Abstract
Background
Total knee arthroplasty (TKA) periprosthetic joint infection (PJI) can be managed with debridement, antibiotic therapy, and implant retention (DAIR). Oral antibiotics can be used after DAIR for an extended time period to improve outcomes. The objective of this study was to compare DAIR failure rates and adverse events between an initial course of intravenous antibiotic therapy and the addition of extended treatment with oral antibiotics.
Methods
A multicenter observational study of patients diagnosed with a TKA PJI who underwent DAIR was performed. The primary outcome of interest was the failure rate derived from the survival time between the DAIR procedure and future treatment failure.
Results
One hundred eight patients met inclusion criteria; 47% (n = 51) received an extended course of oral antibiotics. These patients had a statistically significant lower failure rate compared to those who received only intravenous antibiotics (hazard ratio, 2.47; P = .009). Multivariable analysis demonstrated that extended antibiotics independently predicted treatment success, controlling for other variables. There was no significant difference in failure rates between an extended course of oral antibiotics less or more than 12 months (P = .23). No significant difference in the rates of adverse events was observed between patients who received an initial course of antibiotics alone and those who received a combination of initial and extended antibiotic therapy (P = .59).
Conclusions
Extending therapy with oral antibiotics had superior infection-free survival for TKA PJI managed with DAIR. There was no increase in adverse events, demonstrating safety. After 1 year, there appears to be no significant benefit associated with continued antibiotic therapy.
Keywords: periprosthetic joint infection, antibiotics, adverse events, arthroplasty
An extended course of oral antibiotics for 1 year following debridement and implant retention for patients with total knee arthroplasty periprosthetic joint infection appears to be efficacious and safe and to optimize antibiotic stewardship. Rate of adverse events were low.
Total knee and hip arthroplasty periprosthetic joint infection (PJI) is a devastating complication in orthopedic surgery. Total knee arthroplasty (TKA) PJI is the most common cause of early TKA failure [1–3]. Morbidity and mortality of treatment are high. Five-year mortality is estimated between 15% and 25% and is higher than that of many common cancers including breast, prostate, and melanoma [4–7]. With specific indications, TKA PJI can be managed with debridement, antibiotic therapy, and implant retention of the prosthesis (DAIR). This strategy is based on the logic of salvaging the implant to minimize invasive surgical interventions that could potentially contribute to patient morbidity or mortality. However, the success rate of DAIR has been variable, with failure rates varying from 26% [8] to as high as 84% [9]. Specific organisms such as Pseudomonas and Staphylococcus aureus have been especially associated with higher failure rates [5, 10–12].
A possible solution to decrease the high failure rate associated with DAIR includes the use of oral antibiotics for an extended time period following the initial course of intravenous antibiotic therapy. However, there is limited clinical guidance using this strategy in terms of efficacy, time needed to treat, and rate of adverse events (AEs). Limited studies have suggested that chronic suppression can improve long-term outcomes as defined by reoperation rate [13, 14]. However, the extended use of antibiotics has the potential to be associated with a number of AEs, including allergic reactions, organ injury, and medication intolerance. The optimal duration of chronic antibiotic treatment for patients who undergo DAIR is unknown.
Given this paucity of data, we performed a multicenter observational study to clarify the role of extended antibiotic use in patients who undergo DAIR after TKA PJI. The objectives of the study included comparing the rates of treatment failure between patients who underwent DAIR with and without the use of extended oral antibiotics, assessing the rate and type of AEs associated with extended antibiotic use, and determining if an optimal time period could be identified to discontinue antibiotic use to optimize antibiotic stewardship. We anticipate that the results of this study will help establish treatment guidelines for extended use of oral antibiotics in PJI.
METHODS
Study Design
A retrospective, multicenter cohort study of patients diagnosed with TKA PJI who subsequently underwent DAIR between 2005 and 2015 was performed. Data were acquired through the electronic medical records, and institutional board review approval was obtained. The study was completed at 16 hospitals in a regional health system comprising a variety of academic, hospital-employed, and private practice environments in both urban and rural settings
Study Participants
All participants had a diagnosis of TKA PJI made between 1 January 2005 and 31 December 2015 at any University of Pittsburgh Medical Center institution. Our initial cohort was obtained using the International Classification of Diseases, Ninth Revision code for PJI (996.66). A clinical diagnosis of PJI was based on the Musculoskeletal Infection Society (MSIS) criteria, with modified criteria including synovial nucleated white blood cell count threshold of 2500 cells/µL, and a synovial polymorphonuclear cell percentage of >65% as a secondary analysis of infection status. TKA PJI in particular was identified as the intersection of all patients in the medical system with the above diagnosis code and a concurrent surgical procedure on the knee, as determined by the medical record. Further inclusion criteria included any patient who received debridement with component retention and exchange of the polyethylene liner. Exclusion criteria included any patient who had a subsequent surgical procedure after receiving a TKA on the affected (ipsilateral) knee, or had a musculoskeletal oncological disease.
Selection of Antimicrobial Therapy
Selection of antibiotics was based (when available) on the pathogen identified, and for all patients, infectious disease input was sought. Treatment of PJI was based on the Infectious Diseases Society of America guidelines for prosthetic joint infections [15]. Rifampin was never used as monotherapy.
Definitions and Outcome Measures
PJI was defined using the modified MSIS criteria as outlined above. Perioperative antibiotics included antibiotics that were administered within 72 hours of DAIR. Primary antibiotic therapy was any antibiotic that was administered between 72 hours after DAIR and up to 6 weeks afterward. Suppressive (chronic) antibiotic therapy included any antibiotics that were provided beyond 6 weeks after DAIR. Treatment failure was defined as occurrence of PJI, based on criteria above, that occurred any time after the primary antibiotic therapy period, as well as any further surgical procedure on the operative knee excluding manipulation under anesthesia, periprosthetic fracture, and extensor mechanism disruption. Time to failure was measured as the time between DAIR and the next surgical procedure. Mortality was determined as reported in the electronic medical record. Follow-up was measured as any outpatient visit registered in the medical system, and duration of follow-up was defined as the length of time between the initial debridement procedure and last visit recorded in the medical record.
Statistical Analysis
Descriptive statistics were computed to quantify demographic and clinical characteristics of patients in this study and to assess rates of AEs as a function of antibiotic duration. Means and standard deviations were used for continuous variables with symmetric distributions, and medians and interquartile ranges (IQRs) were reported for continuous variables with skewed distributions. Categorical variables were summarized using counts and percentages. Fisher exact test was employed to compare rates of AEs between patients who received only primary antibiotics and patients who continued on to chronic suppression therapy. To investigate the effects of antibiotic duration (operationalized continuously as well as categorically) on survival probability, Cox proportional hazards models were constructed and direct-adjusted survivor functions were obtained. Similar time-to-event analyses were performed and survival curves calculated to estimate the mortality probability as a function of time from the index procedure. Multivariable Cox proportional hazards models were also constructed to determine if any additional patient or clinical characteristics (age, body mass index [BMI], Charlson comorbidity index [CCMI], length of stay, sex, diabetes mellitus, rheumatoid arthritis, days symptomatic, ASA score, host score, primary organism) were significant predictors of survival probability after adjusting for time on antibiotic therapy. Forward selection with a criterion of P < .05 for variable entry was employed in model building, and time on antibiotics (continuous or categorical) was retained in every model during the model selection procedure.
RESULTS
Study Population
Demographic data of the study participants are presented in Table 1, divided between those patients who received primary antibiotic therapy alone vs primary and chronic suppressive antibiotic therapy. A total of 108 patients were included in the study; 44% were female. The mean age was 67.5 years, 17% of patients had diabetes mellitus, and the mean BMI of the patient population was 33.9 kg/m2. The median CCMI score was 4. Median duration of follow-up was 2.3 years (IQR, 0.9–5.6 years). Acute PJI (defined as PJI occurring within 90 days of the primary TKA surgery) was noted in 33% (36) of our patients. Of the 108 patients included in the study, 52% had staphylococcal infections (58% of these were methicillin-susceptible S. aureus, 29% were methicillin-resistant S. aureus, and 13% were coagulase-negative staphylococci), 8% had streptococcal infections (including groups A, B, C, G, and viridans streptococci), 10% were gram-negative infections (Serratia, Enterobacter, Escherichia coli, Pasteurella, and Pseudomonas species), and 5% had infection with enterococci. One patient (1%) had an infection with diphtheroids. The remainder (24%) were culture-negative infections.
Table 1.
Demographic Data and Clinical Characteristics of Study Population, by Treatment Type
| Characteristics | Primary Alone (n = 57) | Primary + Chronic (n = 51) | Total (N = 108) | P Value |
|---|---|---|---|---|
| Age, y, mean (SD) | 65.7 (10.5) | 68.9 (11.7) | 67.5 (11.1) | .14 |
| BMI, kg/m2, mean (SD) | 34.8 (8.2) | 33.3 (8.1) | 33.9 (8.2) | .34 |
| CCMI, median (IQR) | 3.5 (2–6) | 4 (2–7) | 4 (2–6) | .98 |
| LOS, median (IQR) | 6.3 (4.2–10.2) | 5.6 (4.4–7.3) | 6 (4.4–9.1) | .22 |
| Female sex, No. (%) | 25 (43.9) | 22 (43.1) | 47 (43.5) | .70 |
| DM, No. (%) | 7 (12.3) | 11 (21.6) | 18 (16.7) | .85 |
| RA, No. (%) | 9 (15.8) | 0 (0.0) | 9 (8.3) | .0031 |
Abbreviations: BMI, body mass index; CCMI, Charlson comorbidity index; DM, diabetes mellitus; IQR, interquartile range; LOS, length of stay; RA, rheumatoid arthritis; SD, standard deviation.
Outcome Data
Adverse Events
Table 2 outlines the AEs associated with antibiotic use for perioperative (<72 hours from DAIR), primary (≥72 hours–6 weeks after DAIR), and chronic (>6 weeks from DAIR) suppressive therapy. The most common antibiotics used in the perioperative period were vancomycin (52) and cefazolin (43). The most common agents used in the primary treatment period were vancomycin (72), rifampin (31), cefazolin (26), and ceftriaxone (21); of note, rifampin was never used as monotherapy. The most common antibiotics used for suppressive therapy in the chronic period were cephalexin (23), trimethoprim-sulfamethoxazole (12), and doxycycline (6). All 108 patients received perioperative antibiotic therapy. Fifty-seven received only primary therapy (up to 6 weeks of antibiotics), while 51 received antibiotics for >6 weeks. A total of 7 patients required a switch in their oral suppressive antibiotic therapy due to AEs. In the perioperative period, the most common AEs noted were gastrointestinal (GI) related (non–Clostridioides difficile) with a rate of 9.3%, followed by development of a rash (7.4%). For those who had primary treatment alone, GI intolerance was the only AE recorded (1.8%), and in those who had both primary and chronic treatment, GI intolerance was the single biggest AE, although nonspecific AEs (pancytopenia, dizziness, memory issues, thrush, pruritic) were the biggest reported overall category in this group. Interestingly, the rate of C. difficile colitis was noted to be exceedingly rare, with only 1 patient (1.8%) developing this issue during the treatment period. This patient was on amoxicillin-clavulanate. In addition, the rate of AEs for patients who were on antibiotics for ≤6 weeks was not statistically significantly different from the rate for patients who were on antibiotics for >6 weeks during their treatment course (P = .59).
Table 2.
Adverse Events Associated With Antibiotic Use in the Perioperative, Primary, and Primary + Chronic Treatment Periods
| Adverse Event | Perioperative (n = 108) | Primary Only (n = 57) | Primary + Chronic (n = 51) |
|---|---|---|---|
| Gastrointestinal issue | 10 (9.3) | 1 (1.8) | 2 (3.9) |
| Clostridioides difficile infection | 0 (0) | 0 (0) | 1 (2.0) |
| Rash | 8 (7.4) | 0 (0) | 1 (2.0) |
| Swelling | 2 (1.9) | 0 (0) | 0 (0) |
| Hepatotoxicity | 1 (0.9) | 0 (0) | 0 (0) |
| Renal toxicity | 3 (2.8) | 0 (0) | 0 (0) |
| Leukopenia | 1 (0.9) | 0 (0) | 0 (0) |
| Other | 6 (5.6) | 0 (0) | 3 (5.9) |
Treatment Failure
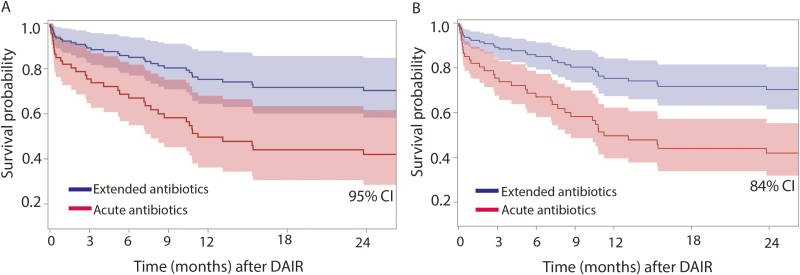
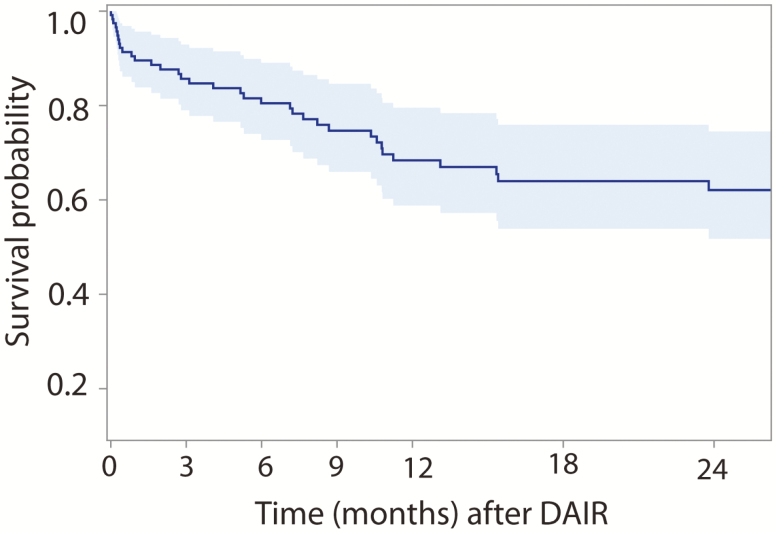
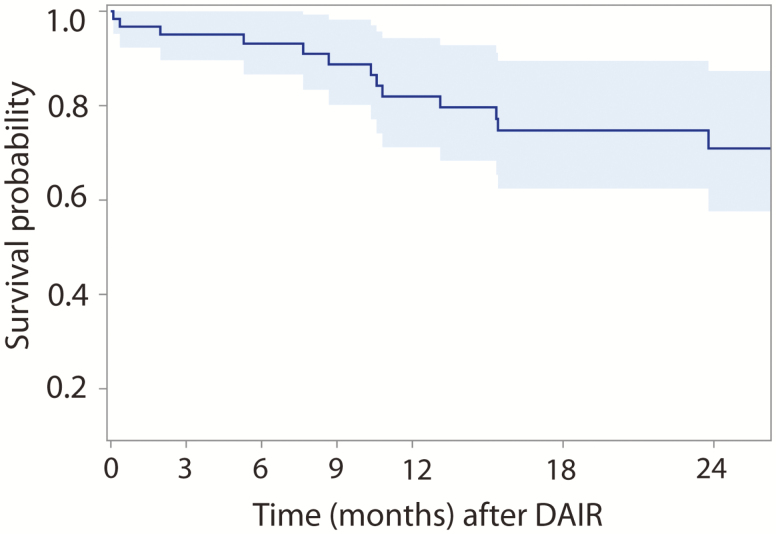
Figure 1A shows the estimated survival probability as a function of time for those patients who completed only primary therapy post-DAIR and for those who went on to chronic therapy (>6 weeks) after their DAIR procedure, along with 95% point-wise confidence intervals (CIs). Overall, those patients who continued on to receive chronic antibiotics had a significantly higher survival probability (68.5%) compared with those who received only primary therapy (39.4%) following their DAIR procedure (hazard ratio [HR], 2.47; P = .009). For ease of comparison, Figure 1B shows the same estimated survival functions but with 84% point-wise CIs, as nonoverlapping 84% point-wise CIs are commonly used in statistics to demonstrate statistically significant differences between curves at the 5% type 1 error rate. Figure 2 analyzed all patients on antibiotic therapy during the study period with respect to survival probability. Regardless of the duration of antibiotic therapy that a patient was on, the estimated hazard of failure decreased by 3.8% every month (P = .44). The estimated survival probability decreased at a steady rate until approximately 12 months, at which point a plateauing effect was observed. In particular, there was no statistically significant difference in failure rates between an extended course of oral antibiotics less or more than 12 months (P = .23). Figure 3 analyzes the probability of survival noted on only those patients who continued with chronic suppressive antibiotics; although a decrease in hazard of failure was noted as months on antibiotics increased, this decrease was not statistically significant (P = .12).
Figure 1.
Total knee arthroplasty periprosthetic joint infection debridement, antibiotic therapy, and implant retention (DAIR) survival with acute vs extended antibiotics. The Kaplan-Meier survival curves of DAIR procedures are shown between groups that received only acute antibiotics as compared to chronic suppressive antibiotics with 95% confidence intervals (CIs) (A) and 84% CIs (B). Areas of the curve that do not overlap at an 84% CI suggest statistical significance.
Figure 2.
Kaplan-Meier survival for total knee arthroplasty periprosthetic joint infection debridement, antibiotic therapy, and implant retention (DAIR) of the entire combined cohort.
Figure 3.
Kaplan-Meier survival for total knee arthroplasty periprosthetic joint infection debridement, antibiotic therapy, and implant retention (DAIR) among patients who received chronic suppressive antibiotics.
A multivariable analysis was completed to ensure extended antibiotic use was an independent predictor of treatment success. Duration of antibiotic therapy and the organism isolated at the time of the original infection were the 2 predictors of treatment success (Table 3). Notably, the hazard for failure in acquiring S. aureus PJI was twice that of all other organisms, and culture-negative PJI interestingly was associated with 3 times the hazard risk compared to S. aureus infections. However, no specific organism affected overall survival probability.
Table 3.
Multivariable Analysis of Predictors of Treatment Success
| Predicting Factors | Unadjusted HR (95% CI) |
Unadjusted P Value | Adjusted HR (95% CI) |
Adjusted P Value |
|---|---|---|---|---|
| Abx duration | .0086 | .018 | ||
| Primary only | Ref | Ref | ||
| Primary + chronic | 0.406 (.207–.795) | 0.427 (.211–.863) | ||
| Age | 0.974 (.947–1.002) | .067 | … | |
| BMI | 1.032 (.989–1.077) | .15 | … | |
| Sex | ||||
| Male | Ref | … | ||
| Female | 1.467 (.763–2.824) | .25 | … | |
| CCMI | 0.865 (.748–.999) | .048 | … | |
| DM (yes) | 0.697 (.246–1.974) | .46 | … | |
| RA (yes) | 2.509 (1.041–6.050) | .041 | … | |
| ASA score | .23 | |||
| 1–2 | Ref | … | ||
| 3 | 0.542 (.265–1.109) | … | ||
| 4 | 0.547 (.176–1.699) | … | ||
| Host score | .54 | |||
| A | Ref | … | ||
| B | 0.640 (.291–1.411) | … | ||
| C | 0.823 (.100–6.787) | … | ||
| Time symptomatic | ||||
| <1 wk | Ref | .33 | … | |
| 2–4 wk | 1.293 (.626–2.671) | … | ||
| >4 wk | 2.456 (.720–8.376) | … | ||
| Primary organism | .0010 | .0049 | ||
| Culture negative | Ref | Ref | ||
| Staphylococcus aureus | 0.259 (.083–.808) | 0.417 (.128–1.359) | ||
| Other | 0.122 (.039–.387) | 0.181 (.056–.586) |
Abbreviations: Abx, antibiotic; BMI, body mass index; CCMI, Charlson comorbidity index; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; RA, rheumatoid arthritis; Ref, reference category.
Unadjusted analyses were completed to identify significant univariate predictors of treatment success, and adjusted analyses were performed to ensure that extended antibiotic use remained a significant predictor of success after controlling for other covariates. Duration of antibiotic therapy (primary + chronic vs primary only), CCMI, rheumatoid arthritis, and primary organism (S. aureus, other, culture negative) were found to be significant predictors of treatment success in unadjusted models (Table 3). After completing adjusted analyses via multivariable modeling, only duration of antibiotic use (P = .018) and primary organism (P = .005) remained significant predictors of treatment success. The hazard of failure from acquiring S. aureus PJI was 2.31 times the hazard associated with all other organisms after adjusting for antibiotic duration, and interestingly, the hazard of failure for culture negative PJI was 2.40 times that of S. aureus infections after controlling for antibiotic duration. Antibiotic duration remained a significant independent predictor of treatment success, however, even after adjusting for the effect of organism. The adjusted hazard of failure for patients who received only primary antibiotic therapy was 2.34 times the hazard for patients who went on to chronic therapy. Accounting for organism decreased the estimated HR by a mere 4.9%, as the unadjusted HR was 2.46. Thus, the effect of antibiotic duration on survival probability remained virtually unchanged after accounting for the effects of other potential predictors.
No statistical differences were noted in this study with regard to mortality among those participants who were on chronic suppressive antibiotic therapy, compared to those who were not (P = .91).
DISCUSSION
Treatment of TKA PJI is challenging, and involves both orthopedic and infectious disease input to optimize results. The 2 main surgical strategies include DAIR vs 2-stage exchange. When appropriate, DAIR has a number of advantages over 2-stage exchange, including limited operative intervention and salvage of implants. The disadvantage of DAIR includes its high failure rate at approximately 55% [5], but there is a large variation in success [16]. Strategies to improve treatment are needed. In this study, we examined the benefits of extended antibiotic treatment in DAIR and associated AEs with this treatment, and assessed whether there was an optimal time to discontinue antibiotics, as well as what factors influenced this timing.
An extended course of oral antibiotics decreased treatment failure rates in DAIR in our study. In patients treated with extended oral antibiotics, long-term infection-free survival was 68.5% compared with 39.4% in patients who did not receive extended antibiotics (HR, 2.5; P = .009). The benefits of extended antibiotic treatment have been previously documented. Siqueira et al completed a retrospective matched cohort study that compared chronic antibiotic suppressive therapy for either 2-stage exchange or DAIR procedures in knee and hip arthroplasty PJI to only an initial course of antibiotics [14]. At 5 years, 64.7% of those on chronic suppressive therapy were free from treatment failure vs 30.4% who were not on chronic suppressive antibiotics (P < .0001). Chaussade et al performed a multicenter retrospective study focusing on duration of antibiotics in patients treated for knee and hip PJI who underwent DAIR. In their study, 6 weeks of antibiotic therapy postprocedure produced similar outcomes to 12 weeks of antibiotics, with a failure rate of approximately 30% [17]. However, an antibiotic course beyond 12 weeks was not monitored, limiting the utility of the study in clinical practice. These studies also did not report time needed to treat or AEs associated with extended antibiotic use.
However, in our study, a finite time period was required to achieve this benefit and after approximately 1 year, no substantial benefit to continuing antibiotics was observed with respect to reducing infection-free survival. Our multivariable analysis showed that both duration of antibiotic therapy as well as organism isolated at the time of infection influenced treatment success, with culture-negative and S. aureus PJI having the highest hazard of failure when compared to all other organisms. However, even with these infections, extending chronic suppressive antibiotic therapy beyond the 1-year mark did not improve survival probability. This suggests potentially important implications for antibiotic stewardship. The time needed to treat with extended oral antibiotics was a finite time period. There was a beneficial effect of extending antibiotic use, with a 3.8% reduction in hazard of failure occurring every month the patient is on antibiotic therapy. After 12 months, this benefit did plateau. There was an inflection point at 12 months on the survival curve as well, suggesting that ongoing antibiotic use beyond this point may not provide any added benefit to the patient in decreasing DAIR failure rates. We are unaware of other studies that have defined a time limit. Further investigations and prospective studies should be completed to confirm these results.
Traditionally, the use of oral antibiotics in the setting of DAIR has been termed “chronic suppression”; this has been in part due to the lack of available evidence to support an appropriate end date for discontinuation of oral antibiotic therapy. However, as stated above, our data suggest that there may be a finite benefit to oral suppressive antibiotic use, which, if reinforced by future studies, may challenge the notion that oral antibiotics following PJI are required for suppressive purposes. The formation of biofilm that is associated with surgical implant infections has been demonstrated to be highly tolerant to antibiotics [18, 19, 20], and perhaps after a fixed-duration antibiotic exposure, the ability for biofilm formation is impaired sufficiently to prevent any meaningful reaccumulation of organisms that would result in reinfection. Again, further studies, both in vitro and in vivo, would be needed to help support these notions.
A concern with long-term antibiotic use is the rate of AEs. This has not been extensively reported previously. Our study demonstrated that the rate of AEs was relatively low in those patients who were placed on antibiotic therapy following DAIR. The most common side effect overall was GI associated (nausea, vomiting, diarrhea), and only 14% required that oral antibiotic suppression therapy be switched or discontinued. The rate of C. difficile was exceedingly rare, occurring in only 1 patient. This may have been due to the fact that the oral antibiotics used in our patient cohort were agents that are historically less likely to be associated with C. difficile acquisition, including trimethoprim-sulfamethoxazole and doxycycline. No significant difference in the proportion of AEs or reported side effects was found between patients on antibiotics >6 weeks when compared to patients on antibiotics for <6 weeks (P = .59); this provides evidence for safe administration of oral antibiotics for extended time periods. It is worth mentioning that although our study found no statistically significant difference in the rate of antibiotic-associated events between those patients who received primary therapy alone and those who went on to have chronic therapy, the differences in AEs among these 2 groups may carry clinical significance with regard to patient care, and should be taken into consideration.
As an observational study, our findings should be interpreted in the context of some limitations. There was no randomization of treatment between populations. We attempted to control for this by completing a multivariable analysis to control for factors that are known to influence DAIR outcomes. When this was completed, extended antibiotic treatment was an independent predictor of treatment success. Second, some medical records contained incomplete documentation, and it was at times difficult to fully determine precise length of therapy on some of the patients. Given the large range in each time period, we believe we have captured an accurate estimate of antibiotic use. As a result, this was unlikely to have an influence on our overall estimate of antibiotic use.
In conclusion, as compared to a limited course of intravenous antibiotics, extended antibiotic use had superior infection-free survival. The continued use of oral antibiotics following DAIR for up to 1 year appears to be safe and efficacious; beyond the 1-year mark, the utility of ongoing antibiotic use may be questionable. There was no statistically significant increase in reported AEs after completion of primary antibiotic therapy, demonstrating relative safety in employing extended antibiotic use up until 1 year. Further studies are needed to reinforce our findings. As noted in our study, both infectious disease and orthopedic input appear to be important in the management of prosthetic joint infections, and when available, both services should be utilized and incorporated into the evaluation and management of these infections.
Notes
Acknowledgments. This study was performed at the Departments of Orthopedic Surgery and Infectious Disease at the University of Pittsburgh, Pennsylvania.
Financial support. K. L. U. is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number K08AR071494); the National Center for Advancing Translational Sciences (grant number KL2TR0001856); the Orthopaedic Research and Education Foundation; and the Musculoskeletal Tissue Foundation.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pitta M, Esposito CI, Li Z, Lee YY, Wright TM, Padgett DE. Failure after modern total knee arthroplasty: a prospective study of 18,065 knees. J Arthroplasty 2018; 33:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SW. Periprosthetic joint infection is the main cause of failure for modern knee arthroplasty: an analysis of 11,134 knees. Clin Orthop Relat Res 2017; 475:2194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozic KJ, Pui CM, Ludeman MJ, Vail TP, Silverstein MD. Do the potential benefits of metal-on-metal hip resurfacing justify the increased cost and risk of complications? Clin Orthop Relat Res 2010; 468:2301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 2006; 42:471–8. [DOI] [PubMed] [Google Scholar]
- 5. Urish KL, Bullock AG, Kreger AM, Shah NB, Jeong K, Rothenberger SD; Infected Implant Consortium A multicenter study of irrigation and debridement in total knee arthroplasty periprosthetic joint infection: treatment failure is high. J Arthroplasty 2018; 33:1154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi HR, Bedair H. Mortality following revision total knee arthroplasty: a matched cohort study of septic versus aseptic revisions. J Arthroplasty 2014; 29:1216–8. [DOI] [PubMed] [Google Scholar]
- 7. Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013; 95:2177–84. [DOI] [PubMed] [Google Scholar]
- 8. Swenson RD, Butterfield JA, Irwin TJ, Zurlo JJ, Davis CM 3rd. Preoperative anemia is associated with failure of open debridement polyethylene exchange in acute and acute hematogenous prosthetic joint infection. J Arthroplasty 2018; 33:1855–60. [DOI] [PubMed] [Google Scholar]
- 9. Bradbury T, Fehring TK, Taunton M, et al. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty 2009; 24:101–4. [DOI] [PubMed] [Google Scholar]
- 10. Shah NB, Osmon DR, Steckelberg JM, et al. Pseudomonas prosthetic joint infections: a review of 102 episodes. J Bone Jt Infect 2016; 1:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuiper JW, Vos SJ, Saouti R, Vergroesen DA, Graat HC, Debets-Ossenkopp YJ, Peters EJ, Nolte PA. Prosthetic joint-associated infections treated with DAIR (debridement, antibiotics, irrigation, and retention): analysis of risk factors and local antibiotic carriers in 91 patients. Acta Orthop 2013; 84:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009; 63:1264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keller SC, Cosgrove SE, Higgins Y, Piggott DA, Osgood G, Auwaerter PG. Role of suppressive oral antibiotics in orthopedic hardware infections for those not undergoing two-stage replacement surgery. Open Forum Infect Dis 2016; 3:ofw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siqueira MB, Saleh A, Klika AK, et al. Chronic suppression of periprosthetic joint infections with oral antibiotics increases infection-free survivorship. J Bone Joint Surg Am 2015; 97:1220–32. [DOI] [PubMed] [Google Scholar]
- 15. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 16. Triantafyllopoulos GK, Poultsides LA, Zhang W, Sculco PK, Ma Y, Sculco TP. Periprosthetic knee infections treated with irrigation and debridement: outcomes and preoperative predictive factors. J Arthroplasty 2015; 30:649–57. [DOI] [PubMed] [Google Scholar]
- 17. Chaussade H, Uçkay I, Vuagnat A, et al. Antibiotic therapy duration for prosthetic joint infections treated by debridement and implant retention (DAIR): similar long-term remission for 6 weeks as compared to 12 weeks. Int J Infect Dis 2017; 63:37–42. [DOI] [PubMed] [Google Scholar]
- 18. Ma D, Shanks RMQ, Davis CM 3rd, Craft DW, Wood TK, Urish KL. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. J Orthop Res 2018; 36:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Urish KL, DeMuth PW, Kwan BW, Craft DW, Wood TK, Davis CM 3rd. Antibiotic tolerant Staphylococcus aureus Biofilm Persists on Arthroplasty Materials. Clin Orthop Relat Res. 2016; 474:1649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mandell JB, Koch J, Nourie B, Bonar DD, Shah N, Urish KL. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]