Abstract
The plant growth hormone auxin controls cell identity, cell division, and expansion. In the primary root of Arabidopsis there is a robust auxin gradient with a peak concentration at the tip of the meristem and a significant decrease throughout the elongation zone. The molecular mechanisms of how such a steep auxin gradient is established and maintained, and how this auxin gradient within the root dynamically adjusts in response to environmental stimuli are still largely unknown. Here, using a large-scale Arabidopsis mutant screening, we described the identification of PIN2 (PIN-FORMED 2), an auxin efflux facilitator, as a key downstream regulator in glucose-TOR (target of rapamycin) energy signaling. We demonstrate that glucose-activated TOR phosphorylates and stabilizes PIN2 and therefore influences the gradient distribution of PIN2 in the Arabidopsis primary root. Interestingly, dysregulation of TOR or PIN2 disrupts the glucose-promoted low auxin region located in the elongation zone that is essential for cell elongation. Taken together, our results shed light on how carbon and metabolic status can be tightly integrated with the hormone-driven processes to orchestrate complex plant growth programs.
Keywords: auxin, glucose, target of rapamycin, root, PIN2
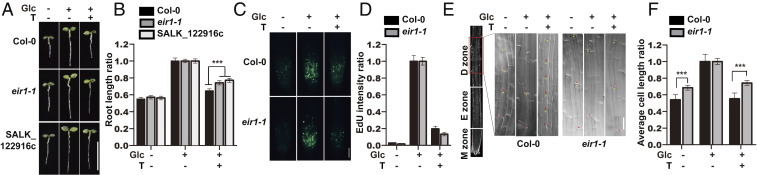
The evolutionarily conserved target of rapamycin (TOR) kinase acts as a master regulator that coordinates cell proliferation and growth by integrating nutrient, energy, hormone, and stress signals in all eukaryotes (1, 2). In plants, TOR senses both the glucose energy signal and the light-auxin hormone signal to promote the rapid plant growth (3–5), although the key downstream effectors are still largely unknown. Our prior studies found that Arabidopsis seedlings geminated in photosynthesis-restrained (with limited CO2) and sugar-free liquid medium entered a mitotic quiescent state with arrested primary root growth due to the depletion of endogenous sugars (4). Exogenously supplied glucose reactivated TOR activity and promoted rapid primary root growth, and this promotion could be abolished by specific TOR inhibitors (e.g., Torin2) (3, 6) (Fig. 1 A and B). Taking advantage of this quantitatively tractable root growth phenotype, we carried out a large-scale mutant screen using collections of confirmed T-DNA insertion lines from the Arabidopsis Biological Resource Center to identify the potential downstream regulators that mediate glucose-TOR promotion of root growth. We isolated one mutant line, SALK_122916c, as it was largely resistant to the inhibition effect of Torin2 on glucose-promoted primary root growth (Fig. 1 A and B). The T-DNA was inserted in the first exon of At5g67090, which was previously designated as PIN-FORMED 2 (PIN2). To exclude possible pleiotropic effects of T-DNA insertion, we examined another well-recognized PIN2 mutant, eir1-1 (7), and found that eir1-1, as compared to WT seedlings, was also less sensitive to Torin2-inhibited root growth (Fig. 1 A and B).
Fig. 1.
PIN2 is a key downstream regulator in glucose-TOR signaling. (A) Images of representative seedlings in WT (Col-0), eir1-1, and SALK_122916c lines with or without glucose (Glc) and Torin2 (T) treatment. (Scale bar, 5 mm.) (B) Relative ratio of root length of A. Means ± SE, unpaired two-tailed t test, ***P < 0.001. (C) EdU staining of root meristem in WT (Col-0) and eir1-1 with or without 2 h glucose (Glc) and Torin2 (T) treatment. (Scale bar, 50 μm.) (D) Quantification of relative EdU intensity in C. (E) Cell length in differentiation zone of Col-0 and eir1-1 with or without glucose (Glc) and Torin2 (T) treatment. Images shown are part of the 10th to 16th epidermal cells after onset of cell elongation. (Scale bar, 50 μm.) D, differentiation; E, elongation; M, meristem. Red and yellow asterisks indicate the bottom and upper boundary of a cell, respectively. (F) Relative ratio of root cell length in E. Means ± SE, unpaired two-tailed t test, ***P < 0.001.
We next investigated how eir1-1 was involved in the glucose-TOR regulation of primary root growth. Previous work has shown that TOR transduces a glucose energy signal to activate cell proliferation in the root meristem. Unexpectedly, using the thymidine analog 5-ethynyl-29-deoxyuridine (EdU) for in situ detection of cell division activity, we found that TOR inhibition could still significantly abolish the glucose-promoted cell division in eir1-1, similar to the response in WT plants (Fig. 1 C and D).
The Arabidopsis primary root can be divided into the meristem, elongation, and differentiation zones. The root length is mainly determined by the cell-proliferation activities within the meristem zone and the final cell length within the differentiation zone. We analyzed the cell length within the differentiation zone in eir1-1 and WT root, with or without glucose and Torin2 treatments. Compared to WT plants, eir1-1 is less sensitive to Torin2- or glucose starvation-inhibited cell elongation within the differentiation zone (Fig. 1 E and F). Together, these data suggested that glucose-TOR signaling controls both cell proliferation and cell elongation in the primary root growth, whereas the resistance to TOR-inhibition phenotype in the eir1-1 mutant was specifically caused by the cell elongation, but not cell-division activity.
PIN2 functions as an auxin efflux facilitator mediating proximal shootward auxin transport in the Arabidopsis root (8). By analyzing the auxin response reporter line DR5v2::ntdTomato (9), we found that in the presence of a glucose supply, there was an auxin-response gradient within the primary root (Fig. 2 A and B). The highest DR5v2::ntdTomato signal observed is in the meristem zone (high auxin region, M zone), followed by an abruptly lower signal within the elongation zone and the beginning of the differentiation zone (low auxin region), and then a significantly increased signal in the middle of the differentiation zone (moderate auxin region). Interestingly, glucose depletion or TOR inhibition resulted in only a very narrow low auxin region adjacent to the meristem zone in WT roots, while the remaining elongation zone and neighboring differentiation zone still showed a relatively high auxin signal (Fig. 2 A and B). In contrast, this narrow auxin-response region under glucose starvation or TOR inhibition was completely blocked in eir1-1 (Fig. 2 A and B), indicating an essential role of PIN2 in this dynamic glucose-TOR regulated auxin gradient establishment.
Fig. 2.
Glucose-TOR modulates auxin and PIN2 distribution regions in root. (A) Expression patterns of DR5v2::ntdTomato in Col-0 and eir1-1 with or without glucose (Glc) and Torin2 (T) treatment. Yellow arrowheads indicate the upper edge of the meristem; white arrowheads indicate the boundary of low auxin region. Maximal projections of z-stacks are presented. (Scale bars, 50 μm.) Each whole root image was assembled by two overlapped confocal images. (B) Quantification of auxin-response regions in A. High auxin region (meristem zone, below the yellow arrowheads), low auxin region (between the yellow and white arrowheads), and moderate auxin region (above the white arrowheads). Means ± SE; n ≥ 8; the letters beside the bars indicate significant different length of low auxin region under different treatments; unpaired two-tailed t test, P < 0.05. The numbers inside the bars indicate the fluorescence intensity of each region. (C) Expression patterns of pPIN2::PIN2-GFP in Col-0 and tor-es with or without glucose (Glc) and Torin2 (T) treatment. The yellow arrowheads indicate the upper edge of the meristem and the white arrowheads indicate the boundary where the expression of PIN2 disappears. Maximal projections of z-stacks are presented. (Scale bar, 50 μm.) Each whole root image was assembled by three to four overlapped confocal images. (D) Quantification of PIN2 expression regions in C. Meristem zone (below the yellow arrowheads), PIN2-declining region (between the yellow and white arrowheads), and no PIN2 region (above the white arrowheads). Means ± SE; n ≥ 8; the letters beside the bars indicate significant different lengths of PIN2-declining region under different treatments, unpaired two-tailed t test, P < 0.05. The numbers inside the bars indicate the fluorescence intensity of each region. (E) The PIN2 expression level under glucose starvation or TOR inhibition conditions. Total RNA was isolated from primary root, and analyzed by qRT-PCR. Means ± SE; n = 3; n.s., no significant difference. (F) TOR inhibition triggers a faster PIN2 degradation. The 4-d-old pPIN2::PIN2-GFP seedlings grown in the presence of a glucose supply were treated with or without Torin2 (T) and CHX for 3 h. Images shown are cells from the elongation zone. (Scale bar, 50 μm.) (G) Quantification of relative fluorescence intensity in F. Means ± SE; n ≥ 8, unpaired two-tailed t test, ***P < 0.001. (H) TOR interacts with PIN2-HL, revealed by semi-in vitro pull-down assay. HL, hydrophilic loop (amino acids 188 to 477). (I) TOR phosphorylates PIN2-HL, revealed by the in vitro kinase assay.
We then investigated the underlying mechanism by which PIN2 is involved in glucose-TOR regulation of the auxin gradient by analyzing the pPIN2::PIN2-GFP transgenic reporter line (10). In the presence of a glucose supply, PIN2-GFP localized apically in the epidermis, with a physical PIN2-GFP gradient distribution pattern of the highest expression within the root meristem zone followed by modest expression in the elongation zone, and a gradual decrease and disappearance of PIN2-GFP in the differentiation zone (Fig. 2 C and D), consistent with prior reports (11). We found that glucose depletion or TOR inhibition (by Torin2 treatment or in estradiol-inducible RNAi tor transgenic plants [tor-es]) down-regulated the PIN2-GFP level in Arabidopsis primary root, and strongly diminished the PIN2-GFP level in the elongation and differentiation zones, while the PIN2 polarized location was still maintained (Fig. 2 C and D). Interestingly, Torin2 treatment did not affect the mRNA level of PIN2 (Fig. 2E), but triggered a faster PIN2 degradation in Arabidopsis primary root in the presence of the protein synthesis inhibitor cycloheximide (CHX) (Fig. 2 F and G), indicating posttranslational regulation of PIN2. Moreover, endogenous TOR kinase immunoprecipitated from Arabidopsis seedlings using a TOR-specific antibody directly interacted and phosphorylated the central hydrophilic loop domain of PIN2 (PIN2-HL) (Fig. 2 H and I). Together these results indicate that PIN2 is a substrate of TOR, and that TOR can phosphorylate and stabilize PIN2.
Auxin forms a steep gradient to maintain stem cell identity in the quiescent center, activate cell proliferation in root meristem, and promote cell expansion in the elongation zone, respectively (12). It is well known that very low auxin concentrations stimulate root elongation, while minute increases in auxin levels can quickly inhibit root elongation (13). Our results suggested that glucose-TOR signaling is essential for maintaining such a low auxin response region within the elongation zone in order to promote cell expansion by regulating the expression of the auxin efflux facilitator PIN2. Auxin can be transported from the meristem through the elongation zone to the differentiation zone via PIN2-mediating shootward polar auxin transport to form a high/low-moderate auxin concentration pattern in the primary root. We propose that when glucose-TOR signaling is inhibited, the region with polarized PIN2 in the elongation zone is largely decreased and, therefore, auxin transport from the elongation zone to the differentiation zone is blocked, leading to a high auxin accumulation in the elongation zone and expansion inhibition in the Arabidopsis root.
Taken together, the data in our study provide an example of how dynamic metabolic energy inputs could influence the preestablished hormone signaling to orchestrate complex growth programs in plants. In the future, identification and functional analyses of the TOR phosphorylation sites in PIN2 will help to uncover additional roles of the glucose-TOR-PIN2 axis in plant growth and development.
Methods
Plant Materials and Growth Conditions.
All plant materials were grown in a plant growth chamber at 23 °C light/21 °C dark, 65% humidity, and 75 µmol/m2 s light intensity under 16-h light/8-h dark photoperiod. Arabidopsis seeds were germinated in six-well plates containing 1 mL of glucose-free liquid medium (1/2 MS, pH = 5.7) for 4 d to enter the mitotically quiescent state. Quiescent seedlings were treated with 15 mM glucose for 1 d with or without Torin2 (0.25 μM, pretreated for 1 h) or estradiol (1 μM, pretreated for 2 d) to reactivate root growth. For monitoring the PIN2 degradation rate, 4-d-old seedlings grown in the presence of a glucose (15 mM) supply were treated with Torin2 (0.25 μM) and CHX (50 μM) for 3 h.
EdU staining assay, semi-in vitro pull-down assay, and in vitro kinase assay was performed as described previously (4).
Acknowledgments
We thank Shingo Nagawa for kindly providing us the DR5v2::ntdTomato, eir1-1, and DR5v2::ntdTomato/eir1-1 seeds. The research included in this report is supported by National Natural Science Foundation of China Grant 31870269 (to Y.X.), and the Basic Forestry and Proteomics Research Center, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University.
Footnotes
The authors declare no competing interest.
Data Availability.
All study data are included in the article.
References
- 1.Liu G. Y., Sabatini D. M., mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu L., Wang P., Xiong Y., Target of rapamycin signaling in plant stress responses. Plant Physiol. 182, 1613–1623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X., et al. , Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc. Natl. Acad. Sci. U.S.A. 114, 2765–2770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong Y., et al. , Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bögre L., Henriques R., Magyar Z., TOR tour to auxin. EMBO J. 32, 1069–1071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q., et al. , Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res. 73, 2574–2586 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luschnig C., Gaxiola R. A., Grisafi P., Fink G. R., EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamowski M., Friml J., PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 27, 20–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao C. Y., et al. , Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12, 207–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Scheres B., Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17, 525–536 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abas L., et al. , Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8, 249–256 (2006).Corrected in: Nat. Cell Biol.8, 424 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Di Mambro R., et al. , Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc. Natl. Acad. Sci. U.S.A. 114, E7641–E7649 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motte H., Vanneste S., Beeckman T., Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 70, 465–488 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.