Significance
In nature, it is extremely rare to observe attraction to fecal matter between wild mammalian species. Horse manure rolling (HMR) behavior described in this study is frequently observed in QIN pandas at low habitat temperature. Based on integrated analysis from climatic data, animal behaviors, and molecular assays, HMR is found as a temperature-, chemical-, and TRPM8-related behavior that may contribute to pandas’ cold tolerance. This study sheds light on how wild animals actively seek and utilize potential chemical resources from their habitat for survival adaptation.
Keywords: giant panda, horse, feces, TRPM8, temperature
Abstract
Attraction to feces in wild mammalian species is extremely rare. Here we introduce the horse manure rolling (HMR) behavior of wild giant pandas (Ailuropoda melanoleuca). Pandas not only frequently sniffed and wallowed in fresh horse manure, but also actively rubbed the fecal matter all over their bodies. The frequency of HMR events was highly correlated with an ambient temperature lower than 15 °C. BCP/BCPO (beta-caryophyllene/caryophyllene oxide) in fresh horse manure was found to drive HMR behavior and attenuated the cold sensitivity of mice by directly targeting and inhibiting transient receptor potential melastatin 8 (TRPM8), an archetypical cold-activated ion channel of mammals. Therefore, horse manure containing BCP/BCPO likely bestows the wild giant pandas with cold tolerance at low ambient temperatures. Together, our study described an unusual behavior, identified BCP/BCPO as chemical inhibitors of TRPM8 ion channel, and provided a plausible chemistry-auxiliary mechanism, in which animals might actively seek and utilize potential chemical resources from their habitat for temperature acclimatization.
Attraction to feces is common in insects, given that the fecal volatiles provide olfactory cues for locating food (1) and oviposition sites (2, 3). Among mammals, fecal odors provide information on sex, reproductive status, individual identity, ownership, competitive ability, and health status of conspecifics, thus play a crucial role in intraspecific communication (4–6). Besides, they act as warning signals for the presence of danger; herbivorous mammals recognize the chemical information available in feces of predators (7–9), suggesting an antipredator function for fecal volatiles in interspecific defense. Consistently, exposure to predator feces to mimic the presence of predators has been reported in small mammals, which led to a reduction of individuals’ body mass (10).
To our knowledge, although some casual cases of attraction to the fecal matter of heterospecifics have been recorded (11, 12), the biological significance is unclear, let alone the related molecular mechanisms. Our 10-y field observations of Qinling giant pandas (also named QIN pandas) indicated that fresh horse manure sometimes exhibits a strong attraction for pandas and leads them to roll in such dunghills. Interestingly, the ancient trade routes (13, 14) connecting Shu kingdom and Chang’an (referred to as Xi'an, the former capital city in Chinese history, SI Appendix, Fig. S1A) made the captive horses for good transit into a stable member in the present panda habitat for thousands of years, thereby keeping horse dunghills and their odor familiar to the populations of QIN pandas (SI Appendix, Fig. S1B). Unlike any novel odor that attracts animals, this type of frequent and stable panda–dunghill interaction triggered our great interest in uncovering its underlying mechanism by combining behavioral observation, compound analysis, climate data, animal assays, molecular cloning, and functional tests to provide further perspective on this behavior.
Results
Horse Manure Rolling Behavior of Wild QIN Pandas.
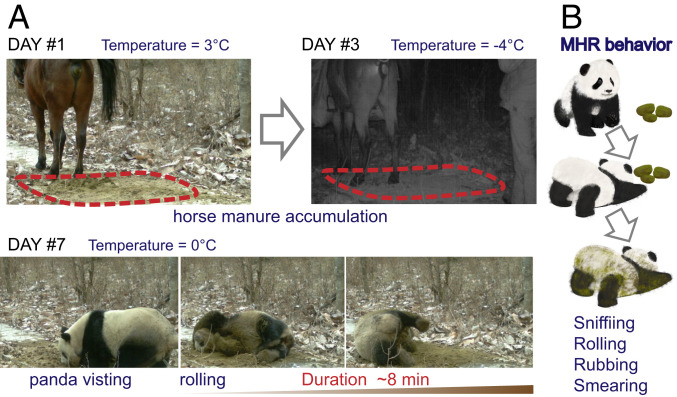
Our behavioral observations showed that QIN pandas tend to seek out and sniff fresh horse manure and then roll over it (Fig. 1A and Movie S1). We made the description of this type of behavior, which consists of four primary behavioral processes: 1) carefully sniffing; 2) rubbing against the horse manure with cheek; 3) rolling in the horse manure; 4) smearing the horse manure over the whole body (Fig. 1B). We named it horse manure rolling (HMR) behavior.
Fig. 1.
HMR behavior of wild giant pandas. (A) Images of HMR behavior captured by an infrared camera at the same spot. Horse manure (indicated by dotted line) was accumulated from the first day to the third day (Top row). A wild QIN panda exhibited HMR behavior on the seventh day. The ambient temperatures and behavior duration are shown. (B) Schematic diagram summarizing the processes and characteristics of HMR behavior.
We captured 38 independent HMR events in both male and female QIN pandas by infrared cameras placed in the wild from July 2016 to June 2017. The duration of these recorded behavioral events ranged from 6 to 499 s (141.3 ± 26.2 s on average; Fig. 2A), which is significantly higher than non-HMR group (Wilcoxon rank sum test, P < 0.001). These findings suggest that HMR is not likely an anecdotal record but rather a frequent behavior.
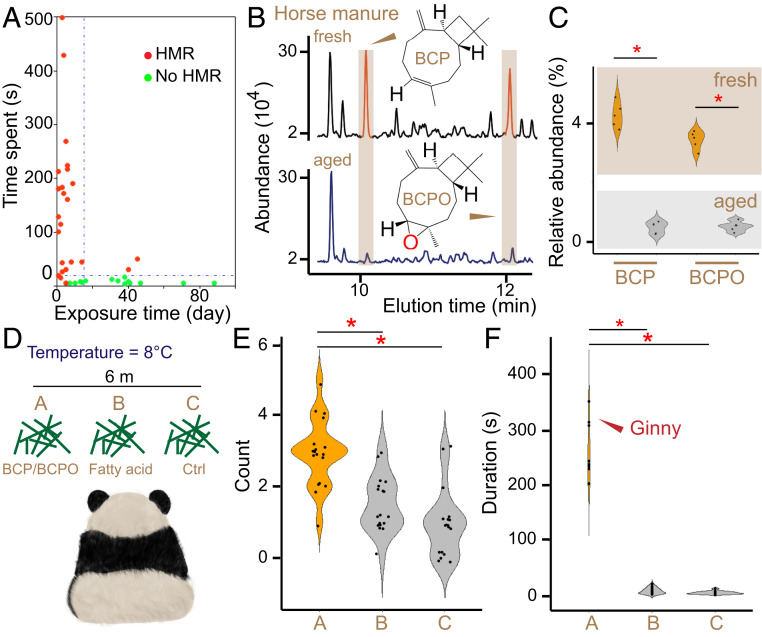
Fig. 2.
BCP/BCPO evokes HMR behavior. (A) Relationship between HMR behavior and exposure time of horse manure. Durations of HMR behaviors were obtained from infrared camera-trap images. (B) Representative gas chromatography-mass spectrometer (GC-MS) analysis of the fresh (Upper) and aged (Lower) horse manure. Chemical structures of BCP and BCPO are indicated side-by-side with their peaks (shown in red and highlighted in yellow). (C) Relative abundance of BCP/BCPO in fresh (in yellow) and aged (in gray) horse manure. *P < 0.01. (D) A sketch indicating the experimental design method for animal behavior observation done in Beijing Zoo. Chemical compounds contained in different types of hay are shown. Control (Ctrl) represents pure water. (E) The number of panda visiting events in front of each hay. Group names are indicated as shown in D. Kruskal–Wallis rank sum test with post hoc Dunn test was used for pairwise comparison, *P < 0.001. (F) Time spent of panda visiting events in front of each hay. HMR behavior exhibited by Ginny is indicated by red arrow. Kruskal–Wallis rank sum test with post hoc Dunn test was used for pairwise comparison, *P < 0.001.
Beta-Caryophyllene/Caryophyllene Oxide Serves as the Key Compound for HMR Behavior.
We found that the freshness of horse manure significantly affected HMR behavior. As shown in Fig. 2A, almost all of the HMR behaviors were recorded when the exposure time of horse manure was less than 10 d. Indeed, fresh horse manure (exposure time <10 d) exhibited a strong attraction for pandas (χ2 = 30.81, df = 1, P < 0.001), evoking HMR behavior in nearly all of the approached individuals (Fig. 2A). Given such a strong freshness dependence, these results encouraged us to identify the crucial compounds that induce HMR behavior by comparing the volatiles in fresh (n = 5) and aged (n = 5) horse manure samples. Among the fecal volatiles and semi-volatiles (SI Appendix, Table S1), we noticed that fresh horse manure is rich in two types of sesquiterpenes (beta-caryophyllene is referred to as BCP and caryophyllene oxide, referred to as BCPO), which are scarce in aged samples (Fig. 2 B and C, Wilcoxon rank sum test, P < 0.01). Accordingly, BCP is a widely spread component in plants, which also shows an oxidized form as BCPO (15–17). These findings prompted us to evaluate the roles of these chemical compounds in HMR behavior of the giant pandas at Beijing Zoo (SI Appendix, Table S2). To mimic the dunghill, we used three separate types of hay containing BCP/BCPO, fatty acids, and pure water, respectively (Fig. 2D and SI Appendix, Table S3). The captive pandas (n = 6) frequently visited the BCP/BCPO-containing hay, but approached the others less frequently (Kruskal–Wallis rank sum test, χ2 = 26.04, df = 2, P < 0.001) (Fig. 2E). It was noteworthy that they spent 3 to 6 min with BCP/BCPO-containing hay by sniffing, rubbing, and smearing it (Fig. 2F). Especially, BCP/BCPO-treated hay induced one of the tested pandas (Ginny) to exhibit HMR behavior for 6 min (Fig. 2F and Movie S2). In contrast, the pandas just stopped over in front of the other hay samples without BCP/BCPO (Kruskal–Wallis rank sum test: χ2 = 38.89, df = 2, P < 0.001) (Fig. 2F). Therefore, these results together demonstrate that BCP/BCPO serves as the crucial component in fresh horse manure to evoke the HMR behavior of giant pandas.
HMR Behavior Correlates with the Ambient Temperature.
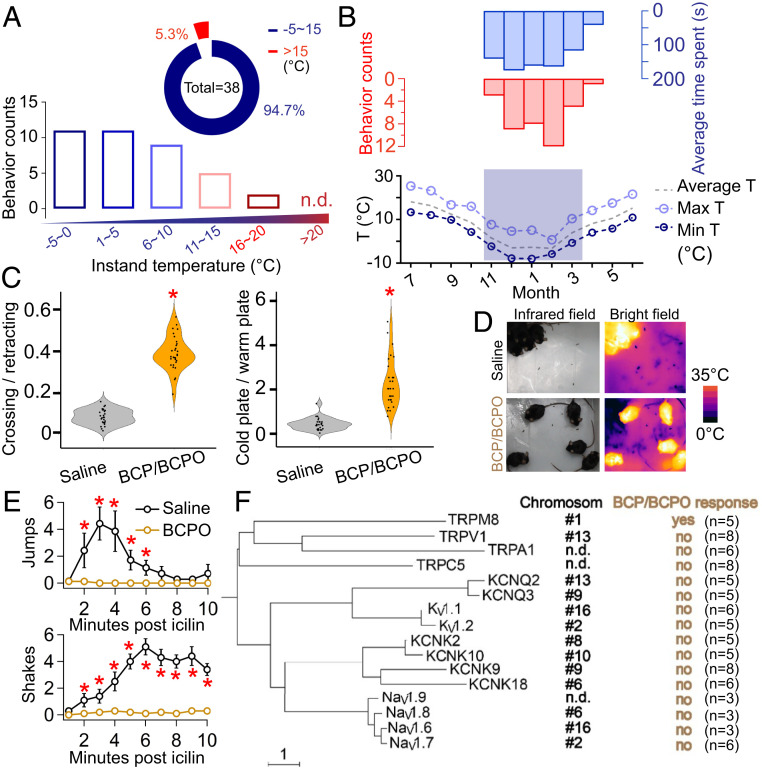
When we analyzed the data from infrared cameras, we were surprised to find that the captured events occurred in certain months but not the whole year, implying a pattern of seasonal behavior. Unlike the mating season lasting from February to April (18), the HMR behavior could be observed for a much longer period (November to April). Furthermore, we measured the current ambient temperature recorded by an infrared camera simultaneously and found a significant correlation between the numbers of captured HMR events and the ambient temperature (Fig. 3A). As illustrated in Fig. 3 A, Inset, 94.7% of HMR events were recorded at −5 to 15 °C. In contrast, there was no record of HMR behavior from an infrared camera when the ambient temperature exceeded 20 °C (Fig. 3A). We examined the relationship between the local monthly climate data (reference to Foping Meteorological Station) and the number of captured HMR events, which allowed us to understand the seasonal and temperature-dependent properties of HMR behavior (Fig. 3B). As illustrated in Fig. 3B, we found a significant correlation between either the frequency (r = 0.59) or duration (r = 0.63) of HMR events and the ambient temperature, whereas there was no significant overlap between the observation window of HMR events and mating seasons. Because of the temperature correlation, we were prompted to test whether BCP/BCPO can affect the thermal sensation of mammals. Limited by the particularity of study on pandas, we used the two-temperature choice assay to compare the cold response between BCP/BCPO-treated and saline-treated mice. We observed that the BCP/BCPO-treated mice exhibited a much higher success rate for crossing from the warm to the cold plate (Wilcoxon rank sum test, P < 0.001), compared to the saline-treated mice (Fig. 3 C, Left and Movie S3). In addition, the BCP/BCPO-treated mice also exhibited a much longer time spent than the saline-treated mice on the cold plate (Wilcoxon rank sum test, P < 0.001) (Fig. 3 C, Right). Moreover, unlike the saline-treated mice, the BCP/BCPO-treated mice (in five repeated experiments) did not show the huddling or other cold-related behavior at 4 °C (Fig. 3D and Movie S4), suggesting an increased cold tolerance of BCP/BCPO-treated mice. As expected, BCPO largely attenuated wet-dog shakes and jumping behaviors induced by icilin (Fig. 3E). Based on these results, we therefore hypothesized that BCP/BCPO directly interacts with a molecular temperature sensor to evoke such a cold tolerance behavior.
Fig. 3.
HMR behavior is temperature related. (A) Relationship between infrared camera-trap number and ambient temperature. (Inset) Ratio of HMR behaviors in two temperature ranges: −5 to 15 °C, in blue; above 15 °C, in red. (B) The relationship between HMR behavior (indicated by behavior number and average time spent) and local climate data. (C) Summary of crossing and retracting behavior when mice were challenged with the cold plate (Left), the y axis represents the ratio of behavior counts. Temperature preference testing for saline-treated and BCP/BCPO-treated mice (Right), the y axis represents the ratio of behavior counts. Warm plate set at 28 °C, cold plate set at 10 °C. *P < 0.001. (D) Images of the representative behavior saline-treated and BCP/BCPO-treated mice at 4 °C. The pictures show representative thermal images (Right) and normal images (Left). (E) Intraperitoneal (i.p.) injection of BCPO reduced jumping behavior of mice (n = 8 for each group) induced by i.p. injection of icilin (Top). The i.p. injection of BCPO reduced shaking behavior of rats (n = 10 for each group) induced by i.p. injection of icilin (Bottom). For mice, icilin and BCPO dissolved in propylene glycol with final concentration of 10 mg/kg and 100 μmol/kg, respectively. For rats, icilin and BCPO dissolved in propylene glycol with final concentrations of 1 mg/kg and 100 μmol/kg, respectively. *P < 0.05. (F) Phylogenetic tree of molecular thermal detectors and transducers in panda. The location on chromosomes (in black), BCP/BCPO responses (in yellow), and sample sizes are shown. The 100 μM menthol, 1 μM capsaicin, 100 μM AITC (allyl isothiocyanate), and 50 μM Gd3+ were used to activate TRPM8, TRPV1, TRPA1, and TRPC5, respectively. n.d., no data, indicating that this gene is absent in the chromosome-level giant panda draft genome (20).
The giant panda genome (19, 20) reveals the full-length sequences of gene-encoded proteins. In combination with the established studies in temperature sensation (21), we are able to test our hypothesis by expressing the potential thermosensors and carrying out functional assays at the molecular level. For thermosensory transduction at each step, multiple ion channel subtypes are involved to first detect and then transmit the temperature information, such as thermosensitive transient receptor potential family (thermo-TRPs) and several other temperature-sensitive channels. Among these primary temperature-related sensors of giant pandas, surprisingly, we found that the BCP/BCPO mixture selectively interacted with the giant panda TRPM8 (gpTRPM8) as well as other species-specific TRPM8 orthologs (Fig. 3F and SI Appendix, Figs. S2 and S3) from amphibians, reptiles, birds, and mammals. The nonselective interaction between BCP/BCPO and TRPM8 made it possible that such molecules can exhibit similar biological significance under certain conditions. At the molecular level, the TRPM8 channel is a prototypical detector located in nociceptive A-delta and C-fiber neurons for cold sensation, which has been validated in knockout mice (22–24) and pharmacological studies (25, 26). This interaction between BCP/BCPO and TRPM8 may enable animals to adjust the cold feeling in peripheral tissues (Fig. 3 C–E and Movie S3). More importantly, the regulation of TRPM8 has been identified to confer temperature adaptation in mammals throughout evolution (27, 28). Since cold-activated TRPM8 is a major excitatory conduit for the decrease in temperature, physiological strategies have been employed by several vertebrates that tuned the cold activation of their own TRPM8 orthologs to achieve temperature adaptation (27–29). Almost without exception, however, these cases of developed temperature adaptation have only been discovered through the genetic evolution of molecular thermosensors. Given the widely used tactic by functional tuning of thermal sensors for habitat temperature adaptation, chemical-induced modulation is plausibly an adopted strategy in mammals.
BCP/BCPO Inhibits Both Chemical and Cold Activation of gpTRPM8.
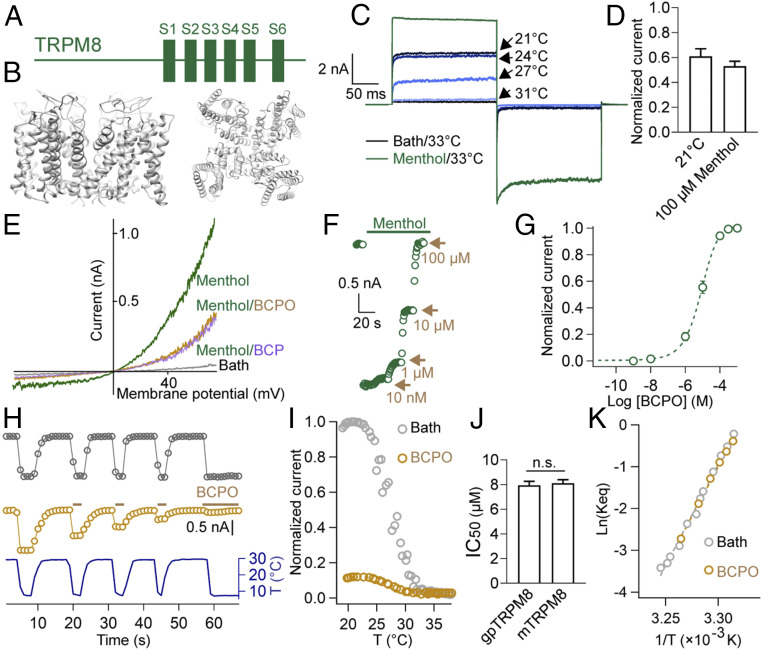
As a tetrameric complex and Ca2+ permeable ion channel with cold sensitivity (25, 30), each subunit of the TRPM8 protein complex contains six transmembrane domains (SI Appendix, Figs. S1–S6 and Fig. 4A). To understand the interaction between BCP/BCPO and gpTRPM8, we first modeled the complex of gpTRPM8 (Fig. 4B) and functionally expressed this ion channel in human embryonic kidney 293 (HEK293) cells for patch-clamp recordings. As expected, gpTRPM8 was activated in the presence of menthol (Fig. 4 C and D), a well-known cooling compound (31). Importantly, the temperature-dependent recordings of gpTRPM8-expressed cells indicated that the cold-induced gating is intact (Fig. 4 C and D). As illustrated in Fig. 4E, we found that BCP and BCPO were equally effective in inhibiting gpTRPM8. Because of the better water solubility of BCPO, we used it as the representative compound to further study the interaction between functional sesquiterpenes and gpTRPM8. As shown in Fig. 4 F and G, raising BCPO concentrations substantially decreased the menthol-activated currents. The half-inhibitory concentration (IC50) of BCPO was 7.8 ± 0.6 μM, yielding a hill slope factor of 0.93 ± 0.02 (Fig. 4G). These results indicate that BCPO acts as a potent inhibitor in the menthol-induced activation of gpTRPM8.
Fig. 4.
BCP/BCPO inhibits the activation of gpTRPM8. (A) Diagram of the subunit of gpTRPM8 illustrating location of the six transmembrane domains. (B) Ribbon diagram of modeled gpTRPM8, showing from side (Left) and top (Right). (C) Representative whole-cell recording of gpTRPM8 in the presence of 1 mM menthol (in green) or bathing solution at different temperatures (in blue or black). (D) Macroscopic currents from five patch recordings were normalized to the amplitude in the presence of 1 mM menthol. (E) Representative whole-cell recordings of gpTRPM8 challenged by menthol (100 μM), menthol (100 μM)/BCPO (3 μM), and menthol (100 μM)/BCP (3 μM). Voltage ramp ranged from −80 to +80 mV. (F) Representative menthol-activated currents in the presence of BCPO. The current amplitude was recorded at −80 mV. (G) Dose–response relationship displaying the BCPO inhibition of gpTRPM8. The curve of the wild-type channel was fitted to a Hill equation. Data points are illustrated as average ±SEM (n = 5 cells). The current amplitude was recorded at −80 mV. (H) Whole-cell recordings of cold-activated gpTRPM8 expressed in HEK293 cells. BCPO application is shown by yellow dots. The current amplitude was recorded at −80 mV. (I) The current–temperature relationships of gpTRPM8 in the presence of bathing solution (in gray, n = 3) and BCPO (in yellow, n = 3). Currents recorded at −80 mV were normalized to the maximum current during gpTRPM8 cold activation in the absence of BCPO. (J) Comparison of IC50 values of TRPM8 orthologs activated by cold (10 °C). n.s., no significance. mTRPM8 represents mouse TRPM8. (K) van ’t Hoff plots of gpTRPM8 in the presence of bathing solution (in gray, n = 3) and BCPO (in yellow, n = 3).
Given that the temperature-activated gating process is likely distinct from the ligand-induced conformational change in TRP channels (28, 32), we tested the cold-induced activation of gpTRPM8 in the absence or presence of BCPO. As shown in Fig. 4 H and I (gray dots), cooling caused rapid and reversible activation of gpTRPM8, while such a cold-induced activation was potently inhibited by BCPO (Fig. 4 H and I, yellow dots). Similarly, BCPO inhibited the cold-activated currents of other tested TRPM8 orthologs (Fig. 4J and SI Appendix, Fig. S3C). When the open probability was brought to an apparently low level by BCPO application, no discernible shift was observed in the current–temperature relationship of gpTRPM8 (Fig. 4I). Consistently, the quantified ΔH (enthalpic change) and ΔS (entropic change) indicated that the cold sensitivity of gpTRPM8 could not be altered by BCPO (Fig. 4K). Our results thus demonstrated that BCP/BCPO served as potent antagonists for both chemical- and temperature-induced gpTRPM8 activation. Together, our findings reveal a chemical-auxiliary mechanism which allows QIN pandas to temporarily cope with low temperatures by seeking chemical resource from their habitat.
Discussion
In conclusion, from accidental discoveries to a rational analysis of our curiosity, our study identified a temperature-dependent HMR behavior of wild giant pandas, which is also a scientific record of consistent heterospecific feces attraction among wild mammalian species. Being the key molecules that evoke the HMR behavior, BCP/BCPO were first identified as the potent inhibitors of the cold-activated TRPM8 ion channel in pharmacology. Although other receptors of BCP/BCPO (33, 34) may also participate in the initiation of HMR behavior through the sense of smell or other unknown mechanisms, our study sheds light on why wild giant pandas frequently roll in horse manure, especially at low ambient temperatures. In this case, TRPM8-expressing nerve endings in the panda skin are considered as a potential target for BCP/BCPO provided by horse manure. Therefore, BCP/BCPO-induced TRPM8 inhibition prevents the generation of action potentials that mediates the cooling sensation. With an extended function for nonselectively targeting species-specific TRPM8 orthologs, BCP/BCPO may possess a similar biological significance in other wildlife. However, given that this chemical resource of BCP/BCPO provided by captive horses is likely rare in habitat, HMR behavior has not been widely observed in other wild animals. As seen in human history for discovering hot sensation or coolness from chili peppers or mints (31, 35), more generally, we assumed that the thermal sensations induced by BCP/BCPO play a role in the life history of giant pandas. Thus far, our current understanding of HMR behavior based on the functional impact of BCP/BCPO may further reveal the origin of this behavior together with other unknown factors required for future efforts.
Materials and Methods
Animals.
All of the animal studies of pandas conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals of Institute of Zoology, Chinese Academy of Sciences. Experimental protocols were approved by the Institutional Animal Care and Use Committees at the Institute of Zoology, Chinese Academy of Sciences (approval ID:IOZ20190051). For mice and rat models, all possible efforts were made to reduce the sample size and also to minimize animal suffering.
Supplementary Material
Acknowledgments
We thank X. Wang, L. Shan, G. Wang, H. Fan, S. Ma, K. Gao, L. Wang, X. Hu, Y. Ma, L. Yu, M. Wang, H. Gao, D. Zhang, X. Lai, and T. Ma for help with the data collection; X. Lu and Y. Han for protein structure simulation; and K. Duan, W. Wei, and L. Qiu for cartographic drawing. We also thank the Foping National Nature Reserve and Beijing Zoo for their assistance on this study. This work was supported by National Natural Science Foundation of China (31821001) and Chinese Academy of Sciences (XDB31020000 and QYZDY-SSW-SMC019) to F.W.; National Natural Science Foundation of China (21761142002 and 31930015), Chinese Academy of Sciences (SAJC201606 and QYZDJ-SSW-SMC012), and Science and Technology Department of Yunnan Province (2019ZF003) to R.L.; National Natural Science Foundation of China (31640071 and 31770835) to S.Y.; and Chinese Academy of Sciences (QYZDB-SSW-SMC047) and National Natural Science Foundation of China (31622012) to Y.N.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. H.X. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004640117/-/DCSupplemental.
Data Availability.
All data needed to evaluate the conclusions are present in this paper and/or the supporting information. Additional data are available from the authors upon request. A detailed version of this study’s materials and methods for the volatiles analysis, mimicked dunghill, transient transfection, gene synthesis, mutation, molecular modeling, and animal experiments is provided in SI Appendix. These assays were all performed using standard approaches.
References
- 1.Wurmitzer C., et al. , Attraction of dung beetles to herbivore dung and synthetic compounds in a comparative field study. Chemoecology 27, 75–84 (2017). [Google Scholar]
- 2.Brodie B. S., Babcock T., Gries R., Benn A., Gries G., Acquired smell? Mature females of the common green bottle fly shift semiochemical preferences from feces feeding sites to carrion oviposition sites. J. Chem. Ecol. 42, 40–50 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Mansourian S., et al. , Fecal-derived phenol induces egg-laying aversion in Drosophila. Curr. Biol. 26, 2762–2769 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Ghosal R., Seshagiri P. B., Sukumar R., Dung as a potential medium for inter-sexual chemical signaling in Asian elephants (Elephas maximus). Behav. Processes 91, 15–21 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Linklater W. L., Mayer K., Swaisgood R. R., Chemical signals of age, sex and identity in black rhinoceros. Anim. Behav. 85, 671–677 (2013). [Google Scholar]
- 6.Wronski T., Apio A., Plath M., The communicatory significance of localised defecation sites in bushbuck (Tragelaphus scriptus). Behav. Ecol. Sociobiol. 60, 368–378 (2006). [Google Scholar]
- 7.Apfelbach R., Blanchard C. D., Blanchard R. J., Hayes R. A., McGregor I. S., The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Melchiors M. A., Leslie C. A., Effectiveness of predator fecal odors as black-tailed deer repellents. J. Wildl. Manage. 49, 358–362 (1985). [Google Scholar]
- 9.Rosen J. B., Asok A., Chakraborty T., The smell of fear: Innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci. 9, 292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettett C. E., et al. , Daily energy expenditure in the face of predation: Hedgehog energetics in rural landscapes. J. Exp. Biol. 220, 460–468 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Reiger I., Scent rubbing in carnivores. Carnivore 2, 17–25 (1979). [Google Scholar]
- 12.Ryon J., Fentress J. C., Harrington F. H., Bragdon S., Scent rubbing in wolves (Canis Iupus): The effect of novelty. Can. J. Zool. 64, 573–577 (1986). [Google Scholar]
- 13.Jupp D. L., Sir Eric Teichman and the Tangluo Shu Road. 5-6 (2014).
- 14.Wang C., et al. , Influence of human activities on the historical and current distribution of Sichuan snub-nosed monkeys in the Qinling Mountains, China. Folia Primatol. (Basel) 85, 343–357 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Jayaprakasha G. K., Jagan Mohan Rao L., Sakariah K. K., Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J. Agric. Food Chem. 51, 4344–4348 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Mockute D., Bernotiene G., Judzentiene A., The essential oil of Origanum vulgare L. ssp. vulgare growing wild in vilnius district (Lithuania). Phytochemistry 57, 65–69 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Orav A., Stulova I., Kailas T., Müürisepp M., Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J. Agric. Food Chem. 52, 2582–2586 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Zhou W., et al. , Seasonal and reproductive variation in chemical constituents of scent signals in wild giant pandas. Sci. China Life Sci. 62, 648–660 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Zhao S., et al. , Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet. 45, 67–71 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Fan H., et al. , Chromosome-level genome assembly for giant panda provides novel insights into Carnivora chromosome evolution. Genome Biol. 20, 267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffstaetter L. J., Bagriantsev S. N., Gracheva E. O., TRPs et al.: A molecular toolkit for thermosensory adaptations. Pflugers Arch. 470, 745–759 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista D. M., et al. , The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Colburn R. W., et al. , Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Dhaka A., et al. , TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 (2007). [DOI] [PubMed] [Google Scholar]
- 25.McKemy D. D., Neuhausser W. M., Julius D., Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Proudfoot C. J., et al. , Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 16, 1591–1605 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Matos-Cruz V., et al. , Molecular prerequisites for diminished cold sensitivity in ground squirrels and hamsters. Cell Rep. 21, 3329–3337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S., et al. , A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc. Natl. Acad. Sci. U.S.A. 117, 8633–8638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers B. R., Sigal Y. M., Julius D., Evolution of thermal response properties in a cold-activated TRP channel. PLoS One 4, e5741 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peier A. M., et al. , A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Patel T., Ishiuji Y., Yosipovitch G., Menthol: A refreshing look at this ancient compound. J. Am. Acad. Dermatol. 57, 873–878 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Yang F., Cui Y., Wang K., Zheng J., Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 7083–7088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khasabova I. A., et al. , CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behav. Pharmacol. 22, 607–616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertsch J., et al. , Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U.S.A. 105, 9099–9104 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilius B., Appendino G., Spices: The savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 164, 1–76 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions are present in this paper and/or the supporting information. Additional data are available from the authors upon request. A detailed version of this study’s materials and methods for the volatiles analysis, mimicked dunghill, transient transfection, gene synthesis, mutation, molecular modeling, and animal experiments is provided in SI Appendix. These assays were all performed using standard approaches.