Abstract
Background:
The risk of transfusion-transmitted viral infections is very low in developed countries. Recent massive migration flows from highly hepatitis B virus (HBV), hepatitis C virus (HCV) and/or HIV endemic regions to Europe may have changed this scenario.
Methods:
During 2017 and 2018, a total of 491,753 blood donations (291,762 donors) were evaluated at the Madrid Regional Transfusion Center. All were tested for hepatitis B surface antigen (HBsAg), anti-HCV and anti-HIV, as well as for HBV-DNA, HCV-RNA and HIV-RNA.
Results:
Overall, 35 donors were positive for HIV-RNA and 26 for HCV-RNA. HBV markers were found in 111 (0.022%) donors, split out into three categories: HBsAg+ (n = 93; 0.019%), occult B infection (OBI) (n = 17; 0.003%), and acute HBV window period (n = 1; 0.0002%). All 17 OBI donors were positive for anti-HBc and confirmed as viremic in repeated testing. Viral load amounts were uniformly below 100 IU/mL. Ten OBI donors were repeated donors and look-back studies could be completed for eight of them. Fortunately, none of all prior recipients experienced transfusion transmitted hepatitis B. Compared with HBsAg+ donors, OBI donors were more frequently native Spaniards (76% versus 40%) and older (median age 52 versus 42 years old).
Conclusion:
Active HBV infection is currently found in 0.022% of blood donations (0.038% of donors) in Madrid. This rate is 3-fold greater than for HIV and/or HCV. On the other hand, HBsAg+ donors are 3-fold more frequent than OBI donors and more often immigrants than native Spaniards. No transfusion-transmitted HBV infections were identified during the study period, including retrospective checking of former recipients of OBI donors.
Keywords: blood donors, hepatitis B, hepatitis C, HIV, immigration, OBI, occult hepatitis B, transfusion
Introduction
Given the well established therapeutic benefit of blood transfusions for several acute and chronic medical conditions,1 prevention of transfusion-transmitted infections must be ensured by all means. Screening of blood donors began in the 1940s with testing for syphilis, followed in the early 1970s by testing for hepatitis B surface antigen (HBsAg). The recognition of higher rates of hepatitis B in paid donors led to conversion to an all-volunteer blood supply in the mid-1970s in most Western countries.
The recognition of transfusion-associated AIDS in 1982 as a threat resulted in a paradigm shift toward more rapid implementation of blood safety interventions, not only for HIV, but also for viral hepatitis B and C. Since then, progressive implementation of nucleic acid-amplification technology (NAT) screening for HIV, hepatitis C virus (HCV) and hepatitis B virus (HBV) has reduced the residual risk of the infectious window period in donations, such that per unit risks are below one per million in Western countries.1
Two major caveats have forced to reconsider the previous scenario. First, the massive arrival of immigration from highly HBV endemic regions to Europe.2 Second, the recognition of occult B infection (OBI) as a potential source for transfusion-transmitted HBV.3 Herein, we provide updated figures for HBV markers in a large and representative population of blood donors in Madrid, Spain.
Methods
All blood donations collected in the region of Madrid, Spain during 2017 and 2018 were retrospectively examined. The area covers a population of roughly 6.5 million habitants. A centralized Regional Transfusion Center is in charge of testing for all transmissible agents and safety of blood donations.
All blood donors completed a questionnaire before donation, in order to exclude potential donations from high risk individuals, for example, injection drug users and persons engaged regularly in unprotected sex with multiple partners. The questionnaire includes consent for the use of this information for anonymous research. Individual samples were tested for HBsAg using Prism® or Alinity® (Abbott). Testing for HBV-DNA was made using mini-pools of six samples using NAT on Cobas 6800® (Roche). All specimens positive for either HBsAg or HBV-DNA were further tested for anti-HBc, anti-HBs, anti-HBe and HBeAg using Architect® (Abbott). All donors harboring HBV markers are referred to the transfusion outclinic, where they undergo further medical assessment.
OBI was defined in the presence of circulating HBV-DNA in the absence of detectable serum HBsAg, with or without anti-HBc. Confirmation of positive HBV-DNA in the same and/or separate specimen was required. Individuals with this profile who subsequently seroconverted for anti-HBs were considered as presenting within the window period.
Statistical analysis
All results are presented as absolute numbers and percentages, and as median values. The comparison of rates was carried out using the Fisher exact test or the Chi-square test. Median ages [and interquartile ranges (IQRs)] were compared using the Wilcoxon test. Only p values below 0.05 were considered as significant. All calculations were performed using SPSS version 21 (SPSS Inc., Chicago, IL, USA).
Results
A total of 491,753 blood donations were evaluated at the Madrid Regional Transfusion Center during the 2-year study period. They corresponded to 291,762 distinct donors. Overall 111 (0.022%) of blood donations (0.038% of donors) had markers of HBV infection, which could be split out into three categories: positive HBsAg (n = 93; 0.019%), OBI (n = 17; 0.003%), and acute HBV window period (n = 1; 0.0002%). As reference, RNA was positive for HIV and HCV in 35 and 26 donors, respectively, during the same study period.
Table 1 records the main features of donors that exhibited HBV markers. The majority (93/111; 83.8%) harbored HBsAg+ and corresponded to individuals unaware of their chronic hepatitis B carrier status. Up to 60% of them were immigrants. Their median age was 42 years (IQR, 22–62) and 59% were male.
Table 1.
Main features of blood donors with hepatitis B virus markers.
| Total | HBsAg-positive | OBI | Window period | |
|---|---|---|---|---|
| n (%) | 111 (0.022) | 93 (0.019) | 17 (0.003) | 1 (0.0002) |
| Rate per 100,000 donors | 22.6 | 18.9 | 3.5 | 0.2 |
| Repeated donors, n (%) | 12 | 1 (1.1) | 10 (59) | 1 |
| Male gender, n (%) | 67 | 55 (59) | 11 (65) | 1 |
| Median age, years (range) | 44 (23–63) | 42 (22–62) | 52 (29–65) | 31 |
| Native Spaniards, n (%) | 34 | 21 (40) | 13 (76) | 0 |
HBsAg, hepatitis B surface antigen; OBI, occult B infection.
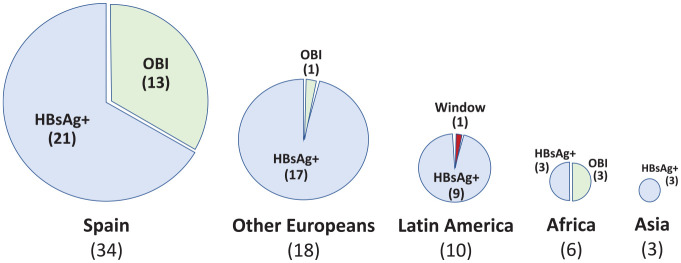
In contrast, OBI donors represented 15.3% of all discharged with HBV markers. Overall 65% were male and their median age was 52 years (IQR, 29–65). In contrast with HBsAg+ donors, OBI donors were mostly native Spaniards (76%) (Figure 1).
Figure 1.
Geographic origin of blood donors with hepatitis B virus markers.
HBsAg, hepatitis B surface antigen; OBI, occult B infection.
All initial OBI donors were positive for HBV-DNA in repeated tests and in all 15 with available separated specimens. Likewise, all were positive for anti-HBc. Viral load amounts were uniformly below 100 IU/mL. Ten OBI donors were repeated donors and look-back studies could be completed for eight of them. Fortunately, none of all prior recipients had evidence of transfusion-transmitted hepatitis B.
One specimen tested positive for HBV-DNA in isolation (44 IU/mL) and was confirmed as viremic 3 weeks later (511 IU/mL), this time along with anti-HBV markers and mild liver enzyme elevations. Further investigation revealed that he was a 31-year old immigrant from an area with a high HBV rate who additionally acknowledged other risk factors for HBV acquisition. This was the only case of acute hepatitis B picked up during the window period in our study.
Discussion
The risk of transfusion-transmitted viral infections is currently low in developed countries, although HBV transmission still represents one of the most important concerns.1 Most HBV transmissions seem to occur from donors tested during the window period and/or with OBI.3 In the United States, these two groups represent 26% of the overall rate of 8 per 100,000 HBV donors.4 Similar figures are not well known in Europe, but certainly the large migration flows from HBV endemic regions in recent times could have changed the former scenario.
A rebound in hepatitis B has been noticed during the last decade in Western Europe, following massive migrations from highly HBV endemic areas.2 Some experts have correctly pointed out that this phenomenon could be viewed as an opportunity for establishing proper HBV screening, vaccination and treatment of refugees.2 Furthermore, prevention strategies should be revised similarly for local Europeans, even acknowledging that universal HBV vaccination programs have been ongoing for many years in most EU countries.
The rate of HBV markers in Madrid’s donors (111; 0.022%) is 2-fold higher than for the rest of Spain (0.011%),5 most likely reflecting the large immigration flow of immigrants from HBV endemic regions to Madrid.
The HBV donor rate in our study is roughly 3-fold greater than for HIV (0.007%) and 4-fold greater than for HCV (0.005%). It is noteworthy that all our donors positive for HBsAg but one were first-time donors, being 60% immigrants. This is in agreement with the claim from Thijssen et al.2 and reports from other large European urban areas, where immigrants are increasingly accounting for new hepatitis B diagnoses.6 In our study, the only donor with acute hepatitis B presenting within the window period was a young male acknowledging unprotected sex with multiple sex partners.
The current rate of HBV markers in our population is lower than in surveys conducted more than one decade ago in Spain. In a study made during 2006–2008 testing half a million blood donors in Barcelona, OBI was found in 56 (one per 9050 donors).7 Another seven donors were identified during the window period and 276 were HBsAg+ carriers. As in our study, OBI donors in that survey were older than HBsAg+ donors (mean age, 52 versus 42 years) and more often male (74%).7 Likewise, in a prior survey in Madrid that we conducted in 2005–2006 testing 383,267 blood units, OBI was found in 13 (one per 30,000 donors), whereas 193 were HBsAg+ carriers and five were donors during the window period.8 Finally, a recent survey conducted in Italy during the last decade over 17 million blood donors acknowledged an estimated residual risk of HBV transmission of one in 2.5 million donors, being only slightly higher for OBI than window period cases.9
The clinical significance of OBI has been a matter of controversy, but recent reports have confirmed its definitive involvement in transfusion-transmitted HBV infection,3 liver disease, including cirrhosis and hepatocellular carcinoma, and occasionally in overt HBV reactivations when such individuals experience immunosuppression for any reason (neoplasms, chemotherapy, corticoids, etc.).10,11
In summary, active HBV infection is currently found in 0.022% of blood donors in Madrid, Spain. HBsAg+ donors are 3-fold more frequent than OBI donors and more often immigrants than native Spaniards. No transfusion-transmitted HBV infections were identified during the study period, including retrospective checking of former recipients of OBI donors.
Footnotes
Author Contribution: RG and LB designed the study. VS wrote the manuscript draft. AA included data and revised the manuscript. All participated in data analysis and interpretation of findings.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval: The Madrid Region Clinical Research Ethics Committee approved both the study and the informed consent procedures used in the study (Ref. FIB UPdH-2018-34).
ORCID iD: Vicente Soriano  https://orcid.org/0000-0002-4624-5199
https://orcid.org/0000-0002-4624-5199
Contributor Information
Rocío González, Regional Transfusion Center, Madrid, Spain.
Luisa Barea, Regional Transfusion Center, Madrid, Spain.
Ana Arruga, Regional Transfusion Center, Madrid, Spain.
Alberto Richart, Regional Transfusion Center, Madrid, Spain.
Vicente Soriano, UNIR Health Sciences School & Medical Center, Calle Almansa 101, Madrid 28040, Spain.
References
- 1. Busch M, Bloch E, Kleinman S. Prevention of transfusion transmitted infections. Blood 2019; 133: 1854–1864. [DOI] [PubMed] [Google Scholar]
- 2. Thijssen M, Lemey P, Amini-Bavil-Olyaee S, et al. Mass migration to Europe: an opportunity for elimination of hepatitis B virus? Lancet Gastroenterol Hepatol 2019; 4: 315–323. [DOI] [PubMed] [Google Scholar]
- 3. Candotti D, Assennato SM, Laperche S, et al. Multiple HBV transfusion transmissions from undetected occult infections: revising the minimal infectious dose. Gut 2019; 68: 313–321. [DOI] [PubMed] [Google Scholar]
- 4. Ramachandran S, Groves J, Xia G, et al. Recent and occult hepatitis B virus infections among blood donors in the United States. Transfusion 2019; 59: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez-Menchero C, Alvarez M, Fernandez P, et al. Evolution of the residual risk of HBV, HCV and HIV transmission through blood transfusion in the Region of Valencia, Spain, during a 15-year period (2003-2017). Blood Transfus 2019; 17: 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brancaccio G, Nardi A, Madonia S, et al. The present profile of chronic hepatitis B virus infection highlights future challenges: an analysis of the Multicenter Italian MASTER-B cohort. Dig Liver Dis 2019; 51: 438–442. [DOI] [PubMed] [Google Scholar]
- 7. Bes M, Vargas V, Piron M, et al. T cell responses and viral variability in blood donation candidates with occult hepatitis B infection. J Hepatol 2012; 56: 765–774. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez R, Torres P, Castro E, et al. Efficacy of hepatitis B virus (HBV) DNA screening and characterization of acute and occult HBV infections among blood donors from Madrid, Spain. Transfusion 2010; 50: 221–230. [DOI] [PubMed] [Google Scholar]
- 9. Velati C, Romano L, Pati I, et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis B virus infection in Italy from 2009 to 2018. Blood Transfus 2019; 17: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yip T, Wong G. Current knowledge of occult hepatitis B infection and clinical implications. Semin Liver Dis 2019; 39: 249–260. [DOI] [PubMed] [Google Scholar]
- 11. Raimondo G, Locarnini S, Pollicino T, et al. Update on the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol 2019; 71: 397–408. [DOI] [PubMed] [Google Scholar]