Abstract
Background
Older adults with chronic kidney disease (CKD)-discordant conditions (comorbid conditions with treatment recommendations that potentially complicate CKD management) have higher risk of hospitalization and death. Our goal is to develop a CKD-Discordance Index using electronic health records to improve recognition of discordance.
Methods
This retrospective cohort study included Kaiser Permanente Southern California patients aged ≥65 years and older with incident CKD (N = 30,932). To guide inclusion of conditions in the Index and weight each condition, we first developed a prediction model for 1-year hospitalization risk using Cox regression. Points were assigned proportional to regression coefficients derived from the model. Next, the CKD-Discordance Index was calculated as an individual’s total points divided by the maximum possible discordance points. The association between CKD-Discordance Index and hospitalizations, emergency department visits, and mortality was accessed using multivariable-adjusted Cox regression model.
Results
Overall, mean (SD) age was 77.9 (7.6) years, 55% of participants were female, 59.3% were white, and 32% (n = 9,869) had ≥1 hospitalization during 1 year of follow-up. The CKD-Discordance Index included the following variables: heart failure, gastroesophageal reflux disease/peptic ulcer disease, osteoarthritis, dementia, depression, cancer, chronic obstructive pulmonary disease/asthma, and having four or more prescribers. Compared to those with a CKD-Discordance Index of 0, adjusted hazard ratios (95% confidence interval) for hospitalization were 1.39 (1.27–1.51) and 1.81 (1.64–2.01) for those with a CKD-Discordance Index of 0.001–0.24 and ≥0.25, respectively (ptrend < .001). A graded pattern of risk was seen for emergency department visits and all-cause mortality.
Conclusion
A data-driven approach identified CKD-discordant indicators for a CKD-Discordance Index. Higher CKD-Discordance Index was associated with health care utilization and mortality.
Keywords: Multimorbidity, Electronic health record, Hospitalization, Mortality, Geriatric nephrology
Routine management of chronic kidney disease (CKD) follows a one-condition-at-a-time approach that often does not anticipate or address the impact of co-occurring conditions on health outcomes (1–3). In this approach to care, clinical practice guidelines (CPGs) recommend measurement of kidney disease biomarkers and disease-specific treatment to prevent the progression of kidney disease (4,5). In the limited cases in which guidance is provided regarding co-occurring conditions, the focus is on those conditions directly related to the treatment of CKD, such as hypertension or diabetes (i.e. concordant conditions). Although this approach may be appropriate in patients for whom CKD is their primary problem, it may be unsuitable for the majority of patients with CKD who are older and have additional chronic conditions not related to CKD (6,7).
Co-occurring chronic conditions with opposing or unrelated treatment goals have been described as discordant conditions (8–10). Examples of CKD-discordant conditions include osteoarthritis requiring nonsteroidal anti-inflammatory drugs, heart failure (HF) requiring diuresis, or dementia that limits capacity for CKD self-management (11). Among older adults with CKD, the presence of one or more CKD-discordant conditions, irrespective of total number of conditions, has been shown to be associated with increased risk of hospitalization, emergency department (ED) visits, and death (7). Furthermore, patients with CKD describe receiving conflicting treatment recommendations for discordant conditions as a major barrier to participating in CKD self-management (11). Reconciling conflicting treatment advice, simplifying self-management tasks, and aligning care with patient preferences may be effective strategies to address discordance (12–14); however, practical tools to help providers identify CKD discordance are not available.
To improve the recognition of CKD discordance and enhance both knowledge and practicality of this concept, our objective is to use a data-driven approach to build a CKD-Discordance Index. We hypothesized that evidence of CKD discordance could be identified from multiple sources of data routinely available in electronic health records (EHRs) and a risk prediction model could be used to guide the selection of conditions for inclusion in a CKD-Discordance Index. To test our hypothesis, we used EHR data from a large, regional, U.S. health system; first, developing a CKD-Discordance Index and then testing for associations with hospitalization, and second, ED visits and mortality.
Materials and Method
Study Population and Data Sources
We conducted a split-sample, retrospective cohort study of Kaiser Permanente Southern California (KPSC) members aged ≥65 years and older, with incident CKD between January 1, 2008, and June 30, 2014. After estimating glomerular filtration rate (GFR) (15), incident CKD was defined as having at least two consecutive estimated (eGFR) measures <45 mL/min/1.73 m2 separated by 90 days and at least one eGFR >60 mL/min/1.73 m2 prior to the first eligible eGFR. The index date was defined as the date of the second eGFR <45 mL/min/1.73 m2. This eGFR cut-point was chosen because at <45 mL/min/1.73 m2, there is a higher likelihood of receiving discordant recommendations. Patients were excluded for the following reasons: preexisting end-stage renal disease, <12 months of continuous membership or a pharmacy benefit, and missing body mass index (BMI), systolic blood pressure, or diastolic blood pressure before study entry. The final analytic cohort included 30,932 patients (Supplementary Figure 1). The study population was randomly split into three data sets: (a) a developing cohort for building a prediction model for 1-year hospitalization risk (n = 10,315), (b) an internal validation cohort for assessing the prediction model’s performance (n = 10,304), and (c) a testing cohort for determining the association of the CKD-Discordance Index (derived from the prediction model) and hospitalization risk (n = 10,313). The study was approved by the KPSC institutional review board. We followed up the TRIPOD statement for reporting (Supplementary Table S1) (16).
Evidence of Discordance
Discordant conditions included HF, osteoarthritis, osteoporosis, hypothyroidism, epilepsy/seizure, Parkinson’s disease, dementia, gastroesophageal reflux disease (GERD)/peptic ulcer disease, depression, chronic obstructive pulmonary disease (COPD), and cancer and were defined using inpatient and outpatient diagnosis codes in the year prior to the index date (Supplementary Table S2) (7). CKD-discordant conditions were defined using a two-step process that included independent nephrologist sorting (concordant vs discordant) and verification by review of current CKD CPGs, details of which have been previously published (7). Inclusion of several conditions, including HF, was further supported by our findings in a qualitative study of older adults with CKD (11). We also broadened our investigation of potential indicators of discordance to include medications and continuity of care. Prescription medications considered potentially renally inappropriate were extracted from outpatient pharmacy records and included two categories: (a) medications contraindicated in CKD and (b) medications that require dose reduction in CKD based on a previously published literature (17). Renally inappropriate prescribing may be evidence of discordance because it indicates the treatment of a co-occurring condition in conflict with appropriate treatment in CKD. The number of medication prescribers was used as a proxy for continuity of care that is easily calculated and shown to be associated with poor outcomes (18). Information on medication usage and the number of prescribers was measured between 1 year prior to and 30 days after the index date to identify prescriptions at the time of the index date.
Covariates
Baseline patient demographic characteristics (such as age, gender, and race/ethnicity), CKD stage, and BMI were obtained from the visit on or most closely preceding the index date. Information on past hospitalizations and ED visits was obtained from the EHR 1 year prior to the index date. Additional comorbidities included the following concordant conditions: hypertension, diabetes, atrial fibrillation, anemia, gout, benign prostatic hypertrophy, peripheral arterial disease, hyperlipidemia, coronary heart disease, and stroke, assessed from 1 year prior to 30 days after the index date. Our goal is to develop a CKD-Discordance Index; therefore, these conditions with overlapping treatment goals that are routinely evaluated for and managed concurrently with CKD were not included in our index, even though they may be associated with our outcome of interest.
Outcomes
The primary outcome was time to first unplanned hospitalization in the year following study entry defined using an established claims-based algorithm (19). Unplanned hospitalization was chosen because it is a potentially preventable consequence of discordance. We chose 1 year of follow-up as a clinically relevant time frame over which future interventions to address discordance may be possible. Secondary outcomes included time to first ED visit and all-cause mortality in the following year.
Statistical Analysis
In a first step, we developed and validated a prediction model for 1-year hospitalization risk using the method applied by Harrell and Steyerberg (20–22). Specifically, after excluding predictors with less than 1% prevalence or more than 40% missing data, we tested a full model containing 40 variables to predict hospitalization in the subsequent first year using Cox proportional hazards regression. Age and BMI were modeled with restricted cubic splines, and the other predictors were modeled as categorical variables. We undertook a step-down method (23), obtaining a simplified model that retained at least 95% of the variation (R2-statistic) explained by the full model (24). Potential indicators of discordance were retained in the model unless they were shown highly unlikely to be related to the hospitalization (p > .7). Validation was performed in the development and validation cohorts. Calibration was assessed by comparing the mean predicted risk and observed risk for each decile of predicted risk in the validation cohort. Discrimination was indicated by bootstrap-corrected C-statistic, measuring the area under the receiver operating characteristic curve in the development and validation datasets (20).
In the second step, we generated a points-based scoring system. Regression coefficients from our simplified model were converted into a score for each indicator of discordance, assigning a higher score to indicators with larger regression coefficients (25). We defined an individual’s total discordance points as the sum of scores for all indicators of discordance that remained in the model. The CKD-Discordance Index was then calculated as an individual’s total discordance points divided by the maximum possible discordance points (range of 0.0–1.0, with higher scores indicating greater discordance).
The CKD-Discordance Index was then applied to the test cohort. To test the associations between the CKD-Discordance Index and the risk for the three outcomes (hospitalizations, ED visits, and all-cause mortality), we calculated hazard ratios (HRs; 95% confidence intervals [95% CIs]) using Cox proportional hazards model, modeling the CKD-Discordance Index first as a continuous and then as a categorical variable. Because the CKD-Discordance Index was newly developed, meaningful cut-points do not exist. Therefore, selection of categories was based on the distribution of the index. These models were adjusted for all other covariates included in the simplified prediction model. Analyses for the three outcomes were then repeated stratifying by level of eGFR (30–44 vs <29 mL/min/1.73 m2).
Analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc, Cary, NC) and RStudio version 1.1.383 (R Foundation for Statistical Computing; www.R-project.org). R was reserved for model development, model discrimination assessment, and the points-based scoring production. All other analyses were performed using SAS. All hypothesis tests were two sided, with a significance level of p < .05.
Results
Cohort Characteristics
Overall, mean (SD) age was 77.9 (7.6) years, 55% of participants were female, and 59%, 7%, 12%, and 20% were white, Asian, black, and Hispanic, respectively (Table 1; Supplementary Table S3). The mean (SD) number of discordant conditions was 1.0 (1.1). Of the 30,932 patients, 32% (n = 9,869) had at least one hospitalization within a year after index date. Compared to those who did not have a hospitalization, those hospitalized had more discordant medical conditions (mean [SD[: 1.3 [1.2] vs 0.9 [1.1]; Table 1).
Table 1.
Baseline Characteristics of Kaiser Permanente Southern California Patients Aged ≥65 Years or Older With Incident CKD, Overall, and for Those With and Without a Hospitalization During 1 Year of Follow-Up
| Characteristic, N (%) or Mean ± SD | Overall (n = 30,932) | Hospitalized During Follow-Up | p Value | |
|---|---|---|---|---|
| No (21,063) |
Yes (9,869) |
|||
| Age | 77.9 ± 7.6 | 77.4 ± 7.5 | 78.8 ± 7.7 | <.001 |
| Female | 17013 (55) | 11747 (55.8) | 5266 (53.4) | <.001 |
| Race/ethnicity | <.001 | |||
| White | 18331 (59.3) | 12326 (58.5) | 6005 (60.8) | |
| Asian | 2191 (7.1) | 1577 (7.5) | 614 (6.2) | |
| Black | 3718 (12) | 2480 (11.8) | 1238 (12.5) | |
| Hispanic | 6055 (19.6) | 4179 (19.8) | 1876 (19) | |
| Other/unknown | 637 (2.0) | 401 (2.3) | 136 (1.5) | |
| BMI, kg/m2 | 28.3 ±6.2 | 28.5 ± 6.1 | 28.0 ± 6.4 | <.001 |
| eGFR (mL/min/1.73 m2) | <.001 | |||
| 30–44 | 27653 (89.4) | 19247 (91.4) | 8406 (85.2) | |
| 15–29 | 3005 (9.7) | 1680 (8.0) | 1325 (13.4) | |
| <15 | 274 (0.9) | 136 (0.6) | 138 (1.4) | |
| Discordant conditions | ||||
| Heart failure | 3940 (12.7) | 1792 (8.5) | 2148 (21.8) | <.001 |
| Osteoarthritis | 5045 (16.3) | 3230 (15.3) | 1815 (18.4) | <.001 |
| Osteoporosis | 4136 (13.4) | 2656 (12.6) | 1480 (15.0) | <.001 |
| Hypothyroid | 3606 (11.7) | 2380 (11.3) | 1226 (12.4) | .004 |
| Epilepsy/seizure | 173 (0.6) | 94 (0.4) | 79 (0.8) | <.001 |
| Parkinson’s disease | 307 (1.0) | 182 (0.9) | 125 (1.3) | <.001 |
| Dementia | 1979 (6.4) | 1143 (5.4) | 836 (8.5) | <.001 |
| GERD/peptic ulcer disease | 2368 (7.7) | 1464 (7) | 904 (9.2) | <.001 |
| Depression | 3639 (11.8) | 2255 (10.7) | 1384 (14) | <.001 |
| COPD/asthma | 2510 (8.1) | 1440 (6.8) | 1070 (10.8) | <.001 |
| Cancer | 4580 (14.8) | 2711 (12.9) | 1869 (18.9) | <.001 |
| Number of discordant conditions | 1.0 ± 1.1 | 0.9 ± 1.1 | 1.3 ± 1.2 | <.001 |
| Other chronic conditions | ||||
| Hypertension | 28059 (90.7) | 19085 (90.6) | 8974 (90.9) | .40 |
| Diabetes | 13325 (43.1) | 8905 (42.3) | 4420 (44.8) | <.001 |
| Atrial fibrillation | 4415 (14.3) | 2342 (11.1) | 2073 (21) | <.001 |
| Anemia | 4574 (14.8) | 2637 (12.5) | 1937 (19.6) | <.001 |
| Gout | 1520 (4.9) | 1010 (4.8) | 510 (5.2) | .20 |
| BPH | 2257 (7.3) | 1468 (7) | 789 (8) | .001 |
| Peripheral arterial disease | 1067 (3.4) | 599 (2.8) | 468 (4.7) | <.001 |
| Hyperlipidemia | 22816 (73.8) | 15554 (73.8) | 7262 (73.6) | .60 |
| Coronary heart disease | 8634 (27.9) | 4757 (22.6) | 3877 (39.3) | <.001 |
| Stroke | 1473 (4.8) | 709 (3.4) | 764 (7.7) | <.001 |
| Taking contraindicated meds | ||||
| Metformin | 5196 (16.8) | 3764 (17.9) | 1432 (14.5) | <.001 |
| Glyburide | 408 (1.3) | 280 (1.3) | 128 (1.3) | .82 |
| Gemfibrozil | 707 (2.3) | 489 (2.3) | 218 (2.2) | .54 |
| Spironolactone | 1783 (5.8) | 1033 (4.9) | 750 (7.6) | <.001 |
| Pentoxifylline | 184 (0.6) | 98 (0.5) | 86 (0.9) | <.001 |
| Taking dose-reduction meds* | ||||
| Ranitidine | 820 (2.7) | 543 (2.6) | 277 (2.8) | .20 |
| Atenolol | 9056 (29.3) | 6442 (30.6) | 2614 (26.5) | <.001 |
| Hydralazine | 2340 (7.6) | 1334 (6.3) | 1006 (10.2) | <.001 |
| Digoxin | 1650 (5.3) | 845 (4) | 805 (8.2) | <.001 |
| Rosuvastatin | 198 (0.6) | 135 (0.6) | 63 (0.6) | .90 |
| NSAID | 2501 (8.1) | 1767 (8.4) | 734 (7.4) | .004 |
| Prescribers | <.001 | |||
| <4 | 22265 (72) | 16212 (77) | 6053 (61.3) | |
| ≥4 | 8667 (28) | 4851 (23) | 3816 (38.7) | |
| Prior hospitalization† | 10192 (32.9) | 5424 (25.8) | 4768 (48.3) | <.001 |
| Prior ED visit† | 8938 (28.9) | 5087 (24.2) | 3851 (39) | <.001 |
Note: CKD = chronic kidney disease; BMI = body mass index; BPH = benign prostatic hypertrophy; COPD = chronic obstructive pulmonary disease; ED = emergency department; eGFR = estimated glomerular filtration rate; GERD = gastroesophageal reflux disease; NSAID = nonsteroidal anti-inflammatory drug.
*Medications that should be given at reduced dose in CKD.
†Prior hospitalizations and ED visits were ascertained within 1 year prior to index date.
Prediction Model for Hospitalizations
In the full model, indicators of discordance associated with statistically significant higher risk of hospitalization included HF, GERD/peptic ulcer disease, COPD/asthma, cancer, and the presence of four or more prescribers (Table 2). Although not statistically significant, osteoarthritis, dementia, and depression had a p value between .5 and .05 and were, therefore, retained in the simplified model (Table 2). Among the contraindicated and dose-reduction medications, only metformin (HR 0.85, 95% CI: 0.76–0.95), hydralazine (HR 1.15, 95% CI: 1.02–1.29), and digoxin (HR 1.17, 95% CI: 0.03) were found to be statistically significant. We excluded medications from the simplified model because of the limited number of medications that were statistically significant, complexity required to incorporate pharmacy data and potential for confounding by indication (e.g. metformin use in individuals with diabetes at lower risk for hospitalization vs metformin use reducing risk of hospitalization).
Table 2.
Hazard Ratios for Variables in the Risk Prediction Models for Hospitalization From the Development Cohort (n = 10,315)
| General Characteristics | HR (95% CI) | |
|---|---|---|
| Full Model | Simplified Model | |
| Age | ||
| Age | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) |
| Age* | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) |
| Female | 0.89 (0.83–0.97) | 0.88 (0.82–0.95) |
| Race/ethnicity | ||
| White | 1 (ref) | 1 (ref) |
| Asian | 0.91 (0.78–1.06) | 0.91 (0.78–1.06) |
| Black | 1.00 (0.90–1.12) | 1.04 (0.93–1.16) |
| Hispanic | 1.02 (0.93–1.12) | 1.02 (0.93–1.12) |
| Other | 0.75 (0.56–1.00) | 0.76 (0.57–1.01) |
| Body mass index (BMI) | ||
| BMI | 0.95 (0.94–0.96) | 0.95 (0.93–0.96) |
| BMI* | 1.06 (1.04–1.08) | 1.07 (1.05–1.08) |
| eGFR (mL/min/1.73 m2) | ||
| 30–44 | 1 (ref) | 1 (ref) |
| 15–29 | 1.47 (1.33–1.63) | 1.48 (1.34–1.64) |
| <30 | 2.60 (1.87–3.60) | 2.60 (1.88–3.60) |
| Covariate/concordant | ||
| Hypertension | 0.94 (0.83–1.07) | – |
| Diabetes | 1.15 (1.05–1.25) | 1.08 (1.00–1.17) |
| Atrial fibrillation | 1.14 (1.03–1.26) | 1.19 (1.08–1.30) |
| Anemia | 1.14 (1.04–1.25) | 1.14 (1.04–1.25) |
| Gout | 1.07 (0.92–1.25) | – |
| Benign prostatic hypertrophy | 0.86 (0.75–0.98) | 0.86 (0.75–0.99) |
| Peripheral arterial disease | 0.98 (0.82–1.17) | – |
| Hyperlipidemia | 0.95 (0.87–1.03) | – |
| Coronary heart disease | 1.29 (1.19–1.40) | 1.29 (1.19–1.39) |
| Stroke | 1.28 (1.12–1.47) | 1.27 (1.11–1.46) |
| Hospitalization in previous 1 year | 1.52 (1.4–1.66) | 1.55 (1.42–1.68) |
| ED visit in previous 1 year | 1.38 (1.28–1.49) | 1.39 (1.29–1.50) |
| Discordant conditions | ||
| Heart failure | 1.33 (1.20–1.47) | 1.37 (1.24–1.51) |
| Osteoarthritis | 1.08 (0.99–1.19) | 1.07 (0.98–1.18) |
| Osteoporosis | 0.99 (0.89–1.09) | – |
| Hypothyroid | 1.01 (0.91–1.13) | – |
| Epilepsy/seizure | 1.06 (0.74–1.51) | – |
| Parkinson’s disease | 1.02 (0.73–1.42) | – |
| Dementia | 1.06 (0.93–1.22) | 1.06 (0.93–1.21) |
| GERD/peptic ulcer disease | 1.19 (1.05–1.34) | 1.18 (1.05–1.33) |
| Depression | 1.05 (0.95–1.17) | 1.05 (0.95–1.16) |
| COPD/asthma | 1.29 (1.16–1.44) | 1.28 (1.15–1.43) |
| Cancer | 1.24 (1.13–1.36) | 1.24 (1.13–1.35) |
| Medications | ||
| Metformin | 0.85 (0.76–0.95) | – |
| Glyburide | 1.27 (0.96–1.69) | – |
| Gemfibrozil | 0.9 (0.71–1.14) | – |
| Spironolactone | 1.02 (0.89–1.16) | – |
| Pentoxifylline | 1.24 (0.81–1.89) | – |
| Ranitidine | 0.89 (0.71–1.11) | – |
| Atenolol | 0.94 (0.87–1.02) | – |
| Hydralazine | 1.15 (1.02–1.29) | – |
| Digoxin | 1.17 (1.02–1.35) | – |
| Rosuvastatin | 0.94 (0.63–1.38) | – |
| NSAID | 0.94 (0.82–1.08) | – |
| Number of medication prescribers ≥4 | 1.21 (1.11–1.31) | 1.22 (1.13–1.32) |
Note: Effect estimates and confidence intervals with statistical significance are indicated in bold. CI = confidence interval; COPD = chronic obstructive pulmonary disease; ED = emergency department; eGFR = estimated glomerular filtration rate; GERD = gastroesophageal reflux disease; HR = hazard ratio; NSAID = nonsteroidal anti-inflammatory drug.
*Cubic splines were applied to age and BMI in the model.
The simplified model had 21 predictors, including eight indicators of discordance (HF, osteoarthritis, dementia, GERD/peptic ulcer disease, depression, COPD/asthma, cancer, and number of prescribers), and explained 98.1% of variance accounted for in the full model. Internal validation using the development cohort showed a C-statistic (95% CI) for the 40-variable full model of 0.70 (0.69–0.71), whereas the C-statistic of the 21-variable simplified model was 0.69 (0.68–0.70). The two models had identical C-statistic (0.69; 0.68–0.70) in the validation cohort (Supplementary Figure 2) and the predicted risk of hospitalization from the 21-variable simplified model was close to the observed risk (within 5%) for all deciles, except for cohort members in the highest deciles for hospitalizations (Supplementary Figure 3).
CKD-Discordance Index Calculation and Associations With Outcomes
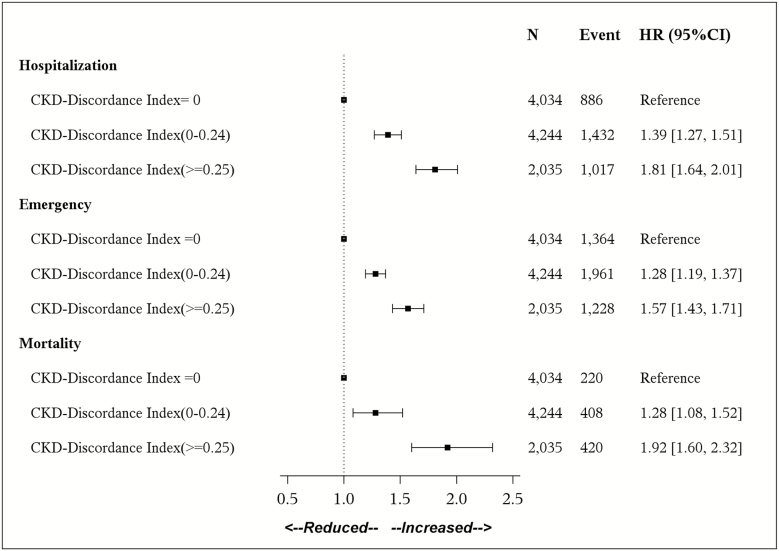
Indicators of discordance regression coefficients were translated to risk scores in which larger HRs were assigned more points (Table 3). For example, HF was assigned 29 points, corresponding to an HR of 1.37 (95% CI: 1.24–1.51), and depression was assigned four points corresponding to an HR of 1.05 (95% CI: 0.95–1.16). The maximum points possible was 124 (i.e. the presence of all eight indicators of discordance). Therefore, a cohort member with HF and depression would have a CKD-Discordance Index of 0.27 ([29 + 4]/124). In the test cohort, median CKD-Discordance Index was 0.08 (interquartile range 0.0–0.22) and 39% of patients had a CKD-Discordance Index of 0. Hazard ratios (95% CI) for a 0.1 increase in the Discordance Index were 1.14 (1.12–1.17), 1.11 (1.09–1.13), and 1.13 (1.09–1.17) for hospitalization, ED visits, and mortality, respectively. Given a large number of cohort members with an index of 0, we chose to classify the Index into three categories: 0, 0.001–0.24, and ≥0.25 (Supplementary Table S4). Compared to those with a CKD-Discordance Index of 0, multivariable adjusted HRs (95% CI) for hospitalization were 1.39 (1.27–1.51) and 1.81 (1.64–2.01) for those with a CKD-Discordance Index of 0.001–0.24 and ≥0.25, respectively (ptrend < .0001; Figure 1). A similar pattern of a higher HR and a higher level of CKD-Discordance Index was found for all-cause mortality and ED visits. Findings were similar in stratified analyses (p interaction >.05; Supplementary Table S5).
Table 3.
Points Assigned to Discordant Indicators Included in CKD-Discordance Index
| Discordance Indicator | Points |
|---|---|
| Heart failure | 29 |
| Osteoarthritis | 7 |
| Dementia | 6 |
| GERD/peptic ulcer disease | 16 |
| Depression | 4 |
| COPD/asthma | 23 |
| Cancer | 20 |
| Number of medication prescribers ≥4 | 19 |
Note: CKD-Discordance Index is calculated as an individual’s points divided by total possible points (total points = 124). COPD = chronic obstructive pulmonary disease; GERD = gastroesophageal reflux disease.
Figure 1.
Association between CKD-Discordance Index and hospitalizations, emergency department visits, and mortality in the test cohort (n = 10,313). Multivariable adjustment includes age, race, sex, BMI, estimated glomerular filtration rate, diabetes, atrial fibrillation, anemia, BPH, coronary artery disease, and stroke. Note: CI = confidence interval; CKD = chronic kidney disease; HR = hazard ratio.
Discussion
In a large retrospective cohort of older adults with incident CKD from a regional integrated U.S. health care system, we used a data-driven approach to select and weight key CKD-discordance indicators in order to develop a novel CKD-Discordance Index. We found that a CKD-Discordance Index >0 was associated with a graded increase in risk of not only 1-year hospitalization but also ED visits and all-cause mortality. These associations persisted even after adjustment for concordant conditions known to be associated with poor outcomes in CKD (e.g. CHD and stroke). This CKD-Discordance Index uses readily available data from EHRs so it may prove to be useful in both population health management and clinical practice for identifying older adults with CKD-discordant conditions at increased risk for hospitalizations, ED visits, and mortality.
The traditional approach to caring for patients with chronic disease is particularly challenging because most CPGs focus on management of a single condition and its related concordant conditions and not the spectrum of comorbidities that often coexist (2). Available comorbidity indices do provide prognostic information and can be used in this population to identify those with a greater burden of disease and thus limited life expectancy. However, many of these tools do not characterize conditions as discordant and therefore do not facilitate clinical care strategies for addressing this challenge. This is a limitation as there is growing evidence of the importance of discordance in CKD. In a Canadian population-based study of more than 500,000 adults with CKD, the presence of a CKD-discordant condition had a graded independent association with hospitalization, length of stay, and mortality (26). In a study of more than 800,000 Veterans, the presence of at least one discordant condition was associated with poor outcomes, at every level of multimorbidity (i.e. two total conditions, three total conditions, four total conditions), suggesting it is not just the total burden of chronic conditions that matters. Highlighting an important patient perspective, a qualitative study of older adults with moderate-to-severe CKD (eGFR < 45 mL/min/1.73 m2) showed that discordant conditions were a major barrier to CKD self-management. Patients were not equipped to manage conflicting self-management tasks, sometimes choosing to follow none of the recommended treatment advice. They expressed concerns that their providers rarely anticipated or proactively discussed the common challenges that arise in the setting of discordance. On the contrary, the task of resolving conflicting recommendations was left on the patient’s “doorstep.” (11)
Building on this prior work, we sought to address provider recognition by developing a tool for identifying important discordant conditions, applying a prediction model approach to guide inclusion and weighting of CKD-discordant indicators. The resulting CKD-Discordance Index was significantly associated with both health care utilization and mortality using the presence of four or more prescribers and seven common CKD-discordant conditions: HF, GERD/peptic ulcer disease, osteoarthritis, dementia, depression, COPD/asthma, and cancer. In this approach, we found the number of prescribers (as a marker of care continuity) to be a key CKD-discordant indicator, which has not been previously reported. In the simplified prediction model used to guide inclusion of conditions in the Index, we found that several concordant conditions, such as CHD and stroke, were significantly associated with hospitalization risk. This finding is consistent with the known association of cardiovascular disease with higher health care utilization and supports the continued identification of these conditions as part of standard care for patients with CKD. The CKD-Discordance Index was not developed to replace any assessment of concordant conditions, but rather to provide potentially actionable information on conditions that otherwise may be overlooked.
The CKD-Discordance Index has potential to facilitate patient-centered care for older adults with CKD and multimorbidity. As a decision support tool, results of the CKD-Discordance Index could be used to individualize care and optimize care of discordant conditions (10). For example, among patients with a low CKD-Discordance Index, traditional CKD care may be adequate. In contrast, a high CKD-Discordance Index may signal the need to consider CKD in the context of their other health problems, their personal health goals, and their capacity to self-manage multiple conditions. As a result, patient-centered care for some patients who have a high CKD-Discordance Index might involve a clinical encounter with less emphasis on CKD management and greater emphasis on managing their other conditions. For other patients, scheduling more frequent appointments or other interactions, such as telehealth encounters, to enhance self-management of CKD could potentially reconcile discordant recommendations. More frequent monitoring may be necessary to identify early signs of illness related to discordant conditions (i.e. elevated creatinine in the setting of nonsteroidal anti-inflammatory drug use for arthritis). Self-management of multiple chronic conditions may also be enhanced by conducting a geriatric assessment for these high-risk patients, uncovering more appropriate care plans based on functional and cognitive limitations (27). Future studies are necessary to determine whether use of the CKD-Discordance Index can improve care for older adults with CKD, and if so, how best to implement its use.
Our study’s strengths lie in both the rich resource available in the KPSC EHR that allows for reliable longitudinal data capture and the use of multiple sources of EHR data readily available at the point of care for the CKD-Discordance Index (28). There are also limitations for consideration. First, we were unable to model potential indicators of discordance that are not captured in the EHR, such as lifestyle recommendations or dietary supplements, functional status, other physiologic indices, or geriatric syndromes (29). This may explain why our prediction model employed in our first step was found to have only moderate predictive ability. Second, we also recognize that multiple indices of multimorbidity exist, which consider a larger number of conditions than was assessed in the current analysis (30–32). Other medications that should be used with caution in CKD, but which have not been previously categorized as renally inappropriate, were not included in our analysis. In addition, the short follow-up time limited our ability to assess disease-specific outcomes such as end-stage renal disease, which only occurred in 190 (0.6%) patients. Patients with high discordance may be vulnerable to acute kidney injury, and future studies with more rigorous ascertainment of acute kidney injury that were possible in the current study, such as measurement of urine biomarkers, may be necessary. Lastly, because the model development occurred in a highly integrated health system, it may not be generalizable to less integrated clinical settings with fragmented information transfer (e.g. clinical practices with separate EHRs).
In conclusion, we developed a novel CKD-Discordance Index from the EHR of a highly integrated health care system that includes CKD-discordant conditions and multiple prescribers. The CKD-Discordance Index was found to be associated with future health care utilization and short-term mortality. Although further validation is needed, the CKD-Discordance Index shows a promising tool to identify patients with CKD-discordant conditions at increased risk for hospitalizations, ED visits, and mortality and/or for investigators pursuing interventional studies targeting this high-risk population.
Supplementary Material
Acknowledgments
The authors thank the patients of Kaiser Permanente Southern California for helping to improve care through the use of information collected through our EHR systems. The authors thank Dr. Eric S. Johnson at Kaiser Permanente Northwest for his advice regarding our statistical approach.
Funding
This work was primarily supported by the Health Care Systems Research Network (HCSRN)-Older Americans Independence Centers (OAICs) AGING Initiative (R24AG045050). Additional support included K76AG059930 (R.K.H), P30AG028716 (R.K.H), KL2TR001115 (R.K.H), R01HL133618 (C.B.B), and I01HX002704 (C.B.B).
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1. Bowling CB, O’Hare AM. Managing older adults with CKD: individualized versus disease-based approaches. Am J Kidney Dis. 2012;59:293–302. doi: 10.1053/j.ajkd.2011.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uhlig K, Leff B, Kent D, et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med. 2014;29:670–679. doi: 10.1007/s11606-013-2659-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vetrano DL, Calderón-Larrañaga A, Marengoni A, et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci. 2018;73:1350–1356. doi: 10.1093/gerona/glx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhlig K, Boyd C. Guidelines for the older adult with CKD. Am J Kidney Dis. 2011;58(2):162–165. doi: 10.1053/j.ajkd.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.77 [DOI] [Google Scholar]
- 6. Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci. 2012;67:1379–1386. doi: 10.1093/gerona/gls173 [DOI] [PubMed] [Google Scholar]
- 7. Bowling CB, Plantinga L, Phillips LS, et al. Association of multimorbidity with mortality and healthcare utilization in chronic kidney disease. J Am Geriatr Soc. 2017;65:704–711. doi: 10.1111/jgs.14662 [DOI] [PubMed] [Google Scholar]
- 8. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078 [DOI] [PubMed] [Google Scholar]
- 9. Magnan EM, Gittelson R, Bartels CM, et al. Establishing chronic condition concordance and discordance with diabetes: a Delphi study. BMC Fam Pract. 2015;16:42. doi: 10.1186/s12875-015-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodman RA, Ling SM, Briss PA, Parrish RG, Salive ME, Finke BS. Multimorbidity patterns in the United States: implications for research and clinical practice. J Gerontol A Biol Sci Med Sci. 2016;71:215–220. doi: 10.1093/gerona/glv199 [DOI] [PubMed] [Google Scholar]
- 11. Bowling CB, Vandenberg AE, Phillips LS, McClellan WM, Johnson TM 2nd, Echt KV. Older patients’ perspectives on managing complexity in CKD self-management. Clin J Am Soc Nephrol. 2017;12:635–643. doi: 10.2215/CJN.06850616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayliss EA, Edwards AE, Steiner JF, Main DS. Processes of care desired by elderly patients with multimorbidities. Fam Pract. 2008;25:287–293. doi: 10.1093/fampra/cmn040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyd C, Smith CD, Masoudi FA, et al. Decision making for older adults with multiple chronic conditions: executive summary for the American Geriatrics Society Guiding Principles on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2019;67:665–673. doi: 10.1111/jgs.15809 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi: 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 17. Chang F, O’Hare AM, Miao Y, Steinman MA. Use of renally inappropriate medications in older Veterans: a national study. J Am Geriatr Soc. 2015;63:2290–2297. doi: 10.1111/jgs.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maciejewski ML, Hammill BG, Voils CI, et al. Prescriber continuity and medication availability in older adults with cardiometabolic conditions. SAGE Open Med. 2018;6:2050312118757388. doi: 10.1177/2050312118757388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horwitz LI, Grady JN, Cohen DB, et al. Development and validation of an algorithm to identify planned readmissions from claims data. J Hosp Med. 2015;10:670–677. doi: 10.1002/jhm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harrell F., Jr Regression Modeling Strategies. 2nd ed Springer; Switzerland; 2015. [Google Scholar]
- 21. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2009. [Google Scholar]
- 22. Steyerberg EW, Moons KG, van der Windt DA, et al. ; PROGRESS Group Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381. doi: 10.1371/journal.pmed.1001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrell FE Jr, Margolis PA, Gove S, et al. Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre Study of Clinical Signs and Etiological agents of Pneumonia, Sepsis and Meningitis in Young Infants. WHO/ARI Young Infant Multicentre Study Group. Stat Med. 1998;17:909–944. doi: 10.1002/(sici)1097-0258(19980430)17:8<909::aid-sim753>3.0.co;2-o [DOI] [PubMed] [Google Scholar]
- 24. Royston P. Explained variation for survival models. Stata J. 2006;6: 83–96. [Google Scholar]
- 25. Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742 [DOI] [PubMed] [Google Scholar]
- 26. Tonelli M, Wiebe N, Guthrie B, et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int. 2015;88:859–866. doi: 10.1038/ki.2015.228 [DOI] [PubMed] [Google Scholar]
- 27. Hall RK, Haines C, Gorbatkin SM, et al. Incorporating geriatric assessment into a nephrology clinic: preliminary data from two models of care. J Am Geriatr Soc. 2016;64:2154–2158. doi: 10.1111/jgs.14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci. 2007;62:281–285. doi: 10.1093/gerona/62.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calderón-Larrañaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72:1417–1423. doi: 10.1093/gerona/glw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi: 10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 32. Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2018;73:225–232. doi: 10.1093/gerona/glx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.