Abstract
Background
Malaysia aims to eliminate malaria by 2020. However, while cases of Plasmodium falciparum and Plasmodium vivax have decreased substantially, the incidence of zoonotic malaria from Plasmodium knowlesi continues to increase, presenting a major challenge to regional malaria control efforts. Here we report incidence of all Plasmodium species in Sabah, including zoonotic P. knowlesi, during 2015–2017.
Methods
Microscopy-based malaria notification data and polymerase chain reaction (PCR) results were obtained from the Sabah Department of Health and State Public Health Laboratory, respectively, from January 2015 to December 2017. From January 2016 this was complemented by a statewide prospective hospital surveillance study. Databases were matched, and species was determined by PCR, or microscopy if PCR was not available.
Results
A total of 3867 malaria cases were recorded between 2015 and 2017, with PCR performed in 93%. Using PCR results, and microscopy if PCR was unavailable, P. knowlesi accounted for 817 (80%), 677 (88%), and 2030 (98%) malaria cases in 2015, 2016, and 2017, respectively. P. falciparum accounted for 110 (11%), 45 (6%), and 23 (1%) cases and P. vivax accounted for 61 (6%), 17 (2%), and 8 (0.4%) cases, respectively. Of those with P. knowlesi, the median age was 35 (interquartile range: 24–47) years, and 85% were male.
Conclusions
Malaysia is approaching elimination of the human-only Plasmodium species. However, the ongoing increase in P. knowlesi incidence presents a major challenge to malaria control and warrants increased focus on knowlesi-specific prevention activities. Wider molecular surveillance in surrounding countries is required.
Keywords: knowlesi, malaria, incidence, elimination, epidemiology
Despite near-elimination of Plasmodium falciparum and Plasmodium vivax malaria in Malaysia, more than 2000 cases of Plasmodium knowlesi occurred in the state of Sabah in 2017, accounting for 98% of malaria diagnoses and representing a major challenge to malaria control.
(See the Editorial Commentary by Karunajeewa and Berman on pages 368–9.)
Human infections with the zoonotic parasite Plasmodium knowlesi occur throughout Southeast Asia in all countries where both the major monkey reservoir (Macaca fascicularis and Macaca nemestrina) and mosquito vector (Anopheles leucosphyrus group) are present [1]. The greatest number of cases have been reported from Malaysia, where P. knowlesi has become the most common cause of malaria [2]. P. knowlesi is also the predominant species in some areas of western Indonesia [3–5].
Within Malaysia, malaria notification data from the eastern state of Sabah during 1992–2014 demonstrated an increase in the incidence of P. knowlesi [6–8]. P. knowlesi (and microscopically indistinguishable Plasmodium malariae) notifications increased from around 100 per year in the early 2000s, to 703 in 2011 [7], and 1325 in 2014 [8]. In contrast, Plasmodium falciparum and Plasmodium vivax notifications decreased from a peak of approximately 50 000 in 1994 to 419 in 2014 [7, 8]. Although microscopy is unreliable for distinguishing P. knowlesi from other human malaria species [9], a true increase in the incidence of P. knowlesi has been suggested by an increase in the proportion of polymerase chain reaction (PCR)–confirmed P. knowlesi cases among all malaria notifications in Sabah [6].
Malaysia is aiming to eliminate malaria by 2020, and World Health Organization (WHO) documents report that only 85 malaria cases occurred nationally in 2017 [10]. However, this figure refers exclusively to the human-only Plasmodium species, with WHO malaria reports including little information on P. knowlesi [10, 11]. P. knowlesi is a highly pathogenic parasite with a risk of severe disease in symptomatic adults of 6–9%, at least as high as that of P. falciparum malaria in co-endemic settings [12–14], and case fatalities occur [14–18]. In this context, WHO has recognized the need for enhanced regional molecular surveillance to further evaluate the epidemiology and distribution of P. knowlesi [19].
We aimed to describe trends in statewide malaria incidence in Sabah using data from the Sabah Department of Health, the State Reference Laboratory, and a prospective malaria surveillance study.
METHODS
Setting
Sabah covers an area of 73 904 km2 and is divided into 5 administrative divisions, encompassing 24 districts (Figure 1, Supplementary Table 1). The Crocker range runs along the northeast–southwest axis, with an elevation of 100–4095 m. A. leucosphyrus group mosquitoes are present throughout Sabah, as are the natural macaque hosts of P. knowlesi [20].
Figure 1.
Map of districts of Sabah. The inset shows the Southeast Asia region with the location of Sabah highlighted.
Malaria control strategies include distribution of insecticide-treated bed nets, indoor residual insecticide spraying, and active and passive case detection [21]. Notification of all microscopy-diagnosed malaria cases to the Department of Health remained mandatory throughout the study period in both the public and private sectors, with species reported according to microscopic diagnosis. PCR for all microscopic diagnoses of malaria is performed at the State Public Health Laboratory using methods described previously [22]. The Department of Health mandated free hospital admission and treatment for all patients with malaria throughout the study period.
Malaria Cases
Microscopy-based malaria notification data and PCR results were obtained from the Sabah Department of Health and State Public Health Laboratory, respectively, from January 2015 to December 2017. From January 2016 this was complemented by a prospective hospital surveillance study, in which standardized data were collected by a nominated hospital staff member for all patients admitted with malaria at every government hospital in Sabah. A unique identifying number was used to match cases across the different data sources. Cross-checking was performed to exclude duplicates and notification entries with a negative Plasmodium PCR result, with data then amalgamated in a de-identified database. Final Plasmodium species was determined by PCR or by microscopy if PCR was not available (~7% of samples).
This study was approved by the ethics committees of the Malaysian Ministry of Health (NMRR-15-168-24821) and Menzies School of Health Research (HREC-2015–2455).
Meteorological Data
Monthly rainfall, humidity, and temperature data at the 6 main meteorological sites (Kudat, Kota Kinabalu, Tawau, Sandakan, Ranau, and Keningau) in Sabah were obtained from the Malaysian Meteorological Department from January 2015 to December 2017.
Statistical Analysis
Data were analyzed using Stata (version 14). Median ages were compared using Wilcoxon rank sum test. Proportions were assessed using chi-square test. Incidence rates were calculated using Malaysian 2010 census district-level population data [23] adjusted by current estimated annual population growth rates [24]. For analysis of age and sex distribution, only PCR-confirmed cases were included. The effects of the average monthly rainfall, humidity, and temperature on the total number of P. knowlesi cases per month were evaluated with a lag period of 3 months using a negative binomial model.
RESULTS
Malaria Incidence
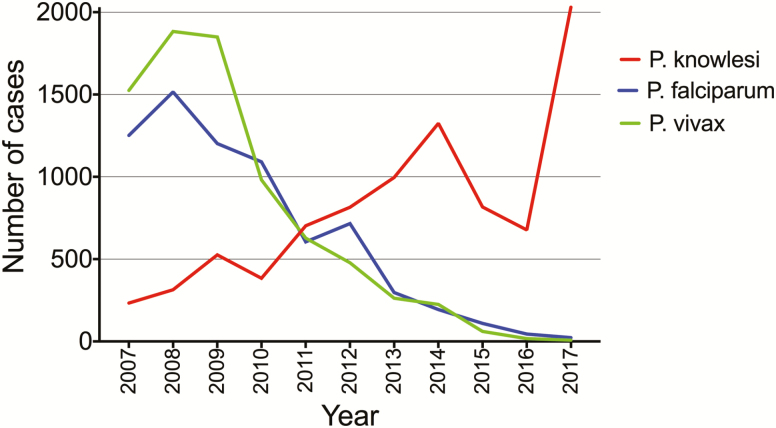
A total of 3867 malaria cases were recorded during 2015–2017: 1018 in 2015, 771 in 2016, and 2078 in 2017 (Figure 2). Of these, PCR was performed in 99%, 93%, and 91%, respectively. Using PCR results, and microscopy if PCR was not available, P. knowlesi accounted for 817 of 1018 (80%), 677 of 771 (88%), and 2030 of 2078 (98%) total malaria cases in 2015, 2016, and 2017, respectively. In contrast, P. falciparum accounted for 110 (11%), 45 (6%), and 23 (1%) cases in 2015, 2016, and 2017, respectively, and P. vivax for 61 (6%), 17 (2%), and 8 (0.4%) cases.
Figure 2.
Cases of Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax in Sabah from 2007 to 2017. Data from 2007 to 2014 have been reported previously [6–8].
The Plasmodium species distribution was similar when only PCR-confirmed results were used. P. knowlesi accounted for 784 of 1001 (78%) PCR-confirmed cases in 2015, 640 of 718 (89%) in 2016, and 1838 of 1880 (98%) in 2017. P. falciparum accounted for 96 (10%), 42 (6%), and 23 (1%) PCR-confirmed cases in 2015, 2016, and 2017, respectively; P. vivax accounted for 58 (6%), 17 (2%), and 7 (0.4%) cases; and P. malariae accounted for 23 (2%), 8 (1%), and 9 (0.5%) cases, respectively. Using PCR, there were 26 (3%) mixed infections in 2015, 9 (1%) in 2016, and none in 2017.
The sensitivity of microscopy for diagnosing P. knowlesi against gold-standard PCR was 96% (95% confidence interval [CI], 95–97%) and the specificity was 68% (95% CI, 62–73%) during 2015–2017 (Supplementary Table 1). The positive-predictive value of a microscopy-diagnosed malaria case being P. knowlesi by PCR was 97% (95% CI, 97–97%).
P. knowlesi Incidence by District
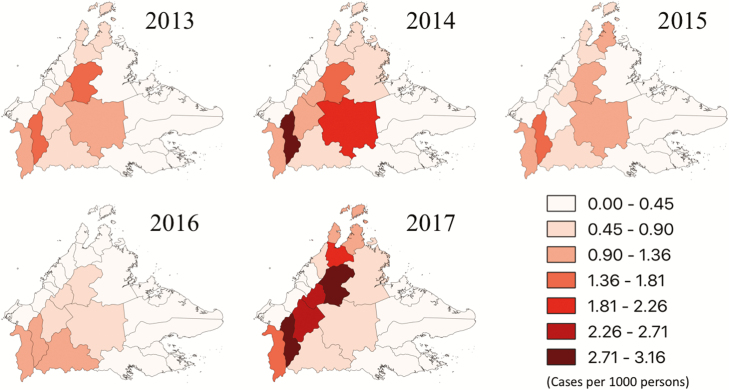
The majority of districts (18/24; 75%) experienced a decrease in P. knowlesi cases from 2015 to 2016; however, the incidence increased in 2017 in all districts except for Kunak (Supplementary Table 2). The highest number of cases occurred in Keningau and Ranau along the Crocker range, with these districts reporting 479 and 358 cases, respectively, in 2017, representing 24% and 18% of the total P. knowlesi cases. In Keningau, this represented a more than 5-fold increase from the number of cases observed in 2015. The P. knowlesi incidence rate was also highest in these districts (Figure 3). This geographical variation became more pronounced in 2017, with the interior districts reporting a median of 2.1 cases (interquartile range [IQR], 1.4–2.7) per 1000 population compared with 0.1 (IQR, 0.1–0.3) per 1000 population among most East and West Coast districts.
Figure 3.
Incidence rate of Plasmodium knowlesi by district, 2013–2017. The key indicates P. knowlesi cases per 1000 persons.
Age and Sex Distribution
The median age of patients diagnosed with PCR-confirmed knowlesi malaria during 2015–2017 was 35 (IQR, 24–47) years compared with 30 (IQR, 16–41) years for P. falciparum (P < .0005) and 30 (IQR, 19–39) years for P. vivax (P = .003). The median age of patients with P. falciparum increased from 25 (IQR, 10–37) years in 2015 to 36 (IQR, 25–45) years in 2016–2017 (P = .0001; n = 96 and 36, respectively). For P. vivax, the median age increased from 28 (IQR, 16–35) years in 2015 to 39 (IQR, 28–55) years in 2016–2017 (P = .0007; n = 58 and 24, respectively). The median age of patients with P. knowlesi did not change over the study period. There was no statistically significant difference in median age by species for the period 2016–2017.
The majority of P. knowlesi cases occurred in adults, with children less than 5 years old accounting for only 25 of 3262 (0.8%) PCR-confirmed infections and children aged 5–13 years accounting for 170 (5%) PCR-confirmed infections. Nonetheless, P. knowlesi was the most common cause of malaria in children younger than 14 years, accounting for 195 of 253 (77%) cases. There were 52 PCR-confirmed P. knowlesi infections in children younger than 14 years of age in 2015, 36 in 2016, and 107 in 2017. In 2015, there were 30 and 12 children younger than 14 years old with P. falciparum and P. vivax, respectively, which decreased to 2 and 0, respectively, in 2017.
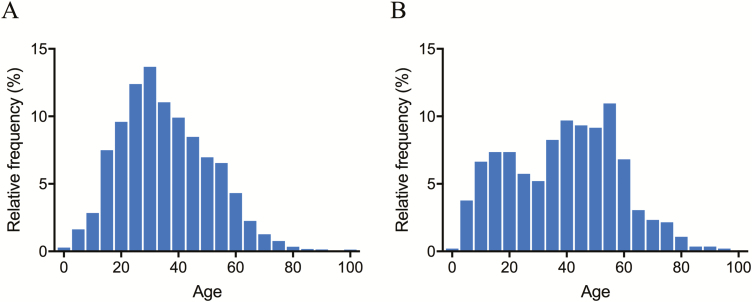
Males accounted for 2757 of 3263 (85%) PCR-confirmed P. knowlesi cases. Females were older, with a median age of 41 (IQR, 23–53) years compared with 34 (IQR, 24–46) years for males (P < .0001). Age was normally distributed among males with knowlesi malaria but appeared to follow a bimodal distribution for females (Figure 4).
Figure 4.
Plasmodium knowlesi age distribution, 2015–2017, in males (A) and females (B).
Meteorological Data
There was a small increase in total rainfall at the 6 meteorological stations, from 11 010 mm in 2015 to 18 060 mm in 2017 ( Supplementary Figure 1 ). In univariate analysis, average monthly rainfall and humidity were both associated with an increased incidence rate ratio of P. knowlesi with a lag time of 3 months; however, they were not independent predictors in the final multivariate model (Supplementary Table 3).
Malaria Deaths and P. knowlesi Case Fatality Rate
Six deaths attributable to malaria were recorded during 2015–2017 [18], all of which were confirmed as P. knowlesi by PCR. This represented a P. knowlesi case fatality rate of 1.7 (95% CI, .6–3.7) per 1000 cases [18].
DISCUSSION
This report highlights the ongoing increase in incidence of P. knowlesi malaria in Sabah, with 2030 cases occurring in 2017, representing 98% of all malaria cases and the highest annual incidence to date. This has coincided with a decline in falciparum and vivax malaria in Sabah, with only a small number of locally acquired infections occurring in 2017. These continuing trends indicate that the interventions successful at reducing transmission of the human-only Plasmodium species do not effectively address the complex factors that are likely contributing to the increase in zoonotic P. knowlesi infections.
As with other emerging zoonotic diseases, the increasing incidence of P. knowlesi in Sabah is likely driven by changes in human land use, leading to complex changes in transmission of the parasite between humans and the mosquito vectors and the macaque hosts [25]. Sabah has undergone extensive deforestation, with approximately 30% of primary forest area lost between 1973 and 2010 [26], with 21% of Sabah’s total area now used for palm oil plantations [27]. P. knowlesi incidence and serological markers of exposure have recently been shown to be associated with higher levels of both forest cover and clearing around households in northern Sabah [28, 29], suggesting that these types of fragmented transition zones are important ecological linkage areas for disease transmission [30]. Modeling has also demonstrated that the relative importance of environmental characteristics associated with P. knowlesi transmission, including forest cover and fragmentation indices, aspect, and slope, varies at different spatial scales [29]. Heterogeneity in land-use change between districts is likely to have contributed to the higher incidence of P. knowlesi observed in the interior regions of Sabah [30]. Finally, deforestation results in reduced biodiversity, and it is possible that the loss of less-efficient or dead-end hosts has contributed to a higher prevalence of P. knowlesi in the highly competent macaque hosts, with consequent spillover of P. knowlesi to humans [25].
Deforestation is known to have complex effects on vector bionomics, with malaria transmission potentially increasing as a result of increased sunlight on breeding sites, changing composition of soil, or by changing Anopheles species distribution or behavior [31]. In Sabah, Anopheles balabacensis is the primary vector of P. knowlesi and is found in village, forest, and farming sites [20]. While vectorial capacity remains highest in forested areas [20], A. balabacensis has recently been shown to be most abundant in logged areas, with host-seeking behavior more prevalent at ground compared to canopy level [32]. Recent population genetic analyses of A. balabacensis across Sabah have demonstrated high genetic variation within subpopulations and a related but currently expanding and growing population overall [33]. Taken together, A. balabacensis can be considered a highly competent vector successfully adapting to ecological changes favoring increased zoonotic P. knowlesi transmission. However, improvements in predictive P. knowlesi risk mapping will also require addressing similar key knowledge gaps regarding the impact of land-use change on macaque distribution and adaptive behavior [25].
Waning immunity to the human-only Plasmodium species in Sabah may also be contributing to the increasing incidence of knowlesi malaria. Heterologous immunity was suggested by data from the early malariotherapy studies, with P. knowlesi more difficult to induce in patients previously infected with P. vivax [34]. This is supported by a study demonstrating that P. vivax antibodies can inhibit P. knowlesi red blood cell invasion [35]. The possibility that declining cross-protective immunity to P. vivax may contribute to rising incidence of knowlesi malaria has important implications for other Southeast Asian countries approaching malaria elimination, highlighting the importance of regional molecular surveillance for P. knowlesi. In addition, concurrent monitoring for the presence of other zoonotic Plasmodium species should be considered, with human Plasmodium cynomolgi infections now reported in West Malaysia, Sarawak in East Malaysia, and Cambodia [36].
In this study, the number of P. knowlesi cases decreased between 2015 and 2016, before increasing in 2017. This initial decrease may in part reflect a short-term impact on vector bionomics due to changing rainfall and weather patterns, with Sabah experiencing unusually low rainfall during this period as part of the worldwide El Niño weather phenomenon. Consistent with previous reports [6], we found an increase in P. knowlesi incidence 3 months after higher rainfall. However, this relationship did not remain statistically significant on multivariate analysis, supporting the relative importance of other biotic land-use–related factors driving P. knowlesi transmission. The substantial increase in incidence of P. knowlesi in 2017 also raises the possibility of whether human-to-human transmission may now be taking place. To date, phylogenetic analyses of P. knowlesi from macaques and humans, in addition to modeling, suggest that transmission remains zoonotic [25, 37]. However, human-to-human transmission has been experimentally demonstrated [38] and, in the context of increasing incidence, cannot be definitively discounted.
As in previous reports, females with knowlesi malaria were older than males [6, 7], and overall, patients infected with P. knowlesi were older than those with P. falciparum or P. vivax [6, 7, 13, 14]. However, in this study there was no difference in the age of those infected with the different Plasmodium species when only the period between 2016 and 2017 was considered. This reflects the recent increase in the median age of patients infected with P. falciparum or P. vivax, as may be expected with waning immunity as these species approach elimination.
Identification of novel interventions targeting specific high-risk population groups or geographical areas will be necessary to control zoonotic P. knowlesi transmission. Individual-level acquisition risk is highest in men involved in farming or agricultural work, particularly in palm oil plantations, with other risk factors including sleeping outside, overnight travel, and to a lesser extent, household construction [28, 39]. However, as A. balabacensis has adapted to predominantly bite outside in the early evening [20, 39], standard malaria-prevention activities such as insecticide-treated bed nets do not appear to be protective [39]. Promotion of current individual methods of vector protection, including personal insect repellent use for high-risk groups and activities, may be a more appropriate interim strategy.
With over 98% of all malaria cases now caused by P. knowlesi, wide dissemination and uptake of case-management protocols for knowlesi malaria are needed in this region. P. knowlesi is more likely to cause severe malaria at lower parasitemias than P. falciparum, the other major species capable of causing severe malaria, with one district study reporting a 16-fold increase in risk with a parasite count of more than 15 000/µL [12]. Management protocols therefore recommend the prompt use of intravenous artesunate at a lower threshold than that recommended for P. falciparum [12, 40]. Longitudinal data from Sabah have previously shown a decrease in case-fatality rate with greater clinical recognition of knowlesi malaria, early referral, and wider use of artesunate [8, 13].
The study had several limitations. Not all patients had PCR performed. However, with nearly all malaria cases now due to P. knowlesi, microscopy had a very high pretest probability for diagnosing P. knowlesi; thus, the lack of PCR results in a small proportion of patients is likely to have had a minimal impact on our overall results. Second, this study may not have captured all malaria cases, thereby underestimating the total number of knowlesi malaria cases in Sabah during the study period. However, with Department of Health–mandated hospital referral and admission policies for all malaria cases in both public and private sectors, mandatory referral for PCR of all microscopy-positive blood to the State Reference Laboratory, and our surveillance linked to both systems, it is likely that the significant majority of symptomatic malaria cases were identified. Furthermore, with these systems consistent across the study period, it is unlikely to have affected the overall malaria trends demonstrated in the report. The near-elimination of P. falciparum and P. vivax is supported by contemporaneous cross-sectional community surveillance studies in northeast Sabah [28].
Despite the near elimination of falciparum and vivax malaria in Sabah, over 2000 cases of P. knowlesi occurred in 2017, the highest annual incidence to date. P. knowlesi thus represents a major challenge to malaria control in Malaysia. With knowlesi malaria now increasingly reported across Southeast Asia, wider molecular surveillance for zoonotic malaria species is needed. Zoonotic P. knowlesi will not be eliminated by current measures targeting the transmission of human-only Plasmodium species. While efforts must continue to ensure the elimination of the human-only malaria species, current malaria-prevention activities may need to be redesigned, or new approaches developed, to mitigate P. knowlesi transmission to humans. In parallel, public health policies need to focus on increasing awareness of knowlesi malaria and ensure that strategies are in place to enable prompt diagnosis and treatment, particularly in high-risk regions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the district-hospital nursing staff for data collection and Clarice James for data entry. They also thank the Sabah Department of Health, Ministry of Health, Malaysia, for providing malaria notification data as well as the State Public Health Laboratory (Makmal Kesihatan Awam) for providing polymerase chain reaction data and the Director-General, Ministry of Health, Malaysia, for permission to publish this manuscript.
Disclaimer. The views expressed in this publication are the authors’ alone and are not necessarily the views of the Australian Government.
Financial support. This work was supported by the US National Institutes of Health (grant number R01 AI116472-03); the Australian National Health and Medical Research Council (grant numbers 1037304 and 1045156; and fellowships to N. M. A. [1042072], B. E. B. [1088738], and M. J. G. [1138860]); “Improving Health Outcomes in the Tropical North: A Multidisciplinary Collaboration ‘Hot North’” (grant number 1131932); and the Australian Centre of Research Excellence in Malaria Elimination. D. J. C. is supported by Prestigious International Research Tuition Scholarship and University Postgraduate Research Scholarship from Charles Darwin University. T. W. Y. is funded by a Singapore National Medical Research Council Clinician Scientist Award (CSA INV 15nov007). The study was also supported by the Tropical Disease Research Regional Collaboration Initiative , which is funded by the Australian Government Department of Foreign Affairs and implemented by the Menzies School of Health Research.
Potential conflicts of interest. The authors have no potential conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shearer FM, Huang Z, Weiss DJ, et al. Estimating geographical variation in the risk of zoonotic Plasmodium knowlesi infection in countries eliminating malaria. PLoS Negl Trop Dis 2016; 10:e0004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Evidence Review Group for Plasmodium knowlesi Available at: http://www.who.int/malaria/mpac/mpac-mar2017-plasmodium-knowlesi-presentation.pdf. Accessed 5 September 2018.
- 3. Lubis IND, Wijaya H, Lubis M, et al. Contribution of Plasmodium knowlesi to multispecies human malaria infections in North Sumatera, Indonesia. J Infect Dis 2017; 215:1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herdiana H, Cotter C, Coutrier FN, et al. Malaria risk factor assessment using active and passive surveillance data from Aceh Besar, Indonesia, a low endemic, malaria elimination setting with Plasmodium knowlesi, Plasmodium vivax, and Plasmodium falciparum. Malar J 2016; 15:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herdiana H, Irnawati I, Coutrier FN, et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar J 2018; 17:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. William T, Jelip J, Menon J, et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J 2014; 13:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. William T, Rahman HA, Jelip J, et al. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl Trop Dis 2013; 7:e2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajahram GS, Barber BE, William T, et al. Falling Plasmodium knowlesi malaria death rate among adults despite rising incidence, Sabah, Malaysia, 2010–2014. Emerg Infect Dis 2016; 22:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar J 2013; 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. World Malaria Report 2018 Available at: https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. Accessed 28 November 2018.
- 11. Barber BE, Rajahram GS, Grigg MJ, William T, Anstey NM. World malaria report: time to acknowledge Plasmodium knowlesi malaria. Malar J 2017; 16:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grigg MJ, William T, Barber BE, et al. Age-related clinical spectrum of Plasmodium knowlesi malaria and predictors of severity. Clin Infect Dis 2018; 67:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barber BE, William T, Grigg MJ, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis 2013; 56:383–97. [DOI] [PubMed] [Google Scholar]
- 14. Daneshvar C, Davis TM, Cox-Singh J, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis 2009; 49:852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J 2012; 11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. William T, Menon J, Rajahram G, et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis 2011; 17:1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox-Singh J, Davis TM, Lee KS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 2008; 46:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajahram GS, Cooper DJ, William T, Grigg MJ, Anstey NM, Barber BE. Deaths from Plasmodium knowlesi malaria: case series and systematic review. Clin Infect Dis 2019; 69:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO Malaria Policy Advisory Committee. Outcomes from the Evidence Review Group on Plasmodium knowlesi. Kota Kinabalu: 2017. Available at: http://www.who.int/malaria/mpac/mpac-mar2017-plasmodium-knowlesi-presentation.pdf. Accessed 22 January 2018. [Google Scholar]
- 20. Wong ML, Chua TH, Leong CS, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis 2015; 9:e0004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Ministry of Health Malaysia, World Health Organization and the University of California, San Francisco Global Health Group. Eliminating malaria: case-study 8—progress towards elimination in Malaysia Available at: https://www.who.int/malaria/publications/atoz/9789241508346/en/. Accessed 1 August 2018.
- 22. Divis PC, Shokoples SE, Singh B, Yanow SK. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar J 2010; 9:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malaysian Department of Statistics. Population distribution and basic demographic characteristics2010. Available at: https://newss.statistics.gov.my/newss-portalx/ep/epFreeDownloadContentSearch.seam?cid=107909. Accessed 1 August 2018.
- 24. Department of Statistics, Malaysia. Current population estimates, Malaysia Available at: https://newss.statistics.gov.my/newss-portalx/ep/epFreeDownloadContentSearch.seam?cid=107904. Accessed 1 August 2018.
- 25. Davidson G, Chua TH, Cook A, Speldewinde P, Weinstein P. The role of ecological linkage mechanisms in Plasmodium knowlesi transmission and spread. Ecohealth 2019. doi:10.1007/s10393-019-01395-6 [DOI] [PubMed] [Google Scholar]
- 26. Gaveau DL, Sloan S, Molidena E, et al. Four decades of forest persistence, clearance and logging on Borneo. PLoS One 2014; 9:e101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malaysian Palm Oil Board. Overview of Malaysian palm oil industry2017. Available at: http://bepi.mpob.gov.my/images/overview/Overview_of_Industry_2017.pdf. Accessed 26 August 2018.
- 28. Fornace KM, Herman LS, Abidin TR, et al. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, The Philippines. PLoS Negl Trop Dis 2018; 12:e0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brock PM, Fornace KF, Grigg MJ, et al. Predictive analysis across spatial scales links zoonotic malaria to deforestation. Proc R Soc Lond B Biol Sci 2019; 286:20182351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fornace KM, Abidin TR, Alexander N, et al. Association between landscape factors and spatial patterns of Plasmodium knowlesi infections in Sabah, Malaysia. Emerg Infect Dis 2016; 22:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol 2006; 100:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brant HL, Ewers RM, Vythilingam I, Drakeley C, Benedick S, Mumford JD. Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malar J 2016; 15:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manin BO, Drakeley CJ, Chua TH. Mitochondrial variation in subpopulations of Anopheles balabacensis Baisas in Sabah, Malaysia (Diptera: Culicidae). PLoS One 2018; 13:e0202905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coatney GR, Collins WE, Warren M, Gontacos PG.. The primate malarias. Atlanta, GA: Centers for Disease Control and Prevention, 1971. [Google Scholar]
- 35. Muh F, Ahmed MA, Han JH, et al. Cross-species analysis of apical asparagine-rich protein of Plasmodium vivax and Plasmodium knowlesi. Sci Rep 2018; 8:5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anstey NM, Grigg MJ. Zoonotic malaria: the better you look, the more you find. J Infect Dis 2019; 219(5): 679-81. [DOI] [PubMed] [Google Scholar]
- 37. Lee KS, Divis PC, Zakaria SK, et al. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog 2011; 7:e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg 1968; 17:355–8. [DOI] [PubMed] [Google Scholar]
- 39. Grigg MJ, Cox J, William T, et al. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: a case-control study. Lancet Planet Health 2017; 1:e97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barber BE, Grigg MJ, William T, Yeo TW, Anstey NM. The treatment of Plasmodium knowlesi malaria. Trends Parasitol 2017; 33:242–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.