Abstract
Background and aims:
Expansion and morphological dysregulation of the bronchial vascular network occurs in asthmatic airways. Interleukin (IL) -17 and Rho-kinase (ROCK) are known to act in inflammation control and remodeling. Modulation of Rho-kinase proteins and IL-17 may be a promising approach for the treatment of asthma through the control of angiogenesis. Our objective was to analyze the effects of treatment with anti-IL17 and/or Rho-kinase inhibitor on vascular changes in mice with chronic allergic pulmonary inflammation.
Methods:
Sixty-four BALB/c mice, with pulmonary inflammation induced by ovalbumin were treated with anti-IL17A (7.5/µg per dose, intraperitoneal) and/or Rho-kinase inhibitor (Y-27632-10 mg/kg, intranasal), 1 h before each ovalbumin challenge (22, 24, 26, and 28/days). Control animals were made to inhale saline. At the end of the protocol, lungs were removed, and morphometric analysis was performed to quantify vascular inflammatory, remodeling, and oxidative stress responses.
Results:
Anti-IL17 or Rho-kinase inhibitor reduced the number of CD4+, CD8+, dendritic cells, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, Rho-kinase 1 and 2, transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF), nuclear factor (NF)-KappaB, iNOS, metalloproteinase (MMP)-9, MMP-12, metalloproteinase inhibitor-1 (TIMP-1), FOXP-3, signal transducer and activator of transcription 1 (STAT1) and phospho-STAT1-positive cells, and actin, endothelin-1, isoprostane, biglycan, decorin, fibronectin and the collagen fibers volume fraction compared with the ovalbumin group (p < 0.05). The combination treatment, when compared with anti-IL17, resulted in potentiation of decrease in the number of IL1β- and dendritic cells-positive cells. When we compared the OVA-RHO inhibitor-anti-IL17 with OVA-RHO inhibitor we found a reduction in the number of CD8+ and IL-17, TGF-β, and phospho-STAT1-positive cells and endothelin-1 in the vessels (p < 0.05). There was an attenuation in the number of ROCK 2-positive cells in the group with the combined treatment when compared with anti-IL17 or Rho-kinase inhibitor-treated groups (p < 0.05).
Conclusion:
We observed no difference in angiogenesis after treatment with Rho-kinase inhibitor and anti-IL17. Although the treatments did not show differences in angiogenesis, they showed differences in the markers involved in the angiogenesis process contributing to inflammation control and vascular remodeling.
The reviews of this paper are available via the supplemental material section.
Keywords: angiogenesis, asthma, interleukin-17, neutralizing antibody, Rho-kinase, vascular remodeling, Y-27632
Asthma is a chronic disorder that results in variable airflow limitation in the airways and is associated with airway responsiveness, active airway inflammation, and tissue remodeling. Although classically considered to be an inflammatory disease induced by T helper 2 (Th2) cells, characterized by eosinophilic inflammation, cytokine production associated with Th2 cells, and hyperresponsiveness, the immune response in asthma is considered highly heterogeneous.1
The remodeling includes subepithelial fibrosis, smooth muscle thickening of the airways, increased number of blood vessels in the walls of the airways, increased numbers of goblet cells in the airway epithelium, and enlargement of submucosal glands.2
One of the factors contributing to the pathology of asthma is increased vascularization in the submucosa. Increased tissue edema may consequently lead to narrowing of the airways, plasma exudation may aggravate local inflammation and remodeling, and the increased blood supply provides the hyperplastic and hypertrophic airway smooth muscle cells with the necessary nutrition and oxygen. Thus, a reduction in vascularization may lead to remodeling and inflammation control.3
Other subsets, such as Th1, Th17, and CD8+ T cells, contribute to the pathophysiology of the disease.4 Asthma is associated with an immune response with known molecular and cellular actors, such as eosinophils, Th2 lymphocytes, and associated cytokines, such as interleukin (IL)-5, IL-4, and IL-17.5 IL-17A and IL-17F are often related to the neutrophilic inflammation in asthma patients.6 IL-17A levels are increased in their airways, and the induced human bronchial fibroblasts produce proinflammatory cytokines.7 There is an increase in IL-17 mRNA levels in the sputum of the asthma patients compared with those in healthy controls.8
Neovascularization is an increasingly reported feature of airway remodeling. Several studies have reported the morphological expansion and dysregulation of the bronchial vascular network in the airways of asthmatics, as well as an increased number, size, density of blood vessels, vascular leakage, and plasma engorgement. Several factors are able to influence these mechanisms, including structural components of the altered extracellular matrix, protease imbalance and its endogenous inhibitors, and unregulated growth factor levels.9
Other mechanisms involved in the formation of new vessels include differentiation of endothelial progenitor cells, mesenchymal progenitor cells, and fibrocytes into endothelial cells that form the bronchial vascular plexuses.10
The importance of Rho-kinase (ROCK) signaling has been reported in various types of cells and diseases, including asthma. Rho-kinases 1 and 2 play a pivotal role in the organization of the smooth muscle cytoskeleton and adhesion plaques as well as in the regulation of transcription factors. ROCK activity regulates cellular contraction, motility, morphology, polarity, division, and gene expression.11
Some mechanical characteristics of vessel leakage and rupture include reduced endothelial barrier function, increased inflammatory cells, abnormal remodeling, and vasoconstriction through overactivation of the Rho signaling pathway.12 Preclinical studies suggest that inhibition of Rho-kinase may be beneficial by reversing these vascular abnormalities and restoring vascular integrity. The Rho-kinase inhibitors Y-27632 and Fasudil competitively inhibit the ATP binding site of ROCK1 and ROCK2.13
In a recent study that we published we verified the effect of the anti-IL17 treatment, both with and without the association with Y-27632, and demonstrated its positive effect as the modulation of hyperresponsiveness, inflammation, extracellular matrix remodeling, and oxidative stress in the airways and lung tissue of mice with chronic allergic lung inflammation.14
In the present study, we investigated the effect of treatment with Rho-kinase inhibitor and anti-IL17 monoclonal antibody on airway vascular remodeling in a mouse experimental model of chronic allergic pulmonary inflammation, with the intention of clarifying the possible mechanisms involved.
Materials and methods
Experimental groups
Research Ethics Committee of the Hospital das Clínicas of the Faculty of Medicine of the University of São Paulo approved the present study (Number 064/15).
The protocol used a total of 64 BALB/c male mice (20–25 g body weight, 6 weeks old), following the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health, and revised in 1985. The research was conducted at the Faculty of Medicine, University of São Paulo.
The mice were divided into eight groups, each group with eight animals (Table 1).
Table 1.
Experimental protocol groups.
| SAL | Received inhalations with sterile saline solution |
| SAL-RHO inhibitor | Received inhalations with sterile saline and 1 h before the challenge they received treatment with Rho-kinase inhibitor (10 mg/kg) |
| SAL-anti-IL17 | Received inhalations with sterile saline and 1 h before the challenge they received treatment with anti-IL17 (7.5 μg/application) |
| SAL-RHO inhibitor-anti-IL17 | Received inhalations with sterile saline and 1 h before the challenge they received treatment with Rho-kinase inhibitor (10 mg/kg) associated with treatment with an anti-IL17 (7.5 μg/application) |
| OVA | Received inhalations of a solution of ovalbumin |
| OVA-RHO inhibitor | Received inhalations of a solution of ovalbumin and 1 h before the challenge they received treatment with Rho-kinase inhibitor (10 mg/kg) |
| OVA-anti-IL17 | Received inhalation of OVA solution and 1 h before the challenge they received treatment with an anti-IL17 (7.5 μg/application) |
| OVA-RHO inhibitor-anti-IL17 | Received inhalations of a solution of ovalbumin and 1 h before the challenge they received treatment with Rho-kinase inhibitor (10 mg/kg) associated with treatment with an anti-IL17 (7.5 μg/application) |
IL, interleukin.
Sensitization protocol
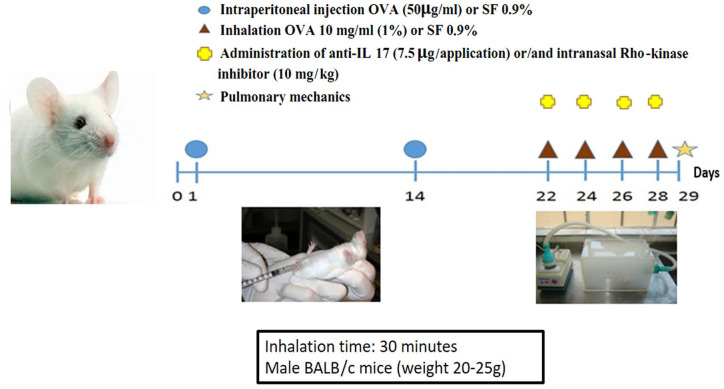
The experimental model of allergic pulmonary inflammation by ovalbumin (OVA) lasted 28 days. On days 1 and 14 of the protocol, the mice were administered a solution of 50 μg ovalbumin via intraperitoneal (i.p.) injection (GRADE IV, Sigma-Aldrich, St. Louis, MO, USA) and 6 mg aluminum hydroxide (Alumen, Pepsamar, Sanofi-Synthelabo SA, Rio de Janeiro, Brazil), both in a total volume of 0.2 ml. On days 22, 24, 26, and 28, the mice of the OVA groups were placed in an acrylic display box coupled to an ultrasonic nebulizer and subjected to aerosol inhalation of OVA diluted in 0.9% NaCl (saline solution), at a concentration of 10 mg/ml (1%), protocol illustrated in Figure 1. The aerosol exposure time was 30 min. The animals in the SAL groups received saline (0.9% NaCl) and 6 mg aluminum hydroxide i.p. on days 1 and 14 and were exposed to aerosolized saline solution (0.9%) for 30 min on days 22, 24, 26, and 28. The histopathological and morphometric studies were performed 24 h after the last antigen challenge, that is, on day 29 of the experiment.15
Figure 1.
Experimental protocol.
Treatment
The anti-IL17 and/or Rho-kinase inhibitors were administered 1 h prior to the ovalbumin or saline aerosol administration on days 22, 24, 26, and 28. The IL-17 neutralizing antibody was administered i.p. at a dose (7.5 μg/application) based on the Barlow Protocol.16 Based on the protocol of Santos et al., we intranasally administered 10 mg/kg of a highly selective Rho-kinase inhibitor (Y-27632), [(+) – (R) -trans-4- (1-aminoethyl) -N- (4-pyridyl) cyclohexanecarboxamide dihydrochloride, monohydrate].14
Immunohistochemistry
On day 29, the animals were euthanized, and their lungs were fixed for 24 h in 4% formalin and dehydrated in a solution of 70% ethanol. Lung tissues were sectioned into 4 μm-thick slices and stained as described and followed.
They were stained for 1 h in Picrosirius red at room temperature, and then washed in running water for 10 min. After this stage, the sections were stained by Harris hematoxylin.
Immunohistochemistry procedures were performed following the study by Camargo et al. The markers and the dilution and specifications of the primary antibodies used in this study are shown in Table 2. After incubation with the primary antibody, the slides were washed in PBS and incubated with the secondary antibody using the ABC Kit by Vectastain (Vector Elite-PK-6105 (anti-goat) or PK-6101 (anti-rabbit) (Vector Laboratories, Burlingame, CA, USA). Digestion or recovery of antigen at high temperature was carried out either in a steam pan for 50 min or in a pressure cooker for 1 min using citrate buffer (pH 6.0). For the visualization of the positive cells, the slides were washed in PBS and proteins were visualized using diaminobenzidine (DAB) chromogen (70 mg DAB in 110 ml of the Tris-HCl, Sigma Chemical Co., St. Louis, MO, USA) and Harris hematoxylin.15
Table 2.
Markers, dilutions, and specifications.
| Markers | Dilution | Secondary antibody | Specifications | Markers | Dilution | Secondary antibody | Specifications |
|---|---|---|---|---|---|---|---|
| Rho-kinase 1 | 1:50 | Anti-goat | SC-1851 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA | CD4+ | 1:300 | Anti-rat | SC-13573 Rat Monoclonal; Santa Cruz Biotechnology, CA, USA |
| Rho-kinase 2 | 1:400 | Anti-goat | SC-6055 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA | CD8+ | 1:600 | Anti-mouse | YTS-169AG Rat Monoclonal; Thermo Fisher Scientific, CA, USA |
| IL-1β | 1:200 | Anti-rabbit | SC-7884 Rabbit Polyclonal; Santa Cruz, Biotechnology, CA, USA | Decorin | 1:600 | Anti-goat | SC-22613 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA |
| TNF-α | 1:100 | Anti-mouse | SC-52746 Mouse Monoclonal; Santa Cruz Biotechnology, CA, USA | NF-KappaB | 1:800 | Anti-rabbit | SC-109 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA |
| IL-4 | 1:300 | Anti-goat | SC-1260 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA | TIMP-1 | 1:400 | Anti-rabbit | LS-C299465 Mouse Polyclonal; LifeSpan BioScience, Inc., WA, USA |
| IL-5 | 1:500 | Anti-rabbit | SC-7887 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA | MMP-9 | 1:50 | Anti-rabbit | SC-6840 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA |
| IL-6 | 1:600 | Anti-goat | SC-1265 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA | MMP-12 | 1:400 | Anti-rabbit | SC-30072 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA |
| IL-10 | 1:300 | Anti-mouse | SC-73309 Rat Polyclonal; Santa Cruz Biotechnology, CA, USA | TGF-β | 1:200 | Anti-rabbit | SC-146 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA |
| IL-13 | 1:600 | Anti-goat | SC-1776 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA | VEGF | 1:400 | Anti-rabbit | SC-152 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA |
| IL-17 | 1:800 | Anti-rabbit | SC-73309 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA | iNOS | 1:200 | Anti-mouse | N-32020 Mouse Monoclonal; BD Transduction Laboratories, CA, USA |
| Isoprostane | 1:1500 | Anti-goat | IS-20 Goat Polyclonal; Oxford Biomedical Research, MI, USA | Fibronectin | 1:600 | Anti-goat | SC-22613 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA |
| FOXP-3 | 1:300 | Anti-mouse | SC-53876 Mouse Monoclonal; Santa Cruz Biotechnology, CA, USA | Biglycan | 1:1200 | Anti-goat | SC-27936 Goat Polyclonal; Santa Cruz Biotechnology, CA, USA |
| STAT1 | 1:2500 | Anti-rabbit | SC-346 Rabbit Polyclonal; Santa Cruz Biotechnology, CA, USA | Phospho-STAT1 | 1:100 | Anti-mouse | SC-8394 Mouse Monoclonal; Santa Cruz Biotechnology, CA, USA |
IL, interleukin; TNF, tumor necrosis factor; STAT-1, signal transducer and activator of transcription 1; MMP, metalloproteinase; TIMP, metalloproteinase inhibitor; NF Kappa-B, nuclear factor Kappa-B; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Quantitative analysis of inflammatory markers
We quantified the expression of the inflammatory cells CD4+, CD8+, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, TNF-α, FOXP-3, STAT1, phospho-STAT1, dendritic cells, NF-KappaB, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF-β) in the airway vascular wall. The point counting technique was used with the reticle of 100 points and 50 lines coupled to the microscope eyepiece with total area of the reticulum of 104μm2 (Nikon Corporation, Tokyo, Japan). The number of points in the positive cells was divided by the total number of points coincident with the analyzed structure.17
Quantitative analysis of remodeling markers
The vascular volume fraction of collagen fibers, and fibronectin, biglycan, decorin, and isoprostane expression levels were evaluated by an Image Analysis System. We analyzed only bronchiolar vessels that can be wholly observed at a magnification of 200×. We captured the images with a Leica DM2500 microscope coupled with a camera (Leica Microsystems, Wetzlar, Germany). Using the Optimas v.4.10 software, the images were processed. Five vessels were analyzed per animal. This software informed about the threshold of the color tones representing the positive areas and, thus, quantified them in the previously determined area. The volumetric fractions of these markers are expressed as percentages of the area.15,18
Quantitative analysis of vascular markers
In the evaluation of vascular markers, we analyzed the expression of actin and endothelin-1. This expression was evaluated using an image analyzer.
Quantitative analysis of angiogenesis
To evaluate the angiogenesis, we used the point counting technique. We counted the numbers of vessels and the number of points that were coincident with lung tissue, and the results were expressed by the number of vessels per area (104 µm2). We analyzed only bronchiolar vessels that can be observed whole at a magnification of 200×.17
To quantify the vascular wall area, we measured the smooth muscle and the adventitious wall areas and the results were expressed in μm2.17
Statistical analysis
Statistical analysis was performed using the Scientific Graphing Software SigmaPlot® Version 11.0. To determine the difference between groups and their statistical significance, we used the unidirectional analysis of variance followed by the Holm–Sidak method for multiple comparisons. All data are represented as display graphs as dot plots, indicating mean and standard deviation. A p < 0.05 was considered statistically significant for all analyses.
Results
Only the results of the SAL group are shown in the graphs to facilitate visualization as there were no differences among the SAL, SAL-RHO inhibitor, SAL-anti-IL17, and SAL-RHO inhibitor-anti-IL17 control groups.
Morphometric evaluation
In relation to the vascular wall, there was an increase in the area of the OVA group compared with the SAL group (p < 0.001). The treated groups were not different compared with the OVA group.
Anti-IL17 and/or Rho-kinase inhibitor treatments have no effect on angiogenesis in experimental asthma induced by ovalbumin
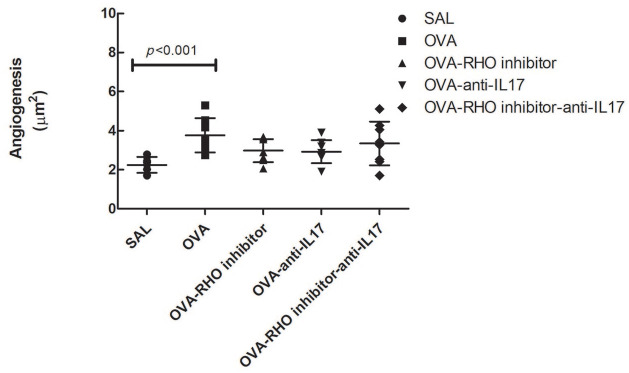
Measurement of angiogenesis showed significant differences among the SAL group (2.2 ± 0.1) and the OVA group (3.7 ± 0.3), as shown in Figure 2 (p < 0.05). There was no difference between the treated groups compared with the OVA and control group, indicating that the treated groups presented an intermediate response.
Figure 2.
In the OVA group there was an increase in angiogenesis compared with the saline group. The inhibitor of Rho-kinase and anti-IL17 has no effect on angiogenesis in chronic allergic pulmonary inflammation induced by ovalbumin in mice. To determine the difference between groups and their statistical significance, we used the unidirectional analysis of variance followed by the Holm–Sidak method for multiple comparisons. The data are presented as dot plots with standard deviations.
N = 8 animals per group.
IL, interleukin; OVA, animals induced to chronic allergic pulmonary inflammation by ovalbumin; OVA-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
Anti-IL17 and/or Rho-kinase inhibitor treatments attenuated vascular markers expression in experimental asthma induced by ovalbumin
Regarding the actin results, there was an increase in the OVA group (10.8 ± 8.9) compared with the SAL group (1.9 ± 1.2), p < 0.001. The treated groups showed attenuation when compared with the OVA group; p < 0.001. Treatment with the association of anti-IL17 to the Rho-kinase inhibitor also attenuated the expression of actin when compared with the OVA group (p = 0.009).
In the evaluation of endothelin 1, the OVA group (18.5 ± 10.0) showed an increase in relation to the SAL group (1.2 ± 1.6). The OVA-RHO inhibitor group showed a decrease compared with the OVA group (p = 0.01). The OVA-anti-IL17 group showed a decrease compared with the OVA group (p < 0.001). Treatment with the association of anti-IL17 to the Rho-kinase inhibitor also attenuated the expression of actin when compared with the OVA group (p < 0.001). The anti-IL17 group and the association of treatments potentiated attenuation when compared with the treatment group that used only Rho-kinase inhibitor (p < 0.001).
Anti-IL17 and/or Rho-kinase inhibitor treatments attenuated Rho-kinase expression in experimental asthma induced by ovalbumin
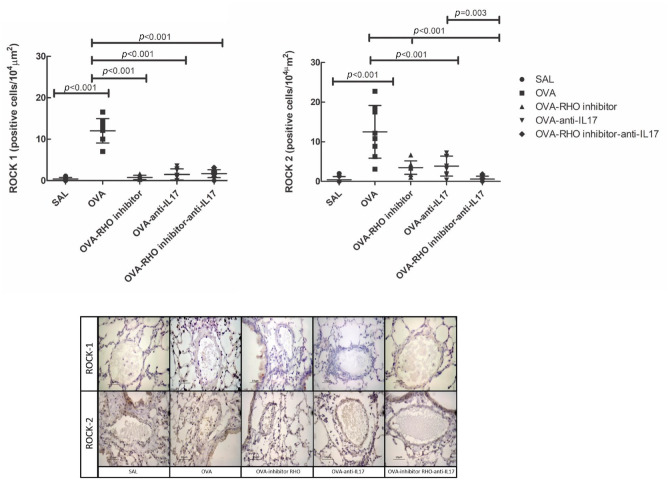
ROCK1- and ROCK2-positive cells in the vessels and representative photomicrographs are presented in Figure 3. There was an increase of ROCK1- and ROCK2-positive cells in the vessels in the OVA group compared with the SAL group (p < 0.05). Rho-kinase inhibitor, anti-IL17 and the combination of the two treatments reduced the number of ROCK1- and ROCK2-positive cells compared with the OVA group (p < 0.05).
Figure 3.
The treatments with the inhibitor of Rho-kinase and anti-IL17 attenuate the expression of ROCK1 and ROCK2 in vessel in chronic allergic pulmonary inflammation induced by ovalbumin in mice. The attenuation of ROCK2 expression in the vessel is enhanced when the treatment of the Rho-kinase inhibitor was associated with anti-IL17 compared with OVA-RHO inhibitor group and OVA-anti-IL17 group. In OVA group there was an increase compared with the saline group. The data are presented as dot plots with standard deviations. Representative photomicrographs of ROCK1 and ROCK2 for each group.
IL, interleukin; ROCK, Rho-kinase; OVA, animals induced to chronic allergic pulmonary inflammation by ovalbumin; OVA-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
In ROCK2 evaluation, in the group that combined the two treatments, there was a potentiation of the reduction in the number of positive cells in relation to anti-IL17 or Rho-kinase inhibitor treatments (p < 0.05).
Anti-IL17 and/or Rho-kinase inhibitor treatments reduced the oxidative stress response in experimental asthma induced by ovalbumin
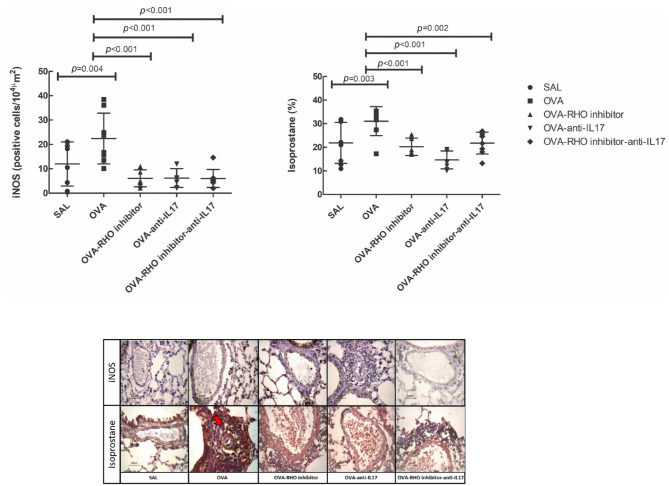
Isoprostane and iNOS were evaluated as markers of oxidative stress, as shown in Figure 4. There was an increase in the number of iNOS-positive cells in the OVA group compared with the control group, and a reduction in the treated groups compared with the OVA group (p < 0.05).
Figure 4.
The treatments with the inhibitor of Rho-kinase and anti-IL17 attenuate the expression of oxidative markers in vessel in chronic allergic pulmonary inflammation induced by ovalbumin in mice. In OVA group there was an increase compared with the saline group. The data are presented as dot plots with standard deviations. Representative photomicrographs of iNOS and isoprostane for each group. The red arrows indicate the marked area in immunohistochemistry.
Eight animals per group.
IL, interleukin; OVA, animals induced to chronic allergic inflammation by ovalbumin; OVA-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
We also observed increased isoprostane content in the OVA group compared with that in the SAL control group, and attenuation of the isoprostane content in the anti-IL17 and Rho-kinase inhibitor-treated groups compared with that in the OVA group (p < 0.05). In both iNOS and isoprostane evaluation, there were no differences among the treated groups.
Anti-IL17 and/or Rho-kinase inhibitor treatments attenuated inflammatory markers in experimental asthma induced by ovalbumin
Inflammatory markers CD4+, CD8+, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, and TNF-α in the vessels are shown in Table 3. There was an increase in the positive cell count of all the inflammatory markers in the OVA group compared with the control group SAL (p < 0.05). The treated groups presented a reduction in the number of positive cells compared with the OVA group (p < 0.05).
Table 3.
Absolute values of the inflammatory markers.
| Inflammatory markers | SAL | OVA | OVA-RHO inhibitor | OVA-anti-IL17 | OVA-RHO inhibitor-anti-IL17 |
|---|---|---|---|---|---|
| CD4 (cells/104μm2) | 0.6 ± 1.2 | 5.5 ± 3.2* | 2.6 ± 5.3** | 2.5 ± 5.1*,** | 2.3 ± 4.4** |
| CD8 (cells/104μm2) | 11.2 ± 12.0 | 40.8 ± 22.6* | 23.9 ± 17.8** | 14.2 ± 15.1** | 9.2 ± 11.2**,& |
| IL-1β (cells/104μm2) | 4.7 ± 5.8 | 35.0 ± 31.2* | 3.9 ± 6.6**,# | 19.1 ± 14.4*,** | 8.5 ± 9.1**,# |
| IL-4 (cells/104μm2) | 1.9 ± 3.6 | 9.2 ± 7.4* | 0.6 ± 1.8** | 2.0 ± 3.4** | 0.6 ± 1.8** |
| IL-5 (cells/104μm2) | 2.5 ± 3.9 | 14.6 ± 15.3* | 2.9 ± 4.0** | 6.2 ± 8.5** | 1.8 ± 3.5** |
| IL-6 (cells/104μm2) | 0.1 ± 0.6 | 5.0 ± 6.2* | 0.5 ± 1.6** | 0.4 ± 1.4** | 0.3 ± 1.2** |
| IL-10 (cells/104μm2) | 0.9 ± 1.4 | 7.8 ± 9.9* | 3.5 ± 4.8** | 4.2 ± 5.3** | 3.2 ± 4.4** |
| IL-13 (cells/104μm2) | 0.8 ± 1.6 | 6.8 ± 7.4* | 2.6 ± 6.3** | 1.5 ± 3.5** | 2.4 ± 3.8** |
| IL-17 (cells/104μm2) | 1.9 ± 2.1 | 12.0 ± 2.8* | 7.2 ± 4.1*,** | 5.3 ± 2.7*,** | 2.9 ± 0.6*,**,& |
| TNF-α (cells/104μm2) | 2.7 ± 4.7 | 7.1 ± 5.2* | 1.6 ± 3.4** | 2.3 ± 4.0** | 1.1 ± 2.5** |
IL, interleukin; TNF, tumor necrosis factor; OVA, animals induced to chronic allergic pulmonary inflammation by ovalbumin; OVA-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
p < 0.05 compared with the SAL group.
p < 0.05 compared with the OVA group.
p < 0.05 compared with OVA-RHO inhibitor group.
p < 0.05 compared with OVA-anti-IL17 group.
The association of the treatments (anti-IL17 and Rho-kinase inhibitor) when compared with treatment with anti-IL17 resulted in potentiation in decreasing the number of IL1-β-positive cells (p < 0.05). When the OVA-RHO inhibitor-anti-IL17 group was compared with the Rho-kinase inhibitor, there was a decrease in the number of CD8+- and IL-17-positive cells (p < 0.05).
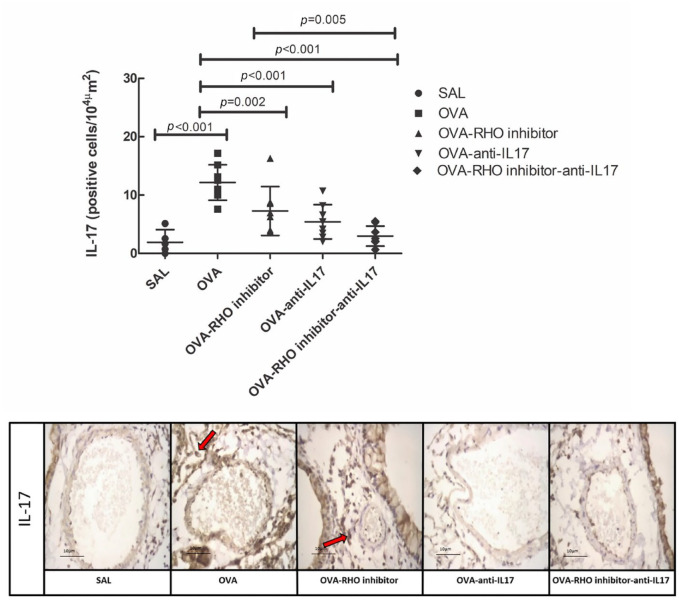
IL-17
The responses of IL-17 and the representative photomicrographs of IL-17 are presented in Figure 5. The number of positive cells in the OVA group was increased when compared with that in the SAL group (p < 0.05). The number of positive cells in the treated groups was reduced when compared with that in the OVA group (p < 0.05). The group with the combined treatment (OVA-RHO inhibitor-anti-IL17) showed reduction in the number of positive cells when compared with the OVA-RHO inhibitor group (p < 0.05).
Figure 5.
The treatments with the inhibitor of Rho-kinase and anti-IL17 attenuate the expression of IL-17 in vessel in chronic allergic pulmonary inflammation induced by ovalbumin in mice. The attenuation of IL-17 expression in the vessel is enhanced when the treatment of the Rho-kinase inhibitor was associated with anti-IL17 compared with treatment of Rho-kinase inhibitor alone. In OVA group there was an increase compared with the saline group. The data are presented as dot plots with standard deviations. Representative photomicrographs of IL-17 for each group. The red arrows indicate the marked area in immunohistochemistry.
IL, interleukin; OVA, animals induced to chronic allergic inflammation by ovalbumin; OVA-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
Anti-IL17 and/or Rho-kinase inhibitor treatments reduced extracellular matrix remodeling in experimental asthma induced by ovalbumin
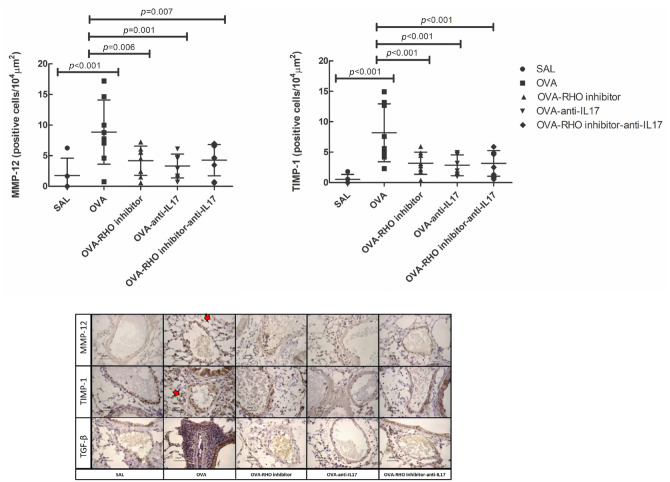
The responses of extracellular matrix remodeling are shown in Table 4. These markers increased in the OVA group compared with the SAL group (p < 0.05). There was a decrease in all markers in the treatment groups compared with the OVA group (p < 0.05). There was potentiation of the reduction of TGF-β-positive cells in the vessels of the mice belonging to the OVA-RHO inhibitor-anti-IL17 compared with the individual treatment with OVA-RHO inhibitor (p < 0.05). The results of MMP-12 and TIMP-1 and the photomicrographs of TGF-β, MMP-12, and TIMP-1 are shown in Figure 6.
Table 4.
Absolute values of the remodeling markers.
| Remodeling markers | SAL | OVA | OVA-RHO inhibitor | OVA-anti-IL17 | OVA-RHO inhibitor-anti-IL17 |
|---|---|---|---|---|---|
| MMP-9 (cells/104 μm2) | 6.3 ± 7.3 | 14.4 ± 11.2* | 3.9 ± 4.9** | 7.0 ± 9.7** | 9.3 ± 7.3** |
| MMP-12 (cells/104 μm2) | 1.7 ± 2.8 | 8.8 ± 5.2* | 4.1 ± 2.3** | 3.3 ± 1.9** | 4.2 ± 2.5** |
| TIMP-1 (cells/104 μm2) | 0.5 ± 0.8 | 8.1 ± 4.7* | 3.1 ± 1.8** | 2.8 ± 1.7** | 3.1 ± 2.0** |
| TGF-β (cells/104 μm2) | 1.4 ± 4.5 | 24.9 ± 15.5* | 4.5 ± 6.6** | 2.5 ± 4.8** | 1.4 ± 4.1**,& |
| VEGF (cells/104 μm2) | 8.1 ± 10.0 | 18.3 ± 14.7* | 5.7 ± 8.9** | 6.1 ± 4.3** | 6.4 ± 7.9** |
| Collagen fibers (%) | 12.7 ± 7.3 | 21.0 ± 9.2* | 9.2 ± 5.8** | 8.5 ± 5.8** | 12.1 ± 7.0** |
| Decorin (cells/104 μm2) | 2.6 ±4.3 | 20.6 ± 17.2* | 11.4 ± 11.9**,* | 8.2 ± 11.1** | 8.2 ± 11.6** |
| Fibronectin (%) | 11.9 ± 16.6 | 41.6 ± 32.2* | 19.3 ± 18.7** | 18.1 ± 15.5** | 15.5 ± 15.1** |
| Biglycan (cells/104 μm2) | 2.7 ± 4.3 | 25.3 ± 22.1* | 7.8 ±9.5** | 9.0 ± 11.4** | 14.7 ± 11.4*,**,& |
IL, interleukin; MMP, metalloproteinase; TIMP, metalloproteinase inhibitor; TGF-β, transforming growth factor; VEGF, vascular endothelial growth factor; OVA, animals induced to chronic allergic pulmonary inflammation by ovalbumin; OVA-anti-IL17, animals induced to chronic allergic inflammation by ovalbumin; OVA-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
p < 0.05 compared with SAL group.
p < 0.05 compared with the OVA group.
p < 0.05 compared with OVA-RHO inhibitor group.
Figure 6.
The treatments with the Rho-kinase and anti-IL17 attenuates the expression of remodeling markers in vessel in chronic allergic pulmonary inflammation induced by ovalbumin in mice. In OVA group there was an increase compared with the saline group in the expression of MMP-12 and TIMP-1. The data are presented as dot plots with standard deviations. Representative photomicrographs of MMP-12, TIMP-1 and TGF-β for each group. The red arrows indicate the marked area in immunohistochemistry.
IL, interleukin; MMP, metalloproteinase; TIMP, metalloproteinase inhibitor; TGF-β, transforming growth factor; OVA, animals induced to chronic allergic inflammation by ovalbumin; OVA-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of anti-IL17; OVA-RHO inhibitor, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals induced to chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
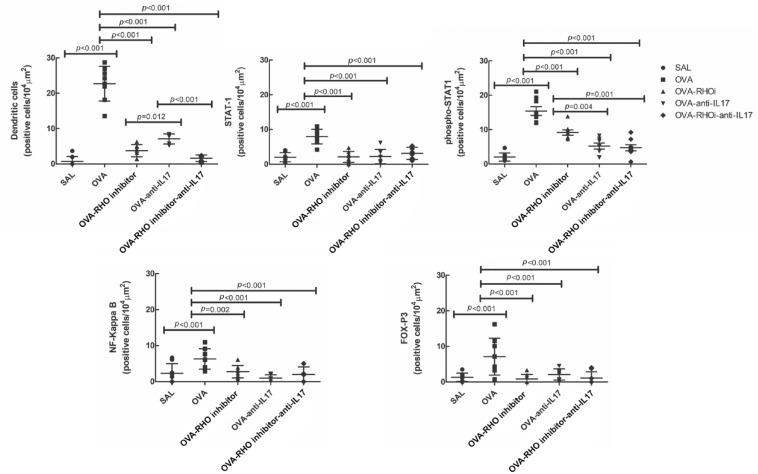
Anti-IL17 and/or Rho-kinase inhibitor treatments attenuated controlling mechanisms including the expression of transcriptors factors in experimental asthma induced by ovalbumin
The effects of anti-IL17 and/or Rho-kinase inhibitor treatment on the controlling mechanisms are shown in Figure 7. We evaluated STAT1, phospho-STAT1, dendritic cells, NF-KappaB, and FOXP-3 and found an increase in the number of positive cells in the OVA group compared with the SAL control group, and decrease of STAT1, phospho-STAT1, dendritic cells, NF-KappaB, and FOXP-3 in the treated groups when compared with the OVA group (p < 0.05).
Figure 7.
The treatments with Rho-kinase inhibitor and anti-IL17 have an effect on the control of mechanisms involved in chronic allergic pulmonary inflammation induced by ovalbumin. In OVA group there was an increase compared with the saline group in the expression of dendritic cells, STAT1, phospho-STAT1, NF-KappaB, and FOX-P3. The data are presented as dot plots with standard deviations.
IL, interleukin; NF, nuclear factor; STAT1, signal transducer and activator of transcription 1; OVA, animals induced to chronic allergic inflammation by ovalbumin; OVA-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use anti-IL17; OVA-RHO inhibitor, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor; OVA-RHO inhibitor-anti-IL17, animals with induction of chronic allergic pulmonary inflammation and use of Rho-kinase inhibitor and anti-IL17; SAL, saline group.
Attenuation of phospho-STAT1 was observed in the anti-IL17 treated groups (OVA-anti-IL17 and OVA-RHO inhibitor-anti-IL17) compared with the OVA-RHO inhibitor group (p < 0.05).
We did not observe differences in STAT1, NF-KappaB and FOXP-3 among the treated groups, while in the evaluation of dendritic cells, we observed potentiation of the attenuation of the number of positive cells in the groups administered with the Rho-kinase inhibitor when compared with the group that was administered only anti-IL17.
Discussion
Asthma is a chronic disorder that results in variable airflow limitation in the airways and is associated with airway hyperresponsiveness, airway inflammation, and tissue remodeling. There are studies that analyze the effect of treatment with anti-IL17 or Rho-kinase inhibitor in the airways and alveolar septa, but there are no studies to date that analyzed the effect of these treatments on the vascular responses. This study, in an asthma model with chronic allergic pulmonary inflammation, analyzed angiogenesis, the expression of ROCK1 and ROCK2, inflammatory cells, and remodeling markers, and the controlling mechanisms. The results of this study showed that when treated with anti-IL17 and/or Rho-kinase inhibitor, we did not observe differences in angiogenesis. There is a reduction in the actin and endothelin-1, and also as attenuation the number of NF-KappaB, FOXP-3, dendritic cells, STAT1, phospho-STAT1, ROCK1, and ROCK2 positive cells. There is also a decrease in the expression of the inflammatory markers, such as TNF-α, CD4+, CD8+, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17 positive cells; remodeling markers, such as TIMP-1, MMP-9, MMP-12, TGF-β and VEGF positive cells; and decorin, fibronectin, biglycan, and collagen fibers. In addition, these treatments also reduced the expression of the oxidative stress markers iNOS and isoprostane.
We noticed that there was a potentiation of the reduction of IL1-β and dendritic cells in the group administered with Rho-kinase inhibitor in association with anti-IL17 when compared with the group administered with anti-IL17. We also observed potentiation of the attenuation of the number of CD8+, IL-17, TGF-β, phospho-STAT1 positive cells and endothelin-1 in the group that combined the two treatments when compared with the group that used only Rho-kinase inhibitor.
We observed that the association of Rho-kinase inhibitor with anti-IL17 potentiated ROCK2 attenuation compared with Rho-kinase inhibitor and anti-IL17 individually.
In our recent study we evaluated the effect of anti-IL17 and/or Rho-kinase inhibitor and demonstrated the beneficial effects of these treatments on the attenuation of hyperresponsiveness, inflammation, tissue remodeling, and oxidative stress in mice with chronic airway inflammation. They evaluated the airways and pulmonary parenchyma.14 In the present study, we analyzed angiogenesis and the vessels. Previous studies demonstrate that it is possible to evaluate angiogenesis with an experimental protocol in which animals receive ovalbumin solution for 4–5 weeks.17,19
For the assessment of myofibroblasts, we considered the analysis of actin. In addition, myofibroblasts produce endothelin 1, a potent vasoconstrictor, but also a factor that stimulates the formation of new myofibroblasts.20 We did not find differences between treatments and the OVA group in relation to the assessment of angiogenesis and vascular wall. When we analyzed actin and endothelin-1, we found that treatments with anti-IL17, Rho-kinase inhibitor and the combination of both were able to attenuate the expression of these markers in the vessel walls. In the evaluation of endothelin-1, the groups that used anti-IL17 (OVA-anti-IL17 and OVA-RHO inhibitor-anti-IL17) demonstrated attenuation of this expression when compared with the group that used only Rho-kinase inhibitor. These data suggest a possible superior effect of anti-IL17 compared with the inhibitor of Rho-kinase. Another study suggests that endothelin regulates the function of the subset of Th17 cells in disease such as multiple sclerosis.21
Researchers demonstrated increased Th1, Th2, and Th17 profile inflammatory markers in mice with chronic allergic inflammation, as well as increased iNOS, isoprostane, and remodeling markers (MMP-9, MMP-12, TIMP-1, biglycan, decorin, fibronectin, collagen fibers) in the airways and parenchyma.14,15,18 We demonstrated that treatment with anti-IL17 and/or Rho-kinase inhibitor attenuates these markers in the vessel walls.
In this study, we demonstrated the attenuation of CD4+ and CD8+ positive cells in the vessel walls of the animals treated with anti-IL17 and/or Rho-kinase inhibitor. These results were similar to our recent studies analyzing airways and pulmonary parenchyma in a model of chronic allergic pulmonary inflammation.14,15
We found a better response in CD8+ cells as our results showed potentiation of attenuation of these cells in the group administered Rho-kinase inhibitor in association with anti-IL17 compared with treatment with Rho-kinase inhibitor alone. We believe this difference was due to the presence of anti-IL17 as previous researchers, using the murine model CD8+ T cells, had reported an increased expression of IL-4 and/or IL-13, possibly under the influence of IL-4 produced by the cells. A previous research suggested that they could contribute to tissue remodeling.22
In this study, there was an increase of the ILs involved in the chronic inflammatory processes, such as TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-10, and IL-13 in an experimental model of asthma. These ILs are directly linked to the angiogenesis process in asthma. We obtained a good response to treatment with anti-IL17, Rho-kinase inhibitor, and the association of both when we evaluated all these cytokines in comparison with the OVA group.
We observed that attenuation of IL-1β positive cells was enhanced in the group that combined anti-IL17 and Rho-kinase inhibitor and in the groups that used Rho-kinase inhibitor alone compared with the group that used anti-IL17 alone. There are no studies evaluating the effect of the Rho-kinase inhibitor on IL-1 and demonstrating its contribution in angiogenesis, but Santos et al. observed that treatment with Y-27632 attenuated the expression of this IL in the airway and alveolar walls compared with the experimental model of asthma.14
The IL-1 family cytokines induce proangiogenic factors, such as VEGF, having a role in mediating angiogenesis directly or indirectly. Better VEGF transcription and neovascularization in the vascular smooth muscle cells of small tumor vessels was observed after treatment with IL-1β.23
Th2 cytokines have been shown to modulate VEGF synthesis and release through different inflammatory and structural airway cells.24 Thus, the attenuation of these markers would be beneficial in vascular remodeling.
It is known that many of the proinflammatory mediators in asthma, such as IL-6, IL-8, TNF-α, and TGF-β, have proangiogenic properties capable of stimulating the vascular expansion or the permeability of endothelial cells, by either direct or indirect contact through stimulation of inflammatory cells.9
Considering that IL-13 must be involved in the process of angiogenesis in asthma, its decrease may allow greater control of angiogenesis.19
We observed an increase in IL-17 in the vessels of the OVA-exposed group compared with the saline control group and attenuation in the treated groups.
Studies have reported the important role of Th17 cells in vascular remodeling. Panariti et al. showed that severe asthmatics have greater vascularity compared with healthy adults, which correlates positively with the concentrations of angiogenic factors in sputum. It was also demonstrated that increased bronchial vascularization correlates positively with the number of IL-17A subepithelial cells, suggesting that the effects of IL-17A on pulmonary vascular remodeling are indirect, affecting endothelial function by stimulating the production of angiogenic factors in bronchial epithelial cells and airway smooth muscle.25 Moreover, Th17 cells contribute to the pulmonary vascular remodeling of asthma in asthmatics by mediating endothelial progenitor cell chemotaxis,26 thus explaining the increase in IL-17, which is the main product of Th17 in the OVA group compared with the control group.
The association of anti-IL17 with the Rho-kinase inhibitor mediated potentiation of this reduction compared with treatment with the Rho-kinase inhibitor only.
Despite its indirect effect, inhibition of IL-17 may be beneficial by directing the effects of IL-17A to the epithelium and smooth muscle cells and interfering with the bronchial vascular remodeling, with beneficial clinical outcomes for asthmatic patients.15 The effect of the combination of anti-IL17 and Rho-kinase inhibitor on IL-17 had been previously demonstrated in the attenuation during airway evaluation, in agreement with this study.14
We demonstrated increased expression of MMP-9, MMP-12, and TIMP-1 in the vessels of animals with chronic airway inflammation and attenuation of these markers when treated with anti-IL17 and/or Rho-kinase inhibitor, corroborating with studies showing the role of MMP-9 in asthma by inducing the migration of inflammatory cells throughout the epithelial basement membrane.14,15,24 The MMP-9 has been identified as a protein associated with angiogenesis.27 Studies have shown the association of MMP-9 with VEGF, demonstrating that VEGF signaling regulates MMP-9 expression and the presence of a VEGF auto-inductive pathway.28,29 It has also been shown that inhibition of VEGF receptors might suppress the remodeling process by regulating MMP-9/TIMP-1 expression.30
There was also an increase in the content of biglycan, decorin, fibronectin, and collagen fibers in the OVA group compared with the control group. These results are similar to those found in our recent study and Camargo et al. in the airway and alveolar walls.14,15 We demonstrated the attenuation of these markers upon treatment with anti-IL17 and/or Rho-kinase inhibitor, indicating that collagen is a potential marker in asthma remodeling and is produced in response to cytokine TGF-β; its attenuation might allow better management of this process.31,32
We observed an increase in the biglycan content in the combination treatment group when compared with the Rho-kinase inhibitor group. These findings are similar to those found in the airway and alveolar septa in the study using the combination of anti-IL17 and Rho-kinase inhibitor in animals with chronic allergic pulmonary inflammation.14
We quantified the number of TGF-β and VEGF positive cells in the vessels after anti-IL17 and Rho-kinase inhibitor treatment. It was found that treatment with anti-IL17 and Rho-kinase inhibitor alone or in association attenuated the expression of TGF-β and VEGF in the vessels compared with the untreated animals. In TGF-β evaluation, we observed potentiation of attenuation in the group that combined the two treatments compared with the group that used the Rho-kinase inhibitor only.
Angiogenesis typically occurs in response to tissue hypoxia, in which hypoxic cells secrete VEGF,33 which is the most potent neovascularization stimulator. VEGF levels are associated with the degree of vascularization.10 It is an important marker of endothelial vascular growth and the major regulator of pathological angiogenesis and its characteristic of migration, proliferation, and tube formation in endothelial cells.34,35
The TH2 cytokines, one of the main pathways of the inflammatory process, and TGF-β stimulate the airway smooth muscle cell release of VEGF. The TGF-β1, in addition to being an extracellular matrix regulator, also plays a key role in bronchial angiogenesis and vascular remodeling via VEGF in asthma.36
The VEGF is also a major contributor to vascular hyperpermeability through ROCK activation, which increases the endothelial permeability by promoting cellular contraction via myosin light chain phosphorylation and formation of contractile fibers. Studies have suggested an important role of Rho-kinase in angiogenesis, which is initiated by endothelial cell activation, followed by endothelial cell migration and proliferation. ROCK is also necessary for the constitution of the proangiogenic microenvironment and regulating gene expression in non-endothelial cells.37
In this study, we observed an increase in the number of ROCK1- and ROCK2-positive cells in the OVA group compared with that in the control group and attenuation of these markers in the augmented groups. According to a previous study, ROCK inhibitor (Y-27632) blocked VEGF-induced microvascular hyperpermeability in a dose-dependent manner.38 The VEGF activates RhoA, which is recruited to the endothelial cytoplasm membrane.39
The specific contributions of each ROCK isoform have been explored. Using small interfering RNA knockdown and mouse heterozygote knockouts of ROCK1 and ROCK2, it was reported that VEGF-induced angiogenesis is largely mediated by ROCK2.37 Our findings are in accordance with this study. We demonstrated attenuation of the number of ROCK2 positive cells in the combination treatment group compared with that in the groups treated individually with anti-IL17 and Rho-kinase inhibitor.
Pharmacological inhibitors of the Rho pathway block angiogenesis. The VEGF rapidly induces RhoA activation in endothelial cells (ECs),33 demonstrating that Rho/ROCK signaling is an important mediator in several angiogenic processes, including cell migration, survival, and permeability, and suggesting that Rho/ROCK inhibition may be useful for treating angiogenesis-related disorders.40
We evaluated iNOS and isoprostane since airway smooth muscle function is modulated by reactive oxygen species (ROS). 8-isoprostane is a marker of oxidative stress that contributes to the greater activity of the airway smooth muscle. ROS produced in the asthmatic airways are involved in vascular/systemic oxidant–antioxidant imbalance.41 The lung has endogenous antioxidants to overcome the damage mediated by ROS to the pulmonary vasculature. However, during asthmatic inflammation, this antioxidant network is weakened, which allows oxidants to leak into the systemic circulation, which may be important in the balance of Th1/Th2/Th17 cytokines in the vasculature.42
This marker has been found in the airway and parenchyma of animals with chronic pulmonary inflammation. To establish the association between IL-17 and ROS, the authors studied the effect of anti-IL17 on the markers of oxidative stress.14,15,43 In agreement with these data, we observed an increase in the oxidative stress markers, such as iNOS and isoprostane, in the group of animals with chronic allergic pulmonary inflammation compared with the animals in the control group. We also observed attenuation in the number of iNOS and isoprostane positive cells in the anti-IL17 and/or Rho- kinase inhibitor treated groups compared with the OVA-exposed group.
The family of signal transducer and activator of transcription (STAT) factors play an important role in the signaling of many cytokines.44 Recent studies on pulmonary adenocarcinoma have revealed the involvement of STAT1 in the vascular remodeling process; IL-17 is also known to directly activate tyrosine phosphorylation in the JAK–STAT pathway and may stimulate the STAT1 pathway.45–47 Therefore, we decided to evaluate the stimulation of the STAT1 pathway in the chronic inflammatory activation of allergic inflammatory disease and examined the effect of anti-IL17 and Rho-kinase inhibitor treatment on STAT1 and phospho-STAT1.
As a result, we found an increase in STAT1 and phospho-STAT1 in the OVA group compared with the SAL control group, suggesting their involvement in the chronic allergic inflammatory process. We also observed attenuation of STAT1 and phospho-STAT1 in the anti-IL17 and/or Rho-kinase inhibitor treated groups when compared with the OVA group. The phospho-STAT1 evaluation demonstrated that the groups administered anti-IL17 showed potentiation of attenuation of the positive cell number when compared with the group that used only the Rho-kinase inhibitor.
We may reflect that the use of anti-IL17 was more effective in controlling the involvement of phosphorylated STAT1 than the Rho-kinase inhibitor treatment. Our findings corroborated with the previous study showing that IL-13 and IL-4 cause a significant increase in the phosphorylated STAT1 levels in pulmonary inflammation.44 This may have been due to the involvement of these ILs in the inflammatory process and their activation by cells through the Th2 and Th17 profile cells.48 However, we have been unable to find studies evaluating the effects of anti-IL17 and Rho-kinase inhibitor on the STAT1 and phospho-STAT1 pathways.
It has been shown that NF-KappaB and STAT1 expression levels increase in children with asthma and are negatively correlated with the lung function indices, suggesting that NF-KappaB and STAT1 are involved in the development and progression of bronchial asthma.49 In the NF-KappaB analysis, we found an increase in the number of positive cells in the OVA group compared with the control group and attenuation in the groups treated with anti-IL17 and/or Rho-kinase inhibitor. The IL-17 activates NF-KappaB and induces the expression of proinflammatory cytokines.50 Camargo et al. demonstrated that the use of anti-IL17 in a model of chronic allergic pulmonary inflammation attenuated the expression of NF-KappaB in alveolar parenchyma.15 Researchers also observed attenuation of NF-KappaB expression in an experimental model of asthma treated with Rho-kinase inhibitor.18
In the evaluation of dendritic cells, we observed an increased expression of these cytokines in the OVA group compared with that in the control group. Activated dendritic cells can have an important impact on the neovascularization process and exert a potent proangiogenic activity that is mediated by VEGF. In turn, pro and anti-angiogenic mediators may affect the biology of dendritic cells (DCs), modulating their differentiation and maturation. These cells can trans-differentiate into endothelial cells, possibly contributing to angiogenesis.51
The treated groups showed attenuation of the dendritic cells when compared with the OVA group. In the Rho-kinase inhibitor-treated groups, we observed an enhancement in dendritic cell attenuation as compared with the group treated with anti-IL17 alone. These data corroborate the findings of researchers who showed that the ROCK inhibitor Y-27632 attenuated OVA-induced lymphocyte proliferation, suggesting that ROCK is required for the dendritic T cell activation process.52
The present study used an experimental animal model, and therefore, has limitations. Although it has similarities in the vessels of humans, we cannot directly extrapolate our findings to those expected in humans. Another limitation of our study was that the results showed no difference in angiogenesis between the treated and OVA groups. We believe that if we used a model with more than 4 weeks of exposure, we would obtain a better response. Although we found no difference in angiogenesis, we presented positive responses of these treatments in important markers involved in the angiogenesis process, such as ROCK1 and ROCK2, remodeling markers, and oxidative stress markers and we could establish the mechanisms involved.
However, our results are in agreement with those of previous studies regarding the therapeutic importance of both Rho-kinase inhibitor and anti-IL17 in the control of inflammation, extracellular matrix remodeling, and oxidative stress management, as well as their involvement in angiogenesis.
Our study also has strengths. We demonstrated attenuation of important markers, such as CD8+, IL-1β, IL-17, ROCK2, TGF-β, and phospho-STAT1, upon combination treatment, considering that when combined they achieve a more potentiated response of this control by these pathways. We performed a detailed analysis of a large number of inflammatory markers for Th1, Th2, and Th17 cytokines, markers of oxidative stress, and the remodeling of the vascular process.
Conclusion
In this study, we showed that Rho-kinase inhibitor and anti-IL17 contributed to the control of inflammation and remodeling. However, we did not observe any difference in angiogenesis after anti-IL17 and Rho-kinase inhibitor treatment.
The treatments proved to be beneficial and attenuated the markers of inflammatory processes, remodeling, vascular markers and oxidative stress and established the mechanisms involved in chronic allergic pulmonary inflammation.
Although the association did not demonstrate differences in angiogenesis, it showed differences in markers involved in the angiogenesis process. The association of anti-IL17 and Rho-kinase inhibitor when compared with treatment with anti-IL17 only resulted in potentiation in decreasing the number of IL-1β and dendritic cells. When we compared the combination treatment with only Rho-kinase inhibitor treatment, there was attenuation of the number of CD8+, IL-17, TGF-β, and phospho-STAT1 positive cells and endothelin-1. There was a potentiation in the attenuation of the number of ROCK2 positive cells in the group administered with the combination treatment compared with the groups treated with anti-IL17 and Rho-kinase inhibitor individually.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Tabata M. dos Santos: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Writing-original draft; Writing-review & editing.
Renato F. Righetti: Funding acquisition; Methodology; Project administration; Supervision; Visualization; Writing-original draft; Writing-review & editing.
Bianca G. Rezende: Data curation; Methodology; Writing-review & editing.
Elaine C. Campos: Formal analysis; Writing-original draft; Writing-review & editing.
Leandro do N. Camargo: Data curation; Formal analysis; Software; Writing-review & editing.
Beatriz M. Saraiva-Romanholo: Conceptualization; Formal analysis; Writing-review & editing.
Silvia Fukuzaki: Data curation; Methodology; Writing-review & editing.
Carla M. Prado: Formal analysis; Methodology; Writing-review & editing.
Edna A. Leick: Conceptualization; Project administration; Writing-original draft.
Milton A. Martins: Conceptualization; Funding acquisition; Project administration; Writing-review & editing.
Iolanda F. L. C. Tibério: Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing-original draft; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: All BALB/c mice were treated according to the Laboratory Animal Care and Use Guideline (National Institutes of Health publication 85-23), revised in 1985. All procedures in this study were approved by the Ethics Committee on the Use of Animals of the School of Medicine of the University of São Paulo (number 064/15).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP) (Grant No. 2018/02537-5), the National Council of Scientific and the Technological Development (CNPq), and Laboratory of Medical Investigations (LIM-20 FMUSP).
ORCID iD: Tabata M. dos Santos  https://orcid.org/0000-0002-5002-5076
https://orcid.org/0000-0002-5002-5076
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Tabata M. dos Santos, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, SP, BR; Hospital Sirio-Libanes, São Paulo, Brazil
Renato F. Righetti, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, SP, BR; Hospital Sirio-Libanes, São Paulo, Brazil
Bianca G. Rezende, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil
Elaine C. Campos, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil; Hospital Sirio-Libanes, São Paulo, Brazil
Leandro do N. Camargo, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, SP, BR. Hospital Sirio-Libanes, São Paulo, Brazil
Beatriz M. Saraiva-Romanholo, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil Department of Medicine, University City of São Paulo (UNICID), São Paulo, Brazil.
Silvia Fukuzaki, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil.
Carla M. Prado, Department of Biosciences, Federal University os Sao Paulo, Santos, SP, Brazil
Edna A. Leick, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil
Milton A. Martins, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, Brazil
Iolanda F. L. C. Tibério, Departamento de Clínica Médica, Faculdade de Medicina da Universidade de São Paulo, Av. Dr. Arnaldo, 455- Sala 1210, São Paulo, SP 01246-903, Brazil.
References
- 1. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther 2018; 186: 98–113. [DOI] [PubMed] [Google Scholar]
- 3. Keglowich LF, Borger P. The three A’s in asthma - Airway smooth muscle, Airway remodeling & Angiogenesis. Open Respir Med J 2015; 9: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xin L, Gao J, Ge X, et al. Increased pro-inflammatory cytokine-secreting regulatory T cells are correlated with the plasticity of T helper cell differentiation and reflect disease status in asthma. Respir Med 2018; 143: 129–138. [DOI] [PubMed] [Google Scholar]
- 5. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50: 975–991. [DOI] [PubMed] [Google Scholar]
- 6. Chenuet P, Fauconnier L, Madouri F, et al. Neutralization of either IL-17A or IL-17F is sufficient to inhibit house dust mite induced allergic asthma in mice. Clin Sci (Lond) 2017; 131: 2533–2548. [DOI] [PubMed] [Google Scholar]
- 7. Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001; 108: 430–438. [DOI] [PubMed] [Google Scholar]
- 8. Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006; 7: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harkness LM, Ashton AW, Burgess JK. Asthma is not only an airway disease, but also a vascular disease. Pharmacol Ther 2015; 148: 17–33. [DOI] [PubMed] [Google Scholar]
- 10. Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001; 107: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 11. Amin E, Dubey BN, Zhang SC, et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem 2013; 394: 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao L, Romero MJ, Toque HA, et al. The role of RhoA/Rho-kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res 2010; 1: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bond LM, Sellers JR, McKerracher L. Rho-kinase as a target for cerebral vascular disorders. Future Med Chem 2015; 7: 1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dos Santos TM, Righetti RF, Camargo LDN, et al. Effect of anti-IL17 antibody treatment alone and in combination with Rho-kinase inhibitor in a murine model of asthma. Front Physiol 2018; 9: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Camargo LDN, Righetti RF, Aristóteles LRCRB, et al. Effects of anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol 2018; 8: 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barlow JL, Flynn RJ, Ballantyne SJ, et al. Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity. Clin Exp Allergy 2011; 41: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 17. Prado CM, Yano L, Rocha G, et al. Effects of inducible nitric oxide synthase inhibition in bronchial vascular remodeling-induced by chronic allergic pulmonary inflammation. Exp Lung Res 2011; 37: 259–268. [DOI] [PubMed] [Google Scholar]
- 18. Righetti RF, Pigati PA, Possa SS, et al. Effects of Rho-kinase inhibition in lung tissue with chronic inflammation. Respir Physiol Neurobiol 2014; 192: 134–146. [DOI] [PubMed] [Google Scholar]
- 19. Zhang FQ, Han XP, Zhang F, et al. Therapeutic efficacy of a co-blockade of IL-13 and IL-25 on airway inflammation and remodeling in a mouse model of asthma. Int Immunopharmacol 2017; 46: 133–140. [DOI] [PubMed] [Google Scholar]
- 20. van Caam A, Vonk M, van den Hoogen F, et al. Unraveling SSc pathophysiology; the myofibroblast. Front Immunol 2018; 9: 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka K, Yoshioka K, Tatsumi K, et al. Endothelin regulates function of IL-17-producing T cell subset. Life Sci 2014; 118: 244–247. [DOI] [PubMed] [Google Scholar]
- 22. Lourenço O, Fonseca AM, Taborda-Barata L. Human CD8+ T cells in asthma: possible pathways and roles for NK-like subtypes. Front Immunol 2016; 7: 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fahey E, Doyle SL. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front Immunol 2019; 10: 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ribatti D, Puxeddu I, Crivellato E, et al. Angiogenesis in asthma. Clin Exp Allergy 2009; 39: 1815–1821. [DOI] [PubMed] [Google Scholar]
- 25. Panariti A, Baglole CJ, Sanchez V, et al. Interleukin-17A and vascular remodelling in severe asthma; lack of evidence for a direct role. Clin Exp Allergy 2018; 48: 365–378. [DOI] [PubMed] [Google Scholar]
- 26. Lu S, Li H, Gao R, et al. IL-17A, but not IL-17F, is indispensable for airway vascular remodeling induced by exaggerated Th17 cell responses in prolonged ovalbumin-challenged mice. J Immunol 2015; 194: 3557–3566. [DOI] [PubMed] [Google Scholar]
- 27. Rivera CG, Bader JS, Popel AS. Angiogenesis-associated crosstalk between collagens, CXC chemokines, and thrombospondin domain-containing proteins. Ann Biomed Eng 2011; 39: 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walters EH, Soltani A, Reid DW, et al. Vascular remodelling in asthma. Curr Opin Allergy Clin Immunol 2008; 8: 39–43. [DOI] [PubMed] [Google Scholar]
- 29. Mira E, Lacalle RA, Buesa JM, et al. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci 2004; 117: 1847–1857. [DOI] [PubMed] [Google Scholar]
- 30. Moon IJ, Kim DY, Rhee CS, et al. Role of angiogenic factors in airway remodeling in an allergic rhinitis murine model. Allergy Asthma Immunol Res 2012; 4: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito JT, Lourenço JD, Righetti RF, et al. Extracellular matrix component remodeling in respiratory diseases: what has been found in clinical and experimental studies? Cells 2019; 8: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ojiaku CA, Yoo EJ, Panettieri RA., Jr. Transforming growth factor β1 function in airway remodeling and hyperresponsiveness. The missing link? Am J Respir Cell Mol Biol 2017; 56: 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barlow HR, Cleaver O. Building blood vessels-one Rho GTPase at a time. Cells 2019; 8: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Peng J, Zhao S, et al. Tussilagone suppresses angiogenesis by inhibiting the VEGFR2 signaling pathway. Front Pharmacol 2019; 10: 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Q, Kan X, Yin J, et al. Chamaejasmine B induces the anergy of vascular endothelial cells to VEGFA pro-angiogenic signal by autophagic regulation of VEGFR2 in breast cancer. Front Pharmacol 2017; 8: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Willems-Widyastuti A, Alagappan VK, Arulmani U, et al. Transforming growth factor-beta 1 induces angiogenesis in vitro via VEGF production in human airway smooth muscle cells. Indian J Biochem Biophys 2011; 48: 262–269. [PubMed] [Google Scholar]
- 37. Liu J, Wada Y, Katsura M, et al. Rho-Associated Coiled-Coil Kinase (ROCK) in molecular regulation of angiogenesis. Theranostics 2018; 8: 6053–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Breslin JW, Sun H, Xu W, et al. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol 2006; 290: H741–H750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Nieuw Amerongen GP, Koolwijk P, Versteilen A, et al. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 2003; 23: 211–217. [DOI] [PubMed] [Google Scholar]
- 40. Bryan BA, Dennstedt E, Mitchell DC, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J 2010; 24: 3186–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al-Harbi NO, Nadeem A, Al-Harbi MM, et al. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int Immunopharmacol 2015; 26: 237–245. [DOI] [PubMed] [Google Scholar]
- 42. Al-Harbi NO, Nadeem A, Al-Harbi MM, et al. Airway oxidative stress causes vascular and hepatic inflammation via upregulation of IL-17A in a murine model of allergic asthma. Int Immunopharmacol 2016; 34: 173–182. [DOI] [PubMed] [Google Scholar]
- 43. Vasconcelos LHC, Silva MDCC, Costa AC, et al. A guinea pig model of airway smooth muscle hyperreactivity induced by chronic allergic lung inflammation: contribution of epithelium and oxidative stress. Front Pharmacol 2018; 9: 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiba Y, Todoroki M, Misawa M. Activation of signal transducer and activator of transcription factor 1 by interleukins-13 and -4 in cultured human bronchial smooth muscle cells. J Smooth Muscle Res 2009; 45: 279–288. [DOI] [PubMed] [Google Scholar]
- 45. Subramaniam SV, Cooper RS, Adunyah SE. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem Biophys Res Commun 1999; 262: 14–19. [DOI] [PubMed] [Google Scholar]
- 46. Peters A, Yosef N. Understanding Th17 cells through systematic genomic analyses. Curr Opin Immunol 2014; 28: 42–48. [DOI] [PubMed] [Google Scholar]
- 47. Murugaiyan G, Beynon V, Pires Da Cunha A, et al. IFN-γ limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. J Immunol 2012; 189: 5277–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choy DF, Hart KM, Borthwick LA, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015; 7: 301ra129. [DOI] [PubMed] [Google Scholar]
- 49. Shi HL, Liu JB, Lu AP. [Expression profiles of PI3K, NF-κB, and STAT1 in peripheral blood mononuclear cells in children with bronchial asthma]. Zhongguo Dang Dai Er Ke Za Zhi 2016; 18: 614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cruz JA, Childs EE, Amatya N, et al. Interleukin-17 signaling triggers degradation of the constitutive NF-κB inhibitor ABIN-1. Immunohorizons 2017; 1: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sozzani S, Rusnati M, Riboldi E, et al. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol 2007; 28: 385–392. [DOI] [PubMed] [Google Scholar]
- 52. Zhu M, Liu PY, Kasahara DI, et al. Role of Rho-kinase isoforms in murine allergic airway responses. Eur Respir J 2011; 38: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466620962665 for Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation by Tabata M. dos Santos, Renato F. Righetti, Bianca G. Rezende, Elaine C. Campos, Leandro do N. Camargo, Beatriz M. Saraiva-Romanholo, Silvia Fukuzaki, Carla M. Prado, Edna A. Leick, Milton A. Martins and Iolanda F. L. C. Tibério in Therapeutic Advances in Respiratory Disease