Abstract
We recently showed in a proof-of-concept study that real-time modeling-based response-guided therapy can shorten hepatitis C virus treatment duration with sofosbuvir-velpatasvir, elbasvir-grazoprevir, and sofosbuvir-ledipasvir without compromising efficacy, confirming our retrospective modeling reports in >200 patients. However, retrospective modeling of pibrentasvir-glecaprevir (P/G) treatment has yet to be evaluated. In the current study, modeling hepatitis C virus kinetics in 44 cirrhotic and noncirrhotic patients predicts that P/G treatment might have been reduced to 4, 6, and 7 weeks in 16%, 34%, and 14% of patients, respectively. These results support the further evaluation of a modeling-based response-guided therapy approach using P/G.
Keywords: hepatitis C virus, direct-acting antivirals, mathematical modeling, response-guided therapy
Retrospective mathematical modeling of early hepatitis C virus kinetics in 44 cirrhotic and noncirrhotic patients predicts that pibrentasvir-glecaprevir treatment might have been reduced to 4, 6, and 7 weeks in 16%, 34%, and 14% of patients, respectively.
An estimated 71 million people are chronically infected with hepatitis C virus (HCV) worldwide [1] and are at risk of developing cirrhosis and/or hepatocellular carcinoma, which can ultimately lead to death if left untreated [2]. Data have shown that direct-acting antivirals (DAAs) for the treatment of HCV can achieve cure rates exceeding 90% after 8–12 weeks of treatment in all patient populations [3]. However, high costs of these medications has limited access to treatment and has placed substantial financial burdens on insurers and national healthcare systems [4].
We have shown in retrospective analyses that viral kinetic modeling might allow for a reduction in the duration of DAA therapy [5–8]. We subsequently demonstrated in a prospective proof-of-concept study (NCT03603327) that real-time modeling-based response guided-therapy (RGT) can shorten treatment duration with sofosbuvir-velpatasvir, elbasvir-grazoprevir, and sofosbuvir-ledipasvir without compromising efficacy (relapse occurred after treatment in only a single noncirrhotic male patient with genotype 3, who was treated with sofosbuvir-velpatasvir for 6 weeks) or patient safety [9]. However, retrospective viral kinetic analysis of pibrentasvir-glecaprevir (P/G) had not been performed, and our prospective study included only 4 patients treated with P/G.
The recent Food and Drug Administration approval of P/G for 8 weeks in treatment-naive compensated cirrhotic as well as noncirrhotic patients with genotype 1–6 infection [10] raises the question whether the duration of P/G therapy can be reduced. The aim of the current study was therefore to retrospectively examine whether mathematical modeling of viral kinetics could be used to guide the duration of P/G therapy in compensated cirrhotic and noncirrhotic patients with chronic HCV infection.
METHODS
Patients
From December 2017 to June 2018, 58 consecutive patients with a diagnosis of chronic HCV infection based on a positive test for HCV RNA (GT3 [n = 2], GT2 [n = 26], GT1 [n = 30]) for >6 months were treated with P/G combination therapy. Blood samples were obtained for HCV RNA measurement at days 0, 1, 7, 14, and every 2 weeks during treatment. Patients who had a diagnosis of cirrhosis based on a Fibrosis-4 index >3.25 [11], ultrasonographic features of cirrhosis, or clinical evidence of portal hypertension were treated with P/G combination therapy for 12 weeks, while patients without cirrhosis were treated for 8 weeks. Patients with decompensated cirrhosis or who were positive for hepatitis B virus or human immunodeficiency virus were not included in this study. All patients provided written informed consent. The study was approved by the ethical committee of Hiroshima University (approval no. E-109).
HCV RNA Measurements
HCV RNA levels were measured at days 0, 1, 7, and 14, every 2 weeks during treatment, and at posttreatment weeks 12 and/or 24, using the COBAS TaqMan assay (v2.0).
Mathematical Modeling
HCV kinetics during therapy was assumed to follow the standard biphasic model [6], as in the following differential equations:
dI/dt = βT0V – δI
and
dV/dt = (1 – ε)pI – cV,
where T0 represents the number of target cells (ie, hepatocytes); I, the number of infected cells; and V, the viral load in blood. Virus, V, infects target cells with rate constant β, generating productively infected cells, I, which produce new virions at rate p per infected cell. Infected cells are lost at a rate δ per infected cell and virions are assumed to be cleared from blood at rate c per virion. Similar to our previous real-time modeling-based RGT approach [12], we assumed that the target cell level remained constant during therapy at pretreatment level T0 = cδ/βp. The DAA effect ε is defined as therapy effectiveness 0 ≤ ε ≤ 1 in preventing viral production and/or secretion.
Parameter Estimations
Viral host parameter estimates were obtained using a constrained optimization by linear approximation (COBYLA) algorithm [13]. The initial viral load was set during fitting based on each patient’s measured pretreatment HCV RNA.
Cure Boundaries
The time to cure was defined as the time to reach <1 HCV particle in the entire extracellular body fluid adjusted to body weight. A value of 1 virus copy in 15 L of extracellular body fluid volume (7 × 10ˉ 5) for V (in international units per milliliter) was used as the threshold for cure. A sensitivity analysis was performed assuming 5–20 L of extracellular body fluid volume corresponding to cure threshold values of 2 × 10ˉ 4 and 5 × 10ˉ 5 IU/mL, respectively.
Statistical Analysis
Associations between patients’ baseline characteristics or prior treatment experience and their viral kinetics, fitted model parameters, and predicted cure times were evaluated with nonparametric tests. Fisher exact tests were used for checking associations between categorical variables, and Wilcoxon rank sum tests were used for continuous variables. For all analyses, differences were considered statistically significant at P ≤ .05. Data analyses were performed using R 3.5.0 software.
RESULTS
Baseline Characteristics
The mean patient age (standard deviation [SD]) was 66 (16) years, and the mean body mass index (SD; calculated as weight in kilograms divided by height in meters squared), 23.6 (3.6); 29 patients (50%) were male. Twenty-one patients were treatment-experienced with interferon (n = 11), DAA (n = 5), or both interferon and DAA (n = 5). Baseline levels for HCV RNA ranged from 3.83 to 7.83 log IU/mL, with a trend toward higher levels among treatment-experienced patients (P = .07). Cirrhosis was also more common among treatment-experienced patients than among treatment-naive patients (67% vs 27%, respectively; P < .01). In total, 25 patients (43%) had cirrhosis (Table 1). Age, body mass index, and baseline levels of the virus did not differ significantly between cirrhotic and noncirrhotic patients (P > .11).
Table 1.
Baseline Characteristics
| Patient Characteristics | Full Cohort (n = 58) | Treatment-Naive Patients (n = 34) | Treatment-Experienced Patients (n = 24) |
|---|---|---|---|
| Age, mean (SD) | 65.97 (15.79) | 63.38 (18.41) | 69.62 (10.38) |
| Weight, mean (SD), kg | 59.42 (12.80) | 56.87 (13.13) | 63.04 (11.62) |
| Male sex, no. (%) | 29 (50) | 13 (38) | 16 (67) |
| Cirrhosis, no. (%)a | 25 (43) | 9 (26) | 16 (67) |
| IL28B genotype, no. (%) | |||
| GG | 3 (6) | 2 (7) | 1 (4) |
| TG | 12 (22) | 5 (17) | 7 (29) |
| TT | 35 (65) | 19 (63) | 16 (67) |
| Unknown | 8 (14) | 8 (24) | 0 (0) |
| Baseline HCV load, mean (SD), log IU/mL | 6.50 (0.87) | 6.28 (1.00) | 6.81 (0.52) |
| HCV genotype, no. (%) | |||
| 1B | 29 (50) | 15 (44) | 14 (58) |
| 1B and 2B | 1 (2) | 1 (3) | 0 (0) |
| 2B | 11 (19) | 6 (18) | 5 (21) |
| 2A | 15 (26) | 11 (32) | 4 (17) |
| 3A | 2 (3) | 1 (3) | 1 (4) |
Abbreviations HCV, hepatitis C virus; SD; standard deviation.
aCirrhosis is more common among treatment-experienced than among treatment-naive patients (P < .01).
Viral Kinetics and Sustained Virological Response Rates
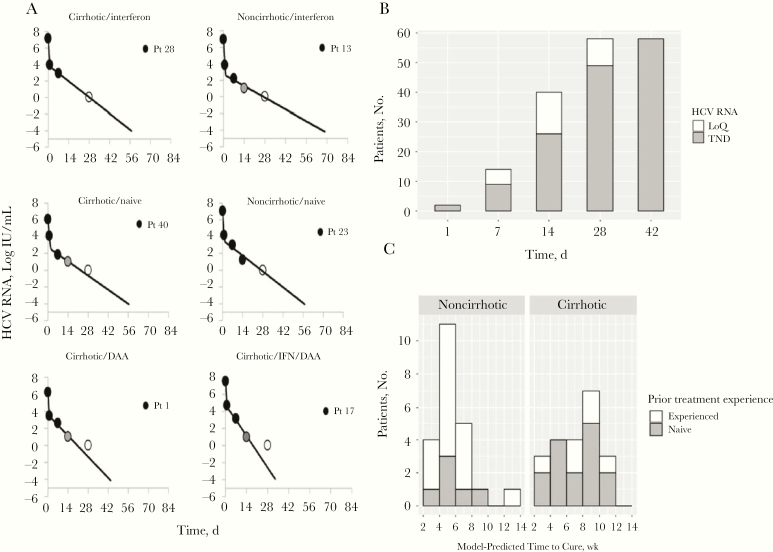
Sustained virological response (SVR) was achieved in 56 patients (97%), 1 patient relapsed, and 1 patient completed therapy but was lost to follow-up. The typical viral decline was biphasic (Figure 1A). The viral load was below the limit of quantification (<15 log IU/mL) for 14 patients after 1 week, 40 after 2 weeks, and all 58 after 4 weeks (Figure 1B). The viral load was not detected in 2 patients after day 1, in 9 after week 1, in 26 after week 2, in 49 after week 4, and in all patients after week 6 (Figure 1B). Notably, the mean time until viral load was undetected (22–23 days) did not differ between treatment-naive and treatment-experienced patients (Supplementary Figure 1).
Figure 1.
Viral kinetics and modeling results. A, Observed viral kinetics and model predicted curves in 6 representative patients. Black circles represent quantifiable hepatitis C virus (HCV); gray circles; lower level of quantification; open circles, observed HCV load below the limit of detection; solid lines, biphasic model best-fit curve, ending when cure threshold is reached. Abbreviations: DAA, direct-acting antiviral experienced: naive, treatment naive: interferon/DAA, interferon and DAA experienced; and Pt, patient. B, Time to reach HCV below the limit of quantification (LoQ; <16 IU/mL) or target not detected (TND) among all 58 patients. C, Projected treatment duration to reach <1 viral copy in entire extracellular fluid. Noncirrhotic patients were treated for 8 weeks, and cirrhotic patients for 12 weeks; a single noncirrhotic patient who was treated for only 3 weeks is excluded.
Viral Kinetic Parameter Estimation
Sufficient viral kinetic sampling data was available for modeling in 44 of the 58 patients (11 interferon experienced, 5 interferon/DAA experienced, 5 DAA experienced, and 23 treatment naive). Of the 44 modeled patients, 1 patient discontinued therapy after 3 weeks, 23 noncirrhotic patients were treated for 8 weeks, and 20 cirrhotic patients were treated for 12 weeks. The biphasic model described the data well (Figure 1A and Supplementary Figure 2). Parameters were estimated for each patient (Supplementary Table 1), and averages of the individual fits were also computed. The mean treatment efficacy in blocking viral infection was ε = 0.998 (standard error [SE], 0.0060). The mean (SE) estimated serum virus clearance rate c was 7.60/d (0.46/d), corresponding to a mean (SE) HCV half-life of 2.4 (SE, 0.095) hours. Across all patients, the mean (SE) death/loss rate of infected cells δ was estimated at 0.45/d (0.030/d), corresponding to a mean (SE) infected cell half-life of 1.87 (0.13) days. Notably, however, the estimates for this parameter differed significantly between cirrhotic (δ = 0.383/d) and noncirrhotic (δ = 0.507/d) patients, giving rise to a longer predicted infected cell half-life for patients in the cirrhotic group (P = .03) (Supplementary Table 2).
Predicting Time to Cure
Using the individual model fits, we calculated the time for each patient to achieve cure. The predicted time to cure ranged from 17 to 96 days of treatment, with a mean (SD) of 6.5 (2.7) weeks (Figure 1C). The predicted time to cure was shorter for noncirrhotic than for cirrhotic patients (mean [SD], 40 [18] vs 52 [18] days, respectively; P = .02) (Supplementary Table 2) with an average 15-day reduction (95% confidence interval, 7–23 days) for noncirrhotic patients treated for 8 weeks and a 32-day reduction (24–40 days) for cirrhotic patients treated for 12 weeks. To be conservative, we stratified the duration of therapy needed to achieve virus eradication based on model predictions as follows: patients with predicted viral eradication in <4 weeks could be assigned to 4 weeks of therapy; those with predicted eradication in 6–8 weeks, to 8 weeks of therapy; those with predicted eradication in 8–10 weeks, to 10 weeks of therapy; and those with predicted eradication in 10–2 weeks, to 12 weeks of therapy.
As such, among the group of noncirrhotic patients, the model predicts that cure would be achieved in 4 patients after 4 weeks of therapy, in 12 after 6 weeks, and in 5 after the full 8 weeks (Figure 1C). For those with cirrhosis, the modeling suggests that cure would be achieved in 3 patients after 4 weeks of therapy, in 4 after 6 weeks, in 4 after 8 weeks, in 7 after 10 weeks, and 3 after the full 12 weeks (Figure 1C). Overall, the model predicts that P/G treatment duration might be reduced to <7 weeks in 64% of the 44 patients (to 4 weeks in 16%, to 6 weeks in 34%, and to 7 weeks in 14%; Supplementary Table 1).
In 4 patients (patients 10, 13, 23, and 50; Supplementary Table 1), the model suggested a treatment duration of 2–40 days longer than the treatment actually received. In cirrhotic patient 10 and noncirrhotic patient 23, the predicted treatment duration was only 2 days longer than required, and the length of treatment fell within the sensitivity range of the model’s predictions. For patients 13 and 50, both noncirrhotic patients treated for 8 weeks, the model overestimated the required length of therapy by 12 and 40 days, respectively. The model predicted a cure time of 30 days of therapy for the single patient who relapsed, likely owing to the development of resistance to pibrentasvir, as our group recently reported [14]. There was no association between predicted time to cure and IL28B genotypes.
DISCUSSION
In a recent study of the efficacy of 8 weeks of P/G therapy in 343 treatment-naive, HCV-infected patients with compensated cirrhosis, a 99.7% SVR rate was achieved [10]. The modeling performed in the current study, which includes both treatment-naive and treatment-experienced cirrhotic patients predicts a cure time of <8 weeks in only about half (11 of 21) of the modeled cirrhotic patients. However, in the majority (67%) of treatment-naive cirrhotic patients (n = 6), the model suggests <8 weeks of P/G therapy (Supplementary Table 1), which is in agreement with the findings by Brown et al [10] and highlights the importance of treatment history when evaluating therapeutic options.
Overall, the current modeling-based RGT approach predicts that 64% of patients (28 of 44) might have been cured with <7 weeks of P/G therapy. The high SVR rate (97%) achieved in this study and by Brown et al [10] suggest that in many patients with chronic HCV infection cure was achieved with <8 weeks of P/G therapy, supporting our modeling predictions. Interestingly, a pilot study of people with recent HCV infection treated with ultrashort P/G therapy (6 weeks) demonstrated a high SVR rate of 96% [15]. A previous kinetic-based RGT study by Lau et al [16]showed that ultrashort (3 weeks) DAA therapy is possible in patients in whom low viral load is reached at day 2. The current study thus underscores the potential usefulness of a personalized modeling-based RGT approach for identifying opportunities to shorten the duration of P/G therapy and indicates that additional prospective studies are warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Japan Agency for Medical Research and Development (grant 19fk0210020h0003) and the National Institutes of Health (grants R01AI078881 and R01GM121600).
Potential conflicts of interest. M. I. received research grant from AbbVie. H. D. has consulted for CoCrystal. K. C. received research funding from AbbVie and Bristol-Myers Squibb and honoraria (lectures fees) from Eizai, AbbVie, Merck Sharp & Dohme, Gilead, Dainippon Sumitomo, Bristol-Myers Squibb, and Mitsubishi Tanabe, Otsuka. None of the other authors has any financial interest or conflict of interest related to this research. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global hepatitis report. Geneva, Switzerland: World Health Organization,2017. https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=7C79007530D4ABFAFFDE9ED91BBF8E2B?sequence=1. Accessed 4 November 2019. [Google Scholar]
- 2. World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva, Switzerland: World Health Organization,2018. https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1. Accessed 4 November 2019. [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69:461–511. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Progress report on access to hepatitis C treatment: focus on overcoming barriers in low- and middle-income countries Geneva, Switzerland: World Health Organization,2018. https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf?sequence=1. Accessed 4 November 2019. [Google Scholar]
- 5. Canini L, Imamura M, Kawakami Y, et al. . HCV kinetic and modeling analyses project shorter durations to cure under combined therapy with daclatasvir and asunaprevir in chronic HCV-infected patients. PLoS One 2017; 12:e0187409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahari H, Canini L, Graw F, et al. . HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol 2016; 64:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gambato M, Canini L, Lens S, et al. . Early HCV viral kinetics under DAAs may optimize duration of therapy in patients with compensated cirrhosis. Liver Int 2019; 39:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandmann L, Manns MP, Maasoumy B. Utility of viral kinetics in HCV therapy—it is not over until it is over? Liver Int 2019; 39:815–7. [DOI] [PubMed] [Google Scholar]
- 9. Etzion O, Dahari H, Yardeni D, et al. . Response‐guided therapy with DAA shortens treatment duration in 50% of HCV treated patients. Hepatology 2018; 68:1468A–9A. [Google Scholar]
- 10. Brown RS Jr, Buti M, Rodrigues L, et al. . Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol 2020; 72:441–9. [DOI] [PubMed] [Google Scholar]
- 11. Vallet-Pichard A, Mallet V, Nalpas B, et al. . FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–6. [DOI] [PubMed] [Google Scholar]
- 12. Dahari H, Shteingart S, Gafanovich I, et al. . Sustained virological response with intravenous silibinin: individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int 2015; 35:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinharz V, Churkin A, Lewkiewicz S, et al. A Parameter Estimation Method for Multiscale Models of Hepatitis C Virus Dynamics. Bull Math Biol 2019; 81:3675–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohya K, Imamura M, Osawa M, et al. . Successful retreatment with 12 weeks of glecaprevir and pibrentasvir for a genotype 2a HCV-infected hemodialysis patient who failed to respond to 8 weeks of prior glecaprevir and pibrentasvir therapy. Clin J Gastroenterol 2020; 13:267–70. [DOI] [PubMed] [Google Scholar]
- 15. Martinello M, Orkin C, Cooke G, et al. . Short duration pan-genotypic therapy with glecaprevir-pibrentasvir for six weeks among people with recent HCV infection. Hepatology doi: 10.1002/hep.31003. [DOI] [Google Scholar]
- 16.Lau G, Benhamou Y, Chen G, et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: a phase 2, open-label, proof-of-concept study. Lancet Gastroenterol Hepatol 2016; 1:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.