Abstract
Background
There have been concerns about reduced adherence and human immunodeficiency virus (HIV) virological suppression (VS) among clinically well people initiating antiretroviral therapy (ART) with high pre-ART CD4 cell counts. We compared virological outcomes by pre-ART CD4 count, where universal ART initiation was provided in the HIV Prevention Trials Network 071 (PopART) trial in South Africa prior to routine national and international implementation.
Methods
This prospective cohort study included adults initiating ART at facilities providing universal ART since January 2014. VS (<400 copies/mL), confirmed virological failure (VF) (2 consecutive viral loads >1000 copies/mL), and viral rebound were compared between participants in strata of baseline CD4 cell count.
Results
The sample included 1901 participants. VS was ≥94% among participants with baseline CD4 count ≥500 cells/µL at all 6-month intervals to 30 months. The risk of an elevated viral load (≥400 copies/mL) was independently lower among participants with baseline CD4 count ≥500 cells/µL (3.3%) compared to those with CD4 count 200–499 cells/µL (9.2%) between months 18 and 30 (adjusted relative risk, 0.30 [95% confidence interval, .12–.74]; P = .010). The incidence rate of VF was 7.0, 2.0, and 0.5 per 100 person-years among participants with baseline CD4 count <200, 200–499, and ≥500 cells/µL, respectively (P < .0001). VF was independently lower among participants with baseline CD4 count ≥500 cells/µL (adjusted hazard ratio [aHR], 0.23; P = .045) and 3-fold higher among those with baseline CD4 count <200 cells/µL (aHR, 3.49; P < .0001).
Conclusions
Despite previous concerns, participants initiating ART with CD4 counts ≥500 cells/µL had very good virological outcomes, being better than those with CD4 counts 200–499 cells/µL.
Clinical Trials Registration
Keywords: HIV/AIDS, early antiretroviral treatment, virological outcomes, baseline CD4 cell count, HPTN 071 (PopART) Trial
There are concerns that people initiating antiretroviral therapy (ART) with high CD4 counts may exhibit reduced HIV virological suppression. In this study, however, participants with CD4 count ≥500 cells/µL had excellent virological outcomes, better than those with lower CD4 counts.
Effective virological suppression (VS) among people living with human immunodeficiency virus (PLHIV) receiving antiretroviral therapy (ART) is essential for both individual benefit and to reduce human immunodeficiency virus (HIV) transmission [1, 2]. Although there has been progress toward the Joint United Nations Programme on HIV/AIDS (UNAIDS) target of 90% of people receiving ART achieving VS by 2020 [3], a number of challenges remain [4]. Concerns have been expressed that ART adherence and VS may be suboptimal among those starting ART with higher CD4 cell counts as they may be asymptomatic [5, 6]. Indeed, higher CD4 count at ART initiation has previously been associated with increased viremia and virological failure (VF) [7–9].
In 2015, the World Health Organization (WHO) recommended ART initiation for all PLHIV [10]. This recommendation was adopted by South Africa in September 2016, with prior CD4 count initiation thresholds being 350 cells/µL and 500 cells/µL, consistent with international recommendations. As part of the HIV Prevention Trials Network (HPTN) 071 (PopART) cluster-randomized trial, all adults living with HIV have been able to access ART at 3 facilities in South Africa since January 2014 [11], almost 3 years prior to the country’s clinical guidelines revision.
There are very few published data of virological outcomes of PLHIV initiating ART with baseline CD4 count >500 cells/µL in sub-Saharan Africa [12, 13]. Implementation research is needed to assess the effectiveness of the expanded HIV treatment strategy and the provision of ART for HIV prevention [5, 6]. The HPTN 071 (PopART) trial provided a unique opportunity to examine outcomes of people initiating ART irrespective of CD4 count in sub-Saharan Africa prior to routine implementation of the revised WHO clinical guidelines. In view of previous concerns regarding virological response during universal test and treatment for HIV, this study aimed to quantify virological outcomes of people initiating ART early (baseline CD4 count ≥500 cells/µL) and to compare these virological outcomes to people initiating ART with lower CD4 counts in the Western Cape Province, South Africa.
METHODS
Setting
A prospective cohort study was conducted among adults initiating ART at 3 public primary healthcare clinics randomly allocated to the full intervention arm of the HPTN 071 (PopART) cluster-randomized trial in South Africa. A full description of the trial design is published elsewhere [11, 14]. Communities surrounding the clinics received household delivery of a combination HIV prevention package, including HIV education and HIV rapid testing in the home by community HIV care providers, referral to the clinics, and active linkage to ART. Study clinics offered ART regardless of CD4 count for all adults living with HIV. Provincial guidelines’ CD4 count ART initiation thresholds during the study period were ≤350 cells/µL until January 2015 and ≤500 cells/µL thereafter [15].
Adults aged ≥18 years initiating ART between January 2014 and November 2015 with available baseline CD4 count measurements and at least 1 on-treatment viral load (VL) were included in analyses. Clients already receiving ART who transferred into the clinics were excluded. Database closure was 31 January 2017.
Outcomes
The outcomes were:
Proportions of participants with measured VL being elevated (≥400 copies/mL) at 6-month intervals after starting ART to a maximum of 30 months.
Cumulative probability of confirmed VF (2 consecutive VLs of >1000 copies/mL) after starting ART.
Cumulative probability of viral rebound (VL ≥400 copies/mL) after initial VS, among participants achieving initial VS who had at least 1 repeat VL >30 days after initial VS.
VS was defined as VL <400 copies/mL. The principal exposure was baseline CD4 count, defined as the most recent CD4 count routinely performed within 6 months before starting ART. CD4 counts were categorized as <200, 200–499, and ≥500 cells/μL. Early ART was defined as a baseline CD4 count of ≥500 cells/μL. The category 200–499 cells/μL was the reference category for all regression analyses.
All participants received standard care and routine follow-up as per Department of Health (DOH) ART guidelines excepting that individuals starting ART outside of DOH guidelines signed informed consent. All participants received standard of care adherence support provided by clinic-based counselors complemented by support from community HIV care providers who were scheduled to see clients a minimum of annually. Guidelines recommended that VL testing be performed 4–6 months after starting ART and at month 12, then annually; when elevated (≥400 copies/mL), the VL was to be repeated 2–6 months later.
Data Collection and Analysis
Clinical data were extracted from sites’ electronic ART patient databases, electronic tuberculosis (TB) registers, and National Health Laboratory Services databases. Baseline characteristics of adults included and excluded from analyses due to absent VL data within the primary exposure categories of interest (baseline CD4 count ≥500 cells/μL and 200–499 cells/μL) were compared using Wilcoxon rank-sum or Pearson χ2 tests as appropriate. Baseline characteristics of the included study sample were compared across strata of baseline CD4 cell count. To estimate relative risks of an elevated VL, crude and adjusted modified Poisson regression with generalized estimating equations to account for clustering by individual was used, with an exchangeable correlation structure [16]. Separate models were constructed for the first year of ART and for the period after the first year to 30 months. All models were controlled for time on ART and healthcare facility. The Wald test was used for global hypothesis tests of the overall effect of multilevel categorical predictor variables.
Kaplan-Meier estimates were used to analyze time to confirmed VF after starting ART, time to viral rebound after initial VS, and time from starting ART to initial VS. Time was measured from the date of starting ART (or date of first VS for viral rebound analyses) to either the event of interest otherwise censored at the most recent available VL result or 24 months following entry, whichever occurred last. The log-rank test was used to compare exposure categories. Cox proportional hazards regression was used for crude and adjusted analyses of predictors of confirmed VF and viral rebound. To account for clustering of observations within facilities, stratified Cox regression was conducted allowing the baseline hazard for each facility to vary. Proportional hazards assumptions were confirmed to be not violated using scaled Schoenfeld residuals.
For the main regression analyses, multiple imputation of missing covariate values using chained equations was conducted assuming missing covariate values were missing at random [17, 18], using 10 imputed datasets. The proportions of missing covariate values that were imputed were low: baseline WHO clinical stage, 1.0%; choice of first nucleoside reverse transcriptase inhibitor drug, 1.1%; choice of nonnucleoside reverse transcriptase inhibitor (NNRTI) drug, 1.1%. Baseline covariates eligible for inclusion in all multivariable models were age, sex, WHO stage, pregnancy, concurrent TB, initial ART regimen, prior ART exposure, and year of starting ART.
As a sensitivity analysis to assess any effect due to missing outcome data, missing VL results were also imputed with fully conditional specification for both included individuals and those excluded from the main analyses due to absent VL data [19]. VL data were imputed as binary values (suppressed/elevated) according to the guidelines’ recommended testing intervals (6, 12, and 24 months after starting ART) for the duration that individuals remained in care at the site during follow-up. Multivariable regression of factors associated with an elevated VL was then repeated on the expanded dataset using the same set of predictor variables included in the main analyses. Statistical analyses were performed with Stata version 15.1. Ethical approval was provided by the Stellenbosch University Health Research Ethics Committee.
RESULTS
During the enrollment period, 2864 individuals started receiving ART, of whom 1901 met inclusion criteria and were included in analyses (Supplementary Figure 1). The proportion of individuals excluded due to absent VL data did not differ by baseline CD4 count strata (Supplementary Table 1). No statistically significant differences in baseline characteristics were found between excluded and included individuals within baseline CD4 count strata, except that excluded individuals had a higher proportion who initiated ART in 2015 (Supplementary Table 2).
Among included participants, the median baseline CD4 count was 331 cells/µL (Table 1). The distribution of participants by baseline CD4 count strata was 477 (25.1%), 1024 (53.9%), and 400 (21.0%) with CD4 count <200, 200–499, and ≥500 cells/µL, respectively. Participants who initiated early ART (baseline CD4 count ≥500 cells/µL) had less advanced baseline WHO clinical stage disease, were less likely to be receiving concomitant TB treatment, and had an appreciably lower proportion of males than participants in lower CD4 count strata. Those with baseline CD4 count <200 cells/µL had a higher proportion with prior ART exposure.
Table 1.
Baseline Characteristics of Study Participants According to Strata of Baseline CD4 Cell Count (N = 1901)
| Characteristic | Baseline CD4 Count Category, Cells/μL | All Participants | ||
|---|---|---|---|---|
| <200 | 200–499 | ≥500 | ||
| No. included (row %) | 477 (25.1) | 1024 (53.9) | 400 (21.0) | 1901 (100) |
| Baseline CD4 count, cells/μL, median (IQR) | 113 (61–158) | 341 (268–407) | 623 (552.9–766) | 331 (199–471) |
| Age, y, median (IQR) | 33.7 (29.3–39.7) | 31.7 (26.4–37.8) | 31.0 (26.2–37.9) | 32.1 (27.1–38.7) |
| Age category, y | ||||
| 18–24 | 43 (9.0) | 187 (19.3) | 80 (20.0) | 310 (16.3) |
| 25–34 | 222 (46.5) | 488 (47.7) | 189 (47.3) | 899 (47.3) |
| 35–49 | 187 (39.2) | 268 (26.2) | 99 (24.8) | 554 (29.1) |
| ≥50 | 25 (5.2) | 81 (7.9) | 32 (8.0) | 138 (7.3) |
| Male sex | 201 (42.1) | 313 (30.6) | 82 (20.5) | 596 (31.4) |
| Baseline WHO stage (n = 1883) | ||||
| I/II | 310 (65.8) | 887 (87.4) | 364 (91.7) | 1561 (82.9) |
| III | 133 (28.2) | 115 (11.3) | 30 (7.6) | 278 (14.8) |
| IV | 28 (5.9) | 13 (1.3) | 3 (0.8) | 44 (2.3) |
| Pregnant | 12 (2.5) | 57 (5.6) | 33 (8.3) | 102 (5.4) |
| Baseline TB treatment | 112 (23.5) | 75 (7.3) | 20 (5.0) | 207 (10.9) |
| First NRTI in ART regimen (n = 1880) | ||||
| Tenofovir | 452 (96.6) | 1002 (98.8) | 396 (99.5) | 1850 (98.4) |
| Zidovudine | 7 (1.5) | 7 (0.7) | 2 (0.5) | 16 (0.9) |
| Stavudine | 9 (1.9) | 5 (0.5) | 0 (0) | 14 (0.7) |
| NNRTI in ART regimen (n = 1881) | ||||
| Efavirenz | 457 (98.1) | 1005 (98.7) | 395 (99.5) | 1857 (98.7) |
| Nevirapine | 9 (1.9) | 13 (1.3) | 2 (0.5) | 24 (1.3) |
| Previous ART exposure for >3 mo | 25 (5.2) | 15 (1.5) | 4 (1.0) | 44 (2.3) |
| Year of starting ART | ||||
| 2014 | 125 (26.2) | 312 (30.5) | 141 (35.3) | 578 (30.4) |
| 2015 | 352 (73.8) | 712 (69.5) | 259 (64.8) | 1323 (69.6) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; TB, tuberculosis; WHO, World Health Organization.
The proportions having available VL results among those remaining in care were high (80%–100%) at all 6-monthly time-points after initiating ART; these did not differ by baseline CD4 count category excepting at 18 months (which was not a recommended time-point for VL testing according to the clinical guidelines) (Supplementary Table 3).
Time to initial VS was shorter with increasing baseline CD4 count: After 12 months, the cumulative probability of VS was 79.7%, 82.9%, and 85.8% among participants with baseline CD4 counts <200, 200–499, and ≥500 cells/µL, respectively (log-rank P for trend = .005).
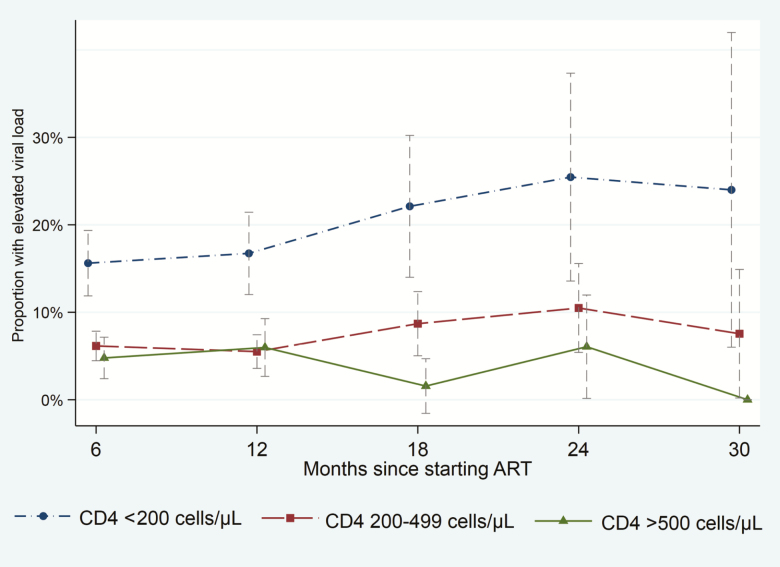
Between 6 and 30 months of ART, VS was higher according to baseline CD4 count strata: 82.2% (653/794), 93.3% (1635/1752), and 95.2% (634/666) among participants with baseline CD4 counts <200, 200–499, and ≥500 cells/µL, respectively (P for trend < .0001). VS was ≥94% at all 6-monthly time-points among participants with baseline CD4 count ≥500 cells/µL up to 30 months. Illustrated in Figure 1, participants with baseline CD4 count <200 cells/µL had substantially increased proportions with elevated VLs, whereas participants with baseline CD4 count ≥500 cells/µL had lower proportions with elevated VLs between months 18 and 30, compared to participants with baseline CD4 count 200–499 cells/µL.
Figure 1.
Proportion of participants with elevated viral load (>400 copies/mL) according to baseline CD4 cell count strata and time on antiretroviral therapy (ART). Vertical bars are 95% confidence intervals.
Table 2 shows predictors of an elevated VL. During the first year of ART, there was no difference in the risk of an elevated VL between participants with baseline CD4 count ≥500 cells/µL vs 200–499 cells/µL. However, between months 18 and 30, participants with baseline CD4 count ≥500 cells/µL had a substantially reduced risk of an elevated VL compared to participants with baseline CD4 count 200–499 cells/µL (3.3% vs 9.2%, respectively; P = .020), with a 70% reduction in multivariable analyses (adjusted relative risk [aRR], 0.30 [95% confidence interval {CI}, .12–.74]; P = .010). Participants with baseline CD4 count <200 cells/µL had a considerably increased risk of an elevated VL during both the first year on ART (16.1%) and during months 18–30 (23.4%).
Table 2.
Proportions With an Elevated Viral Load (>400 Copies/mL) and Baseline Predictors of an Elevated Viral Load After Starting Antiretroviral Therapy
| Elevated VL | Months 3–12 of ART | Elevated VL | Months 18–30 of ART | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Analysis | Multivariable Analysisa | Crude Analysis | Multivariable Analysisa | |||||||||||
| Characteristic | No. (%) | RR | (95% CI) | P Value | Adjusted RR | (95% CI) | P Value | No. (%) | RR | (95% CI) | P Value | Adjusted RR | (95% CI) | P Value |
| CD4 category, cells/μL | <.0001 | <.0001 | <.0001 | <.0001 | ||||||||||
| <200 | 98 (16.1) | 2.73 | (2.03–3.70) | <.0001 | 2.21 | (1.60–3.07) | <.0001 | 43 (23.4) | 2.56 | (1.66–3.93) | <.0001 | 2.40 | (1.52–3.79) | <.0001 |
| 200–499 | 78 (5.9) | Ref | … | Ref | … | 39 (9.2) | Ref | … | Ref | … | ||||
| ≥500 | 27 (5.2) | 0.89 | (.56–1.39) | .61 | 0.96 | (.62–1.48) | .84 | 5 (3.3) | 0.27 | (.10–.70) | .007 | 0.30 | (.12–0.74) | .010 |
| Age, y | .090 | .077 | .97 | .66 | ||||||||||
| 18–24 | 29 (8.3) | 0.82 | (.54–1.24) | 0.99 | (.66–1.48) | 13 (10.7) | 0.92 | (.49–1.71) | 1.13 | (.61–2.08) | ||||
| 25–34 | 103 (8.9) | Ref | … | Ref | … | 40 (11.2) | Ref | … | Ref | … | ||||
| 35–49 | 65 (9.1) | 1.02 | (.73–1.41) | 0.82 | (.58–1.13) | 29 (12.2) | 1.04 | (.62–1.72) | 0.78 | (.47–1.31) | ||||
| ≥50 | 6 (3.3) | 0.37 | (.16–.86) | 0.36 | (.16–.82) | 5 (10.9) | 0.87 | (.31–2.37) | 1.14 | (.42–3.08) | ||||
| Sex | ||||||||||||||
| Female | 123 (7.3) | Ref | … | Ref | … | 58 (10.8) | Ref | … | Ref | … | ||||
| Male | 80 (10.6) | 1.46 | (1.40–1.52) | <.0001 | 1.13 | (.84–1.53) | .41 | 29 (13.0) | 1.22 | (.77–1.91) | .40 | 0.30 | (.55–1.46) | .67 |
| WHO stage | <.0001 | .0001 | .020 | .80 | ||||||||||
| I/II | 132 (6.5) | Ref | … | Ref | … | 58 (9.4) | Ref | … | Ref | … | ||||
| III | 58 (17.5) | 2.71 | (2.48–2.97) | 2.39 | (1.60–3.56) | 23 (19.7) | 2.15 | (1.49–3.11) | 1.18 | (.61–2.30) | ||||
| IV | 11 (20.4) | 3.14 | (1.44–6.84) | 2.01 | (1.06–4.10) | 6 (27.3) | 2.60 | (1.13–5.97) | 1.20 | (.48–3.02) | ||||
| Pregnancy in women | ||||||||||||||
| No | 119 (7.6) | Ref | … | … | Ref | 57 (11.4) | Ref | … | Ref | … | ||||
| Yes | 4 (3.2) | 0.42 | (.13–1.37) | .15 | 0.48 | (.17–1.32) | .156 | 1 (2.7) | 0.18 | (.01–1.34) | .095 | 0.31 | (.03–2.35) | .26 |
| Concurrent TB | ||||||||||||||
| No | 172 (7.8) | Ref | … | … | Ref | 69 (10.1) | Ref | … | Ref | … | ||||
| Yes | 31 (13.3) | 1.72 | (1.17–2.51) | .005 | 0.68 | (.42–1.12) | .13 | 18 (22.0) | 2.29 | (1.30–3.81) | .001 | 1.18 | (.61–2.26) | .61 |
| First NRTI | .013 | .52 | <.0001 | .0037 | ||||||||||
| Tenofovir | 194 (8.1) | Ref | … | Ref | … | 78 (10.6) | Ref | … | Ref | … | ||||
| Zidovudine | 3 (18.8) | 2.18 | (.55–8.70) | 1.51 | (.66–3.41) | 6 (54.6) | 4.90 | (2.45–9.79) | 3.60 | (1.43–9.09) | ||||
| Stavudine | 5 (25.0) | 3.12 | (2.30–4.23) | 1.31 | (.57–3.05) | 3 (42.9) | 4.54 | (1.21–16.92) | 2.49 | (.84–7.38) | ||||
| NNRTI | ||||||||||||||
| Efavirenz | 199 (8.3) | Ref | … | Ref | … | 84 (11.2) | Ref | … | Ref | … | ||||
| Nevirapine | 2 (6.5) | 0.76 | (.41–1.37) | .36 | 0.64 | (.20–2.11) | .47 | 3 (60.0) | 6.40 | (3.41– 12.01) | <.0001 | 1.88 | (.64–5.48) | .25 |
| Prior ART exposure | ||||||||||||||
| No | 194 (8.1) | … | … | … | 82 (11.0) | … | … | … | ||||||
| Yes | 9 (19.2) | 2.36 | (.98–5.72) | .056 | 1.43 | (.67–3.05) | .34 | 5 (27.8) | 4.00 | (1.92–8.40) | < .0001 | 2.85 | (1.41–5.73) | .003 |
| Year starting ART | ||||||||||||||
| 2014 | 70 (8.8) | Ref | … | … | … | 64 (13.5) | Ref | … | … | … | ||||
| 2015 | 133 (8.0) | 0.86 | (.60–1.24) | .42 | … | … | 23 (8.0) | 0.79 | (.39–1.52) | .49 | … | … | ||
Modified Poisson regression with generalized estimating equations to account for clustering was used, including 10 multiple imputed datasets for missing covariate values. Models were controlled for time on ART and healthcare facility.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; RR, risk ratio; TB, tuberculosis; VL, viral load; WHO, World Health Organization.
aMultivariable models included baseline CD4 cell count category, age, sex, baseline WHO stage, pregnancy, concurrent TB, first NRTI in ART regimen, NNRTI in ART regimen, and prior ART exposure.
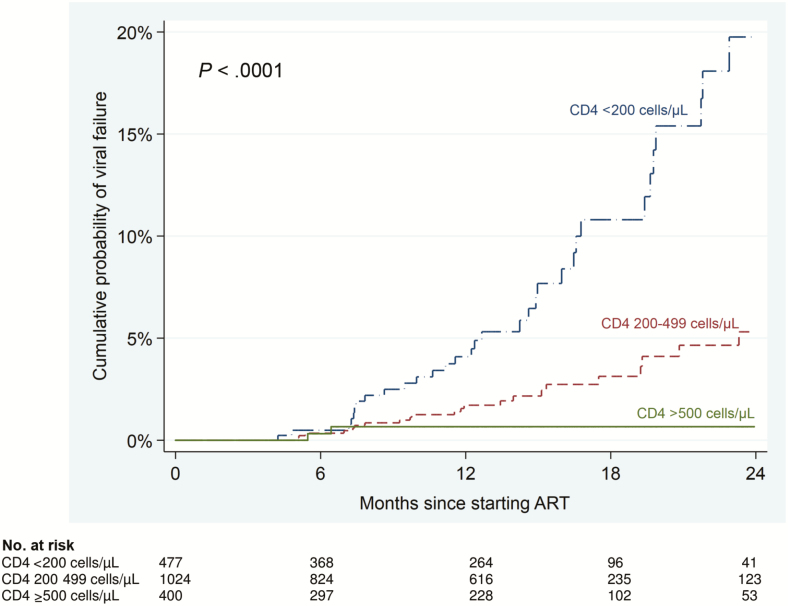
Sixty (3.2%) participants developed confirmed VF during follow-up, with an incidence rate of 2.9 cases per 100 person-years (PY) (95% CI, 2.2–3.7). VF was inversely related to baseline CD4 count. The incidence rate of VF was 7.0, 2.0, and 0.5 cases per 100 PY among participants with baseline CD4 counts of <200, 200–499, and ≥500 cells/µL, respectively (P < .0001). After 24 months, the cumulative probability of VF was 19.8%, 5.3%, and 0.7% among participants with baseline CD4 counts of <200, 200–499, and ≥500 cells/µL, respectively (Figure 2). The cumulative probability of VF among participants with baseline CD4 count >500 cells/µL remained unchanged between months 7 and 24. In multivariable analyses, compared to participants with baseline CD4 count 200–499 cells/µL, those with baseline CD4 count ≥500 cells/µL had a 77% reduced risk of VF (adjusted hazard ratio [aHR], 0.23 [95% CI, .05–.97]; P = .045), while those with baseline CD4 <200 cells/µL had a 3-fold increased risk of VF (aHR, 3.49; P < .0001) (Table 3).
Figure 2.
Kaplan-Meier failure estimates of confirmed virological failure (2 consecutive viral loads >1000 copies/mL) according to baseline CD4 count strata after starting antiretroviral therapy (ART).
Table 3.
Predictors of Confirmed Virological Failure Among Participants Initiating Antiretroviral Therapy
| Crude Analysis | Multivariable Analysisa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Virologic Failure, no./No. (%) | PY | Rate per 100 PY (95% CI) | HR | (95% CI) | P Value | Adjusted HR | (95% CI) | P Value |
| Total sample | 60/1901 (3.2) | 2103.6 | 2.9 (2.2–3.7) | … | … | … | … | ||
| CD4 count, cells/μL | < .0001 | < .0001 | |||||||
| <200 | 35/477 (7.3) | 499.6 | 7.0 (5.0–9.8) | 4.10 | (2.41–7.00) | < .0001 | 3.49 | (2.00–6.14) | < .0001 |
| 200–499 | 23/1024 (2.2) | 1163.2 | 2.0 (1.3–3.0) | Ref | … | Ref | … | ||
| ≥500 | 2/400 (0.5) | 440.8 | 0.5 (.1–1.8) | 0.20 | (.05–.84) | .028 | 0.23 | (.05–.97) | .045 |
| Age, y | .10 | .29 | |||||||
| 18–24 | 6/310 (1.9) | 345.4 | 1.7 (.8–3.8) | 0.54 | (.23–1.26) | 0.75 | (.32–1.78) | ||
| 25–49 | 53/1453 (3.6) | 1613.8 | 3.3 (2.5–4.3) | Ref | … | Ref | … | ||
| ≥50 | 1/138 (0.7) | 144.4 | 0.69 (.1–4.9) | 0.22 | (.03–1.60) | 0.22 | (.30–1.65) | ||
| Sex | |||||||||
| Female | 37/1305 (2.8) | 1456.7 | 2.5 (1.8–3.5) | Ref | … | Ref | … | ||
| Male | 23/596 (3.9) | 651.9 | 3.5 (2.3–5.3) | 1.40 | (.84–2.37) | .196 | 0.85 | (.49–1.48) | .569 |
| Baseline WHO stage | .0001 | … | .085 | ||||||
| I/II | 36/1561 (2.3) | 1736.1 | 2.1 (1.5–2.9) | Ref | … | Ref | … | ||
| III | 19/278 (6.8) | 298.4 | 6.4 (4.1–10.0) | 3.07 | (1.76–5.35) | 2.10 | (1.07–4.10) | ||
| IV | 5/44 (11.4) | 52.9 | 9.5 (3.9–22.7) | 3.48 | (1.35–8.98) | 2.00 | (.70–5.71) | ||
| Pregnancy | |||||||||
| Nonpregnant women | 36/1203 (3.0) | 1341.1 | 2.7 (1.9–3.7) | Ref | … | … | … | ||
| Pregnant woman | 1/102 (1.0) | 110.6 | 0.9 (.1–6.4) | 0.33 | (.05–2.41) | .275 | … | … | |
| Baseline TB treatment | |||||||||
| No | 47/1694 (2.8) | 1886.0 | 2.5 (1.9–3.3) | Ref | … | Ref | … | ||
| Yes | 13/207 (6.3) | 217.7 | 6.0 (3.5–10.3) | 2.46 | (1.33–4.54) | .004 | 0.97 | (.45–2.05) | .927 |
| Regimen first NRTI | .71 | ||||||||
| Tenofovir | 58/1850 (3.1) | 2043.7 | 2.8 (2.2–3.7) | Ref | … | … | … | ||
| Zidovudine | 1/16 (6.3) | 22.9 | 4.4 (.6–31.0) | 1.28 | (.17–9.26) | … | … | ||
| Stavudine | 1/14 (7.1) | 16.7 | 6.0 (.8–42.6) | 2.15 | (.30–15.6) | … | … | ||
| Regimen NNRTI | |||||||||
| Efavirenz | 59/1857 (3.2) | 2062.5 | 2.9 (2.2–3.7) | Ref | … | … | … | ||
| Nevirapine | 1/24 (4.2) | 23.2 | 4.3 (.9–30.6) | 1.72 | (.24–12.47) | .591 | … | … | |
| Previous ART exposure | |||||||||
| No | 58/1857 (3.1) | 2058.9 | 2.8 (2.2–3.6) | Ref | … | … | … | ||
| Yes | 2/44 (4.6) | 44.7 | 4.5 (1.1–17.9) | 1.64 | (.40–6.74) | .491 | … | … | |
| Year of starting ART | |||||||||
| 2014 | 31/578 (5.4) | 893.5 | 3.5 (2.4–4.9) | Ref | … | … | … | ||
| 2015 | 29/1323 (2.2) | 1210.1 | 2.4 (1.7–3.4) | 1.20 | (.66–2.12) | .540 | … | … | |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PY, person-years; TB, tuberculosis; WHO, World Health Organization.
aThe multivariable model included baseline CD4 cell count category, age, sex, baseline WHO stage, and concomitant TB at baseline. Crude and adjusted models were stratified by site.
A total of 1082 participants achieved VS and had a subsequent VL measurement. Amongst these, 81 (7.5%) developed viral rebound at a rate of 7.4 cases per 100 PY. In multivariable analyses, participants with baseline CD4 count <200 cells/µL had a significantly increased risk of viral rebound, but there was no difference between participants initiating early ART compared to those with baseline CD4 count 200–499 cells/µL (Supplementary Table 4; Supplementary Figure 2).
In the sensitivity analysis including imputed VL data for those with missing VLs and including individuals excluded from main analyses due to absent VL data, the association between a reduced risk of an elevated VL among those with baseline CD4 count ≥500 cells/µL compared to 200–499 cells/µL remained apparent during months 18–30 (aRR, 0.35; P = .027; Supplementary Table 5).
DISCUSSION
Individuals with baseline CD4 count ≥500 cells/µL had excellent virological outcomes; VS and VF were better in this group compared to participants with baseline CD4 count 200–499 cells/µL, and viral rebound was equivalent. This suggests that, despite individuals with CD4 count ≥500 cells/µL being less likely to have HIV-related symptoms, expansion of programs to encourage early ART initiation across sub-Saharan Africa will support achieving the UNAIDS third “90-90-90” target of VS by 2020. The results suggest that adherence is good among those initiating ART with CD4 counts ≥500 cells/µL, which aligns with recently reported adherence outcomes from another universal test and treat trial [20], and is encouraging for the scale-up of ART for all PLHIV.
Previous research of virological outcomes has primarily been conducted among people with baseline CD4 count <500 cells/µL, and has shown mixed results. Most studies found that low baseline CD4 count predicted viremia and VF [21–24]; however, a Ugandan study found that baseline CD4 count ≥250 cells/µL independently predicted persistent viremia [7].
Higher baseline CD4 count has also predicted poorer virological outcomes in 2 previous studies that included participants with baseline CD4 counts >500 cells/µL [8, 9]. In contrast, VS was similar up to 6–12 months of ART in the early vs deferred ART arms of the Strategic Timing of Antiretroviral Therapy and TEMPRANO trials [25, 26] and a recent Brazilian observational study [27].
Reasons for increased VF among those with low baseline CD4 count include low CD4 count being a marker of lower HIV-specific host immune responses and higher baseline VL, which are predictors of subsequent viremia and VF [28–31]. HIV drug resistance may also contribute to VF in this group that had a higher prevalence of previous ART exposure. Drug resistance is increasing in sub-Saharan Africa [32, 33]; thus, expanding drug resistance testing (including pre-ART screening) is important considering the historic use of NNRTI single agents for prevention of mother-to-child transmission, the anticipated rollout of tenofovir-based preexposure prophylaxis, and the transition to dolutegravir-based regimens [34].
Potential mechanisms for improved virological outcomes with baseline CD4 count ≥500 cells/µL include greater host immune responses, lower baseline VL, fewer comorbidities, less concomitant medication, and fewer drug–drug interactions. Importantly, the results mitigate concerns that people in southern Africa who initiate ART during earlier stages of HIV infection when not clinically unwell will have reduced adherence [5, 6].
It is possible that previous studies that found less advanced immunosuppression to be associated with poorer virological response had lower proportions of participants having prior ART exposure (thus possible drug resistance) among those with lower baseline CD4 counts compared to our study, with subsequently better virological response in this group compared to our study, explaining differences in results to our study. Our study is among the first to measure longer-term virological outcomes in a prospective cohort study among people initiating ART with baseline CD4 count >500 cells/µL in high-HIV-prevalence settings.
Increased investment is being made in South Africa to initiate 2 million people on ART over 2 years [35]. This study suggests that 20% of these may have baseline CD4 counts ≥500 cells/µL, and that their virological outcomes are potentially favorable. Nevertheless, one-quarter of participants had baseline CD4 counts <200 cells/µL; therefore, efforts to identify and link people to ART early in the disease course remain essential, along with screening and prophylaxis for cryptococcal disease and TB and diagnosis and treatment of severe bacterial infections. The median baseline CD4 count in this cohort (331 cells/μL) was, however, substantially higher than historical levels [36], promisingly suggesting that the rollout of ART regardless of CD4 count will lead to increases in baseline CD4 count and associated improved clinical outcomes.
Study strengths include that data were part of a high-quality routine dataset, strengthened by prospective data quality improvement during the HPTN 071 (PopART) trial. Participant follow-up continued for up to 24–30 months. There were high rates of completeness in key data fields with only a small proportion (6.6%) of eligible clients excluded due to missing baseline CD4 counts. VL test completion of those in care was high (>80%), which is notable as routine VL monitoring in other southern African countries is frequently unavailable [37]. Clinic activities were closely aligned to standard care during the study period, supporting generalizability of findings. Study limitations include that a relatively large number of people were excluded from analyses due to absent VL data. Most of these individuals discontinued follow-up during the initial months of treatment prior to initial VL testing, as commonly occurs in southern Africa [38]. The proportions excluded due to absent VL data did not differ by baseline CD4 count strata, and baseline characteristics of excluded and included individuals did not differ significantly within baseline CD4 count strata, excepting for year of ART initiation, which was not associated with any outcomes. Also, the sensitivity analysis including imputed VL data for those excluded due to absent VLs showed similar results to the main analysis, so it is not likely that sampling bias altered outcome comparisons between baseline CD4 count categories. VL at ART initiation may be associated with on-treatment virological outcomes; however, baseline VL data were not included as baseline VLs were not performed as part of the study and are not performed routinely in South Africa. Although participants with baseline CD4 count ≥500 cells/µL were less likely to be receiving concomitant TB treatment and had a lower proportion of males than participants in lower CD4 count strata, these differences are unlikely to have biased outcome measures as neither factor was associated with any of the outcomes in multivariable analyses.
CONCLUSIONS
The HPTN 071 (PopART) trial has provided a unique opportunity to examine virological outcomes during universal test and treatment for HIV prior to routine implementation. Participants initiating ART with CD4 count ≥500 cells/µL had better (or at least equivalent) VS and reduced VF compared to participants initiating ART with CD4 counts 200–499 cells/µL. These findings provide support for and are encouraging for universal ART implementation in Africa. Expansion of programs targeting early ART initiation are important to reach the UNAIDS second and third “90-90-90” elements of ART coverage and VS. Further research assessing methods to improve VS among those starting ART with CD4 counts <200 cells/µL is warranted, and increased efforts to reduce early ART discontinuation are needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), the US President’s Emergency Plan for AIDS Relief (PEPFAR), the International Initiative for Impact Evaluation (3ie), or the Bill & Melinda Gates Foundation.
Financial support. This work was supported by the NIAID (cooperative agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613), with funding from PEPFAR. Additional funding is provided by 3ie with support from the Bill & Melinda Gates Foundation, as well as by NIAID, NIDA, and NIMH, all part of the US National Institutes of Health (NIH). J. B. N. is supported by research grants from the NIAID/NIH; the AIDS Clinical Trial Group as member of the Scientific Agenda Steering Committee and senior HIV investigator within the Stellenbosch University Clinical Trial Unit (grant number 2UM1AI069521-08); and the Pittsburgh-Stellenbosch University AIDS-comorbidities Training Research Program (Pitt-SU AICoTRP; NIH/Fogarty International Center; grant number 1D43TW010340-01).
Acknowledgments. The authors acknowledge the PopART study team in South Africa, including Kheth’Impilo, Desmond Tutu TB Centre, Anova Health Institute, the City of Cape Town, and Western Cape Government Department of Health, as well as the National Health Laboratory Service. The authors also thank HPTN 071 (PopART) partners in the United States and the United Kingdom (HPTN, FHI 360 North Carolina, London School of Hygiene and Tropical Medicine, Imperial College, and Zambart).
Potential conflicts of interest. H. A. has received personal fees from Global Fund. G. Z. has received an honorarium from ViiV Healthcare. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hamers RL, Sigaloff KC, Kityo C, Mugyenyi P, de Wit TF. Emerging HIV-1 drug resistance after roll-out of antiretroviral therapy in sub-Saharan Africa. Curr Opin HIV AIDS 2013; 8:19–26. [DOI] [PubMed] [Google Scholar]
- 2. Price MA, Wallis CL, Lakhi S, et al. . IAVI Early Infection Cohort Study Group Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in east and southern Africa. AIDS Res Hum Retroviruses 2011; 27:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic Available at: http://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed 13 August 2018.
- 4. Nachega JB, Sam-Agudu NA, Mofenson LM, Schechter M, Mellors JW. Achieving viral suppression in 90% of people living with human immunodeficiency virus on antiretroviral therapy in low- and middle-income countries: progress, challenges, and opportunities. Clin Infect Dis 2018; 66:1487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: what will it take? Clin Infect Dis 2014; 58:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lockman S, Sax P. Treatment-for-prevention: clinical considerations. Curr Opin HIV AIDS 2012; 7:131–9. [DOI] [PubMed] [Google Scholar]
- 7. Adakun SA, Siedner MJ, Muzoora C, et al. . Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr 2013; 62:317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torian LV, Xia Q. Achievement and maintenance of viral suppression in persons newly diagnosed with HIV, New York City, 2006–2009: using population surveillance data to measure the treatment part of “test and treat.” J Acquir Immune Defic Syndr 2013; 63:379–86. [DOI] [PubMed] [Google Scholar]
- 9. Eshleman SH, Wilson EA, Zhang XC, et al. . Virologic outcomes in early antiretroviral treatment: HPTN 052. HIV Clin Trials 2017; 18:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Available at: https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed 18 March 2018. [PubMed]
- 11. Hayes R, Ayles H, Beyers N, et al. . HPTN 071 (PopART) Study Team HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials 2014; 15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tymejczyk O, Brazier E, Yiannoutsos C, et al. . IeDEA Collaboration HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 15:e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song A, Liu X, Huang X, et al. . From CD4-based initiation to treating all HIV-infected adults immediately: an evidence-based meta-analysis. Front Immunol 2018; 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayes R, Floyd S, Schaap A, et al. . HPTN 071 (PopART) Study Team A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med 2017; 14:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Western Cape Department of Health. The Western Cape antiretroviral treatment guidelines 2015 Available at: https://www.westerncape.gov.za/sites/www.westerncape.gov.za/files/the-western-cape-consolidated-guidelines-for-hiv-treatment-2015-v26012016.pdf. Accessed 28 March 2018.
- 16. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol 2011; 174:984–92. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan SR, Oosthuizen C, Stinson K, et al. . Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: a cohort study. PLoS Med 2017; 14:e1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. . Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017; 9:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groenwold RHH, Donders ART, Roes KCB, Harrell JFE, Moons KGM. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2012; 175:210–7. [DOI] [PubMed] [Google Scholar]
- 20. Iwuji C, McGrath N, Calmy A, et al. . Universal test and treat is not associated with sub-optimal antiretroviral therapy adherence in rural South Africa: the ANRS 12249 TasP trial. J Int AIDS Soc 2018; 21:e25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rohr JK, Ive P, Horsburgh CR, et al. . Developing a predictive risk model for first-line antiretroviral therapy failure in South Africa. J Int AIDS Soc 2016; 19:20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007; 146:564–73. [DOI] [PubMed] [Google Scholar]
- 23. Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Doran C, Zhang L. Determinants of virological failure after 1 year’s antiretroviral therapy in Vietnamese people with HIV: findings from a retrospective cohort of 13 outpatient clinics in six provinces. Sex Transm Infect 2014; 90:538–44. [DOI] [PubMed] [Google Scholar]
- 24. Datay MI, Boulle A, Mant D, Yudkin P. Associations with virologic treatment failure in adults on antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010; 54:489–95. [DOI] [PubMed] [Google Scholar]
- 25. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 27. Meireles MV, Pascom ARP, Duarte EC. Factors associated with early virological response in HIV-infected individuals starting antiretroviral therapy in Brazil (2014–2015): results from a large HIV surveillance cohort. J Acquir Immune Defic Syn 2018; 78:e19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siddique MA, Hartman KE, Dragileva E, et al. . Low CD4+ T cell nadir is an independent predictor of lower HIV-specific immune responses in chronically HIV-1-infected subjects receiving highly active antiretroviral therapy. J Infect Dis 2006; 194:661–5. [DOI] [PubMed] [Google Scholar]
- 29. Cartwright EK, Spicer L, Smith SA, et al. . CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity 2016; 45:656–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mellors JW, Muñoz A, Giorgi JV, et al. . Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126:946–54. [DOI] [PubMed] [Google Scholar]
- 31. Maldarelli F, Palmer S, King MS, et al. . ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Sivay MV, Hudelson SE, et al. . Antiretroviral drug use and HIV drug resistance among young women in rural South Africa: HPTN 068. J Acquir Immune Defic Syndr 2018; 79:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinyua JG, Lihana RW, Kiptoo M, et al. . Antiretroviral resistance among HIV-1 patients on first-line therapy attending a comprehensive care clinic in Kenyatta National Hospital, Kenya: a retrospective analysis. Pan Afr Med J 2018; 29:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dorward J, Lessells R, Drain PK, et al. . Dolutegravir for first-line antiretroviral therapy in low-income and middle-income countries: uncertainties and opportunities for implementation and research. Lancet HIV 2018; 5:e400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. President’s Emergency Plan for AIDS Relief. South Africa Country Operational Plan (COP) 2018 strategic direction summary Available at: https://za.usembassy.gov/wp-content/uploads/sites/19/South-Africa-COP18-Strategic-Direction-Summary-SDS-public-version-3.22.18-w-watermark.pdf. Accessed 28 June 2018.
- 36. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fritz CQ, Blevins M, Lindegren ML, et al. . Comprehensiveness of HIV care provided at global HIV treatment sites in the IeDEA consortium: 2009 and 2014. J Int AIDS Soc 2017; 20:20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz IT, Kaplan R, Fitzmaurice G, et al. . Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: a retrospective cohort study. PLoS Med 2017; 14:e1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.