Scavenger receptors (SRs) are a large family of cell-surface receptors that are diverse in their structure and biological function and are divided into different classes. SRs can bind to a range of ligands and enhance the elimination of altered-self or non-self targets. The functional mechanisms that lead to their clearance of harmful substances involve phagocytosis, endocytosis, adhesion, and signaling.
SRs were first described in 1979 by Michael Brown and Joseph Goldstein. As an extension of their work on the native low-density lipoprotein receptor (LDLR), Brown and Goldstein identified receptors in macrophages that could bind and endocytose modified (acetylated or oxidized) but not native LDL. Modified LDL accumulates in plasma and blood vessel walls and is considered to be a damage-associated molecular pattern (DAMP). Functional loss of these SRs can result in hypercholesterolemia, which leads to atherosclerosis and heart disease.
SRs are considered pattern recognition receptors (PRRs) that recognize not only DAMPs but also pathogen-associated molecular patterns (PAMPs), including cell-wall components of Gram-positive and Gram-negative bacteria, such as lipoteichoic acid (LTA) and lipopolysaccharide (LPS), as well as β-glucan from fungal cell walls. In this primer, we discuss fundamental concepts of SR biology, including their structural properties, ligands and functions. We also review the role of SRs in innate immunity, and the link between SRs and degenerative and autoimmune diseases.
Scavenger receptor nomenclature
A consensus formula for SR nomenclature has been developed. As an example, an SR that belongs to class B will be designated as SR-B1, i.e. SR is followed by a hyphen, then a capital letter representing the class of scavenger receptor (A–J), followed by an Arabic numeral representing the type of molecule within the class (the numbering is based on the order in which the molecules were identified). Splice variants of a molecule are designated by a dot and an Arabic numeral following the type of molecule within the class, for example (SR-B1.1). For existing splice variants, the longest variant in terms of amino acid sequence will be given the first number.
Classification of scavenger receptors
In a workshop that was organized by the United States National Institute of Allergy and Infectious Diseases, and included 15 experts in the SR field, a consensus was reached to categorize the metazoan SRs into 12 classes (Table 1 and Figure 1).
Table 1.
Current names of human scavenger receptors and consensus nomenclature.
| Consensus nomenclature | Current names |
|---|---|
| SR-A1 | SCARA1, MSR1, SR-AI |
| SR-A1.1 | SR-AII |
| SR-A3 | SCARA3, MSRL1, APC7 |
| SR-A4 | COLEC12, SCARA4, SRCL, CL-P1 |
| SR-A5 | SCARA5, TESR |
| SR-A6 | MARCO, SCARA2 |
| SR-B1 | SCARB1, SR-BI, CD36L1 |
| SR-B2 | CD36, SCARB3, PAS4 |
| SR-D1 | CD68, SCARD1 |
| SR-E1 | LOX-1, OLR1 |
| SR-E2 | Dectin-1, CLEC7A |
| SR-E3 | Mannose receptor 1, CD206, MRC1 |
| SR-E4 | Asialoglycoprotein receptor 1 (ASGPR1), CLEC4H1, HL-1 |
| SR-F1 | SCARF1, SREC-I |
| SR-F2 | MEGF10, EMARDD |
| SR-G1 | SR-PSOX, CXCL16 |
| SR-H1 | FEEL-1, STAB1, CLEVER-1 |
| SR-H2 | FEEL-2, STAB2 |
| SR-I1 | CD163, CD163A, M130 |
Figure 1. Illustrative cartoons of the structures of the different classes of scavenger receptors.
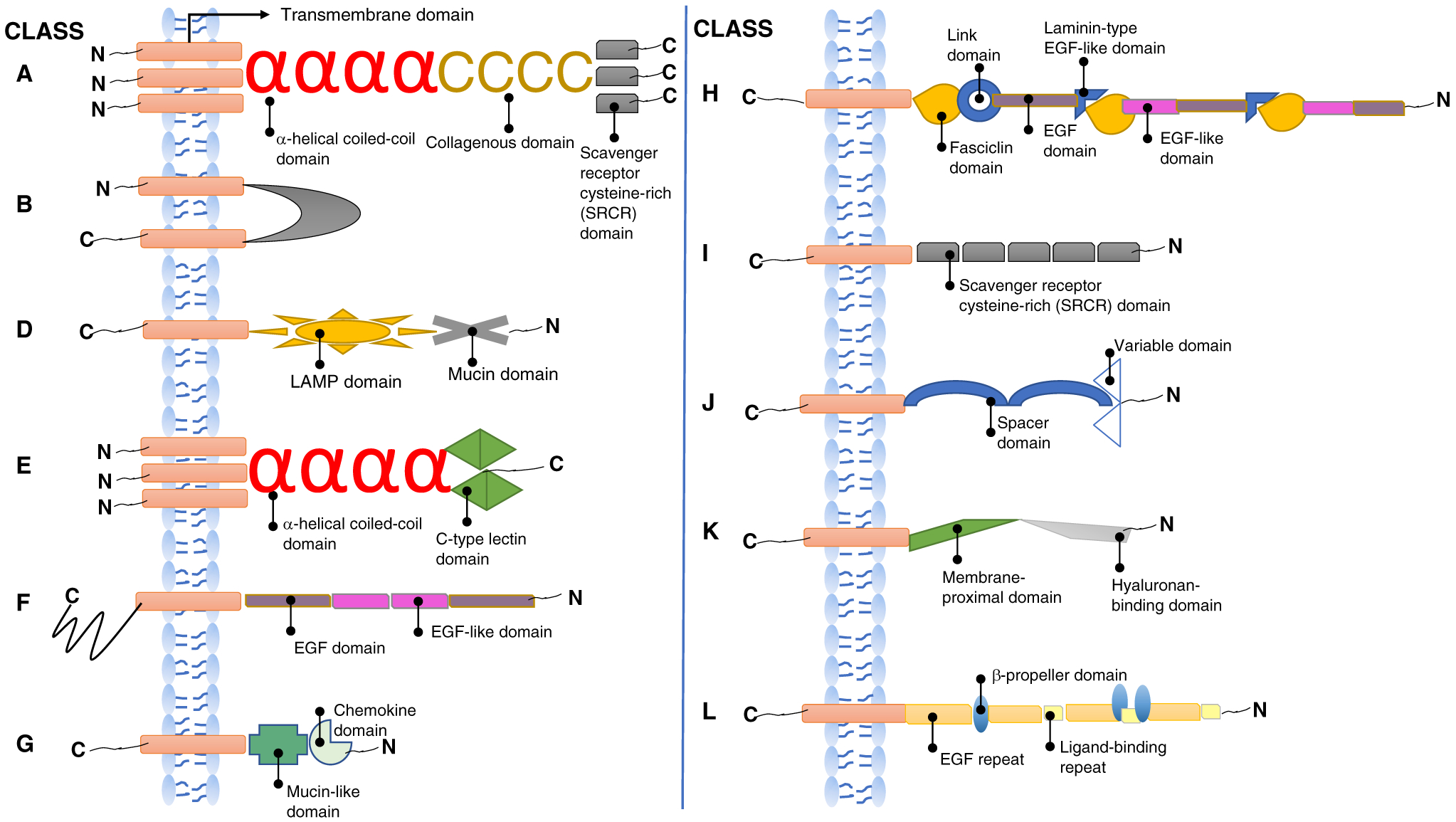
SRs are categorized into 12 classes that are all shown in this figure aside from class C, which is only found in Drosophila melanogaster. The char acteristic protein or carbohydrate domains of each class are indicated by the labels. Most SRs have single-span transmembrane domains except for those of class B, which have two transmembrane domains. All classes have short cytoplasmic tails, except for those of class F, which have long cytoplasmic tails.
C lass A scavenger receptor: SR-A
The class A SRs were the first to be purified and cloned. They are mainly expressed on tissue macrophages, Kupffer cells, and cortical and medullary thymic macrophages, as well as on subpopulations of dendritic cells and high endothelial venules. The structure of these receptors comprises an amino-terminal cytoplasmic tail, a transmembrane domain, a spacer region, an α-helical coiled-coil domain, a collagenous domain, and an ancient conserved carboxy-terminal scavenger receptor cysteine-rich (SRCR) domain (Figure 1).
S R-A1 is expressed on macrophages, mast cells, monocytes and dendritic cells. The variety of ligands that bind to SR-A1 highlight the broad ligand specificity of SRs in general and include heat shock proteins, amyloid-β (Aβ) surface molecules of Gram-negative and Gram-positive bacteria, and acetylated and oxidized low-density lipoprotein (OxLDL). Such variety highlights the potential importance of these receptors under a broad range of conditions. SR-A1.1 is an alternatively spliced variant of SR-A1 that is characterized by a short carboxyl terminus. The collagenous domain of both SR-A1 and SR-A1.1 is considered to be the ligand-binding site as a result of its ability to interact with polyanionic ligands.
Additional members of this class of SR include SR-A3, SR-A4, SR-A5, and SR-A6. SR-A3, SR-A4, and SR-A5 are expressed in different organs, including lung, heart, placenta, intestine, and epithelial cells, while SR-A6 is mainly expressed in macrophages. The role of SR-A3 is different from other class A SRs, as it protects cells against reactive oxygen species. SR-A4 serves as an endothelial receptor for lipoproteins: it is involved in the recognition and degradation of OxLDL and seems to be the endothelial counterpart of the macrophage SR-A1. SR-A5 expression is limited to epithelial cells in the airways, adrenal gland, and thymus. Similar to SR-A1, SR-A5 binds bacteria and is involved in host defense. SR-A6 has the capacity to clear bacteria from the bloodstream and lungs.
Class B scavenger receptors: SR-B
Structurally, these receptors are unique among SRs in that they are characterized by the presence of two transmembrane domains flanking an extracellular loop with amino and carboxyl termini located within the cytoplasm. The extracellular domain of these receptors is subject to extensive N-linked glycosylation, but it is not clear if this affects ligand binding.
SR-B1 was the first identified surface receptor for anionic phospholipids and high-density lipoprotein (HDL) and is a selective lipid transporter. It has a proposed unique transport function that appears to involve a process distinct from endocytosis. SR-B1 is expressed in steroidogenic cells and hepatocytes and also in cells in the arterial wall and macrophages. SR-B1 has a protective effect against atherosclerosis: removal of SR-B1 in apoE-knockout mice resulted in atherosclerosis and myocardial infarction, which can lead to death.
SR-B2 has also been found to bind HDL, but SR-B1 appears to have a more important role in HDL binding. SR-B2 was originally identified as a receptor for thrombospondin and mediates cell-to-cell interactions as well as angiogenesis. Interestingly, a more prominent role of SR-B2 is its ability to bind OxLDL, conferring on SR-B2 a more direct role in the formation of foam cells, a key step in the development of atherosclerotic lesions. SR-B2 is expressed in hematopoietic cells, such as monocytes, platelets, and macrophages, as well as in endothelial cells, testes, liver, and adrenal glands.
Class C scavenger receptors
These scavenger receptors are only present in Drosophila melanogaster: there are currently no known mammalian class C scavenger receptors.
Class D scavenger receptors: SR-D
SR-D1 is a type I transmembrane glycoprotein that contains lysosome-associated membrane protein (LAMP) and mucin-like domains. The 300 amino-acid extracellular domain of SR-D1 is rich in threonine and serine residues to which carbohydrates can attach, and the cytoplasmic tail is short.
SR-D1 is expressed on monocytes and tissue macrophages in the peritoneum, liver, microglia, lungs, and spleen. It functions as a scavenger receptor for OxLDL. However, the relative importance of SR-D1 compared to SR-B2 in OxLDL clearance is not known.
Class E scavenger receptors: SR-E
These receptors are type 2 transmembrane proteins that are characterized by the presence of a C-type lectin-like domain (CLEC). In structural terms, they belong to a subfamily of the natural killer (NK) cell CLEC receptor family. SR-E1 and SR-E2 are the only members of this class that have been well validated and shown to have SR activity.
SR-E1 is expressed on dendritic cells, macrophages, vascular endothelial cells, smooth muscle cells, platelets, and adipocytes. A unique characteristic of SR-E1 is its ability to bind the acute phase reactant C-reactive protein (CRP) and OxLDL. It is not clear if SR-E1 mediates any biological effects of CRP or if it helps to clear CRP during the resolution of inflammation. SR-E1 is known, however, to recognize PAMPs, including those from Gram-negative and Gram-positive bacteria.
SR-E2 is expressed primarily on macrophages, neutrophils, and dendritic cells. It can be regulated by microbial stimuli and cytokines, and serves as an SR for bacterial, fungal, and plant carbohydrates.
SR-E3 is a transmembrane glycoprotein that is characterized by the presence of eight C-type lectin carbohydrate recognition domains. The benefit of having so many domains and the impact of these domains on specific ligand-binding abilities are not clear. SR-E3 is involved in the phagocytosis of a variety of molecules such as mannose-coated particles and glycans on the surface of pathogenic microorganisms.
SR-E4 is a hepatocellular surface receptor that binds asialoglycoproteins, which are glycoproteins that lack terminal sialic acid residues. It plays an important role in binding, internalization and transport of multiple glycoproteins that have N-acetylgalactosamine or galactose residues. This receptor also binds to different plasma proteins, including transferrin and fibronectin, and to apoptotic cells and enzymes such as alkaline phosphatase. SR-E4 plays a role in removing circulating desialylated proteins, which protects the liver against injury.
Class F scavenger receptors: SR-F
SR-F1 was identified as an endothelial receptor for modified LDL. It is characterized by the presence of multiple extracellular epidermal growth factor (EGF) and EGF-like domains. The cytoplasmic tail is long and contains a serine/proline-rich region and a glycine-rich region. SR-F1 is expressed on endothelial cells and macrophages. This receptor binds fungal pathogens and heat shock proteins and clears apoptotic cells. SR-F2 is also involved in the clearance of apoptotic cells by binding complement protein C1q. SR-Fs are evolutionarily conserved receptors with orthologs expressed in Caenorhabditis elegans and Drosophila that also mediate clearance of fungal pathogens and apoptotic cells, attesting to their important role in host defense.
Class G scavenger receptors: SR-G
SR-G1 is a type I transmembrane glycoprotein that contains CXC chemokine and mucin-like stalk domains; there is no structural similarity between SR-G1 and other scavenger receptors. It functions as a receptor for OxLDL and phosphatidylserine and mediates phagocytosis of bacteria by antigen-presenting cells. A unique characteristic of SR-G1 is the presence of a soluble form that is produced by the cleavage of the membrane domain and this form serves as a chemoattractant for bone marrow plasma cells and T cells through its receptor CXCR6. This is the only known protein with combined scavenger receptor and chemokine activity.
Class H scavenger receptors: SR-H
Similar to class F scavenger receptors, SR-H receptors contain EGF and EGF-like domains, but they also have fasciclin and lamin-type EGF-like (FEEL) domains.
SR-H1 is primarily expressed on macrophages, hematopoietic stem cells, mononuclear cells and endothelial cells, whereas SR-H2 is expressed on sinusoidal endothelial cells. SR-H1 and SR-H2 appear to have dual functions, as they are phagocytic receptors and are also involved in the clearance of apoptotic cells and of aged red blood cells by macrophages. In addition, these SRs mediate cell–cell interactions during lymphocyte adhesion, intracellular trafficking, and angiogenesis.
Class I scavenger receptors: SR-I
Class I scavenger receptors are type I transmembrane receptors characterized by multiple ancient extracellular SRCR domains.
The prototypic class I SR is SR-I1: it contains an extracellular domain consisting of nine SRCR domains and a short cytoplasmic tail, and is predominantly expressed on macrophages and monocytes. This unique receptor plays a distinct hematological role in that it binds to haptoglobin–hemoglobin complexes to enhance the clearance of plasma hemoglobin via endocytosis. However, similar to other SRs, it also binds Gram-positive and Gram-negative bacteria. SR-I1 is involved in intracellular signaling through kinases, leading to the release of pro-inflammatory cytokines such as IL-6 and IL-10. This receptor has long been used as a marker for perivascular macrophages in the brain, allowing them to be distinguished from microglia.
SR-I2 bears 12 SRCR domains. It is expressed by cells of the myeloid lineage and is involved in the differentiation of monocytes to macrophages.
SR-I3 contains an extracellular domain with five SRCR domains and a cytoplasmic domain composed of tyrosine and serine phosphorylation motifs as well as a dileucine endocytic motif. Unlike the other members of this class, SR-I3 is expressed by γδ and αβ T cells as well as in the colon and small intestine.
Class J scavenger receptors: SR-J
SR-J1 is a member of the immunoglobulin (Ig) superfamily that can bind advanced glycation end products (AGE), the S-100 protein, and high mobility group protein box-1 (HMGB1). It consists of an extracellular variable domain and a single transmembrane domain that connects the amino-terminal ligand-binding ectodomain with a short cytoplasmic domain. The extracellular domain also contains three Ig-like regions. Several studies have characterized the role(s) of SR-J1 as a PRR that is involved in the recognition of endogenous molecules released during chronic inflammation or infection.
SR-J1 signaling may mediate oxidative stress, inflammation and apoptosis, and these events can be associated with the pathogenesis of several diseases such as neurodegenerative disease, atherosclerosis, stroke, and diabetes. SR-J1.1 is a biologically active, soluble form of SR-J1.
Class K scavenger receptors: SR-K
SR-K1 is the primary receptor for hyaluronan, an important component of the extracellular matrix. There are no common structural features between SR-K1 and other SRs. SR-K1 consists of a single-span transmembrane domain with a modest cytosolic tail with restricted direct signaling abilities. The motifs within the amino-terminal extracellular domain function as docking sites for different ligands such as hyaluronan, cytokines, extracellular glycoproteins, growth factors, and matrix metalloproteinases. The carboxy-terminal cytoplasmic tail binds to cytoskeletal elements and signaling molecules, including Src family kinases. Like other SRs, SR-K1 mediates the clearance of its extracellular matrix ligands through endocytosis.
Class L scavenger receptors: SR-L
SR-L1 is one of the largest members of the LDLR gene family. It functions as the principal clearance receptor for plasma cholesterol.
SR-L2 is characterized by a small cytoplasmic domain. It has a unique distribution, being expressed on the apical membrane of epithelial cells in the lung, kidney proximal tubule, gall bladder, and thyroid gland, as well as in steroid-responsive tissues, such as the uterus, ovary, prostate gland, and epididymis. It is also expressed at the blood–brain barrier where it binds and internalizes many ligands, such as insulin, leptin, and Aβ.
Other potential scavenger receptors: SR-Others
Other receptors have scavenger activity but do not belong to the current SR classes. These receptors include CD11b/CD18α and CD14. CD11b/CD18α is a receptor expressed by macrophages and microglia and can recognize various ligands such as LPS, fibrinogen and Aβ and promote their clearance. CD14 is a glycosylphosphatidylinositol-linked protein expressed on polymorphonuclear leukocytes and monocytes. Like other SRs, CD14 binds multiple ligands, such as LPS and lipidated microbial cell wall fragments, and helps in their clearance.
Scavenger receptors and innate immunity
As a result of their broad ligand specificity, scavenger receptors are key players in innate immunity through their ability to act as PRRs and recognize, phagocytose and clear various PAMPs on microbial surfaces including LPS and LTA. Furthermore, SRs have the ability to cooperate with other PRRs, such as Toll-like receptors (TLRs), in the recognition and phagocytosis of PAMPs and DAMPs and in cytokine production in response to different pathogens and inflammation (Figure 2).
Figure 2. Scavenger receptors cooperate with Toll-like receptors (TLRs) in the recognition and phagocytosis of multiple PAMPs and DAMPs.
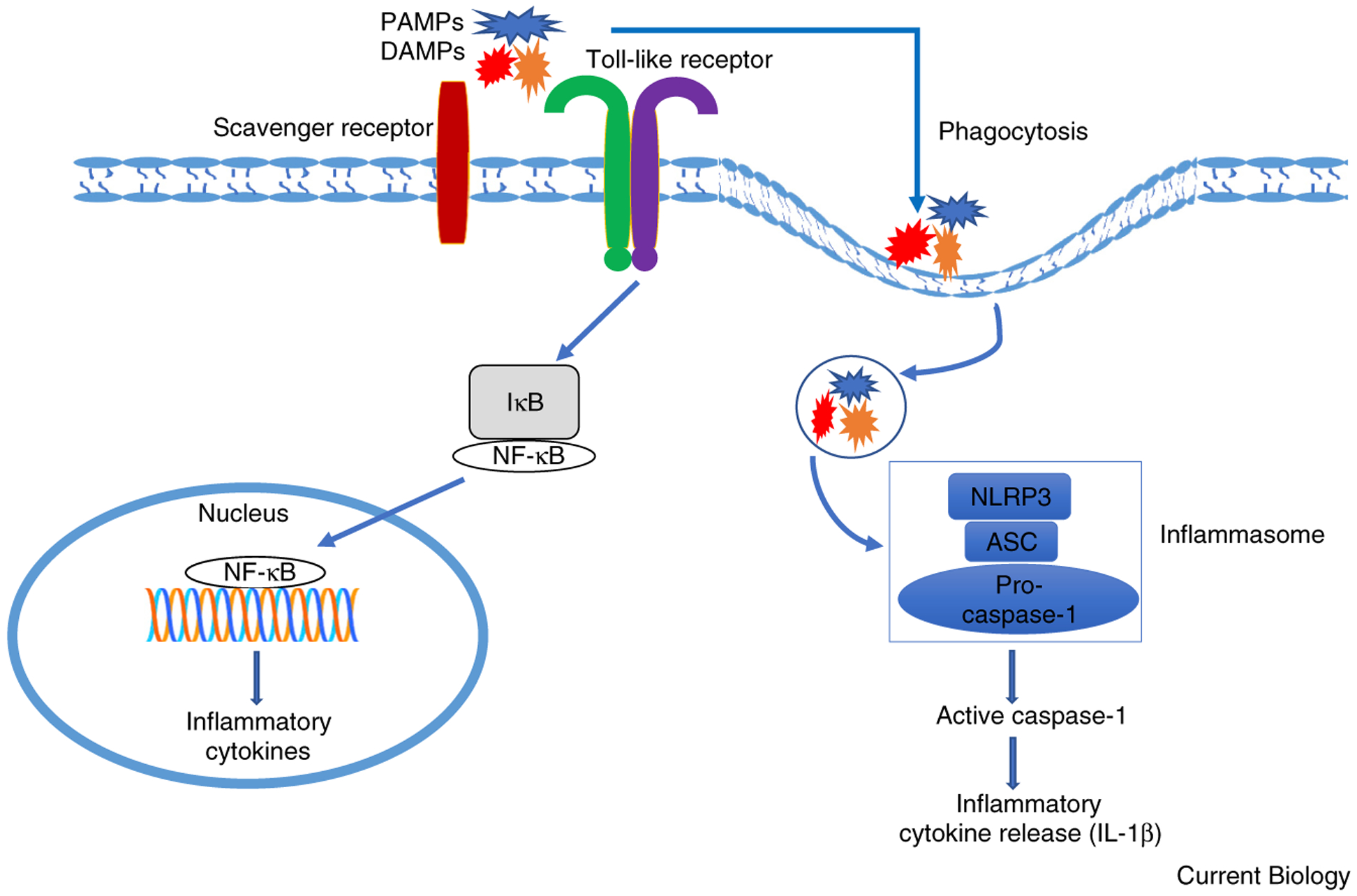
SRs can cooperate with other PRRs, such as TLRs, in the recognition and phagocytosis of PAMPs and DAMPs and in promoting intracellular signaling, which can lead to the production of pro-inflammatory mediators as well as inflammasome activation.
Several examples illustrate these concepts. Indeed, SR-As bind PAMPs such as LTA, LPS and CpG DNA. As a result of the ability of SR-As to recognize these ligands, these receptors mediate the phagocytosis of various bacterial pathogens that express these PAMPs, such as Staphylococc us aureus and Neisseria me ningitidis. This has functional consequences since deficiency in SR-A1 in mice, for example, leads to increased mortality from overwhelming infection with S. aureus.
It is not clear whether binding of SR-As to PAMPs and DAMPS is sufficient to initiate intracellular signaling events. However, there is accumulating evidence that SRs use TLRs as signaling partners. For example, SR-A1 and TLR4 cooperate in the phagocytosis of Escherichia coli, whereas SR-A1 and TLR2 cooperate to enhance the phagocytosis of S. aureus. Furthermore, by mediating the internalization of pathogens, SR-A1 promotes the inflammatory response mediated by endosomally localized TLRs, such as TLR3. SR-A6 also interacts with TLR2 and CD14 to recognize Mycobacterium tuberculosis. Based on these findings, it is tempting to conclude that SRs and TLRs have a cooperative relationship.
SR-B2 plays an important role in host defense against bacteria and fungi, such as S. aureus and Candida albicans. Similar to SR-A’s cooperation with TLR2 and TLR4, SR-B2 cooperates with TLR4 and TLR6 to enhance the innate immune response to ligands that accumulate in Alzheimer’s disease (AD) and atherosclerosis, producing cytokines and chemokines such as IL-1β and RANTES. SR-B2 also interacts with TLR2 to induce cytokines in response to S. aureus.
The common theme of collaboration between SRs and TLRs also applies to SR-E1, which binds Gram-positive bacteria, Gram-negative bacteria, and apoptotic cells. SR-E2 has been implicated in recognizing bacterial, fungal, and plant carbohydrates (β−1,3-glucans and/or β−1→6-glucans), as well as intact fungi and parasites. SR-E2 cooperates with TLR2 to induce pro-inflammatory mediators in response to β-glucan particles.
Along the same theme, SR-F1 and its C. elegans ortholog CED-1 have been implicated in innate immunity by clearing fungal pathogens and engulfing apoptotic cells. Interestingly, both receptors have established binding partners to facilitate their functions. Indeed, like many other SRs, SR-F1 also cooperates with TLRs in performing its host defense and innate immune functions.
SR-K1 is also involved in pattern recognition and innate immunity. SR-K1 binds host and microbial molecules. It interacts with TLR2, TGF-βRI, CD3, and CD14, and thereby is involved in multiple intracellular signaling pathways.
An emerging theme from all these findings is that SRs contribute to the innate immune response to infectious pathogens in two ways. Firstly, some SRs promote the clearance of infectious pathogens, leading to resolution of the infection. Secondly, other SRs collaborate with TLRs to enhance the innate immune response mediated by these receptors, ultimately resulting in resolution of the infection.
Role of scavenger receptors in disease
Over the past decade, several exciting roles for SRs have been described in the pathogenesis of many degenerativ e and au toimmune diseases, such as AD, atherosclerosis and systemic lupus erythematosus (SLE), possibly because SRs are receptors for Aβ, oxidized LDL, an d apoptotic cells.
AD is characterized by extracellular deposition of Aβ in the brain. Microglia, the primary immune cells in the brain, have the ability to activate different inflammatory pathways in response to various toxins and pathogens. In AD, microglia and astrocytes bind Aβ via SR-A1, SR-B2 and SR-F2, leading to the clearance of Aβ by phagocytosis. Deficiency in SR-A1 increases Aβ accumulation and exacerbates AD-like pathology. Furthermore, SR-B2 interacts with TLR4 and TLR6 to promote the activation of microglia by Aβ, which induces the production of reactive oxygen species, IL-1β and other proinflammatory mediators as well as inflammasome activation. These findings indicate that SRs could be both beneficial and detrimental in the progression of AD — beneficial by promoting clearance of the neurotoxic Aβ peptides, yet detrimental by contributing to disease progression and neurotoxicity through mediating the inflammatory response to Aβ. These dichotomous roles of SRs have led to their description as ‘double-edged swords’.
SRs are also associated with atherosclerosis, a chronic inflammatory disease characterized by the accumulation of modified forms of lipoproteins as ‘plaques’ in the arterial wall. The failure of macrophages to process modified lipoprotein efficiently can lead to the formation of foam cells, thereby contributing to atherosclerosis. SR-A1, SR-A4, SR-B1, SR-B2, SRD1, SR-E1, and SR-G1 can recognize OxLDL. Furthermore, upon exposure to modified LDL, SR-B2 interacts with TLR4 and TLR6, resulting in NF-κB activation and contributing to the inflammatory response associated with atherosclerosis. The distinct role of each of these SRs in atherosclerosis is not clear; however, it is possible that the role of each SR depends on the level of its expression in a certain tissue or cell type. This interesting area of investigation remains understudied.
Additionally, SRs are critically important in autoimmune diseases, such as SLE. Removal of apoptotic cells is a crucial process in immunity and in maintaining homeostasis in healthy tissues. Many cells, such as macrophages and dendritic cells, have the ability to clear apoptotic cells. A failure in the clearance of apoptotic cells can lead to their accumulation and facilitate the development of an ‘anti-self’ response to these cells, thereby contributing to autoimmune diseases. SLE is an example of this process, as patients with SLE have high levels of circulating apoptotic cells. SR-F1 binds to and phagocytoses apoptotic cells leading to their clearance, and, in mouse models, SR-F1 deficiency impairs the engulfment of apoptotic cells leading to the development of a syndrome similar to SLE. Because of the limited available options to treat SLE, these findings may have therapeutic implications for this disease.
Conclusions
SRs are phagocytic and innate immune recognition receptors that play a crucial role as regulators of inflammatory signaling. These receptors are involved in multiple physiological and pathological processes, including interactions with TLRs and delivery of ligands to different cellular compartments. Additionally, the roles of these receptors in many degenerative and autoimmune diseases, as well as their potential as targets for therapeutic interventions to treat various disorders, warrant further study.
FURTHER READING
- Areschoug T, and Gordon S (2009). Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol 11, 1160–1169. [DOI] [PubMed] [Google Scholar]
- Canton J, Neculai D, and Grinstein S (2013). Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol 13, 621–634. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, and El Khoury J (2013). Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun 4, 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves DR, and Gordon S (2009). The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res 50, S282–S286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S, Izzy S, Sen P, Morsett L, and El Khoury J (2018). Microglia in neurodegeneration. Nat. Neurosci 21, 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M (1997). The other side of scavenger receptors: pattern recognition for host defense. Curr. Opin. Lipidol 8, 275–280. [DOI] [PubMed] [Google Scholar]
- Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, et al. (2009). Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med 206, 637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas MR, Baldwin CL, Bollyky PL, Bowdish DME, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, et al. (2017). A consensus definitive classification of scavenger receptors and their roles in health and disease. J. Immunol 198, 3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Pendergraft WF, Prasad A, Byrne MH, Iram T, Blanchette CJ, Luster AD, Hacohen N, Khoury J. El., and Means TK (2013). The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat. Immunol 14, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K, and El Khoury J (2012). Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimers. Dis 2012, 489456. [DOI] [PMC free article] [PubMed] [Google Scholar]


