Significance
Gentamicin is a potent aminoglycoside antibiotic with significant ototoxic side-effects. Although it is administered as a mixture of five main C-subtypes and <10% impurities, the significance of mixture is unclear, partly because of the difficulty in chemically separating the individual components. We established methods purifying gentamicin C-subtypes, and found C2b to be the least and sisomicin the most ototoxic, with both antimicrobial comparable to the gentamicin mixture. Ototoxicity varies among C-subtypes and impurities and highly correlates with their affinity for cochlear hair cell mechanotransducer channels, representing a mechanism to alleviate aminoglycoside ototoxicity. Finally, reconstituted mixtures consisting of only C-subtypes remained antimicrobial but were less ototoxic. Our study reveals structure–activity relationships that dissociate ototoxicity from antimicrobial actions of aminoglycosides.
Keywords: aminoglycoside, hair cell, mechanotransducer channel, ototoxicity, gentamicin
Abstract
Gentamicin is a potent broad-spectrum aminoglycoside antibiotic whose use is hampered by ototoxic side-effects. Hospital gentamicin is a mixture of five gentamicin C-subtypes and several impurities of various ranges of nonexact concentrations. We developed a purification strategy enabling assaying of individual C-subtypes and impurities for ototoxicity and antimicrobial activity. We found that C-subtypes displayed broad and potent in vitro antimicrobial activities comparable to the hospital gentamicin mixture. In contrast, they showed different degrees of ototoxicity in cochlear explants, with gentamicin C2b being the least and gentamicin C2 the most ototoxic. Structure–activity relationships identified sites in the C4′-C6′ region on ring I that reduced ototoxicity while preserving antimicrobial activity, thus identifying targets for future drug design and mechanisms for hair cell toxicity. Structure–activity relationship data suggested and electrophysiological data showed that the C-subtypes both bind and permeate the hair cell mechanotransducer channel, with the stronger the binding the less ototoxic the compound. Finally, both individual and reformulated mixtures of C-subtypes demonstrated decreased ototoxicity while maintaining antimicrobial activity, thereby serving as a proof-of-concept of drug reformulation to minimizing ototoxicity of gentamicin in patients.
Gentamicin is an aminoglycoside antibiotic with potent activity against a broad spectrum of gram-positive and gram-negative bacteria. Gentamicin is used to treat patients with sino-pulmonary infections, particularly those with underlying cystic fibrosis, complicated urinary tract infections, and also as an empiric agent in neonates with signs of systemic infection (1–3). However, its usage is severely restricted by its reversible nephrotoxic and irreversible ototoxic side-effects (4, 5). As the use of gentamicin is expected to increase with the rising incidence of antibiotic resistance, there is a pressing need to alleviate its side-effects (6, 7).
The Micromonospora species of bacteria produce gentamicin as a mixture, consisting of five structurally distinct C-subtypes (C1, C1a, C2, C2a, C2b) (Fig. 1 A and B) (8). In the United States, the gentamicin mixture used in hospitals (hospital gentamicin) consists of 10 to 35% C1a, 25 to 50% C1 and C2b, 25 to 55% C2 and C2a, and up to 10% impurities with no individual impurity, consisting of more than 3% (9). In Europe, the approved mixture is comprised of 10 to 30% C1a, 25 to 45% C1, and 35 to 55% as the sum of C2, C2a, and C2b (10). The clinical significance of the mixture is unclear, and it is unknown whether the breadth of antimicrobial activity stems from multiple components. In other words, whether each component operates across a narrower range of bacterial strains or species than the mixture as a whole is unknown. For example, C1 is reported to be equally effective as hospital gentamicin in treating urinary tract infections, while causing less nephrotoxicity (11). In terms of ototoxicity, C2 is more toxic than C1a and C1; however, toxicity of other subtypes is less clear (12, 13). Thus, we currently lack a comprehensive understanding of the ototoxicity and antimicrobial activity of each gentamicin component, including the potential contribution of the impurities. These knowledge gaps hinder the design of new drugs or formulations related to gentamicin. Many previous studies have relied on commercially available gentamicin components or purified compounds that were incompletely validated (12–15). For example, gentamicin B1 has been misidentified by the manufacturer to be the impurity geneticin (G418). To date, a major obstacle is the lack of reliable methods for isolating individual gentamicin components.
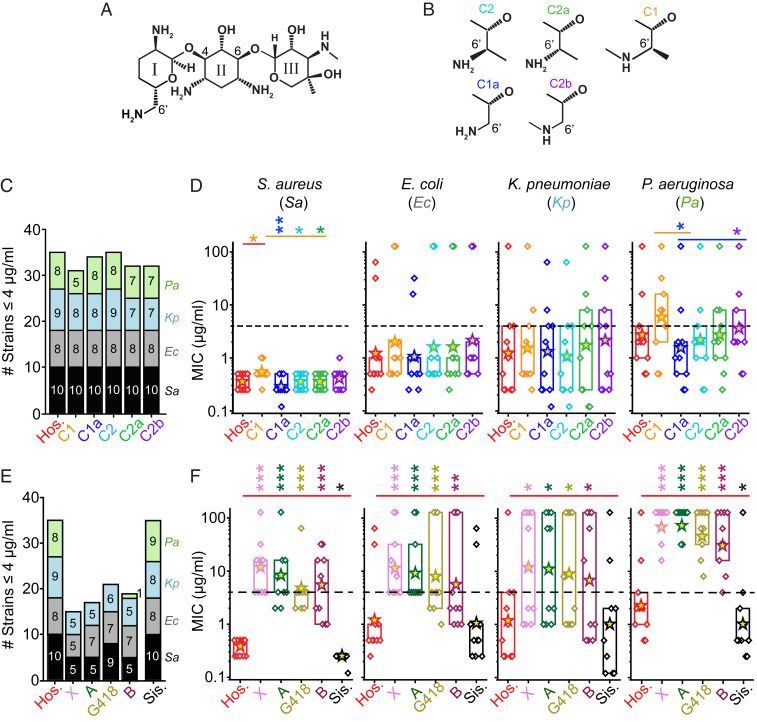
Fig. 1.
Antimicrobial breadths and potencies of hospital gentamicin and its components. (A) Gentamicin C-subtypes are three-ringed structures, containing a central 2-deoxystreptamine with an ammonium group on either side of the deoxy carbon and added at position 4 to ring I, and at position 6 to ring III. (B) The main components of gentamicin differ at the 5′ and 6′ position on ring I. These are termed gentamicin C2 (cyan), C2a (green), C1 (orange), C1a (blue), and C2b (purple); colors used throughout. (C) The antimicrobial breadths of the C-subtypes relative to hospital gentamicin (Hos.), where breadth is defined as the number of strains inhibited by the drug at MIC value ≤ 4 μg/mL. The colors represent different species, the numbers in the columns refer to the number of individual strains inhibited, where black = S. aureus (Sa), gray = E. coli (Ec), blue = K. pneumoniae (Kp), green = P. aeruginosa (Pa); colors used throughout. Hospital gentamicin inhibits 35 of 40 strains tested, with the C-components inhibiting 31 to 35 strains. (D) The MIC values for all strains (diamond symbols) split by species (10 each). The box indicates the 25 to 75 percentiles, asterisks indicate geometric means, dashed line indicates the 4 μg/mL cutoff for susceptibility (*P < 0.05, **P < 0.01 Mann–Whitney U test). Gentamicin C1 is less potent than hospital gentamicin against S. aureus strains only (P < 0.05, Mann–Whitney U test, n = 10). There are also some differences between the C-subtypes against S. aureus and P. aeruginosa strains. (E) The antimicrobial breadths of the impurities relative to hospital gentamicin. Hospital gentamicin inhibits 35 of 40 strains tested, with the impurities gentamicin X, A, B, and G418 inhibiting 15 to 22 strains. The impurity sisomicin inhibited 35 of 40 strains. (F) The MIC values for all strains split by species. The impurities gentamicin X, A, B, and G418 are all significantly less potent than hospital gentamicin (P < 0.05, Mann–Whitney U test). The impurity sisomicin was the same as the hospital gentamicin against E. coli and K. pneumoniae (P > 0.05, Mann–Whitney U test), and was more potent than hospital gentamicin against S. aureus and P. aeruginosa (P < 0.05, Mann–Whitney U test). SI Appendix, Figs. S3 and S4 show individual strains for each drug. ***P < 0.001.
In bacteria, aminoglycosides inhibit protein synthesis by directly binding to a conserved region in the 30S ribosomal subunit (16). In the inner ear, ototoxicity arises from the ability of aminoglycosides to permeate mechanotransducer (MET) channels at the tips of sensory hair cells (17, 18). Both blockers of MET channel and mutations causing defective MET channels are protective against aminoglycoside ototoxicity (17–21). Moreover, studies have shown that sisomicin, a gentamicin impurity, permeates the MET channel and that chemical modification of sisomicin diminished permeation through the MET channel, leading to reduced ototoxicity in vitro and in vivo (22). It is currently unknown how the antimicrobial activity and ototoxicity of the individual gentamicin subtypes relate to their ability to permeate the MET channel.
To determine whether specific properties of antimicrobial activity and ototoxicity were ascribed to the individual constituents, we developed a purification approach allowing investigation of each component of hospital gentamicin. We found that the breadth and potency of antimicrobial activity for each of the five C-subtypes was comparable to hospital gentamicin. The C-subtypes displayed differences in ototoxicity in vitro, with C2b being the least and C2 the most ototoxic. Moreover, C-subtypes both acted as permeant blockers and bound the hair cell MET channel, resulting in different degrees of MET channel blockage that correlated with the severity of ototoxicity. Structure–activity relationships identified sites at the C4′-C6′ region of ring I governing ototoxicity with minimal effects on antimicrobial action. Finally, reformulations containing C2b alone or in combination with other C-subtypes reduced ototoxicity while maintaining antimicrobial breadth and potency, providing proof-of-concept that the hospital gentamicin mixture can be reformulated to reduce ototoxicity while retaining antibacterial action.
Results
Purifying Individual Gentamicin C-Subtypes from Mixtures.
Gentamicin is a mixture of three-ringed compounds consisting of five main subtypes and over 20 impurities (Fig. 1A) (23–25). The C-subtypes (C1, C1a, C2, C2a, and C2b) are required to make up ≥90% of hospital gentamicin for clinical application (Fig. 1B) (9). The biosynthetic pathway for the gentamicin C-subtypes is known (SI Appendix, Fig. S1A) and the specific complement of components in any mixture is dictated by the bacteria and the conditions in which they are grown (26–28).
The C-subtypes of gentamicin differ in the C6′ positions on ring I (Fig. 1 A and B). The relatively minor structural differences between the C-subtypes are determined by the presence or absence of methyl groups on N6′ and C6′ and the relative stereochemistry of the C6′-methyl, rendering C-subtype separation technically challenging, particularly between C2, C2a, and C2b. Unlike prior studies using only one starting material, we took a unique approach by examining the natural separation of gentamicin mixtures that occurs simply based on the different strains of bacteria from which the compounds were synthesized. Variation occurs based on different Micromonospora strains and growth conditions employed during manufacturing (SI Appendix, Fig. S1A) (24, 28). We compared the composition of gentamicin from five commercial vendors using high-performance liquid chromatography (HPLC) (SI Appendix, Fig. S1B). The five gentamicin C-subtypes were purified from vendors B and E because these lots had the largest quantities of C1, C1a, C2, C2a, and C2b (see methods for purification details in SI Appendix). The identity of each subtype is confirmed using HPLC (SI Appendix, Fig. S1C) by matching the known NMRs (which indicate the presence or absence of methyl groups) to those we obtained, and the mass spectral fragmentation patterns, which give differing molecular weight according to the presence or absence of methyl groups in ring I (SI Appendix, Fig. S2 and Table S2).
Gentamicin C-Subtypes Have Similar Antimicrobial Activity to Hospital Gentamicin.
The antimicrobial activity of the C-subtypes was compared to a single bottle of hospital gentamicin (Fresenius Kabi, Cat #401896G) purchased from Stanford Hospital. This bottle is referred to as “hospital gentamicin” hereon. Using a microbroth dilution assay, 10 different strains each from 4 different species of bacteria—Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus—were tested by the Clinical Microbiology Institute (Oregon, United States). The breadth of coverage, measured as the number of strains with minimum inhibitory concentration (MIC) ≤ 4 μg/mL, and the potency (the MIC value for each bacterial strain) were compared (29). The ≤4 μg/mL value is the recommended cutoff for clinical susceptibility (29).
Each C-subtype had similar breadth to hospital gentamicin, with the hospital gentamicin inhibiting growth of 35 of 40 strains and each C-subtype inhibiting growth in 31 to 35 strains (Fig. 1C). The strains inhibited are similar across C-subtypes (SI Appendix, Fig. S3). Thus, the mixture of compounds found in the hospital gentamicin is not necessary for its broad-spectrum activity. These data suggest that a single C-subtype might be used therapeutically or as the starting material for new antibiotic development.
We next examined antimicrobial potency by assessing MIC values and found that most C-subtypes (C1a, C2, C2a, and C2b) were not significantly different from hospital gentamicin across all four species tested (Fig. 1D). Gentamicin C1 was comparable to hospital gentamicin against E. coli, K. pneumoniae, and P. aeruginosa, but showed slightly higher MICs against S. aureus strains (P < 0.05, Mann–Whitney U test, n = 10 strains). Small differences were also observed between the C-subtypes, with C1a showing lower MIC values than C1 against S. aureus and P. aeruginosa strains (P < 0.05, Mann–Whitney U test, n ≥ 8 strains per species). Since the differences in MICs were small and significantly below 4 μg/mL for three of the four strains tested (Fig. 1D), these differences are unlikely to be clinically significant. In support of this interpretation, C1 has been used alone clinically as an antimicrobial agent (5, 11). Taken together, these data further suggest that the breadth and potency of the hospital gentamicin are not derived from being a mixture.
Most Impurities Display Inferior Antimicrobial Activities Relative to Hospital Gentamicin.
Hospital gentamicin contains several impurities (e.g., sisomicin, G418, gentamicin A, B, and X), which account for ≤10% of the mixture, each comprising ≤3% (9). We compared the antimicrobial activities of commercially obtained impurities (SI Appendix, Table S1). Impurity composition was confirmed using NMR (SI Appendix, Fig. S2 and Table S2) except for gentamicin X, where there was limited compound.
The antimicrobial activity of five impurities was compared to the hospital gentamicin (Fig. 1E). Four impurities (G418, gentamicin A, B, and X) displayed lower breadths than the hospital gentamicin. Each strain inhibited by these impurities was also inhibited by the C-subtypes, suggesting that these impurities are unlikely to increase the breadth of the hospital gentamicin mixture (SI Appendix, Fig. S4). Unlike the other impurities, sisomicin has the same antimicrobial breadth as the hospital gentamicin (Fig. 1F). Among the 35 species inhibited by hospital gentamicin, 33 were inhibited by both sisomicin and hospital gentamicin. Notably, sisomicin was effective against one multidrug-resistant P. aeruginosa strain against which neither the C-subtypes nor the hospital gentamicin inhibited (SI Appendix, Fig. S4). Given the MIC value of sisomicin for this specific strain was 4 μg/mL, and that sisomicin can only make up 3% of hospital gentamicin, it is unlikely to contribute to the antimicrobial breadth of hospital gentamicin. Additionally, hospital gentamicin and the C-subtypes were effective against one K. pneumoniae isolate against which sisomicin was not (SI Appendix, Fig. S4). As expected, the four impurities showing lower breadth (G418, gentamicin A, B, and X) were also significantly less potent than hospital gentamicin (P < 0.05, Mann–Whitney U test, n ≥ 8 strains) (Fig. 1F). In contrast, sisomicin was more potent than hospital gentamicin against S. aureus and P. aeruginosa (P < 0.05, Mann–Whitney U test, n ≥ 8 strains) (Fig. 1F). Collectively, the impurities were less broad and potent than hospital gentamicin and are unlikely to contribute to the antimicrobial breadth and potency of the hospital gentamicin mixture.
Subtypes of Gentamicin Have Different Degrees of Ototoxicity.
Sensory hair cells in the cochlea are selectively targeted by aminoglycosides due to the characteristics of the hair cell MET channel, which allows for drug entry and subsequent cell death (17, 18, 30). We examined the toxicity of C-subtypes and impurities on rat cochlear hair cells using an in vitro damage model (Fig. 2 A and B) (31). Data points for each drug were fitted with Hill functions from which EC50s and Hill coefficients were derived. In comparison to hospital gentamicin, C2 is more ototoxic and C2b is less ototoxic (P < 0.05, F-test, n = 7 to 16 cochleae) (Fig. 2C and SI Appendix, Table S3), while C1, C1a, and C2a were not significantly different (P > 0.05, F-test, n = 5 to 8). Overall, C2 has the lowest and C2b the highest EC50 values (Fig. 2D). As ototoxicity and antimicrobial activity do not directly correlate for C-subtypes, we postulate that it is possible to reformulate hospital gentamicin with less ototoxic components without altering antimicrobial activity.
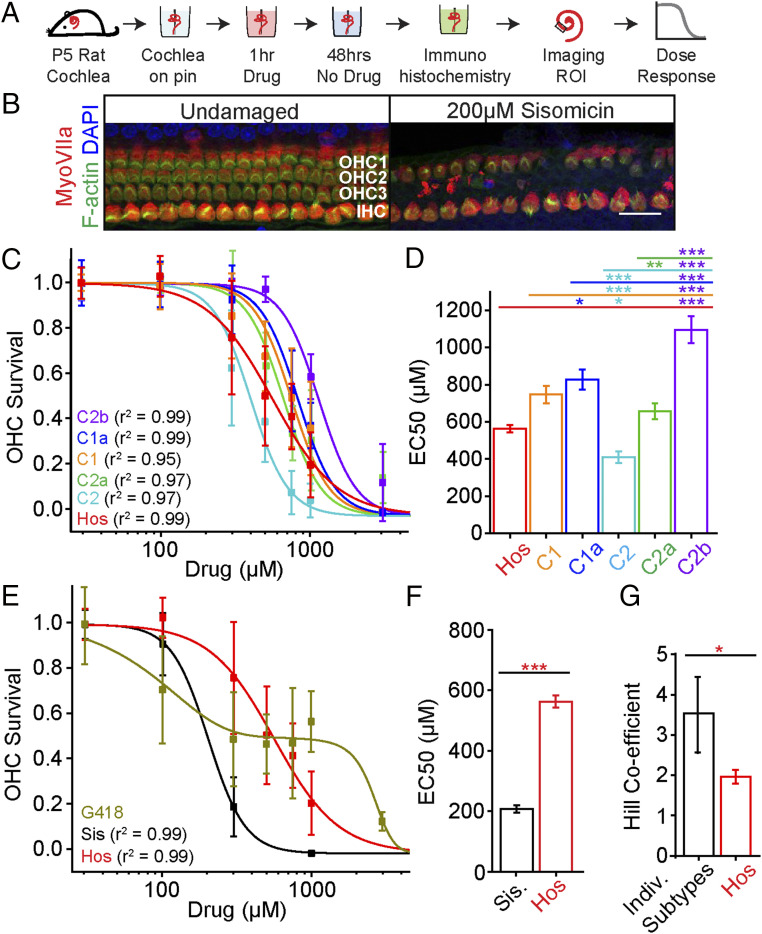
Fig. 2.
Components of hospital gentamicin have different degrees of ototoxicity. (A) Schematic of the experimental method using cochlear explants from P5 rats to study ototoxicity. ROI, region of interest. (B) Representative images of the midapical turn of undamaged and sisomicin-damaged cochlear tissues showing inner hair cells (IHC) and three rows of outer hair cells (OHC) (Scale bar, 25 μm.) (C) Dose–responses of the C-subtypes and hospital gentamicin (red) and OHC survival in the midapical region of the cochlea. Data are shown as mean ± SD, n = 5 to 16 for each data point (SI Appendix, Table S3). Hill function best fits were used for each dataset and were used to derive EC50s and Hill coefficient values. (D) Relative to hospital gentamicin (Hos), C2 is more and C2b is less ototoxic (P < 0.05, F-test; EC50 ± SE, C2 = 403 ± 23 μM, Hos. = 563 ± 28 μM, C2b = 1,130 ± 22 μM). (E) Dose–responses of the impurities (sisomicin and G418) and hospital gentamicin. A biphasic function was used to fit the G418 dose–response results. (F) Sisomicin (Sis) is more ototoxic than the hospital gentamicin (P < 0.05, F-test, n = 8 to 60; Sis. = 199 ± 17 μM, Hos. = 563 ± 28 μM. (G) Hospital gentamicin has a lower Hill coefficient than the individual components (excluding G418) (P < 0.05, F-test, n = 8 to 60), global fit used for sisomicin and the C-subtypes. *P < 0.05, **P < 0.01, ***P < 0.001.
Of the two impurities tested, sisomicin was more ototoxic than hospital gentamicin (P < 0.05, F-test, n = 8 to 60) (Fig. 2 E and F). G418 was also ototoxic at lower doses, but in contrast to all other drugs tested it showed a biphasic dose–response (Fig. 2E). It is possible that the ototoxicity at lower doses of hospital gentamicin is attributed to sisomicin and G418 since they are more toxic than gentamicin C-subtypes. Since some impurities were more ototoxic and showed inferior antimicrobial activity relative to hospital gentamicin (Fig. 2F), we hypothesize that a gentamicin mixture without impurities may be less ototoxic and may have unimpaired antimicrobial activity. This idea is further supported by analysis of the hospital gentamicin ototoxicity dose–response curve, which has a smaller Hill coefficient (Fig. 2G) than the individual components. The smaller Hill coefficient is likely due to the difference in EC50s between the components.
Structure–Activity Relationships of Gentamicin C-Subtypes.
Thus far, data suggest that antimicrobial activity is comparable across hospital gentamicin constituents (C-subtypes and sisomicin impurity), but that there are differences in ototoxicity. Here, we analyze the individual differences between these components, and identify four structure–activity relationships for both ototoxicity and antimicrobial activity (Fig. 3).
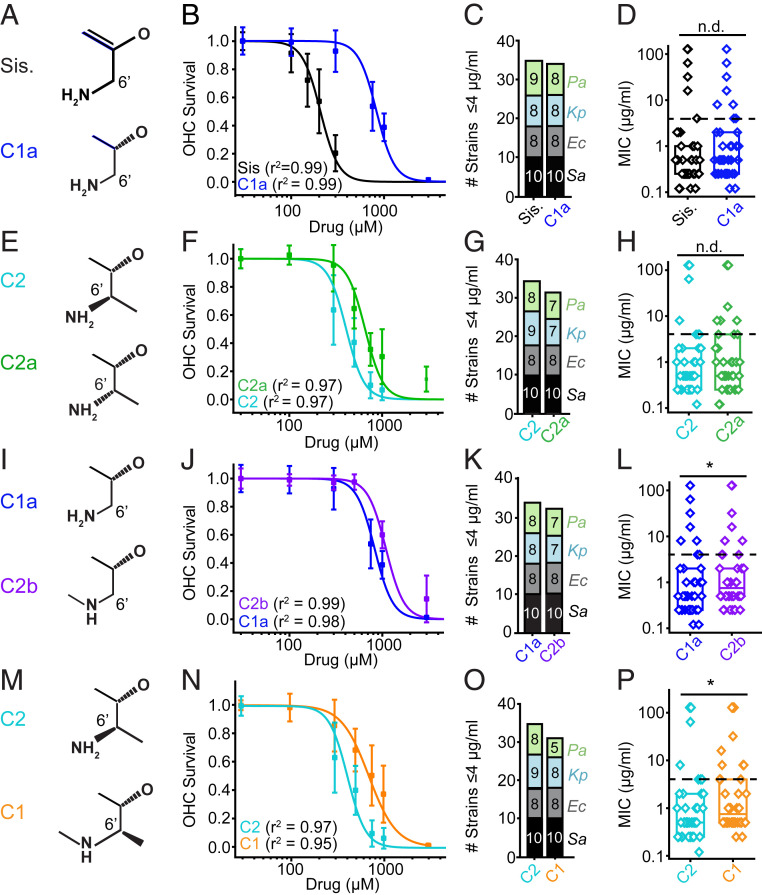
Fig. 3.
Structure–activity relationships between gentamicin subtypes: Ototoxicity and antimicrobial activity. (A) Sisomicin and C1a differ at the 4′ and 5′ position. (B) C4′-5′ saturation alters ototoxicity. Sisomicin has a lower EC50 than C1a (P < 0.05, F-test, n = 8 to 60; Sis. = 199 ± 17 μM, C1a = 821 ± 24 μM). (C). Sisomicin and C1a have similar antimicrobial breadths (35 vs. 34 stains). (D) There is no difference in potency between sisomicin and C1a (P > 0.05, Mann–Whitney U test, all strains). (E) Gentamicin C2 and C2a are epimers at the C6′ position. (F) C2 is more ototoxic than C2a (P < 0.05, F-test, n = 7 to 16; C2 = 403 ± 23 μM, C2a = 656 ± 36 μM). (G) C2 and C2a have similar antimicrobial breadths (35 vs. 32 strains). (H) C2 and C2a show no difference in antimicrobial potency (P > 0.05, Mann–Whitney U test, all strains). (I) Gentamicin C1a and C2b differ at the N6′ site. (J) C1a has a lower EC50 than C2b (P < 0.05, F-test, n = 8; C2b = 1,130 ± 22 μM, C1a = 821 ± 24 μM). (K) C1a and C2b have similar antimicrobial breadths (34 vs. 32 strains). (L) There is a detectable difference in potency between C1a and C2b (P < 0.05, Mann–Whitney U test, all strains). Fig. 2 shows this is related to P. aeruginosa strains. (M) Gentamicin C2 and C1 differ at the N6′ position. (N) C2 has a lower EC50 than C1 (P < 0.05, F-test, n = 8; C2 = 403 ± 23 μM, C1 = 728 ± 59 μM). (O) C2 has a slightly higher breadth than C1 (four strains). (P) C2 is more potent than C1 (P < 0.05, Mann–Whitney U test, all strains). Fig. 1 shows breakdown by species for all compounds. *P < 0.05, n.d., no difference.
Sisomicin has a double bond at the C4′-C5′ position that C1a lacks (Fig. 3A). This chemical difference led to a twofold increase in the EC50 for ototoxicity (Fig. 3B) (P < 0.05, F-test, n = 5 to 8; sisomicin = 199 ± 17 μM, C1a = 821 ± 24 μM), with no difference between the Hill coefficients (global fit, r2 > 0.95). However, this difference did not alter antimicrobial activity, as both C1a and sisomicin have comparable breadth (35 vs. 34 strains) and potency (P > 0.05, Mann–Whitney U test, all strains) (Fig. 3 C and D and SI Appendix, Figs. S3 and S4). Data suggest that the presence of a double bond leads to differential ototoxicity but not antimicrobial activity. Structurally, the double bond alters the conformation of ring I from a chair to a more flattened conformation (32). There is a slight increase in the pKa of the N6′ amine but one unlikely to alter the relative charge. Given that aminoglycosides are permeant blockers that enter the MET channel based on a function of drug size and charge, one would not expect this structural change to cause significant differences in their ability to permeate and block the MET channel (33). We thus hypothesize that the changes in ototoxicity are caused by other factors that impact channel permeation or differential toxicity inside the hair cell.
Gentamicin C2 and C2a are isomers where the primary amine and methyl orientation around C5′ differs (Fig. 3E). This chiral difference alters ototoxicity, with gentamicin C2 being 50% more ototoxic than C2a (Fig. 3F) (P < 0.05, F-test, n = 5 to 8; C2 = 403 ± 23 μM, C2a = 656 ± 36 μM). Chirality had no detectable effect on the Hill coefficient values (global fit, r2 > 0.95). In bacteria, the chiral difference between C2 and C2a had little effect on antimicrobial breadth (35 vs. 32 strains) (Fig. 3G and SI Appendix, Figs. S3 and S4). In addition, no significant effect on antimicrobial potency was identified (P > 0.05, Mann–Whitney U test, all strains) (Fig. 3H). As before, since there are no differences in size or charge between C2 and C2a, the difference in ototoxicity likely arises from factors other than their properties as permeant blockers of the MET channel or by a mechanism of intracellular toxicity.
The third structure–activity relationship we investigated is between C1a and C2b, which is distinguished by N6′ methylation (Fig. 3I). Gentamicin C2b N6′ methylation results in decreased ototoxicity relative to C1a (P < 0.05, F-test, n = 5 to 8; C2b = 1,130 ± 22 μM, C1a = 821 ± 24 μM) (Fig. 3J). The N6′ methylation had no effect on the Hill coefficients (global fit, r2 > 0.95) (Fig. 3K). This structural difference had little effect on antimicrobial breadth (34 vs. 32 strains) (Fig. 3K and SI Appendix, Fig. S3) and led to a small difference in potency, mainly in the P. aeruginosa species (both ≤4 μg/mL) (Figs. 1D and 3L). The N6′ methylation results in a secondary amine with a slightly reduced pKa, which is unlikely to significantly alter charge. It is plausible that the reduced ototoxicity is a function of the amine being shielded, implying that the compound is interacting directly with the MET channel.
A fourth structure–activity relationship investigated was between C1 and C2 (Fig. 3M), where again the difference is the presence of N6′ methylation in C1. Consistent with the previous comparison between C1a and C2b, N6′ methylation reduced ototoxicity (Fig. 3N) (P < 0.05, F-test, C2 = 402 ± 23 μM, C1 = 748 ± 59 μM). It also had a limited effect on antimicrobial activity, a small reduction in breadth (35 vs. 31 strains) and potency (Fig. 3 O and P) (P < 0.05, Mann–Whitney U test, all strains). Given the similar effects of N6′ methylation on C1 and C2b in alleviating ototoxicity with minimal effects on antimicrobial efficacy, this represents a candidate site for further modification to reduce ototoxicity. As above, these data could similarly argue for an effect on permeation of the MET channel resulting from the drug binding to the channel while traversing.
Gentamicin Subtypes Binding the MET Channel Are Otoprotective.
Impeding aminoglycoside uptake via the hair cell MET channel decreases ototoxicity (17, 19, 22). Aminoglycosides are traditionally considered permeant blockers of the MET channel as they prevent current flow, particularly at negative potentials across the hair cell membrane (30, 33). At positive potentials when the driving force across the channel is less, no or minimal current blockage is expected as drugs are driven away from the channel. In addition, factors that hinder drug passage through the MET channel, such as a decrease in charge and increase in size, predictively increase the extent of MET channel block by classic permeant blockers, including gentamicin (33). As noted previously, we detected changes in ototoxicity among gentamicin C-subtypes that are unaccounted for based on minimal changes in charge and size, such that a simple permeant blocking model incompletely describes the mechanism of decreased ototoxicity. To explore the possibility of alternative factors altering MET channel permeation by gentamicin C-subtypes, we used whole-cell voltage-clamp recordings from outer hair cells in postnatal day (P) 7 to 10 rat cochlea.
A protocol that elicited the total MET current by using a strong positive and negative hair bundle deflection (∆X) (Fig. 4A) was coupled with a series of voltage steps between −140 and +140 mV (∆V) (Fig. 4A). The control current responses are presented in Fig. 4A after having the non-MET currents subtracted. Measurements in the presence of either 1 mM sisomicin or gentamicin C2b (the most- and least-ototoxic gentamicin constituents, respectively) demonstrated a large reduction of inward MET current with considerably less reduction of the outward MET current (Fig. 4A). Even at this these high concentrations, the current was not completely blocked by either drug. Moreover, the block appeared reduced at more depolarized, negative potentials (Fig. 4B), which is unexpected for a permeant channel blocker because the reduced driving force should be less effective at moving the drug through the channel, thereby resulting in more blockage. These data suggest that neither C2b nor sisomicin behaved only as permeant blockers of the MET channel.
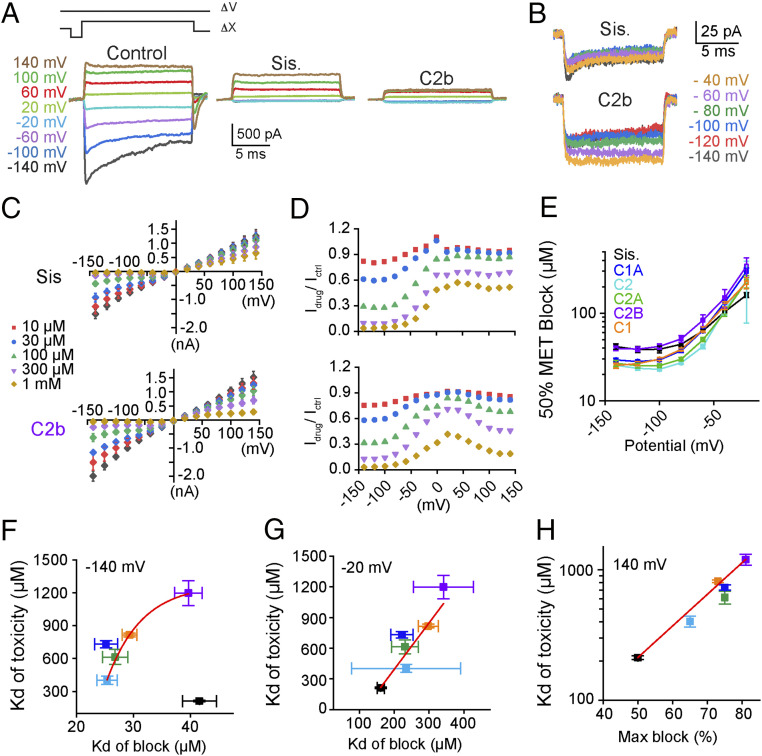
Fig. 4.
Components of hospital gentamicin bind and block the hair cell MET channel. (A, Upper) The timing of the voltage steps (∆V) and mechanical stimulus (∆X) used to evoke maximal MET currents. (Lower) Control currents are showed in (Left) with responses in the presence of sisomicin or gentamicin C2b presented Center and Right, respectively. The currents shown are net mechanosensitive currents where color indicates voltage step. (B) Negative potential responses in the presence of sisomicin (Upper) and gentamicin C2b (Lower, both 1 mM). (C) Summary voltage (mV)–current (nA) plots for sisomicin and gentamicin 2b, where color denotes drug concentration as indicated. Data from C were used to identify half-blocking drug concentrations used for plots in E–G. Data from C were also used to generate plots of drug block of MET channel normalized at each voltage (D). (D) Data were derived from the corresponding plot in (C and SI Appendix, Fig. S5A) for each drug. (E) Plots showing membrane potential against the half blocking dose, derived from the data in C and SI Appendix, Fig. S5A, of each tested aminoglycoside as indicated by the color code. (F) Plots showing the half-blocking concentration obtained at −140 mV against the Kd for ototoxicity obtained in Fig. 3. (G) Plots showing half-blocking dose at −20 mV against the ototoxicity Kd. (H) The maximal block at +140 mV against the ototoxicity Kd at 1 mM. n = 8 for each drug.
We next generated voltage–current relationships for the five gentamicin C-subtypes and sisomicin and found a reduced block at more negative potentials and a greater block at higher doses (Fig. 4C and SI Appendix, Fig. S5A). Inspection of the 100- and 300-µM dosages shows an unexpected flattening of the response between −100 and −20 mV, where the channel block is reduced rather than enhanced by the reduced driving force. To further characterize this unusual response, we plotted the ratio of the current in the presence of the drug over the control current at each potential and dose for each drug tested (Fig. 4D and SI Appendix, Fig. S5B). As expected, the channel block was reduced between −100 and 0 mV, which were potentials more negative than predicted for a permeant blocker. These data suggest that a larger than expected driving force is required to move compounds into the channel vestibule or, alternatively, a voltage-dependent interaction between the compounds and the channel (34). Importantly, dose–response curves made at negative potentials likely do not reflect the true permeation of the channel.
Permeant blockers typically display lower half-blocking doses for the MET channel at more negative, depolarizing voltages. As a group, gentamicin C-subtypes and sisomicin displayed half-blocking doses for the MET channel that increase with depolarization (Fig. 4E). This relationship further supports the concept that C-subtypes and sisomicin are not behaving only as traditional permeant blockers. We next compared the ability of C-subtypes and sisomicin to block MET channels to their in vitro ototoxicity. At both −140 and −20 mV, the half-maximum (Kd) of MET channel block correlated well with ototoxicity for all gentamicin C-subtypes (Fig. 4 F and G). However, at −140mV, sisomicin was considerably more toxic than its blocking efficacy suggests, but its Kd of MET channel block correlated with ototoxicity at −20 mV (Fig. 4G). However, the variance of different components was quite high likely because of the smaller current amplitudes (Fig. 4G).
The hypothesis that gentamicin C-subtypes and sisomicin bind to the MET channel was further investigated by exploring MET currents in the presence of drugs at positive potentials. Permeant blockers are expected to not block the channel when presented from the outside at positive potentials because they will be electrically driven away from the channel. Comparison between sisomicin and C2b showed that the results were more complex: the sisomicin block was mostly but not completely relieved, while C2b maintained a strong block at positive potentials (Fig. 4A). Among the compounds tested, sisomicin showed the most and C2b the least complete loss of block at positive potentials (Fig. 4C and SI Appendix, Fig. S5A). Indeed, the apparent block was not removed, but often increased at positive potentials (Fig. 4D and SI Appendix, Fig. S5B). Given our primary interest is in determining the relationship between drug interactions and ototoxicity, we examined the maximal drug block at +140 mV against the Kd of ototoxicity (Fig. 4H) and found a clear positive correlation.
Reformulating Gentamicin to Reduce Ototoxicity.
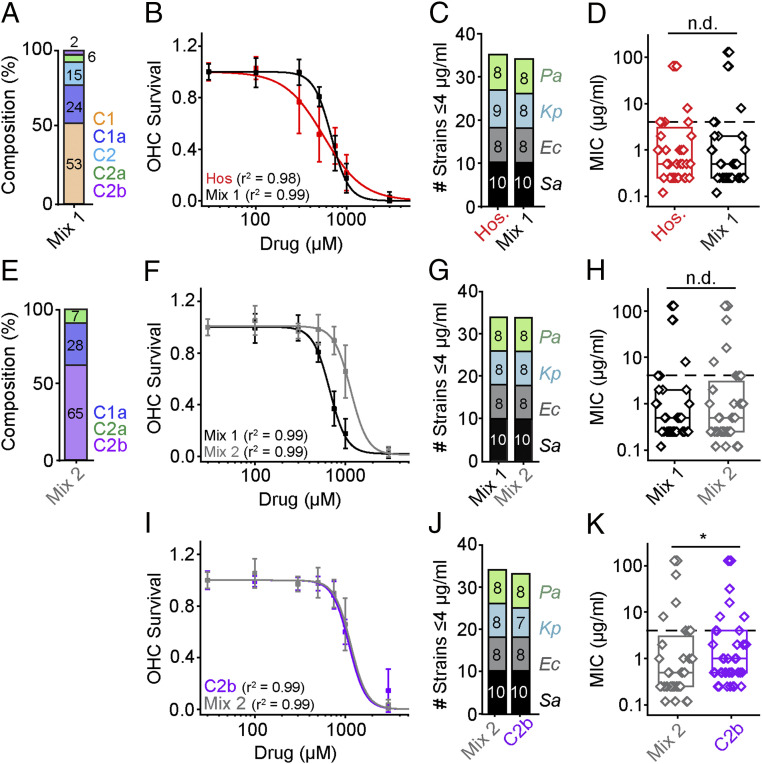
Because of the differences in ototoxicity (Fig. 2) but minimal differences in antimicrobial breadth or potency among the C-subtypes (Fig. 1), we postulate that it is possible to reformulate the gentamicin mixture to minimize ototoxicity and preserve antimicrobial actions. To do this, we prepared a reference mixture consisting of a defined composition of C-subtypes devoid of impurities (mix 1) (Fig. 5A). Mix 1 consisted of 53% C1, 24% C1a, 15% C2, 6% C2a, and 2% C2b, where the mixture totals 100% of C-subtypes only. A similar ratio of C-subtypes is found in hospital gentamicin plus an additional 10% impurities. Comparing mix 1 (no impurities) to hospital gentamicin (Fig. 5B) revealed no difference in ototoxicity (P > 0.05, F-test, n = 5 to 8, hospital gentamicin = 562 ± 28 μM, mix 1 = 673 ± 19 μM) but a difference in Hill coefficients (r2 = 0.88 when the same fit was used, cutoff r2 > 0.95). Given that the lower Hill coefficient is driven by increased toxicity at lower doses, we ascribe this change to the loss of impurities, as only sisomicin and G418 were toxic at these lower doses (Fig. 2E). In support of this interpretation, gentamicin C-subtypes have higher Hill coefficients than hospital gentamicin (Fig. 2G). Removal of the impurities had little effect on antimicrobial activity, reducing breadth (Fig. 5C) by just one K. pneumoniae strain and no differences in potency (Fig. 5D) (P > 0.05, Mann–Whitney U test, all strains). Thus, removing the impurities increases the therapeutic window between ototoxicity and antimicrobial activity.
Fig. 5.
Reformulations of hospital gentamicin showing reduced ototoxicity with unimpaired antimicrobial activities. (A) A formulation of individual C-subtypes without impurities (mix 1). The percentage composition of this mixture was 53% C1, 24% C1a, 15% C2, 6% C2a, and 2% C2b. (B) There is no difference between the EC50 of hospital gentamicin and mix 1 (P > 0.05, F-Test, n = 8; mix 1 = 673 ± 19 μM, Hos. = 563 ± 28 μM). The Hill coefficient for hospital gentamicin and mix 1 are significantly different (P < 0.05, F-test). (C) Mix 1 and hospital gentamicin have comparable breadths (one strain difference). (D) There is no difference in potency between mix 1 and hospital gentamicin (P > 0.05, Mann–Whitney U test, all strains). (E) A formulation of C-subtypes, where C1 and C2 was replaced with C2b (mix 2). (F) The EC50 of mix 1 is lower than mix 2 (P < 0.05, F-test, n = 8; mix 1 = 673 ± 19 μM, mix 2 = 1,229 ± 44 μM). (G) There is no difference in breadth between mix 1 and mix 2. (H) There is no detectable difference in potency between mix 1 and mix 2 (P > 0.05, Mann–Whitney U test, all strains). (I) Next, we compared gentamicin C2b and mix 2. There is no difference in ototoxicity between C2b and mix 2 (P > 0.05, F-test, C2b = 1,130 ± 22 μM, mix 2 = 1,229 ± 44 μM). (J) There is a one-strain difference in breadth between mix 2 and C2b. (K) There is a small difference in potency between mix 2 and C2b (P < 0.05, Mann–Whitney U test, all strains). See SI Appendix, Fig. S6 for the breakdown by species. *P < 0.05, n.d., no difference.
To further explore reformulation, we removed C2 as it was the most ototoxic C-subtype that provides no additional antimicrobial activity (SI Appendix, Figs. S3 and S4). In addition, we removed C1, which is the most abundant component contained in hospital gentamicin bottles and yet the least antimicrobial component with comparable ototoxicity (SI Appendix, Figs. S3 and S4) (24). Both components were replaced by C2b because it was the least ototoxic component but retained antimicrobial activity. Termed mix 2, we compared ototoxicity and antimicrobial activity to mix 1 (a hospital formulation without impurities). Relative to mix 1, mix 2 is less ototoxic (Fig. 5 E and F) (P < 0.05, F-test, n = 8, mix 1 = 673 ± 19 μM, mix 2 = 1,229 ± 44 μM). Mix 2 also demonstrated comparable antimicrobial breadth and potency (Fig. 5 G and H) (P > 0.05, Mann–Whitney U test, all strains). Thus, these data demonstrate that hospital gentamicin can be reformulated to reduce ototoxicity while retaining antimicrobial breadth and potency.
A final question addressed is whether the mix 2 provides added value over an individual C-subtype. We compared mix 2 to its major component C2b and found that ototoxicity is comparable (Fig. 5I) (P > 0.05, F-test, n = 8; C2b = 1,130 ± 22 μM, mix 2 = 1,229 ± 44 μM). Even though all of the other subtypes in mix 2 are more toxic than C2b at comparable concentrations, no difference in ototoxicity was detectable between mix 2 and C2b. Antimicrobial breadth was comparable, and potency was slightly better for mix 2 (P < 0.05, Mann–Whitney U test, all strains) (SI Appendix, Fig. S6). These data further support the concept that reformulating the hospital gentamicin can yield a less ototoxic aminoglycoside antibiotic.
Discussion
Gentamicin is a broad-spectrum aminoglycoside antibiotic mixture that is associated with ototoxicity and nephrotoxicity (3, 5). These side-effects are common to most aminoglycosides, limiting the use of this class of antibiotics. The ultimate goal of this work is to alleviate ototoxicity in order to maximize the usability of these potent antibiotics. Developing a purification approach that takes advantage of the variance in gentamicin composition allowed for the characterization of the individual components. First, we found that antimicrobial activity is not derived from the mixture or its impurities, but from the individual C-subtypes. A range of ototoxicity was found among C-subtypes that was independent of antimicrobial activity, and removal of the impurities produced a less ototoxic mixture while preserving antimicrobial activity. As the least ototoxic C-subtype, gentamicin C2b represents a less toxic alternative to hospital gentamicin, and also a candidate compound for further modifications for drug development. Moreover, structure–activity relationships reveal that four different modifications of the C4′-C6′ region of ring I underlie decreases in ototoxicity without affecting antimicrobial activity. Finally, these structural changes limited penetration of C-subtypes through the hair cell MET channel, likely via direct binding to the channel, resulting in reduced hair cell toxicity. Collectively, these results provide proof-of-concept of reducing ototoxicity and preserving antimicrobial function of aminoglycosides.
Minimal Variations in Antimicrobial Activity.
Among the gentamicin C-subtypes, we observed small differences in potency (e.g., C1 is less potent than C1a); however, these are unlikely to be clinically significant given their MIC values were well below 4 μg/mL for most species. Consistent with this interpretation, treatment with C1 alone appeared equally efficacious as hospital gentamicin in treating infection in patients, implying that the small differences in MIC values below 4 μg/mL are unlikely to reduce their clinical value (5, 11). In terms of mechanisms accounting for the differences in potency between C1 and C1a, while it is not known if gentamicin C-subtypes similarly enter and bind to intracellular targets, our observation that C1 is less potent than C1a is consistent with kinetic bacterial ribosome binding data (35). Previous ribosomal data show that C1 has a lower affinity for the intracellular bacterial ribosomal binding site than C1a (35). However, it remains an open question if all C-subtypes and impurities bind to the ribosome in the same manner, and to what extent ribosome binding and cellular entry are responsible for the differences we detected in antimicrobial activity.
With the exception of sisomicin, all of the impurities had reduced breadth of activity (e.g., gentamicin A, B, X, and G418), resulting from a >10-fold decrease in potency across all species (Fig. 1). Thus, it is unlikely that impurities in hospital gentamicin provide any positive antimicrobial value. Furthermore, since the impurities are more ototoxic and removal increases the therapeutic window in vitro, our data imply that that removing the impurities could reduce ototoxicity. Notably, the concentration of impurities (10%) allowed in hospital gentamicin is high relative to newer drugs on the market where the maximum is 1% (36). While X-ray crystallography data recently showed that sisomicin interacts with the decoding center of bacterial ribosomes, whether gentamicin C-subtypes show similar interactions remains unclear (35, 37, 38). Future experiments to delineate this relationship can further enhance our understanding of the structure–activity relationships between gentamicin structure and ribosomal binding, which is necessary for antimicrobial activity.
Variations in Ototoxicity.
Previous in vitro and in vivo studies have reported that commercially available gentamicin C-subtypes display different levels of ototoxicity (12, 13, 16). To ensure purity, we developed an approach to isolate gentamicin C-subtypes in order to perform a comprehensive assessment, showing that gentamicin C2b is the least ototoxic C-subtype and is 50% less toxic than hospital gentamicin. C2b maintains antimicrobial breadth and potency relative to hospital gentamicin and so may be a viable, less toxic alternative to the current hospital formulation. Further in vivo testing as well as investigations of nephrotoxicity are needed for this potential alternative.
Data further demonstrated that gentamicin C2 was 50% more ototoxic than hospital gentamicin and can be removed without loss of antimicrobial activity. The impurities sisomicin and G418, and gentamicin C2, are more ototoxic than either hospital gentamicin or the other components alone. Finally, we showed that replacing both C1 for its slightly lower antimicrobial activity and C2 because of its enhanced ototoxicity with the C2b component reduced ototoxicity while maintaining antimicrobial activity. These data underscore the potential to make safer alternatives to the existing formulation of hospital gentamicin; notably some of these changes could be made within existing regulations since ranges of the C-subtypes are permitted (9).
Structure–Activity Relationship of Gentamicin C-Subtypes and Sisomicin.
Aminoglycoside ototoxicity arises from drug entering hair cells via the MET channel and causing dysfunction (17). The structural differences among gentamicin C-subtypes and sisomicin allowed for four distinct structure–activity relationships to be investigated. A comparison of C1a with sisomicin isolates the double bond in ring 1, which flattens the conformation of this ring minimal change in charge distribution. The presence of the double bond decreases the ability of N6′ to form hydrogen bonds internally, and thus more able to interact externally. The presence of the double bond did not alter antimicrobial activity but, surprisingly, greatly increased ototoxicity in a manner suggestive of altered interaction with the MET channel. A second comparison between C2 and C2a provided a stereochemistry change that reduces ototoxicity for C2a. Surprisingly, these data suggest an interaction between the drug and the MET channel that is more complex than a simple permeant channel blocker might imply. The final two comparisons are of N6′ methylation and both resulted in decreased ototoxicity with little effect on antimicrobial activity. These structure–activity relationship data collectively underscore structural changes in the C4′-6′ region on ring 1 markedly reduced ototoxicity without significantly affecting the size or charge of the aminoglycoside compound. In support of this structure–activity relationship, plazomicin and propylamicin are both potent aminoglycosides modified in the C4′-C6′ region displaying less ototoxicity (39, 40). Plazomicin is less ototoxic than gentamicin in vitro and in vivo, yet remains an effective antimicrobial (40, 41).
In recent years, relationships between intracellular cytosolic and mitochondrial aminoglycoside ribosome-binding and ototoxicity have garnered attention (42–44). Upstream of these intracellular events, aminoglycoside entry into the hair cell via the MET channel is required for toxicity resulting in cell death (17, 19). Aminoglycosides are traditionally viewed as permeant blockers of the MET channel whose transport is directly affected by size and charge. Since the gentamicin C-subtypes and sisomicin do not significantly differ in terms of size or charge, the structure activity data were not simply accounted for by the permeant blocker model; we thus hypothesize an alternative model of drug-MET channel interaction (i.e., drug-channel binding).
An alternative model is supported as the current voltage plots show unusual behavior by gentamicin C-subtypes and sisomicin at several levels (Fig. 4). First, the hair cell MET channel block is reduced as when the membrane potential is varied between −140 and −20 mV, opposite to what is predicted by the driving force simply affecting a permeant blocker. Second, the hair cell MET channel block increases between +20 and 140 mV, again counter to responses typical of a permeant blocker, where the block should be relieved or absent at positive potentials as drug permeation is unlikely to take place under these conditions. The voltage dependence of the block has multiple interpretations, including the concept that charge and size are not the only variables impacting permeation but that compounds may bind to the MET channel and thereby limit permeation. The degree of blockage correlates with ototoxicity for gentamicin C-subtypes and sisomicin at both negative and positive potentials, with the positive potential correlation being the strongest (Fig. 4 F–H). Notably, drugs that respond more like a permeant blocker (i.e., sisomicin and C2) were more ototoxic than those that did not. These unexpected data predict that the drugs act both as permeant blockers and may also interact with the channel in a voltage-dependent manner. This interaction reduces currents through the channel in the presence of aminoglycosides, making the correlation between channel block and permeation weaker and thus not necessarily a good indicator of toxicity. Interestingly, more block at positive potentials, which indicates channel interaction exclusively since permeation is typically reversed at this point, is a strong indicator of toxicity. What this suggests is that drug binding within the channel limits permeation through the channel, such that there is less intracellular accumulation and so less toxicity. Binding might occur in the vestibule where dehydration is thought to occur, or it might take place more deeply within the pore at sites where calcium ions are interacting. Further characterization of this binding may provide additional targets for modification that could further reduce ototoxicity.
Once inside the hair cell, aminoglycosides can induce cell death by altering calcium trafficking between the endoplasmic reticulum and mitochondrion (45, 46). In addition, aminoglycosides can cause dysfunction of mitochondrial and cytosolic ribosomes in hair cells, leading to generation of reactive oxygen species and eventually cell death (42, 47, 48). At present, there is no evidence that aminoglycosides cause hair cell loss by selectively activating one mechanism. It is possible that differential intracellular effects of individual gentamicin subtypes contribute to their different degrees of ototoxicity.
Conclusion
In summary, we demonstrate that individual gentamicin C-subtypes sufficiently provide equivalent antimicrobial breadth and potency to the hospital mixture of gentamicin. Impurities provide no additional antimicrobial value but increase ototoxicity. Structure–activity relationships show that different modifications of the C4′-C6′ region on ring I can reduce ototoxicity while preserving antimicrobial activity. Moreover, reduced ototoxicity results from decreased drug penetration via the hair cell MET channel likely because of drug binding to the channel. Finally, using the least-ototoxic subtype gentamicin C2b to reformulate the gentamicin mixture also decreases ototoxicity while preserving antimicrobial action. Together, our data lay the groundwork for the discovery of safer antibiotic alternatives via future drug development or reformulation.
Methods
Compound Purification.
Gentamicin C-subtypes were isolated in pure form from two different batches of commercially available gentamicin mixtures (SI Appendix, Table S1) by Nanosyn. See SI Appendix, Supplemental Methods for details for separation methods.
Antimicrobial Testing.
Ten bacterial strains were tested from four species: E. coli, S. aureus, K. pneumoniae, and P. aeruginosa. The MIC was established for each drug using a microdilution test. See SI Appendix, Supplemental Methods for details.
Cochlear Cultures.
Cochleae were harvested from P5 Sprague–Dawley rats. The cartilaginous capsule surrounding the cochlea was removed and a stainless-steel minutien pin was placed through the modiolus, all contained in a custom-made sterile plastic tissue chamber containing 1 mL of DMEM/F12 supplemented with 10 µM sodium pyruvate and 20 µM penicillin G.
Housing conditions and experimental procedures used in this study were in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (49) and in accordance with Stanford University Administrative Panel on Laboratory Animal Care. See SI Appendix, Supplemental Methods for details.
NMR, Image Acquisition and Analysis, Immunohistochemistry, Electrophysiology.
See SI Appendix, Supplemental Methods for details.
Supplementary Material
Acknowledgments
We thank our laboratory for insightful comments and fruitful discussion on this project throughout its maturation; Y. Shi, E. Huang, L. Becker, A. Sajjadi, and Z. Jawadi for excellent technical support; M. Tracezewski (Clinical Microbiology Institute) for antimicrobial testing; the Nanosyn staff for drug purification/validation; and S. Russell Lynch for NMR experiments. The Core is supported by the Stanford Initiative to Cure Hearing Loss through generous gifts from the Bill and Susan Oberndorf Foundation. This work was supported by NIH/National Institute on Deafness and Other Communication Disorders Grant R01DC014720 (to A.J.R. and A.G.C.).
Footnotes
Competing interest statement: R.G., J.P.M., Z.S., and D.L. are employed by Nanosyn.
This article is a PNAS Direct Submission. U.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2013065117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
Change History
September 7, 2021:The text of this article, Figs. 1 and 3, and the SI Appendix have been updated; please see accompanying Correction for details.
References
- 1.Fuchs A., Bielicki J., Mathur S., Van Den Anker J. N., Antibiotic use for sepsis in neonates and children: 2016 evidence update. WHO-Reviews (2016), https://www.who.int/selection_medicines/committees/expert/21/applications/s6_paed_antibiotics_appendix4_sepsis.pdf. Accessed 29 March 2020.
- 2.Prayle A., Smyth A. R., Aminoglycoside use in cystic fibrosis: Therapeutic strategies and toxicity. Curr. Opin. Pulm. Med. 16, 604–610 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Huth M. E., Ricci A. J., Cheng A. G., Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol. 2011, 937861 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend P. L., Fink M. P., Stein K. L., Murphy S. G., Aminoglycoside pharmacokinetics: Dosage requirements and nephrotoxicity in trauma patients. Crit. Care Med. 17, 154–157 (1989). [PubMed] [Google Scholar]
- 5.Forrey A. W., et al., Nephrotoxicity: A comparison in humans of gentamicin and gentamicin C1 administration. Toxicol. Appl. Pharmacol. 44, 453–462 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Becker B., Cooper M. A., Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 8, 105–115 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Craig W. A., Optimizing aminoglycoside use. Crit. Care Clin. 27, 107–121 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Weinstein M. J., et al., Gentamicin, a new antibiotic complex from Micromonospora. J. Med. Chem. 6, 463–464 (1963). [DOI] [PubMed] [Google Scholar]
- 9.Anonymous, Gentamicin Sulfate, United States Pharmacopeia (USP), USP40-NF Page 4391. https://www.uspnf.com/. Accessed 1 May 2020. [Google Scholar]
- 10.Eren E., et al., Toward understanding the outer membrane uptake of small molecules by Pseudomonas aeruginosa. J. Biol. Chem. 288, 12042–12053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosegaard A., Welling P. G., Madsen P. O., Gentamicin and gentamicin C1 in the treatment of complicated urinary tract infections: Comparative study of efficacy, tolerance, and pharmacokinetics. Antimicrob. Agents Chemother. 7, 328–332 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi M., et al., Comparisons of cochleotoxicity among three gentamicin compounds following intratympanic application. Acta Otolaryngol. 128, 245–249 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M., Umemura M., Sone M., Nakashima T., Differing effects on the inner ear of three gentamicin compounds: GM-C1, -C2 and -C1a. Acta Otolaryngol. 123, 916–922 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Friesen W. J., et al., The minor gentamicin complex component, X2, is a potent premature stop codon readthrough molecule with therapeutic potential. PLoS One 13, e0206158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox K. E., Brummett R. E., Brown R., Himes D., A comparative study of the ototoxicity of gentamicin and gentamicin C1. Arch. Otolaryngol. 106, 44–49 (1980). [DOI] [PubMed] [Google Scholar]
- 16.Ogle J. M., Ramakrishnan V., Structural insights into translational fidelity. Annu. Rev. Biochem. 74, 129–177 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Alharazneh A., et al., Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One 6, e22347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vu A. A., et al., Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One 8, e54794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly M., et al., Design, synthesis, and biological evaluation of a new series of carvedilol derivatives that protect sensory hair cells from aminoglycoside-induced damage by blocking the mechanoelectrical transducer channel. J. Med. Chem. 62, 5312–5329 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood N. K., et al., d-Tubocurarine and berbamine: Alkaloids that are permeant blockers of the hair cell’s mechano-electrical transducer channel and protect from aminoglycoside toxicity. Front. Cell. Neurosci. 11, 262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitcher S. R., et al., ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight 4, e126764 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huth M. E., et al., Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. J. Clin. Invest. 125, 583–592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraisintu K., Parfitt R. T., Rowan M. G., A high-performance liquid chromatographic method for the determination and control of the composition of gentamicin sulphate. Int. J. Pharm. 10, 67–75 (1982). [Google Scholar]
- 24.White L. O., Lovering A., Reeves D. S., Variations in gentamicin C1, C1a, C2, and C2a content of some preparations of gentamicin sulphate used clinically as determined by high-performance liquid chromatography. Ther. Drug Monit. 5, 123–126 (1983). [DOI] [PubMed] [Google Scholar]
- 25.Grahek R., Zupančič-Kralj L., Identification of gentamicin impurities by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 50, 1037–1043 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Guo J., et al., Specificity and promiscuity at the branch point in gentamicin biosynthesis. Chem. Biol. 21, 608–618 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., et al., Delineating the biosynthesis of gentamicin x2, the common precursor of the gentamicin C antibiotic complex. Chem. Biol. 22, 251–261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D., Li H., Ni X., Zhang H., Xia H., Construction of a gentamicin C1a-overproducing strain of Micromonospora purpurea by inactivation of the gacD gene. Microbiol. Res. 168, 263–267 (2013). [DOI] [PubMed] [Google Scholar]
- 29.FDA , National antimicrobial resistance monitoring system–Enteric bacteria (NARMS): 2005 Executive report (U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, MD, 2009).
- 30.Marcotti W., van Netten S. M., Kros C. J., The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 567, 505–521 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krey J. F., et al., Mechanotransduction-dependent control of stereocilia dimensions and row identity in inner hair cells. Curr. Biol. 30, 442–454.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondo J., Koganei M., Kasahara T., Crystal structure and specific binding mode of sisomicin to the bacterial ribosomal decoding site. ACS Med. Chem. Lett. 3, 741–744 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farris H. E., LeBlanc C. L., Goswami J., Ricci A. J., Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J. Physiol. 558, 769–792 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Netten S. M., Kros C. J., Insights into the pore of the hair cell transducer channel from experiments with permeant blockers. Curr. Top. Membr. 59, 375–398 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Yoshizawa S., Fourmy D., Puglisi J. D., Structural origins of gentamicin antibiotic action. EMBO J. 17, 6437–6448 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cass R. T., et al., Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob. Agents Chemother. 55, 5874–5880 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Sullivan M. E., et al., Aminoglycoside ribosome interactions reveal novel conformational states at ambient temperature. Nucleic Acids Res. 46, 9793–9804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.François B., et al., Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: Role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 33, 5677–5690 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsushita T., et al., Design, multigram synthesis, and in vitro and in vivo evaluation of propylamycin: A semisynthetic 4,5-deoxystreptamine class aminoglycoside for the treatment of drug-resistant enterobacteriaceae and other gram-negative pathogens. J. Am. Chem. Soc. 141, 5051–5061 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhanel G. G., et al., Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev. Anti Infect. Ther. 10, 459–473 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Sonousi A., et al., Effects of the 1- N-(4-Amino-2 S-hydroxybutyryl) and 6′- N-(2-Hydroxyethyl) substituents on ribosomal selectivity, cochleotoxicity, and antibacterial activity in the sisomicin class of aminoglycoside antibiotics. ACS Infect. Dis. 4, 1114–1120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobbie S. N., et al., Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proc. Natl. Acad. Sci. U.S.A. 105, 20888–20893 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sati G. C., et al., N6′, N6′′′, and O4′ modifications to neomycin affect ribosomal selectivity without compromising antibacterial activity. ACS Infect. Dis. 3, 368–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Fernandez D., et al., 4′-O-substitutions determine selectivity of aminoglycoside antibiotics. Nat. Commun. 5, 3112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esterberg R., et al., Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Invest. 126, 3556–3566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hailey D. W., Esterberg R., Linbo T. H., Rubel E. W., Raible D. W., Fluorescent aminoglycosides reveal intracellular trafficking routes in mechanosensory hair cells. J. Clin. Invest. 127, 472–486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis S. P., et al., A novel role of cytosolic protein synthesis inhibition in aminoglycoside ototoxicity. J. Neurosci. 33, 3079–3093 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shulman E., et al., Designer aminoglycosides that selectively inhibit cytoplasmic rather than mitochondrial ribosomes show decreased ototoxicity: A strategy for the treatment of genetic diseases. J. Biol. Chem. 289, 2318–2330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.