Abstract
Background
Persistent Staphylococcus aureus bacteremia (SAB) is defined based on varying duration in literature. The primary objective was to determine the risk of poor outcomes in relation to bacteremia duration.
Methods
Multicenter, prospective, observational study of adult hospitalized patients with SAB. Medical records were reviewed for pertinent data. Patients were grouped by bacteremia duration: short (1–2 days), intermediate (3–6 days), and prolonged (≥7 days) and compared for risk factors and outcomes.
Results
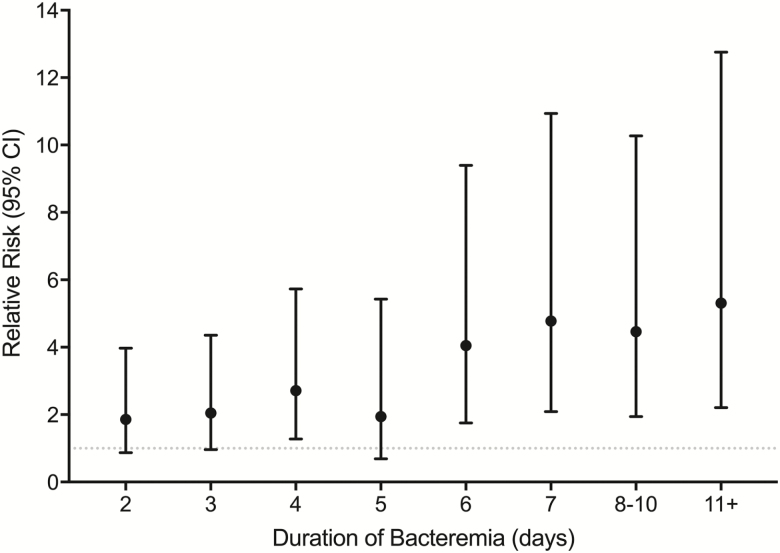
Of 884 patients, 63% had short, 28% intermediate, and 9% prolonged bacteremia. Overall mean age was 57 years, and 70% were male. The prolonged group had the highest proportion of methicillin-resistant SAB (P < .0001). Choice of antibiotic therapy did not significantly affect bacteremia duration; however, time to source-control procedure was delayed in the prolonged and intermediate groups compared with the short group (3.5 vs 3 vs 1 day, P < .0001). Metastatic complications, length of stay, and 30-day mortality were progressively worse as bacteremia duration increased (P < .0001). Every continued day of bacteremia was associated with a relative risk of death of 1.16 (95% confidence interval, 1.10–1.22; P < .0001), with a significant increase in risk starting at 3 days as determined by receiver operating characteristic analysis.
Conclusions
Optimal management of SAB should target bacterial clearance as soon as possible to minimize incremental risk of mortality with each day of positive blood culture. Delay in source control but not type of antistaphylococcal therapy was significantly associated with prolonged bacteremia and worse outcomes.
Keywords: S. aureus bacteremia, persistence, mortality
Every continued day of persistent Staphylococcus aureus bacteremia is associated with a 16% increased risk of death. Bacteremia duration of 3 days was found to best distinguish patients who died versus those who survived.
Staphylococcus aureus bacteremia (SAB) affects an estimated 50 in 100 000 persons, with mortality rates of 19–57% in adults [1]. Microbial persistence occurs in approximately one-third of cases despite appropriate antibiotic administration [2–4]. The 2011 Infectious Diseases Society of America (IDSA) treatment guidelines for methicillin-resistant S. aureus (MRSA) infections recommend consideration of alternative management if blood cultures remain positive for 7 days [5].
Prior studies have defined persistence as ≥3 days [6–8], >5 days [9], or ≥7 days [3, 5, 10–15] either arbitrarily or through limited analysis and evaluated outcomes compared with a control group with shorter bacteremia durations. However, they did not assess the duration of bacteremia as a continuous variable to define the breakpoint at which risk of poor outcome significantly increased. One study [16] evaluated the duration of bacteremia as a continuous variable and found a 15.9% increased risk of death per day of continued bacteremia; however, this study was limited by a small sample size of 59 patients, only 10 of whom had bacteremia durations of more than 4 days. Thus, our primary objectives were to determine (1) the risk of poor outcomes in relation to bacteremia duration in a large cohort of 884 patients with SAB and (2) a precise and meaningful outcome-based definition of persistence. The results could provide a reference time point by which treatment efficacy can be evaluated and compared across future studies.
METHODS
This was a prospective, observational study conducted at 3 university-affiliated institutions in Los Angeles, California. All patients with first occurrence of positive blood cultures for S. aureus along with signs and symptoms of infection were identified between 2012 and 2017. Exclusion criteria were as follows: 1) age less than 18 years, 2) receipt of less than 48 hours of antistaphylococcal therapy, 3) delayed initiation of antistaphylococcal therapy of more than 48 h from bacteremia onset, 4) polymicrobial blood culture, and 5) not having repeat blood cultures drawn and had clinical failure (death or lack of clinical resolution in the hospital). The study protocol was approved by institutional review boards at each study site. Informed consent was waived. Medical charts were reviewed for relevant data and entered into a secure database software.
Study Definitions
Sources of bacteremia were grouped relative to mortality risk: low (<10%), intermediate (10–20%), and high (>20%), as previously defined [17]. Bacteremia duration was calculated as the number of days between the first and last positive blood culture with S. aureus [13]. Patients without repeat blood cultures and who experienced clinical success were categorized as having 1 day of bacteremia. Clinical success was defined as resolution of white blood cell count and vital signs towards the normal range and subjective well-being as documented by the physician. Nosocomial SAB was the first positive blood culture date 48 hours or more after the admission date. Concurrent infection is the presence of a non–S. aureus infection diagnosed within 48 hours of SAB onset. Definitive SAB therapy was the primary directed therapy prescribed for the longest duration. Metastatic complication was presumptive or confirmed S. aureus infection at a distant focus anatomically unrelated to the implicated source. Mortality was evaluated within 30 days from the first positive blood culture for S. aureus via review of the patient’s medical chart.
Data Analysis
The primary endpoint was all-cause 30-day mortality, and secondary outcomes were hospital length of stay (LOS) and metastatic complications. A receiver operating characteristics (ROC)–based cut-point method was utilized first to estimate the optimal cutoff for the number of days of bacteremia for predicting 30-day mortality. The Youden index J (J = sensitivity + specificity – 1 is maximized) was chosen as the most appropriate summary measure. To identify the incremental risk of 30-day mortality with each day of bacteremia, a modified Poisson regression analysis using robust error variance was performed, with 1 day of bacteremia as the reference group. A sensitivity analysis was additionally performed for the ROC and modified Poisson regression analysis, which included only patients with blood cultures drawn on consecutive days to confirm bacteremia duration. Patients were then grouped by bacteremia duration and compared for clinical data and outcomes: short (1–2 days of positive blood cultures), intermediate (3–6 days), and prolonged (≥7 days). These durations were chosen based on considerations of the ROC analysis performed above as well as published data using 3 days [13] or 7 days [5] as persistence definitions.
Continuous data were analyzed using Kruskal-Wallis or analysis of variance for 3 or more group comparisons with Dunn's or Tukey multiple comparison posthoc test; and Wilcoxon rank sum or Student t test for 2 groups. Categorical data were analyzed by chi-square or Fisher’s exact test. A P value <.05 was considered significant. A univariate analysis was performed with the following variables: age, gender, bacteremia duration of 3 or more days, source-control procedure, source risk category (low-/intermediate- vs high-risk source), Pitt bacteremia score, infection with MRSA versus methicillin-sensitive S. aureus (MSSA), and initial vancomycin with β-lactam therapy, followed by multivariable logistic regression analysis to determine the predictors of 30-day mortality. Multicollinearity was assessed among possible predictors by calculating the variance inflation factor for each variable. The final model was derived in a stepwise fashion by removing/adding variables until a model was reached with all predictors jointly significant at P ≤ .05, after adjustment for age and gender. Statistical analyses were performed using GraphPad Prism version 4.0 (GraphPad Software) or SAS version 9.4 (SAS Institute).
RESULTS
Of 1186 patients screened, 884 were included. Reasons for exclusion were as follows: age less than 18 years (n = 12), receipt of less than 48 hours of therapy (n = 126), delay of more than 48 hours to initiate therapy (n = 22), polymicrobial bacteremia (n = 100), unavailable medical chart (n = 29), and lack of repeat blood cultures drawn (n = 13) and expiring (n = 8) or clinically failing (n = 5). Overall, the mean age was 57 years, 70% were male, and 82% had community-onset SAB (Table 1). Bacteremia duration ranged from 1 to 26 days; 63% had short (1–2 days; n = 555), 28% had intermediate (3–6 days; n = 250), and 9% had prolonged (≥7 days; n = 79) duration. Comorbidities were similar across groups, except hypertension (P = .0019) and diabetes (P = .024) were more frequent in the prolonged group.
Table 1.
Patient Characteristics
| Duration of Bacteremia | P Value | |||
|---|---|---|---|---|
| Short (1–2 d) (n = 555) | Intermediate (3–6 d) (n = 250) | Prolonged (≥7 d) (n = 79) | ||
| Age (mean ± SD), y | 56 ± 16.6 | 58 ± 15.3 | 57 ± 14.4 | .14 |
| Female | 168 (30) | 71 (28) | 23 (29) | .86 |
| Race/ethnicity | ||||
| Non-Hispanic white | 170 (31) | 78 (32) | 26 (33) | .82 |
| Hispanic | 224 (40) | 103 (42) | 29 (37) | |
| Asian | 49 (9) | 18 (7) | 7 (9) | |
| African American | 60 (11) | 34 (14) | 12 (15) | |
| Other | 20 (4) | 7 (3) | 3 (4) | |
| Not reported | 23 (4) | 6 (2) | 1 (1) | |
| Residence prior to admission | ||||
| Home | 400 (72) | 186 (74) | 56 (71) | .104 |
| Skilled nursing facility | 44 (8) | 20 (8) | 1 (1) | |
| Othera | 111 (20) | 44 (18) | 22 (28) | |
| Comorbid conditions | ||||
| None | 46 (8) | 16 (6) | 7 (9) | .61 |
| Intravenous drug use | 51 (9) | 37 (15) | 9 (11) | .056 |
| Hypertension | 249 (45) | 139 (56) | 48 (61) | .0019 |
| Dyslipidemia | 100 (18) | 48 (19) | 16 (20) | .850 |
| Diabetes | 222 (40) | 87 (35) | 41 (52) | .024 |
| Congestive heart failure | 63 (11) | 33 (13) | 5 (6) | .25 |
| Malignancy | 61 (11) | 31 (12) | 7 (9) | .66 |
| Liver disease | 94 (17) | 44 (18) | 14 (18) | .97 |
| Renal disease | 142 (26) | 78 (31) | 25 (32) | .18 |
| End-stage renal disease on dialysis | 100 (18) | 57 (23) | 19 (24) | .18 |
| Other | 246 (44) | 123 (49) | 29 (37) | .13 |
| 3 or more comorbid conditions | 300 (54) | 138 (55) | 44 (56) | .93 |
| History of MRSA infection | 52/512 (10) | 18/223 (8) | 9/72 (13) | .49 |
| History of MSSA infection | 59/516 (11) | 15/220 (7) | 10/68 (15) | .08 |
| Microbiology | ||||
| MSSA | 387 (70) | 173 (69) | 33 (42) | <.0001 |
| Vancomycin MIC | .31 | |||
| ≤1 mg/L | 45/170 (26) | 21/68 (31) | 4/8 (50) | |
| >1 mg/L | 125/170 (74) | 47/68 (69) | 4/8 (50) | |
| MRSA | 167 (30) | 77 (31) | 46 (58) | |
| Vancomycin MIC | .90 | |||
| ≤1 mg/L | 41/125 (33) | 23 / 68 (34) | 14/ 38 (37) | |
| >1 mg/L | 84/125 (67) | 45/68 (66) | 24/38 (63) | |
| Nosocomial bacteremia | 106 (19) | 43 (17) | 13 (16) | .73 |
| Duration of hospital stay prior to SAB for patients with nosocomial bacteremia (median, IQR), d | 7 (4, 16) | 5 (3, 12) | 10 (5, 15) | .34 |
| Source risk of SAB | ||||
| Low | 121 (22) | 60 (24) | 16 (20) | 0.0057 |
| Intermediate | 338 (61) | 122 (49) | 42 (53) | |
| High | 96 (17) | 68 (27) | 21 (27) |
Data are no. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; SAB, Staphylococcus aureus bacteremia; SD, standard deviation.
a“Other” includes other hospital, rehabilitation center, or homeless.
MRSA caused 33% (290/884) of bacteremia. Compared with MSSA, MRSA was associated with a nearly 3-fold increased risk of prolonged bacteremia duration (relative risk [RR], 2.85; 95% confidence interval [CI], 1.86–4.36; P < .0001). Bacteremia duration did not differ by Etest (bioMérieux, Durham, NC) vancomycin minimum inhibitory concentration (MIC) of isolates, with an MIC greater than 1 mg/L being similarly prevalent across all groups.
Patients with intermediate and prolonged bacteremia had significantly worse initial clinical presentation and high-risk sources (27%, 89/329) compared with the short group (17%, P = .0008) (Table 1). Endocarditis or other endovascular cause was least common in the short group (short vs intermediate/prolonged: 5% [25/555] vs 23% [75/329]; P < .0001). Among patients with endocarditis, MRSA had longer bacteremia duration than MSSA [median (interquartile range [IQR]): 5 (3, 8) days vs 4 (2, 5) days; P = .04]. A higher rate of concurrent infections (prolonged vs intermediate: 33% vs 20%; P= .02) and intensive care unit admission during SAB (prolonged vs intermediate: 56% vs 36%; P= .0024) were differentiating characteristics between the prolonged and intermediate groups.
Management
The proportion of patients with infectious diseases (ID) consultation increased with bacteremia duration (short, intermediate, and prolonged: 50%, 66%, and 89%, respectively; P < .0001) (Table 2). Notably, the time to consultation was 1 day later (median) in the prolonged group compared with the other groups (P= .005). Source-control procedures to eradicate the foci of SAB (incision and drainage/surgical procedure, infected hardware or catheter removal) were performed at similar rates in all groups (short vs intermediate vs prolonged: 44% vs 44% vs 56%; P = .13); however, the procedure was delayed by 2 days in the intermediate and prolonged bacteremia groups compared with the short group (median [IQR] after onset of SAB: prolonged, 3.5 [2, 9.75] days, vs intermediate, 3 [1, 5] days, vs short, 1 [1, 3] day; P < .0001).
Table 2.
Clinical Presentation and Management of Staphylococcus aureus Bacteremia
| Duration of Bacteremia | P Value | |||
|---|---|---|---|---|
| Short (1–2 d) (n = 555) | Intermediate (3–6 d) (n = 250) | Prolonged (≥7 d) (n = 79) | ||
| Pitt bacteremia score (median, IQR) | 0 (0, 2) | 1 (0, 2) | 1 (0, 2) | .019 |
| ≥2 | 169 (30) | 96 (38) | 31 (39) | .046 |
| ≥4 | 58 (10) | 30 (12) | 12 (15) | .42 |
| ICU stay during SAB | 158 (28) | 89 (36) | 44 (56) | <.0001 |
| Duration of ICU stay (median, IQR), d | 5 (2, 12) | 5 (2, 11) | 11 (4, 22.5) | .028 |
| Need for vasopressors | 72 (13) | 60 (24) | 25 (32) | <.0001 |
| Concurrent infections | 127 (23) | 50 (20) | 26 (33) | .064 |
| ID consultation | 278 (50) | 165 (66) | 70 (89) | <.0001 |
| Time to receipt of ID consult from SAB onset (median, IQR), d | 2 (1, 4) | 2 (1, 4) | 3 (2, 6) | .0005 |
| Procedure performed for source control | 245 (44) | 109 (44) | 44 (56) | .13 |
| Incision and drainage | 123 (50) | 42 (38) | 23 (52) | .099 |
| Catheter removal | 94 (38) | 54 (50) | 15 (34) | .088 |
| Time to source-control procedure (median, IQR), d | 1 (1, 3) | 3 (1, 5) | 3.5 (2, 9.75) | <.0001 |
| Initial active antibiotic therapy | .85 | |||
| Vancomycin monotherapy | 170 (31) | 76 (30) | 30 (38) | |
| Vancomycin and β-lactam | 273 (49) | 118 (47) | 35 (44) | |
| Antistaphylococcal β-lactam | 21(4) | 12 (5) | 3 (4) | |
| Other | 91 (16) | 44 (18) | 11 (14) | |
| Time to start active antibiotic therapy | .66 | |||
| Prior to SAB onset | 51 (9) | 18 (7) | 4 (5) | |
| On day of SAB onset | 355 (64) | 159 (64) | 47 (59) | |
| 1-day delay from onset of SAB | 131 (24) | 65 (26) | 25 (32) | |
| 2-day delay from onset of SAB | 18 (3) | 8 (3) | 3 (4) |
Data are no. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; ID, infectious diseases; IQR, interquartile range; SAB, Staphylococcus aureus bacteremia.
There were similar rates across the 3 groups (P = .66) of use of an antibiotic with in vitro activity against the infecting pathogen initiated on or before SAB onset (overall, 72%; 634/884) (Table 2). Delay in initiation of effective therapy by 1 day (n = 221, 25%) or 2 days (n = 29, 3%) after SAB onset was not associated with longer duration of bacteremia (P = .70). Vancomycin was the most common empiric therapy initiated (79%, 702/884); the choice of agent did not differ by MRSA versus MSSA or by bacteremia duration. Nearly half (48%) of all patients received a β-lactam with vancomycin empirically. Among those with MSSA bacteremia initiated on a vancomycin-containing empiric regimen (n = 458), 89% (407/458) were changed to an antistaphylococcal β-lactam alone within a median of 2 days (IQR: 1.5, 3 days). The duration to transition from vancomycin to an antistaphylococcal beta-lactam was not delayed in the prolonged or intermediate group compared to the short group (prolonged vs intermediate vs short: median 2 days vs 2 days vs 3 days, P = .025). For patients infected with MSSA, higher proportions in the intermediate (81%) and prolonged (97%) groups received definitive therapy of antistaphylococcal β-lactam compared with the short group (61%) (P < .0001). The proportion of patients with MSSA bacteremia experiencing 3 or more days of bacteremia was similar regardless of whether patients were initiated on vancomycin and then de-escalated to an antistaphylococcal β-lactam (100/309, 32%) or were on an antistaphylococcal β-lactam on the first day of bacteremia (14/35, 40%) (P = .45) (Supplementary Material).
Among patients infected with MRSA, empiric therapy was initiated with vancomycin (86%), linezolid (5%), daptomycin (4%), or other (5%); the choice of agent was similar among the 3 SAB duration groups (P = .67) (Supplementary Material). As the duration of bacteremia increased, patients were more likely to be receiving daptomycin therapy as definitive therapy (prolonged vs intermediate vs short: 52% vs 36% vs 24%; P < .0001), whereas the short group remained on vancomycin therapy (prolonged vs intermediate vs short: 26% vs 56% vs 63%; P < .0001) (data not shown). Time to change from empiric therapy to daptomycin was the longest at a median of 4 days (IQR: 3, 7 days) in the prolonged group compared with 3 days in the intermediate (IQR: 1, 4 days) and short (IQR: 1, 3 days) groups (P = .0017). Excluding patients in the short group, MRSA patients who were initiated on vancomycin empirically (n = 106) but switched to daptomycin within 72 hours following SAB onset (n = 31) had a similar duration of bacteremia (median: 6 days; IQR: 3, 8 days) compared with those who remained on vancomycin (n = 67) (median: 5 days; IQR: 4, 8 days; P = .98). The prolonged group was more likely to receive combination regimens as definitive therapy (prolonged vs intermediate vs short: 57% vs 34% vs 16%; P < .0001).
Clinical Outcomes
The primary (30-day mortality) and secondary (LOS and metastatic complications) outcomes were progressively worse as bacteremia duration increased (Table 3). The highest proportion of the patients who died had sources of endocarditis (20%) and pneumonia (18%). When bacteremia duration was considered as a continuous variable, each additional day of bacteremia increased mortality risk by 16% compared with those with 1 day of bacteremia (RR, 1.16; 95% CI, 1.10–1.22; P < .001) (Table 4, Figure 1). The sensitivity analysis of only patients with blood cultures drawn on consecutive days (n = 338) showed a higher risk of 20% per day of positive blood culture (RR, 1.20; 95% CI, 1.12–1.27; P < .001) (Supplementary Material).
Table 3.
Clinical Outcomes
| Duration of Bacteremia | P Value | |||
|---|---|---|---|---|
| Short (1–2 d) (n = 555) | Intermediate (3–6 d) (n = 250) | Prolonged (≥7 d) (n = 79) | ||
| Metastatic complication | 69 (12) | 58 (23) | 26 (33) | <.0001 |
| 30-day mortality | 29 (5) | 28 (11) | 17 (22) | <.0001 |
| Hospital length of stay for survivors (median, IQR), d | 9 (6, 18) | 12 (8, 21) | 24 (14.75, 37.5) | <.0001 |
| Disposition | <.0001 | |||
| Home | 328/548 (60) | 130/249 (52) | 28 (35) | |
| Skilled nursing facility | 100/548 (18) | 43/249 (17) | 11 (14) | |
| Outside hospital | 37/548 (7) | 20/249 (8) | 8 (10) | |
| Rehab center | 28/548 (5) | 9/249 (4) | 10 (13) | |
| Homeless/other | 24/548 (4) | 19/249 (8) | 4 (5) | |
| Died | 31/548 (6) | 28/249 (11) | 18 (23) |
Data are no. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
Table 4.
Relative Risk of 30-Day Mortality by Duration of Bacteremia
| No. of Days of Bacteremia | Total N | Mortality, % | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| 1 | 446 | 4.5 | Reference | Reference |
| 2 | 108 | 8.3 | 1.86 (0.87–3.97) | .11 |
| 3 | 98 | 9.2 | 2.05 (0.96–4.36) | .06 |
| 4 | 74 | 12.2 | 2.71 (1.28–5.73) | .01 |
| 5 | 46 | 8.7 | 1.94 (0.69–5.43) | .21 |
| 6 | 33 | 18.2 | 4.05 (1.75–9.40) | .001 |
| 7 | 28 | 21.4 | 4.78 (2.09–10.94) | <.001 |
| 8–10 | 30 | 20.0 | 4.46 (1.94–10.27) | <.001 |
| 11+ | 21 | 23.8 | 5.31 (2.21–12.76) | <.001 |
| Per day | … | … | 1.16 (1.10–1.22) | <.001 |
N = 884. The numbers of days of infection at 8–10 and 11+ were collapsed to account for the observed sample sizes.
Abbreviation: CI, confidence interval.
Figure 1.
Relative risk (95% confidence interval) of mortality by duration of bacteremia (N = 884). The numbers of days of infection at 8–10 and 11+ were collapsed to account for the observed sample sizes.
Of the patients who received a source-control procedure (n = 398), a delay of 48 hours or more in obtaining source control from SAB onset was associated with prolonged LOS (median [IQR]: 14 [8, 24] days vs 9 [6, 19] days; P < .0001) and higher 30-day mortality (10% vs 4%, P = .052) compared with those with an early source-control procedure.
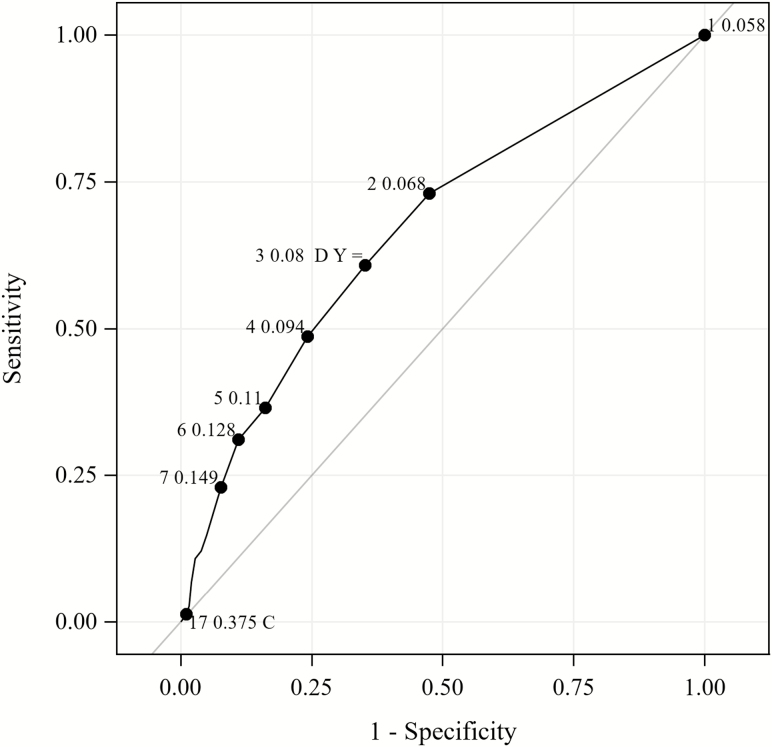
Three days of bacteremia were found to best distinguish patients who died versus those who survived (J = 0.26) by ROC analysis and Youden index (Figure 2). By multivariable logistic regression analysis, after controlling for age and gender, 2 independent predictors for 30-day mortality were as follows: duration of bacteremia of 3 days or more (adjusted odds ratio, 1.17; 95% CI, 1.06–1.29; P = .002) and Pitt bacteremia score (adjusted odds ratio, 1.48; 95% CI, 1.33–1.65; P < .001). The final prediction model showed acceptable goodness-of-fit with the Hosmer-Lemeshow test (χ2-statistic = 8.00, P = .43).
Figure 2.
ROC curve to determine the cutoff to differentiate 30-day mortality versus survivors. The ROC curve with corresponding labels for the number of days of infection for several optimality criteria plotted for 30-day mortality. The cut-point at 3 days was found to minimize the absolute distance between sensitivity and specificity (diff = 0.04) and the distance to the (0,1), or “ideal” point (D = 0.53). Label C is the cut-point with the maximum correct classification rate. Label Y is the cut-point with the maximum Youden index. Abbreviation: ROC, receiver operating characteristics.
DISCUSSION
In this prospective, observational study of a large cohort of 884 patients with SAB, longer durations of bacteremia were associated with worse clinical outcomes; prompt achievement of source control was strongly associated with earlier bacterial clearance and better clinical outcomes. Surprisingly, in contrast, choice of antistaphylococcal therapy did not affect the duration of bacteremia or outcome, as long as the agent demonstrated in vitro sensitivity against the infecting strain.
Prior studies have defined persistence of bacteremia a priori as 3 days or longer [6, 7], more than 5 days [9], or 7 days or more [3, 5, 10–15]. We were interested in characterizing the risk of mortality with every continued day of bacteremia, as well as determining a time point predictive of outcome that may be used to evaluate treatment efficacy across studies.
The overall median duration of bacteremia observed in our study was relatively short, with 1 day for MSSA and 2 days for MRSA SAB. The incidence of duration of 3–6 days of bacteremia was 28% and for durations of 7 days or more was 9%, which is similar to previously reported incidence of 8–38% for 3 or more days [6, 7], 4–20% for more than 5 days [9, 18, 19], and 8–16% for 7 days or more [3, 10, 13–15]. We have shown that bacteremia caused by MRSA is significantly prolonged compared with MSSA, which is similar to prior studies [7, 20]. Fowler et al [20] found the median time to clearance to be longer with MRSA than MSSA bacteremia (8–9 days vs 3–4 days, respectively), although their study included a much higher proportion of patients with endocarditis (77%) compared with ours (11%), which likely explains the differences in duration. In our patients with endocarditis, the duration of bacteremia was 3 days longer than in patients without endocarditis, and the median duration was 4 and 5 days for MSSA and MRSA endocarditis, respectively. Because more patients with endocarditis had persistence for 3 days or more compared with those with early resolution, persistence for 3 days or more can be used as an additional early indication for obtaining ID consultation.
Although definitions of persistence have varied across prior studies, risk factors found to be associated with prolonged bacteremia, include the following: diabetes [7], heart failure [14], chronic renal failure [3], septic shock [9], community-onset bacteremia [10], intravascular catheter or hardware [3, 7, 10, 11, 21], osteoarticular infection [10], metastatic infection [3, 7, 10, 11, 14], endocarditis [3, 7, 9], MRSA [3, 10, 22], vancomycin MIC of 2 μg/mL [9, 11], and vancomycin treatment [7].
It has long been debated whether an elevated vancomycin MIC (defined as either ≥1.5 or ≥2 μg/mL) contributes to worse outcomes [9, 11]. Because most of our MRSA isolates (67%) had Etest MICs ≥1.5 μg/mL, vancomycin MIC was not found to significantly influence the duration of bacteremia. Although use of vancomycin over cloxacillin for MSSA bacteremia was attributed to persistence (>3 days) in a prior study [6], treatment with vancomycin was unlikely to have significantly contributed to persistence in our MSSA cohort because only 18% in the intermediate group and 6% of the prolonged group remained on vancomycin past 72 hours from SAB onset. The comparative effectiveness of different anti-MRSA regimens was beyond the scope of this observational study. Nonetheless, anti-MRSA therapy either at onset or at 72 hours was not a significant determinant in the duration of bacteremia.
Receipt of ID consultation is known to significantly improve clinical outcome of SAB [23]. Most (89%) patients with prolonged bacteremia received ID consultation but this was significantly delayed compared with the other groups. Thus, obtaining an ID consult early during SAB appears to be a target area for improvement in clinical practice. In addition, although the proportion of patients receiving a source-control procedure was similar among the 3 groups, patients in the short group underwent their procedure significantly earlier than the persistent groups by 2 days. This is similar to findings by Chong et al [10], where a delay of more than 3 days in source-control procedure was associated with persistence. Early source control continues to be an important cornerstone in SAB management to promote bacterial clearance.
Persistent bacteremia has been shown to significantly increase the risk of morbidity and/or mortality [3, 7, 9–11, 18]. We evaluated the duration of persistence as a continuous variable and found the risk of mortality significantly increased by 16% per day of positive blood cultures. This confirms findings by Rose et al [16] of 15.9% in a limited sample of 59 SAB patients. In our sensitivity analysis of 338 patients with blood cultures drawn on consecutive days, we found an even greater risk of an increase of 20% per day. These findings underscore the urgency to intensify management efforts aimed at sterilizing blood cultures as soon as possible rather than awaiting results from subsequent blood cultures. These efforts include surgical interventions for foci eradication and prescribing rapidly bactericidal antibiotics.
By ROC analysis, we found 3 days to be the most significant duration to differentiate survival versus death. In support of our findings, Khatib et al [13] observed that any complication (metastatic foci, relapse, or attributable mortality) (P = .05) was significantly more common for patients with 3 or more days of bacteremia. Rose et al [16] found significant mortality differences with bacterial persistence of 4.5 days by Classification and Regression Tree analysis, although only 10 of 59 total patients had more than 4 days of bacteremia. The 2011 IDSA guidelines for treatment of MRSA currently note that persistence of 7 or more days should prompt an assessment to change therapy after considering clinical response and attainment of source control [5]. However, results from our large cohort of 884 patients support a much earlier time point of 3 days for assessing the need to alter management approach. Because rapid diagnostic technology has significantly shortened time to SAB diagnosis [24, 25], early optimization of management may be achieved as soon as 3 days to effect positive outcomes. In settings where SAB diagnosis may not be feasible within 3 days, early optimization of management should be made as soon as possible.
This study has several limitations worth noting. Due to the observational design, we could not control for confounding factors of antibiotic selection and how quickly optimal therapy was initiated, although these factors did not differ between the 3 groups. Also, obtaining repeat blood cultures was at the discretion of the treating physician; thus, some patients did not have daily repeat blood cultures drawn. This may have affected the bacteremia duration; however, obtaining repeat blood cultures is often driven by suboptimal clinical response in practice, thus categorizing patients without repeat blood cultures into the “short” group was likely appropriately placed. Recognizing this limitation, we conducted a sensitivity analysis of patients who had blood cultures drawn on consecutive days to allow calculation of definitive durations of bacteremia. Results from this subgroup yielded a similar ROC breakpoint of 3 days to best differentiate survival versus death.
Conclusions
Duration of persistence adversely affects outcomes of SAB, with higher rates of mortality, metastatic complications, and prolonged hospitalization. Because each day of positive blood cultures increases mortality risk by 16%, early intervention for foci removal, optimization of therapy, and ID consultation should be undertaken. Persistent SAB can be defined as positive cultures for 3 or more days, at which mortality significantly increased. Our findings warrant future prospective interventional studies to evaluate whether early recognition of persistence at 3 days to prompt a change in management could improve outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Anne Au and the laboratory personnel at Huntington Hospital, Los Angeles County–University of Southern California (USC) Medical Center, and Keck Hospital of USC for assistance with laboratory specimen collection; and Karen Tan, Daniel Salas, Nicole Pepe, Stephanie Mac, and Vanessa Delayo for assisting with data collection.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Part of this work was supported by the National Center for Advancing Translational Science of the US National Institutes of Health (grant numbers UL1TR001855 and UL1TR000130).
Potential conflicts of interest. In the last 12 months, B. S. has received consulting fees from Alexion, Synthetic Biologics, Paratek Pharmaceuticals, TheoremDx, and Acurx Pharmaceuticals and is a shareholder for Motif, BioAIM, Synthetic Biologics, Mycomed Technologies, and ExBaq. A. W.-B. has received grants from Merck and Allergan and consulting fees from Nabriva Therapeutics, Insmed, Rempex Pharmaceuticals, Paratek Pharmaceuticals, Achaogen, Inc, Bayer Healthcare, SIGA Technologies, and GlaxoSmithKline. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chua T, Moore CL, Perri MB, et al. Molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J Clin Microbiol 2008; 46:2345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 2007; 167:1861–7. [DOI] [PubMed] [Google Scholar]
- 4. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 6. Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand J Infect Dis 2005; 37:572–8. [DOI] [PubMed] [Google Scholar]
- 7. Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis 2006; 38:7–14. [DOI] [PubMed] [Google Scholar]
- 8. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 2016; 44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis 2010; 67:228–33. [DOI] [PubMed] [Google Scholar]
- 10. Chong YP, Park SJ, Kim HS, et al. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine (Baltimore) 2013; 92:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother 2010; 65:1015–8. [DOI] [PubMed] [Google Scholar]
- 12. Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006; 50:3039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis 2009; 41:4–9. [DOI] [PubMed] [Google Scholar]
- 14. Lin SH, Liao WH, Lai CC, et al. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J Antimicrob Chemother 2010; 65:1792–8. [DOI] [PubMed] [Google Scholar]
- 15. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 2015; 350:h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200. [DOI] [PubMed] [Google Scholar]
- 18. Liao CH, Chen SY, Huang YT, Hsueh PR. Outcome of patients with meticillin-resistant Staphylococcus aureus bacteraemia at an emergency department of a medical centre in Taiwan. Int J Antimicrob Agents 2008; 32:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah MD, Wardlow LC, Stevenson KB, Coe KE, Reed EE. Clinical outcomes with penicillin versus alternative β-lactams in the treatment of penicillin-susceptible Staphylococcus aureus bacteremia. Pharmacotherapy 2018. doi: 10.1002/phar.2124. [DOI] [PubMed] [Google Scholar]
- 20. Fowler VG Jr, Boucher HW, Corey GR, et al. ; S. aureus Endocarditis and Bacteremia Study Group Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–65. [DOI] [PubMed] [Google Scholar]
- 21. Ok HS, Lee HS, Park MJ, et al. Predictors and clinical outcomes of persistent methicillin-resistant Staphylococcus aureus bacteremia: a prospective observational study. Korean J Intern Med 2013; 28:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fowler VG Jr, Miro JM, Hoen B, et al. ; ICE Investigators Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–21. [DOI] [PubMed] [Google Scholar]
- 23. Pragman AA, Kuskowski MA, Abraham JM, Filice GA. Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim Md) 2012; 20:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Box MJ, Sullivan EL, Ortwine KN, et al. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 2015; 35:269–76. [DOI] [PubMed] [Google Scholar]
- 25. Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis 2016; 84:159–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.