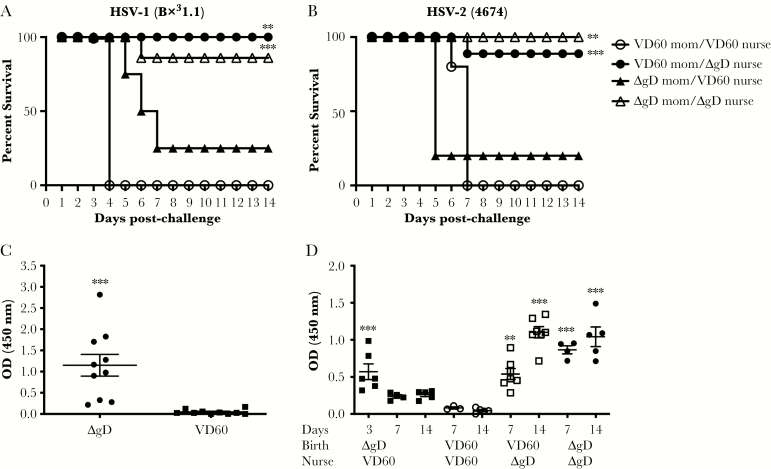
Acquisition of Abs from breastmilk could contribute to the greater protection observed when pups were challenged on Day 7 or 14 compared with Day 1 of life. To explore this possibility, pups born to ∆gD-2-immunized mothers were switched at birth and nursed by control-immunized mothers (transplacentally acquired Abs only), or, conversely, mice born to control-immunized mothers were nursed by ∆gD-2-immunized mothers (breastmilk-acquired Abs only). Positive controls were mice that were born to and nursed by ∆gD-2-immunized-mothers; negative controls were mice that were born to and nursed by control-immunized mothers. The pups were challenged intranasally on Days 7 or 14 of life and monitored for 2 weeks. Compared with the negative controls, pups born to a ∆gD-2-immunized dam were significantly protected from HSV-1 or HSV-2 challenge only if also nursed by a ΔgD-2-immunized dam, but not if the were switched at birth and nursed by a control-immunized dam (P < .001, Kaplan-Meier with correction for multiple comparisons) (Figure 4A and B). In contrast, pups born to a control-immunized dam, but nursed by a ΔgD-2-immunized mouse, were also significantly protected (P < .001).
Figure 4.
Optimal protection requires both transplacental and breastmilk-acquired antibodies (Abs). Female mice were prime-boost vaccinated with either ΔgD-2 or VD60 cell lysate, mated, and monitored for birth. Pups born to ΔgD-2-immunized mothers were switched and nursed by VD60-immunized mothers (only transplacental maternal Ab transfer), and mice born to VD60-immunized mothers were nursed by ΔgD-2-immunized mothers (only breastmilk-acquired Abs). Pups were challenged intranasally on Day of life 7 or 14 with (A) 105 plaque-forming units (pfu) of herpes simplex virus HSV-1(B3x1.1) or (B) 103 pfu of HSV-2(4674) and monitored for survival. (C). Breastmilk was obtained from ΔgD-2- or VD60-immunized mothers on postgestational day 8, and HSV-specific immunoglobulin G (IgG) was determined using enzyme-linked immunosorbent assay (ELISA) with HSV-2 infected cell lysates. (D). Serum was obtained on Days 3, 7, and 14 from pups born to and nursed by the indicated dams (without being inoculated with virus), and HSV-specific IgG was determined using ELISA with HSV-2-infected cell lysates; results are presented as optical density units (OD) with 1:1000 dilution of serum. Each point represents results for single pup. Survival was compared by Kaplan-Meier test (A and B) and Ab response by Student’s t test (C) or analysis of variance with Tukey’s multiple comparison test (D) relative to VD60 control (**, P < .01; ***, P < .001).
Results correlated with the HSV-binding Abs detected in the serum of uninfected pups on Day of life 3, 7, or 14 (Figure 4C). Herpes simplex virus-specific IgG rapidly declined if mice were born to but not nursed by a ∆gD-2-immunized mother, which is consistent with the short half-life of murine IgG [24]. In contrast, HSV-specific IgG increased rapidly in mice that were only nursed by a ∆gD-2-immunized mother. The presence of HSV-specific Abs in breastmilk was confirmed in samples collected on day 8 postparturition (Figure 4D).
Protection Correlates With Fcγ Receptor IV-Activating Antibodies
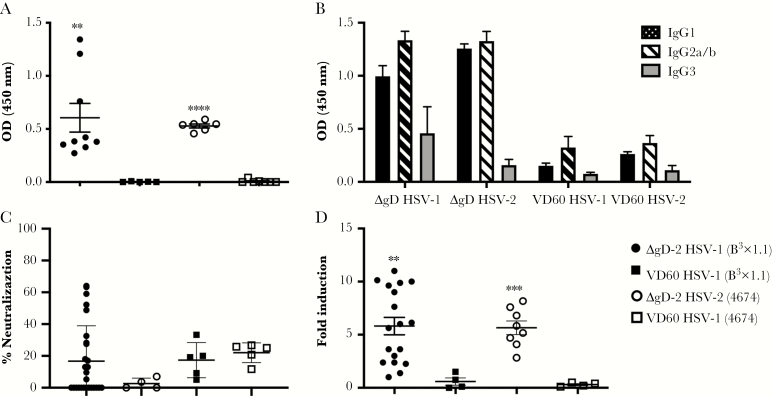
In addition to the quantity of Abs transferred from dam to pup, the functionality of the Abs may contribute to protection. Therefore, we compared the functionality of HSV-specific Abs in the serum of pups obtained at time of euthanasia for disease or at the end of the 2-week observation period in mice that were challenged on Day 7. Herpes simplex virus-specific IgG was readily detected in pups born to ΔgD-2-vaccinated dams (1:1000 dilution) (Figure 5A) with a slight predominance of IgG2 compared with IgG1 subtypes (Figure 5B). Consistent with previously published studies in ΔgD-2-vaccinated adult mice [17, 20, 21], the HSV-specific Abs in the pups’ serum were weakly neutralizing (and not significantly different from control serum) (Figure 5C) but displayed significant FcγRIV activation (Figure 5D) [17, 20, 21].
Figure 5.
Characterization of herpes simplex virus (HSV)-specific antibodies transferred from immunized dams to 7-day-old pups. Seven-day-old pups born to either ΔgD-2 or VD60 lysate-immunized dams were challenged intranasally with 105 plaque-forming units (pfu) of HSV-1(B3x1.1) or 103 pfu of HSV-2(4674). Serum samples were collected at time of sacrifice or Day 14 postchallenge and assessed as follows: (A) HSV-1- (closed symbols) or HSV-2- (open symbols) specific immunoglobulin G (IgG) was quantified by enzyme-linked immunosorbent assay (ELISA) at 1:1000 dilution with results presented as optical densitometry (OD) units. (B). The subtypes (IgG1, IgG2, or IgG3) of the HSV-specific antibodies (Abs) were quantified by a similar ELISA assay but with subtype-specific secondary Abs. Results for 1:1000 dilution of murine serum are shown as mean + standard error of the mean ([SEM] n = 4–6 mice per group). (C) The ability of the serum to neutralize HSV-1 or HSV-2 was determined by plaque reduction assay; results with 1:5 dilution of serum are shown as percentage inhibition of viral plaques relative to control (no immune serum), and each dot represents results for an individual mouse. (D) Murine FcγRIV activation was measured using a reporter bioassay with HSV-1- or HSV-2-infected targets and 1:5 dilution of serum. Each point represents results for an individual pup, and lines are mean ± SEM. Fold induction was compared by t test for immune serum from ΔgD-2- or VD60-immunized mice challenged with the indicated serotype (**, P < .01; ***, P < .001; ****, P < .0001).
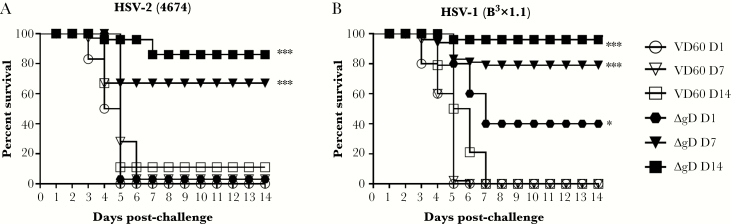
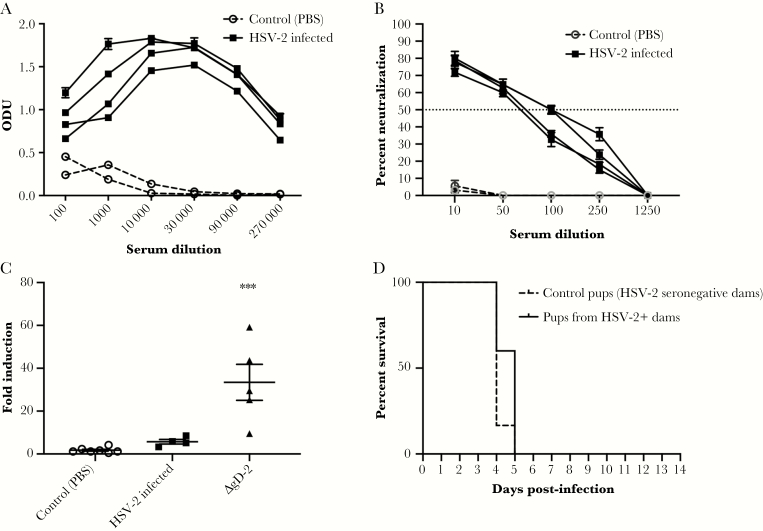
To assess whether convalescent immune serum provided similar protection in this model, we intranasally infected adult mice with a sublethal (105 pfu of HSV-2(4674)) dose or with PBS as control and assayed maternal serum 2 weeks later for total, neutralizing, and FcγRIV-activating Abs. Sublethal infection induced an HSV-2 IgG response that was predominantly neutralizing with significantly less FcγRIV activation compared with ΔgD-2-vaccinated mice (Figure 6A–C). Seropositive or control mice were then mated, and pups were challenged intranasally on Day 7 with HSV-2(4674). No significant protection was observed (Figure 6D).
Figure 6.
Antibodies generated in response to sublethal infection do not protect 7-day-old pups from herpes simplex virus (HSV). Female C57BL/6 mice were intranasally infected with 1 × 105 plaque-forming units (pfu) of HSV-2(4674) or phosphate-buffered saline (PBS) as a control and monitored for 2 weeks. (A) Blood was obtained from surviving mice and assayed for HSV-specific immunoglobulin G by enzyme-linked immunosorbent assay with results presented as optical densitometry units (ODU) at the indicated serum dilutions for n = 4 infected and n = 2 control mice. (B) The ability of the serum to neutralize HSV-2 was determined by plaque reduction assay; results for individual mice at each dilution are shown as percentage inhibition of viral plaques relative to cells infected in the absence of any mouse serum; the dotted line at 50% indicates the 50% neutralization titer. (C). Murine FcγRIV activation was measured using a reporter bioassay with HSV-2-infected targets and 1:5 dilution of serum from control, sublethally infected, or ΔgD-2 prime-boost immunized mice. Each point represents results for an individual mouse, and lines are mean ± standard error of the mean. Fold induction was compared by one-way analysis of variance (***, P < .001 for ΔgD-2-vaccinated versus control). (D) Pups born to control or HSV-2-seropositive dams were challenged intranasally on Day 7 with 103 pfu of HSV-2(4674) or PBS (n = 5 each).
Decreased Protection on Day 1 Is Associated With Reduced Effector Cell Killing
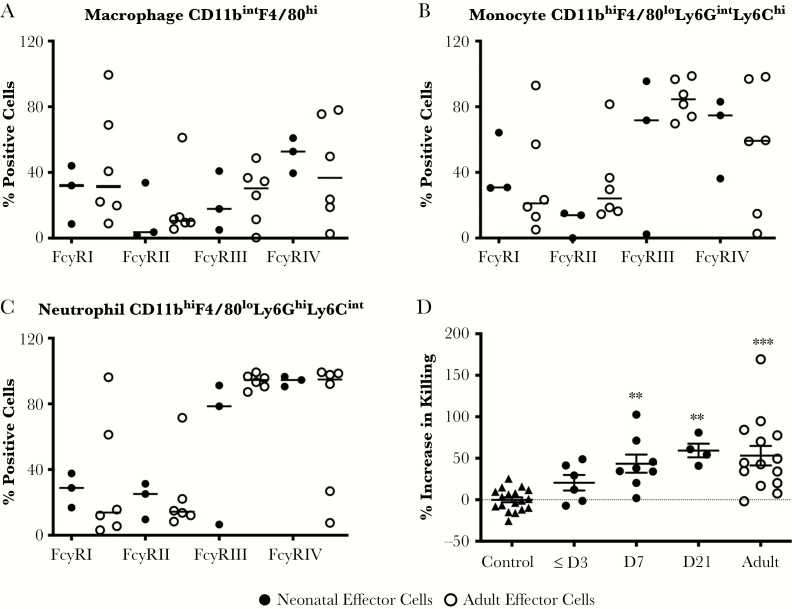
The levels of HSV-specific Abs found in blood in 3-day-old pups born to ΔgD-2-vaccinated dams even without nursing suggest that the lack of protection on Day 1 of life cannot be solely attributed to Ab levels, but it could reflect differences in the expression of FcγRs (primarily FcγRIV) and/or defects in ADCK function. No significant differences in the expression of any of the FcγRs were observed in immune cell subpopulations isolated from the livers and spleens of 1- to 3-day-old compared with adult mice (Figure 7A–C). However, the ADCK activity of the immune cells isolated from 1- to 3-day-old pups was significantly reduced compared with Day 7 or adult cells (Figure 7D).
Figure 7.
Fcγ receptor (FcγR) expression and ability of neonatal and adult immune cells to mediate antibody-dependent killing of herpes simplex virus (HSV)-infected target cells. Single-cell suspensions of pooled hepatocytes and splenocytes from adult and 7-day-old neonatal mice were obtained, and FcγR expression was quantified by flow on (A) macrophages, (B) monocytes, and (C) neutrophils. Populations were defined by expression of indicated markers and results are presented as percentage of positive cells. (D) Antibody-dependent cellular killing was quantified using single-cell suspensions of immune cells from different aged pups or adults (6–8 weeks) as effectors and HSV-2(333)ZAG-infected cells as targets and pooled heat-inactivated immune serum from ΔgD-2 or VD60 (control) immunized adult mice. The results are presented as percentage of killed targets (GFP+ [HSV-2-infected] and FVD450+ [dead cells]) relative to FVD450+ dead cells in the absence of serum. Each point represents results for a single mouse, and results were compared with controls (VD60 immune serum) by analysis of variance with Tukey’s multiple comparison (**, P < .01; ***, P < .001).
DISCUSSION
The extent to which maternal neutralizing and/or nonneutralizing Abs contribute to protection against neonatal disease is controversial. The observation that the highest risk of neonatal herpes was observed in pregnant women who had not seroconverted by the time of delivery supports a role for transplacental Abs in providing some protection, although this also could reflect neonatal exposure to higher viral loads [5]. Using a murine model, the current study demonstrates that female mice immunized with ΔgD-2, a candidate vaccine that primarily induces FcγR-activating Abs that mediate ADCC rather than neutralizing Abs, provide significant protection and was associated with decreased viral dissemination when pups were challenged on Day 7 or 14 of life with clinical isolates of HSV-1 or HSV-2. In contrast, sublethal infection, which induces a predominantly neutralizing Ab response in adult mice, was not protective.
These results are consistent with human studies, which demonstrated a correlation between ADCC and protection against HSV dissemination in neonates [6], as well as earlier murine studies [25]. Using a panel of murine monoclonal Abs (mAbs), Kohl [25] found that the ability of the Abs to mediate ADCC in vitro, but not their neutralizing activity, correlated with in vivo protection when the Abs were administered intraperitoneally in combination with human leukocytes to 7-day-old pups before challenge with 104 pfu of HSV-1. The protective ADCC Abs were all of the IgG2a subclass. Neutralizing Abs only protected against a lower (102–103 pfu) dose challenge. Protection from the ADCC Abs in that model also required administration of human leukocytes, which presumably provided additional effector cell killing, because murine Abs also bind human FcγRs [26]. In the current studies, maternal vaccination without transfer of human leukocytes was sufficient to protect. This difference may reflect greater breadth or titer of the ADCC Abs elicited by active immunization with ΔgD-2 compared with passive transfer of individual mAbs.
Other murine studies, as well as the failed clinical trials with recombinant protein vaccines, also suggest that neutralizing Abs alone may not provide sufficient protection. For example, maternal immunization with an attenuated, thymidine-kinase negative virus, HSV-2(186ΔKpn), or a replication-defective vaccine candidate, HSV-2 dl5-29, reduced viral dissemination, but it did not prevent spread of virus to the trigeminal ganglia or CNS, and all of the pups succumbed to infection after high-dose oral challenge on Day 1 and Day 7 of life [27]. The protection against dissemination correlated modestly with HSV-specific and neutralizing Abs, but ADCC activity was not assessed. Greater protection was observed in a more recent study in which female mice were immunized intramuscularly with dl5-29. Maternal vaccination resulted in significant protection against CNS disease when pups were challenged intranasally on Days 1–2 of life with 103 pfu of HSV-1(17) or HSV-2(G) [28]. The differences between the 2 studies with dl5-29 may reflect the route of vaccination (intramuscular elicits higher Ab responses than subcutaneous immunization [29]) as well as differences in the challenge dose and strains. The authors proposed that the protection was mediated by neutralizing antibodies, although again ADCC was not assessed.
Maternal vaccination with ΔgD-2 provided less protection when pups were challenged on Day 1 with greater loss of vaccine efficacy against HSV-2 compared with HSV-1. Studies with additional clinical isolates are needed to determine whether this observation is generalizable. Serotype differences in neurological disease and mortality were observed in one study of adult mice challenged by multiple routes with different clinical isolates [30]. The decreased protection against either serotype on Day 1, which immunologically models a preterm infant, was associated with decreased effector killing cell function and not differences in FcγR expression. Similar findings have been described in humans. For example, the risk of mother-to-infant human immunodeficiency virus (HIV) transmission is higher in preterm compared with term infants and has been linked, in part, to differences in natural killer (NK) cell activity. In a study that compared functionality of preterm, term, and adult cells, the immune cells from term neonates exhibited levels of NK cytolytic activity equal to adults, but the cytolytic activity of preterm neonatal cells was significantly diminished. Overnight stimulation with interleukin (IL)-12 augmented the NK cytolytic activity of both infant groups and adults [31]. Other studies have shown that neonatal ADCC effector cell function may be augmented by treatment with IL-15 [32].
The ΔgD-2-elicited protection was greatest when pups received both transplacental and breastmilk-acquired Abs as evidenced by switching nursing dams at birth. This may reflect the relatively short half-life of murine IgG, which is ~6–8 days for IgG1, IgG2a/c, and IgG3 and 4–6 days for IgG2b [24]. In addition, mice receive the majority of their IgG through the gut because of the high levels of FcRn expression on the brush border of the proximal small intestine. In contrast, humans express high levels of FcRn in the placental syncytiotrophoblasts and receive the majority of maternal IgG transplacentally [33]. The greater transplacental transport and the longer half-life of human IgG suggest that active immunization of young women (ideally before age of sexual debut) with ΔgD-2 or passive transfer of ADCC Abs would protect neonates from perinatal or postnatal exposure. Human breast milk Abs may also contribute to protection as suggested by a recent study in rhesus macaques in which polyfunctional HIV envelope-specific Abs with ADCC activity isolated from the milk of HIV-infected women, but not Abs with only neutralizing activity, provided partial protection to macaques when administered at the time of challenge [34].
CONCLUSIONS
In summary, the current study highlights the potential for a vaccine that elicits FcγR-activating Abs capable of mediating ADCC to prevent HSV-1 and HSV-2 neonatal disease. Future studies are needed to determine whether the ADCC Abs could be used with current antivirals as adjunctive therapy to treat neonatal disease. Although the murine model does not fully recapitulate human responses, the results are consistent with clinical observations and support further development of this novel vaccine and a focus on ADCC and other nonneutralizing antibody functions to prevent HSV and other perinatally acquired infections.
Notes
Acknowledgments. We thank Mei Cong, Aileen Paguio, and Vanessa Ott from Promega for providing murine Fcγ receptor activation bioassays.
Financial support. This work was funded by the National Institutes of Health (R01 AI117321) and the Price Family Foundation. C. M. K. was supported by the Pichichero Family Foundation Vaccines for Children Initiative Research Award in Pediatric Infectious Diseases.
Potential conflicts of interest. B. C. H. is an inventor on patents on the HSV-2 ΔgD-2 vaccine and is a scientific consultant for X-VAX, Technologies. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. James SH, Kimberlin DW. Neonatal herpes simplex virus infection: epidemiology and treatment. Clin Perinatol 2015; 42:47–59, viii. [DOI] [PubMed] [Google Scholar]
- 2. Looker KJ, Magaret AS, May MT, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health 2017; 5:e300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernstein DI, Bellamy AR, Hook EW 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 2013; 56:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003; 289:203–9. [DOI] [PubMed] [Google Scholar]
- 5. Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997; 337:509–15. [DOI] [PubMed] [Google Scholar]
- 6. Kohl S. The neonatal human’s immune response to herpes simplex virus infection: a critical review. Pediatr Infect Dis J 1989; 8:67–74. [PubMed] [Google Scholar]
- 7. Kohl S. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev Infect Dis 1991; 13(Suppl 11):S950–2. [DOI] [PubMed] [Google Scholar]
- 8. Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis 1989; 160:770–6. [DOI] [PubMed] [Google Scholar]
- 9. Mertz GJ, Ashley R, Burke RL, et al. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J Infect Dis 1990; 161:653–60. [DOI] [PubMed] [Google Scholar]
- 10. Corey L, Langenberg AG, Ashley R, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999; 282:331–40. [DOI] [PubMed] [Google Scholar]
- 11. Stanberry LR, Spruance SL, Cunningham AL, et al. ; GlaxoSmithKline Herpes Vaccine Efficacy Study Group Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652–61. [DOI] [PubMed] [Google Scholar]
- 12. Bernstein DI, Aoki FY, Tyring SK, et al. ; GlaxoSmithKline Herpes Vaccine Study Group Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin Infect Dis 2005; 40:1271–81. [DOI] [PubMed] [Google Scholar]
- 13. Belshe RB, Leone PA, Bernstein DI, et al. ; Herpevac Trial for Women Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belshe RB, Heineman TC, Bernstein DI, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 2014; 209:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leroux-Roels G, Clément F, Vandepapelière P, Fourneau M, Heineman TC, Dubin G. Immunogenicity and safety of different formulations of an adjuvanted glycoprotein D genital herpes vaccine in healthy adults: a double-blind randomized trial. Hum Vaccin Immunother 2013; 9:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Awasthi S, Belshe RB, Friedman HM. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis 2014; 210:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burn C, Ramsey N, Garforth SJ, Almo S, Jacobs WR Jr, Herold BC. A herpes simplex virus (HSV)-2 single-cycle candidate vaccine deleted in glycoprotein D protects male mice from lethal skin challenge with clinical isolates of HSV-1 and HSV-2. J Infect Dis 2018; 217:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kohl S, Charlebois ED, Sigouroudinia M, et al. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J Infect Dis 2000; 181:335–9. [DOI] [PubMed] [Google Scholar]
- 19. Cheshenko N, Trepanier JB, Stefanidou M, et al. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J 2013; 27:2584–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petro C, Gonzalez PA, Cheshenko N, et al. et al. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife. 2015; 4. doi:10.7554/eLife.06054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petro CD, Weinrick B, Khajoueinejad N, et al. HSV-2 DeltagD elicits FcgammaR-effector antibodies that protect against clinical isolates. JCI Insight 2016; 1:e88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol 1988; 62:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004; 4:553–64. [DOI] [PubMed] [Google Scholar]
- 24. Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 1988; 18:313–6. [DOI] [PubMed] [Google Scholar]
- 25. Kohl S. Protection against murine neonatal herpes simplex virus infection by lymphokine-treated human leukocytes. J Immunol 1990; 144:307–12. [PubMed] [Google Scholar]
- 26. Lubeck MD, Steplewski Z, Baglia F, Klein MH, Dorrington KJ, Koprowski H. The interaction of murine IgG subclass proteins with human monocyte Fc receptors. J Immunol 1985; 135:1299–304. [PubMed] [Google Scholar]
- 27. Evans IA, Jones CA. Maternal immunization with a herpes simplex virus type 2 replication-defective virus reduces visceral dissemination but not lethal encephalitis in newborn mice after oral challenge. J Infect Dis 2002; 185:1550–60. [DOI] [PubMed] [Google Scholar]
- 28. Patel CD, Backes IM, Taylor SA, et al. et al. Maternal immunization confers protection against neonatal herpes simplex mortality and behavioral morbidity. Sci Transl Med 2019; 11:eaau6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diaz F, Gregory S, Nakashima H, Viapiano MS, Knipe DM. Intramuscular delivery of replication-defective herpes simplex virus gives antigen expression in muscle syncytia and improved protection against pathogenic HSV-2 strains. Virology 2018; 513:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richards JT, Kern ER, Overall JC Jr, Glasgow LA. Differences in neurovirulence among isolates of herpes simplex virus types 1 and 2 in mice using four routes of infection. J Infect Dis 1981; 144:464–71. [DOI] [PubMed] [Google Scholar]
- 31. Merrill JD, Sigaroudinia M, Kohl S. Characterization of natural killer and antibody-dependent cellular cytotoxicity of preterm infants against human immunodeficiency virus-infected cells. Pediatr Res 1996; 40:498–503. [DOI] [PubMed] [Google Scholar]
- 32. Choi SS, Chhabra VS, Nguyen QH, Ank BJ, Stiehm ER, Roberts RL. Interleukin-15 enhances cytotoxicity, receptor expression, and expansion of neonatal natural killer cells in long-term culture. Clin Diagn Lab Immunol 2004; 11:879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715–25. [DOI] [PubMed] [Google Scholar]
- 34. Himes JE, Goswami R, Mangan RJ, et al. Polyclonal HIV envelope-specific breast milk antibodies limit founder SHIV acquisition and cell-associated virus loads in infant rhesus monkeys. Mucosal Immunol 2018; 11:1716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]