Abstract
Early phase clinical trials are evaluating the feasibility, safety and therapeutic potential of ex vivo expanded regulatory T cells (Treg) in transplantation. A limitation is the paucity of naturally-occurring Treg numbers in peripheral blood. Hence, protracted ex vivo expansion is required to obtain sufficient Treg in order to meet target cell doses. Since cytokine administration has been used successfully to mobilize immune cells to the peripheral blood in experimental and clinical studies, we hypothesized that granulocyte-macrophage-colony stimulating factor (GM-CSF) and granulocyte-CSF (G-CSF) administration would enhance Treg percentages in leukapheresis products of rhesus monkeys. Following combined GM-CSF and G-CSF administration, the incidence of Treg in peripheral blood and leukapheresis products was elevated significantly, where approximately 3.7x106/kg CD4+CD25hiFoxp3hi or 6.8x106/kg CD4+CD25hiCD127lo Treg can be collected from individual products. Mobilized Treg expressed a comparable repertoire of surface markers, chemokine receptors and transcription factors to naïve monkey peripheral blood Treg. Furthermore, when expanded ex vivo, mobilized leukapheresis product and peripheral blood Treg exhibited similar ability to suppress autologous CD4+ and CD8+ T cell proliferation. These observations indicate that leukapheresis products from combined GM-CSF and G-CSF-mobilized individuals are a comparatively rich source of Treg and may circumvent long-term ex vivo expansion required for therapeutic application.
1 |. INTRODUCTION
Regulatory T cells (Treg), a rare subset of CD4+ T cells, play a crucial role in the maintenance of self-tolerance,1 as well as in the induction and maintenance of tolerance to organ allografts.2, 3 Several clinical trials are currently testing the feasibility, safety and efficacy of ex vivo expanded Treg (www.clinicaltrials.gov) (supplemental references). The rarity of naturally-occurring (thymic-derived) Treg represents a major limitation for Treg therapeutic application. Hence, large-scale production is a prerequisite for Treg therapy in the clinic. Furthermore, low starting numbers of purified Treg necessitate extended periods of ex vivo expansion (up to several weeks) to obtain sufficient numbers for adoptive cell transfer.
Cytokine administration has been used routinely to mobilize immune cells, particularly hematopoietic stem cells, to the peripheral blood.4, 5 Granulocyte-colony stimulating factor (G-CSF) (= colony stimulating factor [CSF3]) is a hematopoietic growth factor that modulates the generation and differentiation of the myeloid lineage. Several studies have demonstrated its immunoregulatory influence in vitro6 and in vivo,7, 8 when administered either alone or in combination with other cytokines. In addition, G-CSF administration is associated with a tolerant gene signature in T cells9 and enhanced incidences of Treg10 in humans. Similarly, granulocyte-macrophage (GM)-CSF (= CSF2) promotes Treg expansion in experimental graft-versus-host disease (GVHD)11 and autoimmune disorders.11, 12 Under these conditions, GM-CSF administration promotes the incidence of suppressive Treg, either directly,13 or indirectly through the induction of regulatory dendritic cells (DCreg).12
Leukapheresis products have been used as a rich source of Treg from non-mobilized humans.14–16 However, in these studies, extensive ex vivo expansion of isolated Treg was still required in order to achieve sufficient Treg numbers for therapeutic application. We hypothesized that combined GM-CSF and G-CSF administration would improve the Treg yield in leukapheresis products, which in turn may reduce the need for extended periods of ex vivo expansion. We used a clinically-relevant nonhuman primate (NHP) model to evaluate the impact of combined GM-CSF and G-CSF administration (compared with either GM-CSF or G-CSF alone) on the incidence of peripheral blood Treg, and on the phenotype and function of leukapheresis product Treg, compared with steady-state, naturally-occurring peripheral blood Treg, in rhesus macaques.
2 |. METHODS
2.1 |. Animals
Captive-bred, male Indian juvenile rhesus monkeys (weight 6-8 kg) were obtained from the NIAID–sponsored NHP colony (Alpha Genesis, Inc., Yemassee, SC). All animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and conducted under a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Environmental enrichment was provided.
2.2 |. Cytokine mobilization
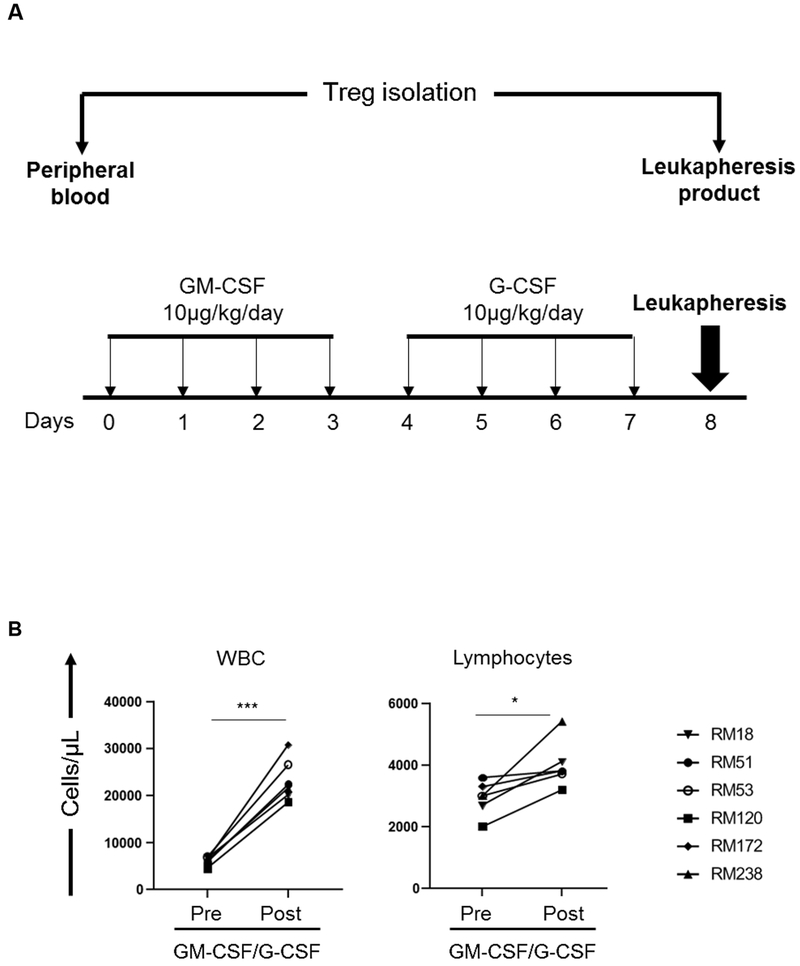
Recombinant human (rh) GM-CSF (Leukine; 10 μg/kg/day), or rh G-CSF (Neupogen; 10 μg/kg/day) were administered by subcutaneous injection. Animals received either rh G-CSF only for 4 days (n=2), or rh GM-CSF for 4 days followed by rh G-CSF for an additional 4 days (n=2) (Figure 1). In an additional group of animals (n=6), combined GM-CSF and G-CSF administration was followed by leukapheresis on day 8 (Figure 2), using a dedicated COBE® Spectra Apheresis System (Lakewood, CO, USA), as described.17
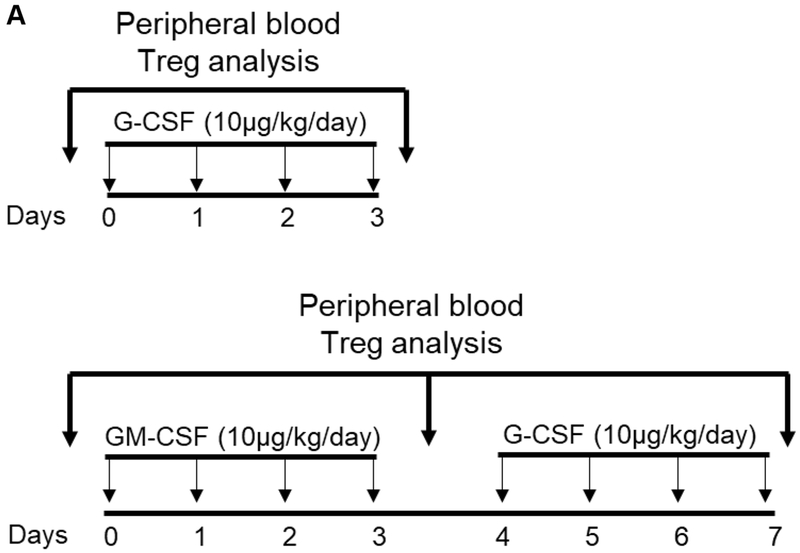
FIGURE 1. Impact of G-CSF, GM-CSF, or combined GM-CSF/G-CSF administration on the percentages of peripheral blood Treg in rhesus monkeys.
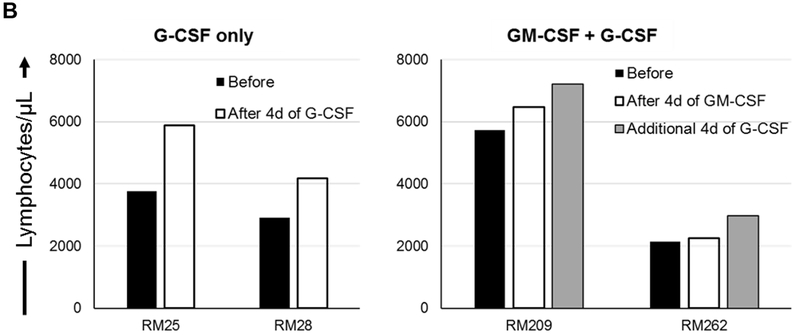
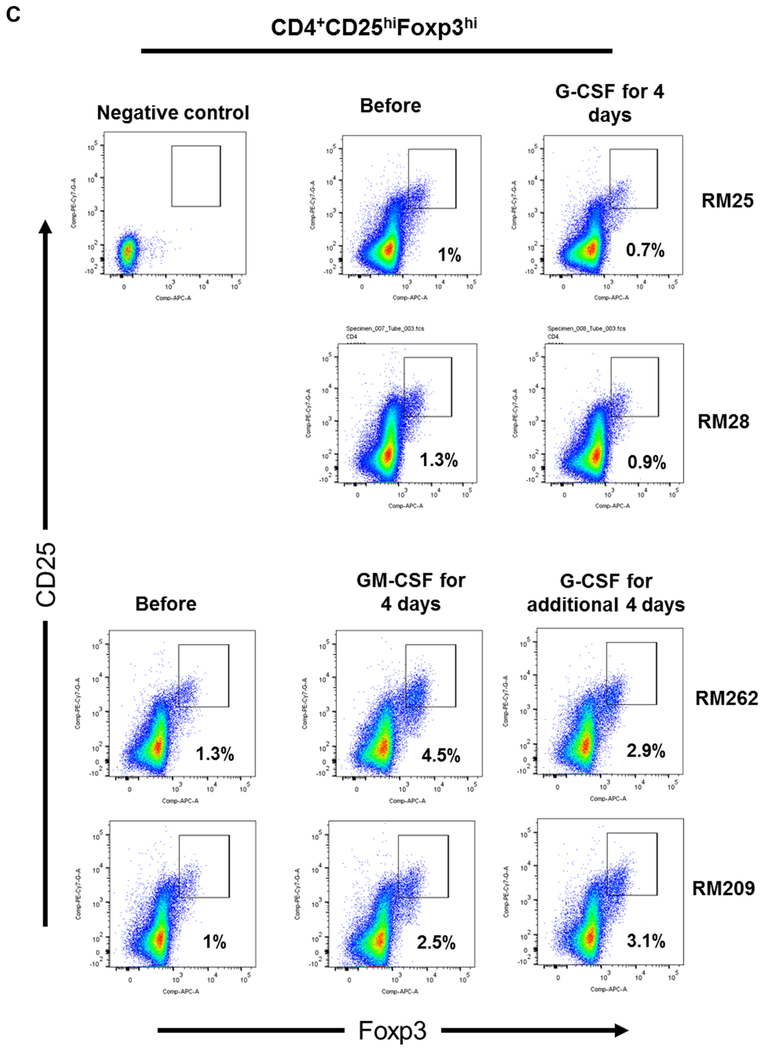
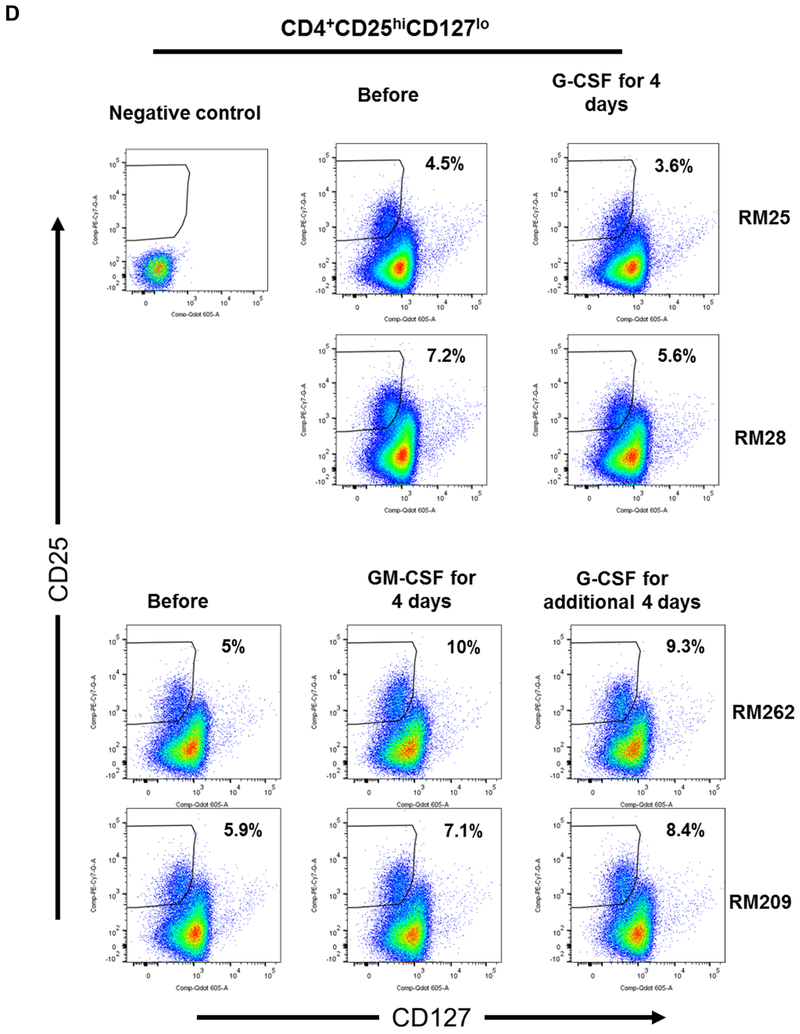
(A) Monkeys received subcutaneous injections of either recombinant (r) human G-CSF (Neupogen 10 μg/kg/day) only for four days (n=2), or r human GM-CSF (Leukine; 10 μg/kg/day) for four days followed by r human G-CSF (Neupogen 10 μg/kg/day) for an additional four days (n=2). (B) Absolute numbers of lymphocytes in peripheral blood before and after G-CSF, GM-CSF, or GM-CSF/G-CSF administration. (C) Percentages of CD4+CD25hiFoxp3hi Treg in peripheral blood before and after G-CSF, GM-CSF, or GM-CSF/G-CSF administration. (D) Percentages of CD4+CD25hiCD127lo Treg before and after G-CSF, GM-CSF, and GM-CSF/G-CSF administration. Peripheral blood samples were collected before, after G-CSF administration, after GM-CSF administration, and after combined GM-CSF/G-CSF administration. Top left dot plots indicate isotype (negative) controls.
FIGURE 2. Impact of GM-CSF/G-CSF administration on the absolute numbers of WBC and lymphocytes and the incidences of T cell subsets in peripheral blood and in leukapheresis products.
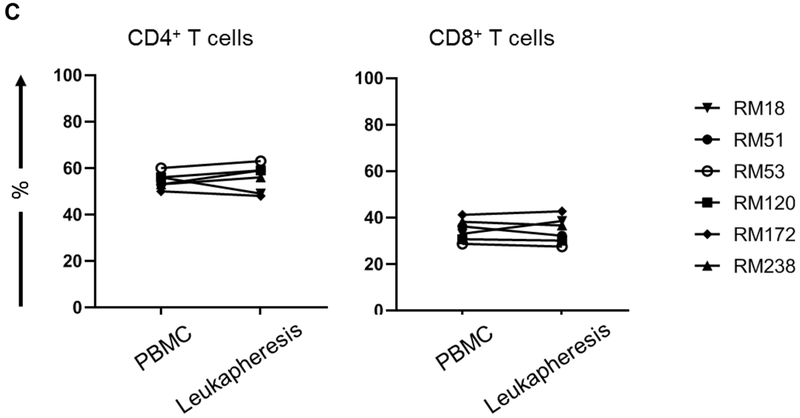
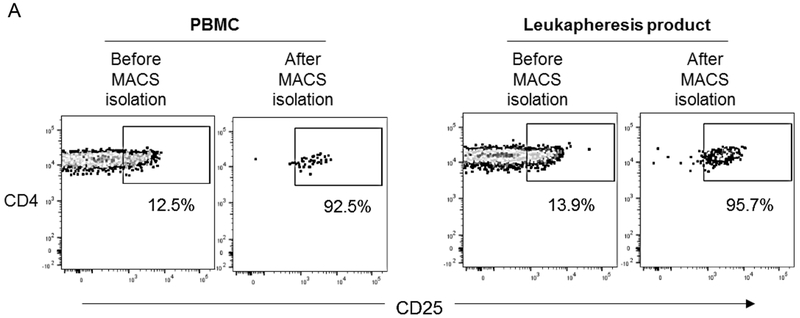
(A) Monkeys received subcutaneous injections of r human GM-CSF (Leukine; 10 μg/kg/day for four days), followed by r human G-CSF (Neupogen 10 μg/kg/day for an additional four days). Leukapheresis was performed on day 8. Peripheral blood mononuclear cells (PBMC) were collected from the peripheral blood and leukapheresis product, respectively, before and after GM-CSF/G-CSF administration for subsequent Treg analysis. (B) Absolute numbers of WBC and lymphocytes. Peripheral blood samples were collected before (Pre) and after (Post) GM-CSF/G-CSF administration (n=6). (C) Percentages of CD4+ and CD8+ T cells in normal PBMC (before GM-CSF/G-CSF administration) and leukapheresis products (after GM-CSF/G-CSF administration) were determined by flow cytometry (n=6). * p<0.05, *** p<0.001.
2.3 |. Quantitation of immune cell subsets
Absolute numbers and percentages of total white blood cells (WBC) and lymphocytes were analyzed at the clinical laboratory of the University of Pittsburgh Medical Center. Rhesus monkey CD3+, CD4+, CD8+, CD4+CD25hiCD127lo and CD4+CD25hiFoxp3hi T cell subsets in peripheral blood and leukapheresis products were assessed by flow cytometry, as described.18
2.4 |. PBMC isolation
Briefly, peripheral blood was diluted with PBS at 1:1 volume ratio, then overlaid (30 ml) on 12 ml Ficoll-Paque Plus (GE Healthcare Life Sciences AB), spun for 20 min at 1500 rpm at room temperature, and the buffy coat collected. PBMC were treated with red blood cell (RBC) lysis buffer (150 mM NH4C; 1 mM KHCO3; 0.1 mM Na2EDTA) and absolute cell number and cell viability evaluated with trypan blue. PBMC collected from peripheral blood and leukapheresis products were cryopreserved (in 70% RPMI-1640; 20% v/v fetal calf serum; 10% DMSO) until further analysis.
2.5 |. Isolation and ex vivo expansion of CD4+CD25hiTreg
Treg were isolated from stored PBMC using NHP CD4+CD25hi cell isolation kits (Miltenyi Biotech, Auburn, CA), following the manufacturer’s protocol. For ex vivo expansion of Treg, artificial antigen-presenting cells (aAPC; L-32) that express CD32 (Fc receptor), CD58 (LFA-3; CD2 binding) and CD80 (kindly provided by Dr. M.K. Levings, University of British Columbia, Vancouver, Canada) were used to promote polyclonal Treg expansion, as we have described in NHP . Isolated Treg were cultured on irradiated (80 Gy) and anti-CD3 monoclonal antibody (mAb)-preloaded L-32 cells at 1:1 ratio in X-VIVO 15 medium (BioWhittaker, Lonza, Allendale, NJ) supplemented with 10% v/v heat-inactivated human AB serum in the presence of 2000 U/ml r human IL-2 (R&D Systems, Minneapolis, MN). Half of the media was changed every 3 days. On day 6, 12, and 18, non-adherent Treg were harvested and re-stimulated with anti-CD3 mAb-preloaded L-32 cells.
2.6 |. Treg phenotype analysis
The following fluorochrome-labeled mAbs were used: BUV395-anti-CD3 (clone number SP34-2), FITC-anti-CD45RA (5H9), PECF594-anti-CTLA4 (BNI3), AF488-anti-GATA3 (L50-823), BUV737-anti-CD8 (SK1), Alexa Fluor®647-Anti-Stat3 (pY705) (4/P-STAT3), and Alexa Fluor®488 Anti-Stat5 (pY694) (47/Stat5) from BD Biosciences (San Jose, CA). APCCy7-anti-CD4 (OKT4), PerCP-anti-CD8α (RPA-T8), BV605-anti-CD127 (A019D5), PE-anti-Helios (22F6), FITC-anti-CXCR3 (G025H7), PETexRed-anti-CCR4 (L291H4), PE-anti-CCR7 (G043H7), PETexRed-anti-Tbet (4B10), and Brilliant Violet 605™ anti-CD25 (BC96) were from BioLegend (San Diego, CA). PECy7-anti-CD25 (4E3), APC-anti-Foxp3 (PCH101), PE-anti-RORγt (AFKJS-9), eFluor450-Foxp3 (PCH101) were from Invitrogen (Carlsbad, CA).
Following live/dead staining with Zombie Aqua™ Fixable Viability Kit (BioLegend) at 4°C for 15 min, cell suspensions were stained with CD3, CD4, CD8, CD25, CD127, CD45RA, CXCR3, CCR4 and CCR7 Abs at 4°C for 20 min. The cells were then fixed and permeabilized for 45 min at 4°C using Fixation/Permeabilization buffer (eBioscienceTM; Invitrogen). Thereafter, intracellular staining was performed for Foxp3, CTLA4, Helios, GATA3, T-bet and RORγt Abs at 4°C for 40 min.
For analysis of pSTAT3 and pSTAT5 expression, after live/dead staining, the cells were stained with CD3, CD4, CD8, and CD25 Abs at 4°C for 20 min. They were then fixed and permeabilized using PerFix Reagent Kits (Beckman Coulter Life Sciences, Indianapolis, IN). After fixation/permeabilization, the cells were stained with Foxp3, pSTAT5 and pSTAT3 Abs at room temperature for 30 min.
Data were acquired on a LSR FORTESSA (BD Bioscience) and analyzed by Flowjo software version 10 (TreeStar Inc., Ashland, OR). Relative mean fluorescence intensity (MFI) was determined by dividing the MFI value of the stained sample with that of the negative (isotype) control (obtained from unstained T cell subsets; actual MFI values are shown in supplemental Figure 4).
2.7 |. TSDR methylation analysis
Bisulfite pyrosequencing was performed by EpigenDx, inc. Methylation analysis of the Treg-specific demethylated region (TSDR) within the Foxp3 locus (−2361 bp to −2253 bp from ATG) was performed. The methylation status of 8 CpG sites was evaluated. The samples are 24-days expansion of PBMC-isolated Treg (n=3) and Leukapheresis-isolated Treg (n=3). Effector CD4+CD25−T cells were used as control (n=2).
2.8 |. Treg suppressive function
Autologous CD2+T cells were isolated from stored rhesus PBMC using CD2 MicroBeads NHP (Miltenyi) and labeled with 0.625μM CFSE (Invitrogen) for 10min at 37°C, then stimulated with NHP-specific anti-CD2/CD3/CD28 microbeads (T Cell Activation/Expansion Kit, NHP, Miltenyi) at a cell:bead ratio of 1:2 for 3 days. Expanded autologous Treg were labeled with 1 μM Violet Proliferation Dye 450 (VPD450, BD Biosciences) at 37°C for 15 min and then added at the start of cultures at the indicated ratios. The percentages of divided CD4+ and CD8+T cells were calculated by Flowjo software. Percent suppression was determined as (percent divided T cells without addition of Treg – percent divided T cells with Treg)/percent divided T cells without addition of Treg x 100%.
2.9 |. Statistical analysis
Differences between means were evaluated using Student’s ‘t’ test. Statistical analysis was conducted using the standard formula in Prism GraphPad Software (San Diego, CA).
3 |. RESULTS
3.1 |. Impact of G-CSF, GM-CSF, or combined GM-CSF/G-CSF administration on the percentage of peripheral blood Treg in rhesus monkeys
The impact of combined GM-CSF/G-CSF administration on the incidence of peripheral blood Treg, in comparison to either G-CSF alone or GM-CSF alone was evaluated. Two animals received G-CSF (10 μg/kg/day) for 4 days, and two animals received GM-CSF (10 μg/kg/day) for 4 days followed by G-CSF (10 μg/kg/day) for additional 4 days (Figure 1A).
Following the administration of G-CSF alone, peripheral blood lymphocyte absolute numbers were increased. Similarly, following the administration of GM-CSF alone, lymphocyte absolute numbers were increased, and continued to increase after additional 4 days of G-CSF administration (Figure 1B). The percentages of peripheral blood CD4+CD25hiFoxp3hi (Figure 1C) and CD4+CD25hiCD127lo (Figure 1D) Treg populations did not increase after the administration of G-CSF alone. In contrast, Treg percentages were increased after GM-CSF alone, with higher percentages of Treg maintained after an additional 4 days of G-CSF administration, compared to before GM-CSF administration.
These observations suggest that while GM-CSF administration might be superior to G-CSF in enhancing the incidence of peripheral blood Treg, G-CSF administration promotes total peripheral blood lymphocyte numbers. Furthermore, G-CSF administration following GM-CSF administration enhances peripheral blood Treg percentages in rhesus monkeys.
3.2 |. Impact of combined GM-CSF/G-CSF administration on total WBC and lymphocytes in peripheral blood and the incidence of CD4+ and CD8+ T cells in leukapheresis products
In response to combined GM-CSF/G-CSF administration (Figure 2A), WBC and absolute lymphocyte numbers increased significantly in the peripheral blood. WBC increased from 6.1±0.9 x 103 in normal blood to 23±4.5 x 103 cells/μL (p<0.001), while lymphocytes increased from 2.9±0.5 x 103 to 4.0±0.7 x103 cells/μL (p<0.05) (Figure 2B). However, the incidences of CD4+ and CD8+ T cells did not differ significantly before and after GM-CSF/G-CSF administration (55±4% and 56±6%; 35±5% and 35±6%, respectively) (Figure 2C). Thus, although total WBC and lymphocyte numbers increased significantly in peripheral blood after GM-CSF/G-CSF administration, the incidences of CD4+ and CD8+ T cells in peripheral blood (before GM-CSF/G-CSF) and leukapheresis products (after GM-CSF/G-CSF) were comparable.
3.3 |. Combined GM-CSF/G-CSF administration enhances the incidence and yield of Treg in leukapheresis products
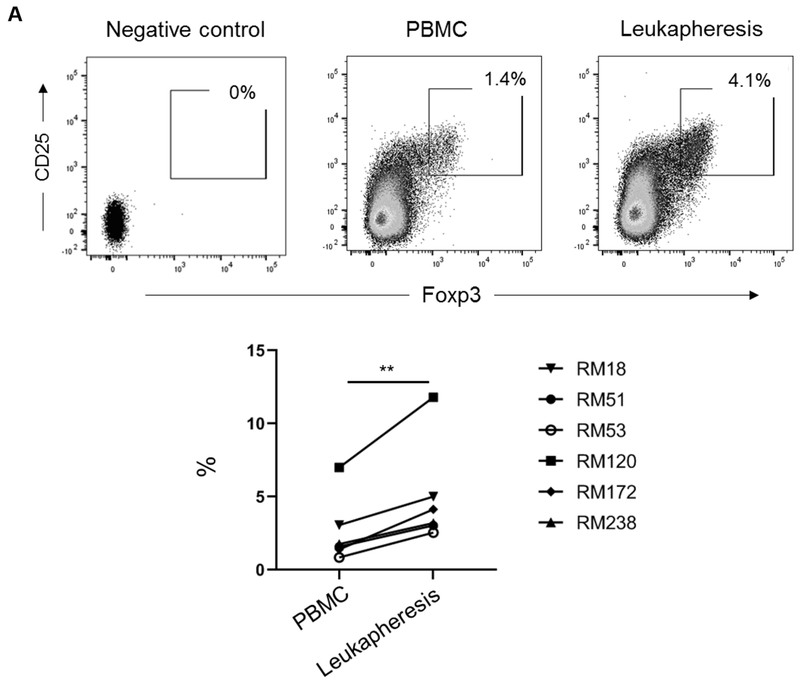
Before GM-CSF/G-CSF administration, the mean incidence of CD4+CD25hiFoxp3hi Treg in normal peripheral blood was 2.6±2.3%. After GM-CSF/G-CSF administration, the mean incidence of CD4+CD25hiFoxp3hi Treg in leukapheresis products was 5±3.5% (p<0.01) (Figure 3A). Since average of 2.5 billion cells were collected in individual whole leukapheresis, the mean absolute number of CD4+CD25hiFoxp3hi Treg that can be isolated from individual leukapheresis product was 26 ± 7.3 x 106 (i.e. 3.7 ± 1.2 x 106/kg) (Table 1).
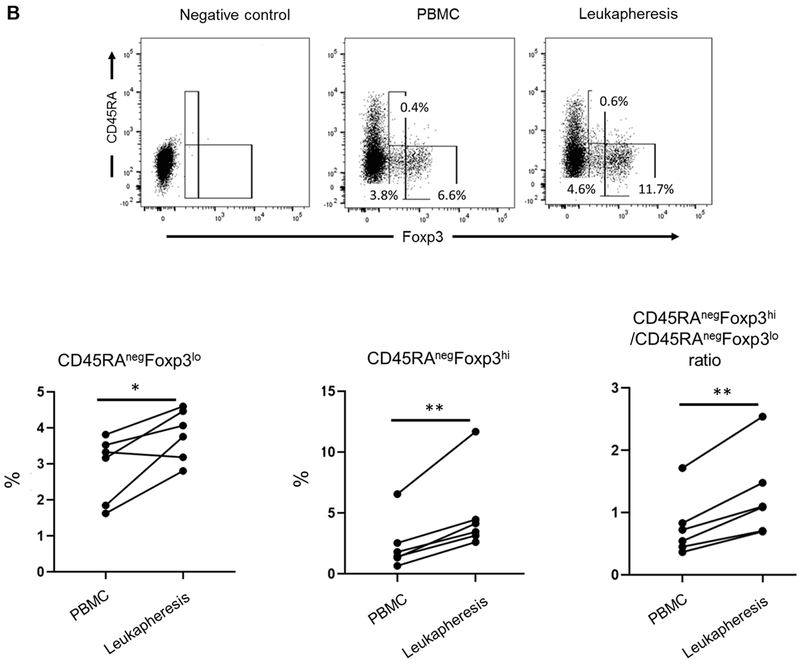
FIGURE 3. Incidence and phenotype of CD4+CD25hiFoxp3hi Treg in peripheral blood before and in leukapheresis products after GM-CSF/G-CSF administration.
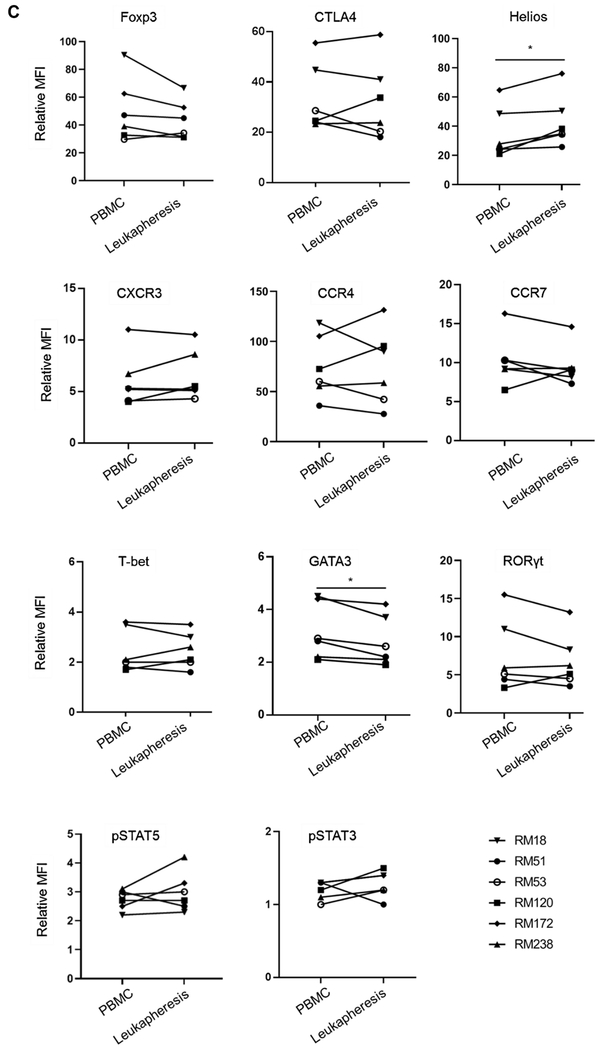
(A) Percentages of CD4+CD25hiFoxp3hi Treg in PBMC and leukapheresis products were determined by flow cytometry. Dot plots represent data from one animal (upper panel) representative of six animals. The percentage of CD4+CD25hiFoxp3hi Treg in all six animals are shown in the lower panel. (B) Percentages of CD4+CD45RAnegFoxp3lo cells and CD4+CD45RAnegFoxp3hi cells (upper panel), and the ratios of CD4+CD45RAnegFoxp3hi / CD4+CD45RAnegFoxp3lo cells in all six animals (lower panel) are shown. Top left dot plots indicate isotype (negative) control. (C) Treg-associated markers (Foxp3, CTLA4 and Helios), chemokine receptors (CXCR3, CCR4 and CCR7), transcription factors (T-bet, GATA3 and RORγt) and pSTAT5 and pSTAT3 expression on Treg (n=6). * p<0.05, ** p<0.01. MFI = mean fluorescence intensity (Actual MFI values are shown in supplemental figure 4).
Table 1.
Incidences and absolute numbers of Treg in leukapheresis products (n=6)
| Animal # | Body weight | Total cell number (PBMC) | CD4+CD25hiCD127lo cells* | CD4+CD25hiFoxp3hi cells* | ||||
|---|---|---|---|---|---|---|---|---|
| (kg) | (x109) | (%) | (x106) | (x106/kg) | (%) | (x106) | (x106 /kg) | |
| RM18 | 6.2 | 3.0 | 8.8 | 51.8 | 8.4 | 5.0 | 29.6 | 4.8 |
| RM51 | 6.9 | 1.7 | 6.6 | 44.1 | 6.4 | 3.1 | 20.3 | 2.9 |
| RM53 | 8.3 | 2.8 | 6.7 | 46.5 | 5.6 | 2.6 | 17.9 | 2.2 |
| RM120 | 7.1 | 1.6 | 14.5 | 47.2 | 6.7 | 11.8 | 38.4 | 5.4 |
| RM172 | 7.1 | 2.9 | 9.0 | 55.4 | 7.8 | 4.1 | 25.4 | 3.6 |
| RM238 | 7.0 | 2.9 | 5.8 | 43.7 | 6.2 | 3.2 | 24.2 | 3.5 |
| Mean ± SD | 7.1 ± 0.7 | 2.5 ± 0.6 | 8.6 ± 3.2 | 48.1 ± 4.6 | 6.8 ± 1.0 | 5.0 ± 3.5 | 26.0 ± 7.3 | 3.7 ± 1.2 |
Cell numbers were calculated based on the percentages of total CD3+ cells in leukapheresis products.
Next, we evaluated CD45RA expression in conjunction with Foxp3 expression on Treg before and after GM-CSF/G-CSF administration. The percentages of CD45RAnegFoxp3hi cells increased significantly (p<0.01) in the leukapheresis products (after GM-CSF/G-CSF) compared to peripheral blood (before GM-CSF/G-CSF). Also, the percentages of CD45RAnegFoxp3lo population increased significantly (p<0.05) in the leukapheresis products compared to peripheral blood. However, the ratios of the CD45RAnegFoxp3hi cells to the CD45RAnegFoxp3lo cells were significantly higher (p<0.01) in the leukapheresis products compared to PBMC (Figure 3B). This was associated with a significant increase in the CD4+CD25hiFoxp3hi T cell population relative to the CD4+CD25loFoxp3lo population (Supplementary Figure 2).
We also ascertained the incidence of Treg in the leukapheresis products based on CD25 and CD127 expression, since these are the most commonly-used cell surface markers for Treg isolation/sorting (Supplementary Figure 3). The mean incidence of CD4+CD25hiCD127lo Treg was significantly higher in leukapheresis products after GM-CSF/G-CSF administration (8.6 ± 3.2%) than normal peripheral blood before GM-CSF/G-CSF administration (6.7 ± 2.1%) (p<0.05). Hence, the mean absolute number of CD4+CD25hiCD127lo Treg that can be isolated from individual leukapheresis product was 48.1 ± 4.6 x106 cells (i.e. 6.8 ± 1.0 x 106 cells/kg) (Table 1).
In our experience, up to 1.5 x 106 (0.2 x 106/kg) CD4+CD25hiFoxp3hi, or 2.8 x 106 (0.4 x 106/kg) CD4+CD25hiCD127lo Treg can be isolated from peripheral blood of a 7 kg naïve monkey. Accordingly, these data indicate that larger numbers of Treg can be isolated from GM-CSF/G-CSF-mobilized leukapheresis products on a body weight basis, in comparison to maximal peripheral blood draws from naïve subjects.
3.4 |. Phenotypic analysis of Treg in leukapheresis products following GM-CSF/G-CSF administration
The phenotype of Treg in leukapheresis products compared with PBMC collected before GM-CSF/G-CSF administration is shown in Figure 3C. No significant differences were found in the relative MFI of Foxp3 and CTLA4 expression. However, Helios expression by Treg was significantly higher after GM-CSF/G-CSF administration (p<0.05). Chemokine receptor (CCR4, CCR7 and CXCR3) expression was comparable. Also, expression of the transcription factors T-bet and RORγt, as well as phosphorylated signal transducer and activator of transcription 3 and 5 (pSTAT3 and pSTAT5) by Treg, was unaffected. However, the transcription factor GATA3 was minimally reduced after GM-CSF/G-CSF administration.
These data indicate that, while GM-CSF/G-CSF administration enhances Treg frequency and numbers, leukapheresis product Treg exhibit a similar phenotype to naturally-occurring rhesus peripheral blood Treg.
3.5 |. Ex vivo expansion and suppressive function of Treg obtained from GM-CSF/G-CSF-mobilized leukapheresis products
Next, we assessed the impact of GM-CSF/G-CSF administration on the suppressive function of peripheral blood Treg. CD4+CD25hi Treg were isolated simultaneously using MACS from peripheral blood PBMC obtained before and leukapheresis products obtained after GM-CSF/G-CSF administration (n=3).
Initially, comparable numbers of cells from peripheral blood PBMC and leukapheresis product were used for CD4+CD25hi Treg isolation. After MACS isolation, average of 170 ± 70 x 103 Treg were obtained from peripheral blood PBMC, and average of 270 ± 30 x 103 Treg were obtained from leukapheresis products. The purity of the isolated Treg was slightly but not significantly lower before, compared with after GM-CSF/G-CSF administration (87.9 ± 6.6% and 94.9 ± 2.3%, respectively) (Figure 4A and Table 2).
FIGURE 4. Isolation, expansion and phenotype of Treg isolated from PBMC before and in leukapheresis products after GM-CSF/G-CSF administration.
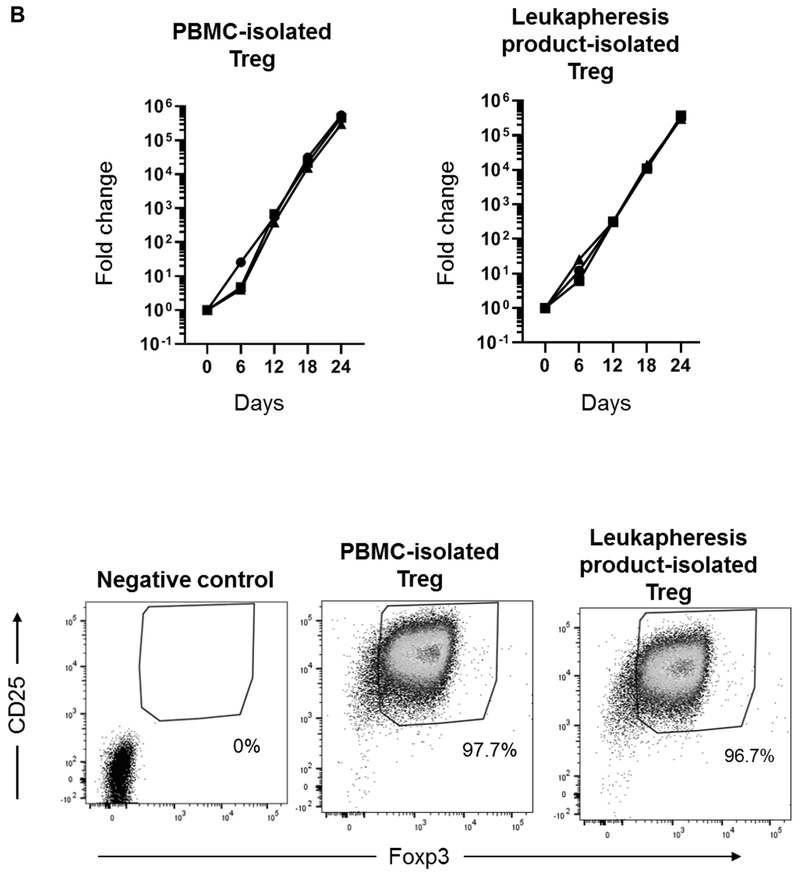
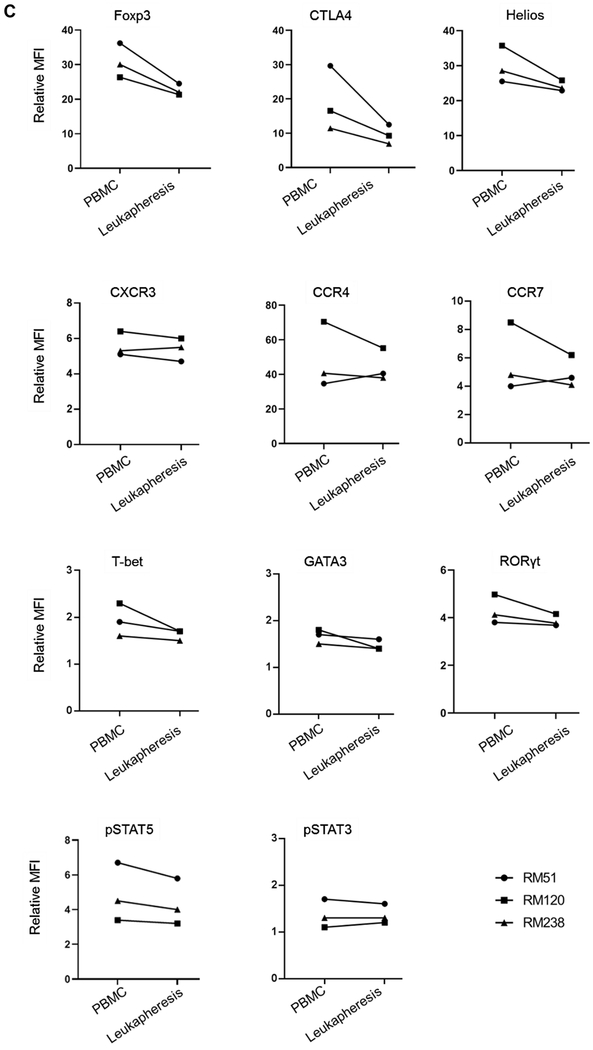
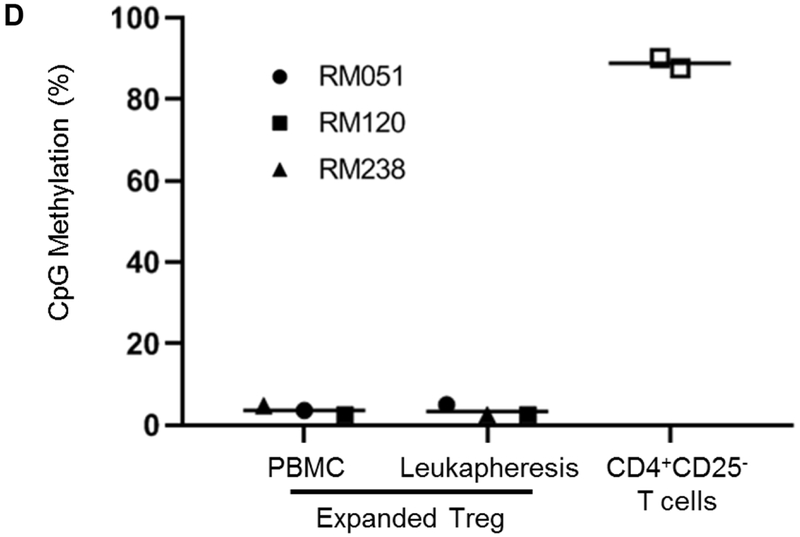
(A) Following MACS isolation, the purity of isolated Treg was evaluated. The percentages of CD4+CD25hi Treg (in PBMC and leukapheresis products) were evaluated before and after MACS isolation. Dot plots are from one animal representative of three animals. (B) Isolated Treg were expanded ex vivo for 24 days in co-culture with L-cells (upper panels), and the fold-increase determined (n=3). Percentages of CD4+CD25hiFoxp3hi expanded Treg were evaluated at 24 days by flow cytometry. Left dot plot indicates isotype (negative) control (C) Expanded Treg were evaluated for expression of Treg-associated markers (Foxp3, CTLA4 and Helios), chemokine receptors (CXCR3, CCR4 and CCR7), transcription factors (T-bet, GATA3 and RORγt) and pSTAT5 and pSTAT3 expression levels (n=3). MFI = mean fluorescence intensity. (D) The CpG methylation status of Foxp3 TSDR (Treg-specific demethylation region) in expanded Treg on day 24. (n=3). CD4+CD25− effector T cells were used as controls (n=2). The y axis shows methylation status of 8 CpG sites on average.
Table 2.
Purity, yield and ex vivo expansion of Treg from normal PBMC or leukapheresis products (n=3)
| Animal # | Total cell number | MACS-isolated CD4+CD25hi Treg | Expanded CD4+CD25hiFoxp3hiTreg† | ||||
|---|---|---|---|---|---|---|---|
| (x106) | Purity (%) | Yield (%) | (x103) | Fold change (x103) | Purity (%) * | ||
| RM51 | PBMC | 28.8 | 90.9 | 0.55 | 160 | 540 | 97.8 |
| Leukapheresis | 31.5 | 96.6 | 0.86 | 270 | 319 | 96.0 | |
| RM120 | PBMC | 23.3 | 92.5 | 0.46 | 110 | 462 | 97.7 |
| Leukapheresis | 42.0 | 95.7 | 0.59 | 250 | 370 | 96.7 | |
| RM238 | PBMC | 30.2 | 80.4 | 0.79 | 240 | 299 | 93.6 |
| Leukapheresis | 36.3 | 92.3 | 0.83 | 300 | 304 | 93.1 | |
| (Mean±SD) | PBMC | 27.4 ± 3.6 | 87.9 ± 6.6 | 0.60 ± 0.17 | 170 ± 70 | 434 ± 123 | 96.4 ± 2.4 |
| Leukapheresis | 36.6 ± 5.3 | 94.9 ± 2.3 | 0.76 ± 0.15 | 270 ± 30 | 331 ± 35 | 95.3 ± 1.9 | |
| p value | 0.200 | 0.116 | 0.181 | 0.047 | 0.257 | 0.101 | |
Determined by flow cytometry
Cells were expanded for 24 days
Ex vivo expansion of Treg was performed as described in the Materials and Methods and the suppressive function of the expanded Treg was assessed. After 24 days of expansion, Treg isolated from peripheral blood and GM-CSF/G-CSF-mobilized leukapheresis products exhibited comparable fold increases of 434 ± 123 x 103 and 331 ± 35 x 103, respectively (Figure 4B and Table 2). Next, we evaluated the phenotype of the expanded Treg. The expression (relative MFI) of Foxp3, CTLA4 and Helios in leukapheresis product-expanded Treg was reduced, but not significantly compared to peripheral blood PBMC-expanded Treg. CCR4, CCR7 and CXCR3 expression was similar. Expression of the transcription factors T-bet, RORγt, GATA3, pSTAT3 and pSTAT5 by Treg was also similar (Figure 4C). To assess the stability of Foxp3 expression, DNA isolated from normal PBMC- and leukapheresis product-expanded Treg was evaluated for CpG methylation of eight CpG sites in the TSDR at the Foxp3 locus. CD4+CD25− T cells obtained at the time of MACS isolation were used as controls. As shown in Figure 4D, both PMBC- and leukapheresis product-expanded Treg exhibited low levels of methylation compared to CD4+CD25− T cells, that displayed high levels of methylation (Figure 4D).
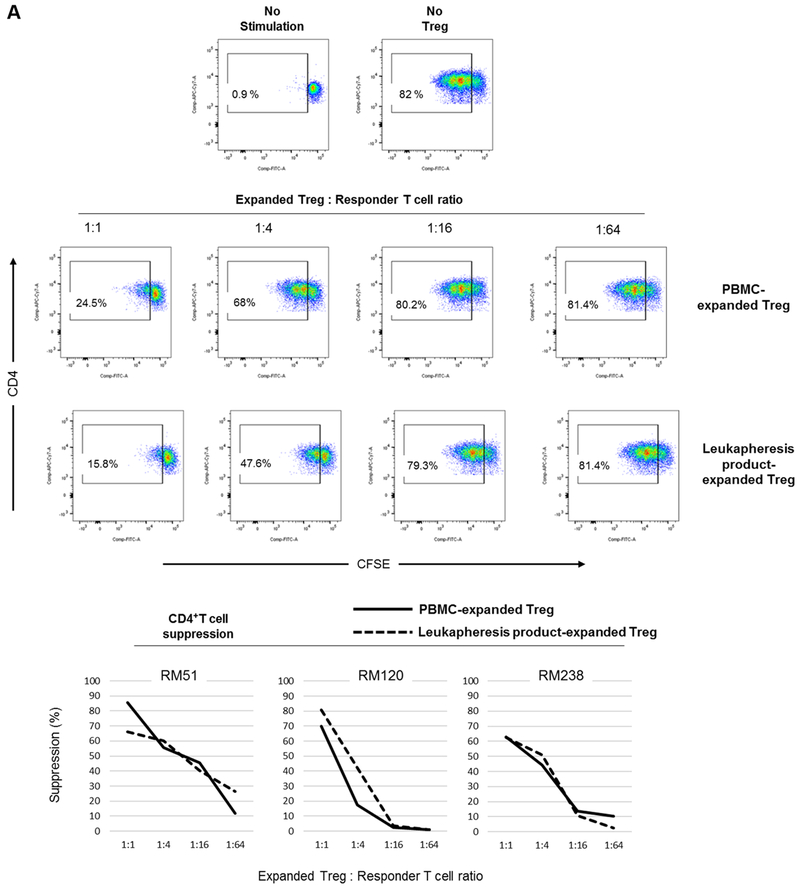
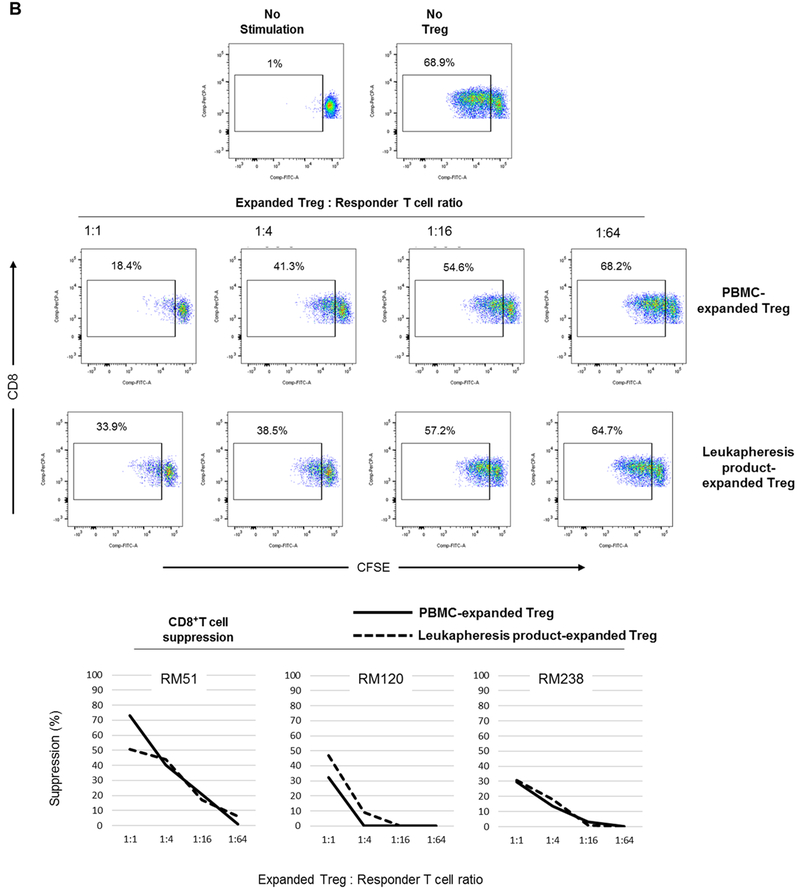
We then evaluated the suppressive function of the expanded Treg populations (Figure 5). Autologous T cells were used as responders to assess the suppressive function of the expanded Treg. Following 3 days of αCD2/CD3/CD28 bead stimulation, peripheral blood PMBC-expanded and leukapheresis product-expanded Treg suppressed autologous CD4+ (Figure 5A) and CD8+ (Figure 5B) T cell proliferation in a dose-dependent manner. Furthermore, the extent of suppression of CD4+ and CD8+ T proliferative responses was comparable.
FIGURE 5. Suppressive function of Treg expanded from normal steady state PBMC before and in leukapheresis products after GM-CSF/G-CSF administration.
Treg were evaluated for their suppressive effect on autologous CD4+ (A) and CD8+ (B) T cell proliferative responses following polyclonal stimulation (n=3 monkeys). Autologous CFSE-labeled responder CD2+ T cells were stimulated by αCD2/CD3/CD28-coated microbeads at a cell:bead ratio of 1:2 for three days in the presence or absence of VPD450-labeled Treg at the indicated ratios. Percent T cell proliferation was determined by CFSE-dilution. Dot plots (upper panels) are from one animal representative of three monkeys. Graphs (lower panels) depict data from all three animals.
4 |. DISCUSSION
Clinical application of Treg-based therapeutic approaches is constrained by the paucity of these cells that can be recovered from peripheral blood. Hence, ex vivo expansion of Treg is required to obtain sufficient numbers for clinical testing. Studies in rodents have indicated that peripheral blood Treg can be mobilized following cytokine (G- or GM-CSF) or IL-2-mAb complex administration,11, 12, 19 either directly, or through mobilization of antigen-presenting cells with a regulatory phenotype, e.g., DCreg.12 Several human studies have also documented Treg mobilization into the peripheral blood after cytokine administration. Thus, low-dose IL-2 administration enhances peripheral blood Treg in patients with type-1 diabetes,20 while in chronic graft-versus-host disease, low-dose IL-2 administration is associated with enhanced incidences of Treg and amelioration of clinical symptoms.21 Similarly, G-CSF administration induces Treg, particularly CD4+CD25hiCD45RO+Treg mobilization in healthy volunteers,9 likely through the downregulation of bone marrow CXCL12.22
NHP have proven valuable as critical pre-clinical models for evaluation of promising novel therapeutic approaches. In rhesus macaques, AMD3100, a CXCR4 antagonist (with or without G-CSF) administration, has been used to mobilize peripheral blood T cells.23 In this latter study, using FDA-approved dosages, AMD3100 was administered either alone, or at the end of a 5-days course of G-CSF administration, followed by leukapheresis. Two hours after AMD3100 administration, lymphocyte numbers increased significantly in the peripheral blood, compared to either G-CSF alone, or combined AMD3100 and G-CSF administration. Fold increase of absolute numbers of the peripheral blood lymphocyte subset (including conventional and regulatory T cells) were significantly higher after AMD3100 administration alone, compared to G-CSF alone. In mobilized leukapheresis products, combined AMD3100 and G-CSF administration was superior to either alone in enhancing lymphocyte subpopulations. AMD3100 administration alone (or in combination with G-CSF) enhanced the percentages of Treg in leukapheresis products (up to 3 - 4 fold increase), compared to G-CSF alone (1 fold increase). However, in this study, the ratios of Treg to effector T cells were not determined. In cynomolgus monkeys, low-dose IL-2 administration significantly expands peripheral blood CD4+CD45RA−Foxp3hi activated Treg, with limited expansion of non-Treg cells. Previously, we have shown24 that an 8-day course of GM-CSF/G-CSF in rhesus monkeys results in the accumulation of functional myeloid-derived suppressor cells in leukapheresis products.25 In the current study, we evaluated the impact of combined GM-CSF and G-CSF administration on the incidences of peripheral blood Treg in rhesus monkeys, in comparison to either GM-CSF or G-CSF alone. Following GM-CSF (for four days) and G-CSF administration (for an additional four days), we observed significant increases in the incidences of peripheral blood CD4+CD25hiFoxp3hi Treg. These increases were associated with significantly elevated CD4+CD25hiFoxp3hi Treg to CD4+CD25loFoxp3lo non-Treg ratios. Notably, CD45RA expression has been used to delineate suppressive from non-suppressive human Treg.26 In that study, human CD4+CD45RAnegFoxp3hi Treg were suppressive in vitro, while CD4+CD45RAnegFoxp3lo Treg were non-suppressive and comprised cells with Th17 potential. In our study, the ratios of CD4+CD45RAnegFoxp3hi cells to CD4+CD45RAnegFoxp3lo cells increased significantly in the leukapheresis products (after combined GM-CSF/G-CSF administration), compared to the peripheral blood (before combined GM-CSF/G-CSF administration). Of note, the average number of CD4+CD25hiFoxp3hi Treg that could be isolated from a single mobilized leukapheresis product was approx. 3.7 x 106/kg. When calculated based on CD25 and CD127 expression (commonly used for Treg sorting), approx. 6.8 x 106/kg CD4+CD25hiCD127lo Treg could be isolated.
In some reports, prolonged ex vivo Treg expansion has been associated with gradual loss of Foxp3 expression, as well as a decline in their suppressive function.27–29 Obtaining sufficient, naturally-occurring Treg before their ex vivo expansion might reduce the overall expansion time required to obtain therapeutic target cell numbers. Non-mobilized leukapheresis products have been used as a source of Treg in humans,14 and from which 6-10 x 109 total leukapheresed PBMC can be obtained. Of these, approx. 6% were CD4+CD25hiCD127lo Treg, corresponding to approx. 1.6 x 106/kg for an average 70kg adult human (total 114 million Treg). Also, this observation suggests that leukapheresis alone may not enhance the incidence of peripheral blood Treg. In humans given G-CSF (10 mg/kg/day for 4 days) followed by leukapheresis,30 CD4+CD25hiCD127lo Treg constituted < 1% of the leukapheresis product. However, the authors observed a 1.6-fold increase in the percentage of Treg in the G-CSF mobilized compared to the non-mobilized leukapheresis products. In the present study, the percentages of CD4+CD25hiFoxp3hi and CD4+CD25hiCD127lo Treg in peripheral blood in 2 animals increased by 2.6 and 2.1-, and 1.4 and 1.9 folds, respectively, after combined GM-CSF/G-CSF administration (Figure 1). Meanwhile, CD4+CD25hiCD127lo Treg constituted average of 8.6% of the leukapheresis product after combined GM-CSF and G-CSF administration (6.8 x 106/kg) (Table 1). In comparison to PBMC-expanded Treg, mobilized Treg exhibited a similar phenotype, TSDR demethylation status and suppressive function to peripheral blood Treg obtained from normal blood (before GM-CSF/G-CSF administration). However, slightly lower Foxp3 expression by leukapheresis-expanded Treg (compared to PBMC-expanded Treg) was observed. This might be due to additional in vivo GM-CSF/G-CSF administration prior to the ex vivo expansion (for 24 days). With sufficient Treg being obtained from leukapheresis products, lengthy ex vivo expansion can be avoided.
Our results indicate that, combined GM-CSF and G-CSF administration efficiently enhances the Treg yield from leukapheresis products in rhesus macaques. Our data further show that leukapheresis products from GM-CSF/G-CSF-mobilized individuals can be used as a comparatively rich source for peripheral blood Treg. This may allow acquisition of sufficient Treg numbers to allow reduction in the time required to obtain sufficient number of functional Treg for therapeutic application.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grant U19 AI131453. grants U01 AI51698 and U19 AI131453, part of the NIH NHP Transplantation Tolerance Study group and sponsored by the NIAID and NIDDK.
Abbreviations:
- aAPC
artificial antigen-presenting cell
- G-CSF
granulocyte colony-stimulatory factor
- GM-CSF
granulocyte-macrophage colony-stimulatory factor
- NHP
Non-human primate
- PBMC
peripheral blood mononuclear cell
- Treg
regulatory T cell
- TSDR
Treg-specific demethylated region
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nature reviews Immunology 2010;10(7):490–500. [DOI] [PubMed] [Google Scholar]
- 2.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nature reviews Immunology 2003;3(3):199–210. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer IR, Hester J, Bushell A, Wood KJ. Induction of transplantation tolerance through regulatory cells: from mice to men. Immunological reviews 2014;258(1):102–116. [DOI] [PubMed] [Google Scholar]
- 4.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 2003;102(4):1249–1253. [DOI] [PubMed] [Google Scholar]
- 5.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. Journal of cellular biochemistry 2006;99(3):690–705. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti M, Gregori S, Roncarolo MG. Granulocyte-colony stimulating factor drives the in vitro differentiation of human dendritic cells that induce anergy in naive T cells. European journal of immunology 2010;40(11):3097–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4(+)CD25(+) regulatory T-cells. Diabetes 2005;54(1):78–84. [DOI] [PubMed] [Google Scholar]
- 8.Layseca-Espinosa E, Korniotis S, Montandon R, Gras C, Bouillie M, Gonzalez-Amaro R et al. CCL22-producing CD8alpha-myeloid dendritic cells mediate regulatory T cell recruitment in response to G-CSF treatment. Journal of immunology 2013;191(5):2266–2272. [DOI] [PubMed] [Google Scholar]
- 9.Toh HC, Sun L, Soe Y, Wu Y, Phoon YP, Chia WK et al. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clinical immunology 2009;132(1):83–92. [DOI] [PubMed] [Google Scholar]
- 10.Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E et al. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. Journal of immunology 2006;176(11):6631–6639. [DOI] [PubMed] [Google Scholar]
- 11.Hotta M, Yoshimura H, Satake A, Tsubokura Y, Ito T, Nomura S. GM-CSF therapy inhibits chronic graft-versus-host disease via expansion of regulatory T cells. European journal of immunology 2019;49(1):179–191. [DOI] [PubMed] [Google Scholar]
- 12.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. International immunology 2009;21(3):269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. Journal of immunology 2007;179(6):3638–3647. [DOI] [PubMed] [Google Scholar]
- 14.Golab K, Grose R, Trzonkowski P, Wickrema A, Tibudan M, Marek-Trzonkowska N et al. Utilization of leukapheresis and CD4 positive selection in Treg isolation and the ex-vivo expansion for a clinical application in transplantation and autoimmune disorders. Oncotarget 2016;7(48):79474–79484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Guo H, Lu L, Zahorchak AF, Wiseman RW, Raimondi G et al. Sequential monitoring and stability of ex vivo-expanded autologous and nonautologous regulatory T cells following infusion in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15(5):1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukena SN, Velaga S, Geffers R, Grosse J, Baron U, Buchholz S et al. Human regulatory T cells in allogeneic stem cell transplantation. Blood 2011;118(13):e82–92. [DOI] [PubMed] [Google Scholar]
- 17.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013;13(8):1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzelarab MB, Lu L, Shufesky WF, Morelli AE, Thomson AW. Donor-Derived Regulatory Dendritic Cell Infusion Maintains Donor-Reactive CD4(+)CTLA4(hi) T Cells in Non-Human Primate Renal Allograft Recipients Treated with CD28 Co-Stimulation Blockade. Frontiers in immunology 2018;9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. The Journal of experimental medicine 2009;206(4):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenzwajg M, Churlaud G, Mallone R, Six A, Derian N, Chaara W et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. Journal of autoimmunity 2015;58:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. The New England journal of medicine 2011;365(22):2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer research 2004;64(22):8451–8455. [DOI] [PubMed] [Google Scholar]
- 23.Kean LS, Sen S, Onabajo O, Singh K, Robertson J, Stempora L et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood 2011;118(25):6580–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoyama A, Klarin D, Yamada Y, Boskovic S, Nadazdin O, Kawai K et al. Low-dose IL-2 for In vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2012;12(9):2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahorchak AF, Ezzelarab MB, Lu L, Turnquist HR, Thomson AW. In Vivo Mobilization and Functional Characterization of Nonhuman Primate Monocytic Myeloid-Derived Suppressor Cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2016;16(2):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30(6):899–911. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. European journal of immunology 2009;39(4):1088–1097. [DOI] [PubMed] [Google Scholar]
- 28.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 2009;113(21):5125–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 2009;58(3):652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ukena SN, Velaga S, Goudeva L, Ivanyi P, Olek S, Falk CS et al. Human regulatory T cells of G-CSF mobilized allogeneic stem cell donors qualify for clinical application. PloS one 2012;7(12):e51644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.