Abstract
Background and aims:
Early prediction of the effect of vedolizumab (VDZ) in inflammatory bowel disease (IBD) is of paramount importance to guide clinical decisions. This study assessed whether early fecal calprotectin (FC) can predict endoscopic response and histologic remission after VDZ initiation.
Methods:
This was a prospective study. Inclusion criteria were endoscopic inflammation and FC >100 µg/g. FC was determined at baseline and weeks 2, 4, 8 and 16. At week 16, endoscopies with ileal and colonic biopsies were performed. FC changes were assessed with Wilcoxon Rank Sum tests. ROC statistics were used to assess the diagnostic accuracy of FC.
Results:
In total, 45 patients [27 Crohn’s disease (CD), 16/2 ulcerative colitis (UC)/IBD-unclassified] [40% males, median age 39 (28–51) years] were included. Week 16 endoscopic response and histologic remission rates were 58% and 33%. A median 37% decline in FC at week 2 was observed only in endoscopic responders, p = 0.025. FC <250 µg/g at week 8 predicted endoscopic response in both UC and CD (positive predictive value 100%), whereas absence of FC decline at week 8 corresponded with absence of endoscopic response in CD [negative predictive value (NPV) 82%] and absence of histologic remission in both UC and CD (NPV 90%).
Conclusion:
The onset of a decline in FC as early as week 2 is associated with endoscopic response to VDZ induction. FC <250 µg/g at week 8 is associated with endoscopic response, whereas absence of FC decline at week 8 is associated with absence of both endoscopic response and histologic remission. FC levels 8 weeks after the start of VDZ could be used to guide clinical decisions and might substitute for endoscopic response evaluation.
Keywords: endoscopy, fecal calprotectin, vedolizumab
Introduction
Vedolizumab (VDZ) is a gut-selective humanized monoclonal antibody inhibiting the α4β7 integrin, which is registered for the treatment of adult patients with ulcerative colitis (UC) and Crohn’s disease (CD). Clinical efficacy has been demonstrated in the GEMINI I and II registration trials and afterwards confirmed in real-world cohorts.1–5 The GEMINI I trial reported clinical response rates of 47% in UC at week 6. A strategy combining VDZ with steroids during induction has been adopted widely in CD, as the GEMINI II trial showed an improved clinical response rate at week 6 for VDZ in combination with steroids, from 28% to 36%.1,2,6 Data on objective response to VDZ confirm clinical efficacy and show a significant decline of fecal calprotectin (FC) levels of 40% directly after induction at week 14, and endoscopic response rates of 40% at week 26.7,8 A clinically important question for every new therapeutic intervention is when and how to evaluate response. Suboptimal timing of response evaluation to the treatment effect might lead to either over- or under-treatment in a considerable proportion of patients, with subsequently unnecessary cessation of therapy in patients with a delayed response, delayed treatment optimization in partial responders or unnecessary prolonged treatment of (primary) non-responders. The ECCO and STRIDE guidelines recommend endoscopic response evaluation after the start of inflammatory bowel disease (IBD) therapy to objectify mucosal improvement.9–12 However, standardized endoscopic evaluation has not been implemented widely after the start of medication, due to the drawbacks of endoscopy associated with invasiveness of the procedure and costs. As timely recognition of response to VDZ will more effectively guide treatment optimization, early (non-invasive) prediction of the response to induction therapy is warranted and should reflect mucosal improvement.9,10,13 FC is a useful surrogate marker for monitoring mucosal improvement during IBD treatment and is increasingly used in IBD care to predict disease relapse.12,14–18 To date, the potential of FC to early predict response to VDZ induction therapy is largely unknown as studies have mainly focused on a single FC measurement.19 To investigate whether early FC level measurements are indicative for mucosal improvement, as observed by endoscopy and histology at week 16, FC levels at predefined time points were assessed after the initiation of VDZ therapy.
Materials and methods
The study design concerns a real world, prospective cohort study. All consecutive adult patients with UC, IBD–unclassified (IBD-U) and CD who started VDZ between December 2016 and November 2018 at the Erasmus Medical Center were prospectively included.
Inclusion criteria were baseline active endoscopic disease defined as Mayo endoscopic score of ⩾1 or simple endoscopic score in CD (SES-CD) ⩾3 or Rutgeerts’ score ⩾i2, and FC > 100 µg/g. The concomitant use of corticosteroids and immunomodulators (IMs) was allowed. After first vedolizumab infusion, systemic corticosteroids were tapered to zero at a rate of 5 mg per 1–2 weeks, and budesonide was tapered at a rate of 3 mg every 2–6 weeks. Only if tapering failed, re-introduction of the lowest effective dose of corticosteroid was allowed and left to the discretion of the treating physician. VDZ induction therapy consisted of four 300 mg infusions for UC patients and was scheduled at baseline (week 0), week 2, week 6 and week 14. All CD patients received an additional VDZ infusion at week 10.
Data collection
Clinical and demographic characteristics were collected at baseline (age, gender, smoking status, disease characteristics and treatment history). Clinical disease activity scores [the Harvey Bradshaw Index (HBI) for CD and the Simple Clinical Colitis Activity Index (SCCAI) for UC] were collected at baseline, week 8 and week 16. At baseline, week 2, week 4, week 8 and week 16 FC was determined using the QuantOn Cal (QoC) FC home test (Preventis, Germany) or a quantitative enzyme-linked immunosorbent assay (ELISA) (Bühlmann Laboratories AG, Schönenbuch, Switzerland). All patients were offered the QoC FC home test. When patients were not able to use the FC home test (due to various reasons), they were offered the ELISA laboratory tests. During follow-up, measurements were scheduled with the same FC measurement technique. C-reactive protein (CRP), hemoglobin, leukocytes, platelets and albumin were assessed at baseline, week 6 and week 16. Endoscopies were performed at baseline and week 16. Ileal and segmental colonic biopsies for histology were collected at week 16, whenever possible from inflamed and non-inflamed mucosa per biopsy location. Endoscopic inflammation was determined using the endoscopic Mayo score for UC and IBD-U patients, the SES-CD and the Rutgeerts’ score for postoperative CD patients.
Outcome measures and definitions
The primary outcome was the predictive value of serial FC levels for endoscopic response at week 16. Secondary outcomes included the predictive value of serial FC levels for clinical response, and (steroid free) clinical, (steroid free) biochemical, endoscopic and histologic remission at week 16.
Clinical response was defined as a decline of ⩾3 points in SCCAI or HBI as compared to baseline. Biochemical remission was defined as a FC <150 µg/g and CRP< 5 mg/L. Clinical remission was defined as a SCCAI <3 or HBI <5.20,21
Endoscopic remission was defined as an endoscopic Mayo score of 0, SES-CD ⩽2 and a Rutgeerts’ score of i0 or i1.22,23 Endoscopic response was defined as a decline of one or more points in the endoscopic Mayo score, ⩾50% decline in SES-CD score or a decline of one or more points in the Rutgeerts’ score. In patients with an ileostomy, ileoanal pouch anastomosis or ileorectal anastomosis endoscopic disease activity was classified as no, mild, moderate or severe as judged by the endoscopist. In this subgroup, endoscopic remission was defined as “no endoscopic disease activity” and response as a decline of ⩾1 point on the four-grade scale mentioned in Supplemental material Table 1 online. Histologic disease activity was scored on a four-point scale based on pathology reports: 0, no active histologic disease activity; 1, mild active inflammation (cryptitis, but no crypt abscesses); 2, moderate active inflammation (few crypt abscesses); 3, severe active inflammation (numerous crypt abscesses).
Ethical considerations
Each patient signed Informed Consent. The study was conducted according to the World Medical Association Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee (METC) Rotterdam (MEC 2004-168 2012), The Netherlands.
Statistical analysis
Normally distributed data were presented as mean ± standard deviation (SD) and continuous data with a skewed distribution as median and the first and third quartile (Q1–Q3). Categorical data were presented as numbers and percentages. Chi-square tests and Wilcoxon Rank Sum tests were used to evaluate differences in categorical and continuous not-normally distributed data, between endoscopic responders and non-responders for UC and CD patients. IBD-U patients were included in the UC group for analyses. Differences in clinical scores (HBI/SCCAI), biochemical serologic markers (CRP, hemoglobin, leukocytes, platelets, albumin) and FC levels [or the relative (%) change in FC between two time points] between responders and non-responders were assessed with the Wilcoxon Rank Sum test. Differences in the course of FC over time between responders and non-responders were visualized and additionally tested with Friedman’s ANOVA. The predictive value and optimal (the best discriminatory performance and clinical relevance) cut-off levels of FC or relative change in FC on endoscopic response were assessed using receiver operating characteristic (ROC) statistics. The ROC derived area under the curve can range from 0.5 to 1.0, with 1.0 denoting perfect discriminative performance and a value of 0.5 meaning that FC is equivalent to a coin toss in predicting endoscopic response. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FC to predict endoscopic response and histologic remission were calculated by cross-tabulation. A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPS Statistics version 25.0 (IBM Corp. Released 2013, IBM Corp., Armonk, NY, USA).
Results
A total of 51 consecutive patients were included, of whom six were excluded from further analysis since these patients were not evaluated with endoscopy at week 16 (Supplemental Figure 1). The study population comprised 45 patients [16 UC (36%), two IBD-U (4%) and 27 CD patients (60%)] with a median age of 39 years (Q1–Q3 28–51) (Table 1). In total 38/45 (84%) patients were exposed to one or more anti-tumor necrosis factor (anti-TNF) drugs prior to the start of VDZ, of whom 35/38 (92%) were anti-TNF refractory defined as clinical primary failure or secondary clinical loss of response, and 3/38 (8%) ceased anti-TNF due to side-effects. In 28/45 patients (62%) VDZ induction was combined with corticosteroid induction therapy (nine prednisone; 19 budesonide), which was completely tapered at week 16 in 21/28 (75%) patients. No difference was observed in corticosteroid prescription rate between UC and CD patients (p = 1.00). In total 15/45 patients (33%) were on concomitant therapy (9 on IM; three thiopurines, six tacrolimus and six on 5-aminosalicylic acid) during VDZ induction. In the majority of patients, 36/45 (80%), FC was analyzed with the same test at all time points (in n = 21: ELISA assay (Bühlmann Laboratories AG, Schönenbuch, Switzerland); in n = 15: QuantOn Cal test). In 9/45 (20%) patients the FC assay changed during the time points. At all time points, no statistical differences were observed between FC values measured with the ELISA assay as compared with the FC home test.
Table 1.
Baseline patient characteristics.
| Male, n (%) | 18 (40) |
| Median age, years (Q1–Q3) | 39 (28–51) |
| Smoking, n (%) | 9 (20) |
| Median disease duration, years (Q1–Q3) | 11 (4–19) |
| Diagnosis, n (%) | |
| UC | 16 (36) |
| IBD-U | 2 (4) |
| CD | 27 (60) |
| CD disease location, n (%) | |
| L1 ileal | 1 (4) |
| L2 colonic | 6 (22) |
| L3 ileocolonic | 20 (74) |
| +L4 upper GI disease | 2 (7) |
| CD disease behavior, n (%) | |
| B1 non stricturing, non penetrating | 9 (33) |
| B2 stricturing | 16 (59) |
| B3 penetrating | 2 (8) |
| +Perianal disease | 5 (19) |
| UC disease location, n (%) | |
| E2 left sided colitis | 5 (28) |
| E3 pancolitis | 13 (72) |
| Previous IBD-related surgerya, n (%) | 15 (33) |
| Anti-TNFα exposed, n (%) | |
| Naive | 7 (15) |
| 1 | 16 (36) |
| ⩾2 | 22 (49) |
All 15 patients had CD; 12 patients received an ileocecal resection (of whom three patients twice), one patient underwent a small bowel (jejuna)l resection, one patient with ileostomy, and one with ileoanal pouch.
B, behavior; CD, Crohn’s disease; E, extent; GI, gastrointestinal; IBD, inflammatory bowel disease; IBD-U, inflammatory bowel disease–unclassified; L, location; n, number; Q, quartile; TNFα, tumor necrosis factor alpha; Q, quartile; UC; ulcerative colitis.
Clinical, biochemical, endoscopic and histologic outcome
At week 16, clinical response was observed in 21/45 patients (47%) (11/18 UC versus 10/27 CD, p = 0.138), clinical remission in 17/45 patients (38%) (6/18 UC versus 11/27 CD, p = 0.351) and steroid free clinical remission in 15/45 patients (33%) (4/18 UC versus 11/27 CD, p = 0.333). Biochemical remission was documented in 9/40 patients (23%) (4/14 UC versus 5/26 CD, p = 0.694) and steroid free biochemical remission in 8/40 patients (20%) (4/14 UC versus 4/26 CD, p = 0.416) at week 16. The week 16 endoscopic response rate was 58% (26/45 patients; 13/18 UC versus 13/27 CD, p = 0.134) and endoscopic remission rate was 29% (13/45 patients; 6/18 UC versus 7/27 CD, p = 0.739). Seven patients used corticosteroids at week 16, of whom 4/7 (57%) were endoscopic non-responders (p = 1.00). The mean SES-CD declined from 12 points (SD 5.0) at baseline to 9.1 points (SD 7.3) at week 16 (p = 0.07). The Rutgeerts’ score declined from 2.9 points (SD 0.6) at baseline to 1.8 points (SD 1.0) at week 16 (p = 0.016). Mayo endoscopic score declined from 2.2 points (SD 0.5) at baseline to 1.2 points (SD 1.2) at week 16 (p = 0.003). Histologic remission was observed in 12/36 (33%) patients (7/15 UC versus 5/21 CD, p = 0.175) at week 16. No significant differences between patients with or without concomitant IM therapy in clinical response, (steroid free) clinical and biochemical, endoscopic and histologic remission were observed.
Association between serial FC measurements and endoscopy
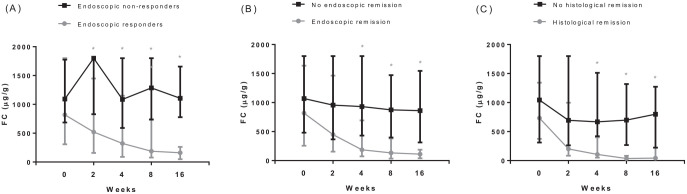
Patients with an endoscopic response had significantly lower FC levels at all predefined time points after baseline as compared with patients without an endoscopic response (Figure 1A). Median FC levels declined significantly in patients with an endoscopic response at week 16 from baseline to all subsequent time points (Figure 1A). In contrast, in patients without an endoscopic response FC levels did not show a significant decline from baseline to subsequent serial FC levels (Figure 1A). A significant steep decline in median FC was observed in patients with an endoscopic response from baseline to week 2, 37% (p = 0.025); to week 4, 51% (p = 0.035); to week 8, 73% (p < 0.001) and to week 16, 83% (p < 0.001). Week 8 was the early time point with highest decline in FC levels s as compared with baseline. Patients with endoscopic and histologic remission had significantly lower FC levels at weeks 4, 8 and 16 as compared with patients without endoscopic and histologic remission (Figure 1B and C). In addition, Friedman’s ANOVA test showed a significant difference in the complete course of FC between patients with and without endoscopic response (p < 0.001 versus p = 0.56), endoscopic remission (p < 0.001 versus p = 0.09) and histological remission (p = 0.001 versus p = 0.36). Baseline FC levels were significantly higher in patients with UC (median 1800 µg/g, Q1–Q3 563–1800) as compared with CD (median 775 µg/g, Q1–Q3 398–1525), p = 0.04. No differences in FC levels at all predefined time points were observed between patients with and without corticosteroids at week 16. At weeks 2, 4, 8 and 16 no significant differences of FC levels (or changes in FC levels) were observed between UC and CD patients (Figure 2).
Figure 1.
Serial fecal calprotectin levels in IBD patients during VDZ induction, specified for patients with and without endoscopic response (A), endoscopic remission (B) and histological remission (C)Number of patients (FC) with/without endoscopic response at week 0: 26/18, week 2: 25/17, week 4: 25/16, week 8: 26/16, week 16: 24/16Number of patients (FC) with/without endoscopic remission at week 0: 13/31, week 2: 12/30, week 4: 13/28, week 8: 13/29, week 16: 13/27Number of patients (FC) with/without histological remission at week 0: 12/23, week 2: 10/23, week 4: 11/21, week 8: 11/22, week 16:11/20Boxplots describe median and first and third quartile of FC at the predefined serial time points
*Indicates statistically different FC levels between patients with and without response or remission the two evaluated groups in analysis as tested by the Wilcoxon Rank testFC; fecal calprotectin
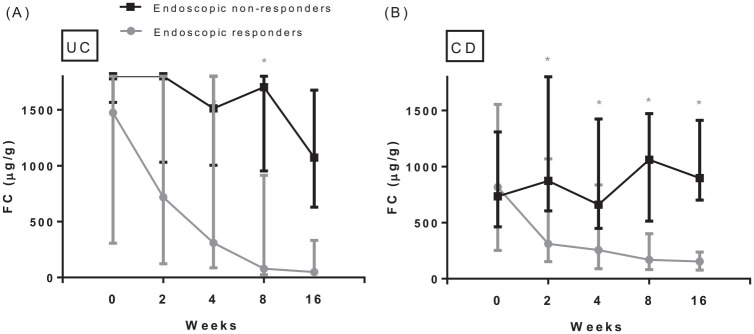
Figure 2.
Serial FC levels in UC (A) and CD (B) patients separately during vedolizumab induction, specified for patients with and without an endoscopic response.
Number of UC patients (FC) with/without endoscopic response at week 0: 13/5; week 2: 13/5; week 4: 12/4; week 8: 13/4; week 16: 11/4. Number of CD patients (FC) with/without endoscopic response at week 0: 13/13; week 2: 12/12; week 4: 13/12; week 8: 13/12; week 16: 13/12. Boxplots describe median and first and third quartile of FC at the predefined serial time points. FC values for UC patients with an endoscopic response: baseline: 1474 µg/g (Q1–Q3 306–1800); week 2: 718 µg/g (Q1–Q3 122–1800); week 4: 309 µg/g (Q1–Q3 86–1800); week 8: 78 µg/g (Q1–Q3 25–914); week 16: 49 µg/g (Q1–Q3 25–332). FC values for UC non-responders: baseline: 1800 µg/g (Q1–Q3 1566–1800); week 2: 1800 µg/g (Q1–Q3 1032–1800); week 4: 1513 µg/g (Q1–Q3 1004–1800); week 8: 1704 µg/g (Q1–Q3 954–1800); week 16: 1073 µg/g (Q1–Q3 630–1676). FC values for CD patients with an endoscopic response: baseline: 816 µg/g (Q1–Q3 252–1555); week 2: 310 µg/g (Q1–Q3 152–1068); week 4: 255 µg/g (Q1–Q3 89–835); week 8: 169 µg/g (Q1–Q3 82–401); week 16: 154 µg/g (Q1–Q3 77–238). FC values for CD non-responders: baseline: 734 µg/g (Q1–Q3 463–1309); week 2: 872 µg/g (Q1–Q3 605–1800); week 4: 661 µg/g (Q1–Q3 449–1424); week 8: 1062 µg/g (Q1–Q3 513–1472); week 16: 897 µg/g (Q1–Q3 701–1412).
*Indicates statistically different FC levels between patients with and without response as tested by the Wilcoxon Rank test.
CD, Crohn’s disease; FC, fecal calprotectin; Q, quartile; UC, ulcerative colitis.
Optimal FC levels to predict endoscopic response, and endoscopic and histologic remission
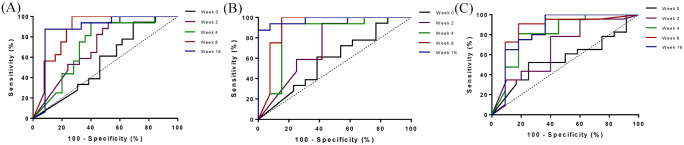
During VDZ induction, the diagnostic accuracy for FC levels to predict endoscopic response at week 16 increased over time with a corresponding area under the curve (AUC) of 0.503 at baseline, 0.683 at week 2, 0.699 at week 4, 0.854 at week 8 and 0.866 at week 16 (Figure 3A). All patients with FC <250 µg/g at week 8 had endoscopic response at week 16, hence this value may serve as a cut-off to identify responders (sensitivity 65.4%, specificity 100%, PPV 100% and NPV 64.0%). The diagnostic accuracy was comparable in both UC and CD (AUC UC 0.833, CD 0.886).
Figure 3.
Receiver operating characteristic curves on the association between fecal calprotectin (µg/g) levels at weeks 0, 2, 4, 8 and 16 to predict endoscopic response (A), endoscopic remission (B) and histologic remission (C) after vedolizumab induction (at week 16).
Area under the curve for fecal calprotectin to predict endoscopic response (A): baseline: 0.503; week 2: 0.683; week 4: 0.699; week 8: 0.854; week 16: 0.866. For endoscopic remission (B) these were 0.568, 0.720, 0.824, 0.908, 0.970 and for histologic remission (C) these were 0.606, 0.706, 0.806, 0.875 and 00.839 respectively.
Absence of a decline in FC level (defined as zero decline or an increase) from baseline to week 8 predicted endoscopic non-response (AUC 0.898, sensitivity 84.6%, specificity 68.8%, PPV 81.5%, NPV 73.3%) (Supplemental Table 2). Despite comparable diagnostic accuracy in both UC and CD (AUC UC 0.864, CD 0.886), absence of a decline in FC was a better predictor for endoscopic non-response in CD as compared with UC (CD: sensitivity 85%, specificity 75%, PPV 79%, NPV 82%; UC: sensitivity 85%, specificity 50%, PPV 85%, NPV 50%). A cut-off of FC ⩾450 µg/g at week 8 showed a lower sensitivity and higher specificity as compared with absence of FC decline (sensitivity 76.9%, specificity 87.5%, PPV 91% and NPV 70%).
A high diagnostic accuracy was demonstrated for FC at week 8 to predict endoscopic remission (Figure 3B). However, a relevant cut-off to differentiate between endoscopic response and endoscopic remission could not be defined in our data (Supplemental Figure 2). In a sensitivity analysis on 32 patients (patients with only an endoscopic response were excluded), FC <250 µg/g at week 8 predicted endoscopic remission at week 16 (AUC 0.908, sensitivity 77%, specificity 100%, PPV 100%, NPV 84%).
Similar high diagnostic accuracy was observed for FC and histologic remission (Figure 3C). FC of 250 µg/g at week 8 predicted histologic remission (sensitivity 82%, specificity 77%, PPV 64%, NPV 90%). Absence of FC decline from baseline to week 8 corresponded to absence of histological remission (sensitivity 89%, specificity 50%, PPV 47%, NPV 90%). FC of ⩾450 µg/g at week 8 also corresponded to absence of histologic remission (sensitivity 91%, specificity 59%, PPV 52%, NPV 93%).
Clinical scores and serologic biochemical parameters to predict endoscopic response, and endoscopic and histologic remission
Clinical (SCCAI and HBI) and biochemical scores (CRP, leucocyte count, hemoglobin level, platelet count and albumin at baseline or during follow-up) showed lower diagnostic accuracy as compared with FC to predict endoscopic response and no added value to FC was demonstrated to predict endoscopic response (Supplemental Figures 3 and 4).
Discussion
To define optimal timing of VDZ response evaluation is important to guide effective treatment in IBD patients. This real world prospective cohort study with a high percentage of anti-TNF exposed IBD patients demonstrates that the onset of a decline in FC levels as early as week 2 is associated with endoscopic response to VDZ induction therapy in IBD patients. FC <250 µg/g 8 weeks after the initiation of VDZ is associated with an endoscopic response at week 16 in both UC and CD patients (PPV 100%, NPV 64%), with high diagnostic accuracy. These findings imply that endoscopic response evaluation is not strictly necessary in this subgroup. In this study, the absence of a FC decline at week 8 prognosticates the absence of endoscopic response in CD (NPV 82%, PPV 79%) and the absence of histologic remission in both UC and CD (NPV 90%, PPV 47%). In these latter cases, trough (or empiric) level guided dose optimization or medication switch out of class might be considered prior to endoscopic evaluation.24–27
Early FC levels are an attractive alternative to monitor mucosal healing as compared with invasive endoscopic evaluation, especially since standardized endoscopic evaluation has not been implemented widely after the start of (new) IBD medication. Previous reports observed that FC shows the best diagnostic accuracy to predict response to VDZ as compared with clinical disease activity scores or biochemical serological markers, which is in line with our study results.28 FC data from the GEMINI I trial have shown only a moderate predictive value of FC at week 6 to predict endoscopic remission at week 6.19 Possible explanations for the discrepancy between these findings and the high diagnostic accuracy of low FC and endoscopic response in our study are not only the earlier timing of the endoscopy in the GEMINI trial but, most importantly, the endpoint of endoscopic remission instead of endoscopic response. Taken together, the GEMINI and our study suggest that a response to VDZ with improvement of mucosal inflammation may be evaluated as early as week 8, whereas the optimal timing to evaluate further improvement towards mucosal healing requires further research. Since in CD the inflammation is often transmural, transmural healing in CD may be a new treatment target.29 The correlation between a decline of FC and the findings at diffusion-weighted magnetic resonance imaging and/or abdominal ultrasound requires further investigation.30 In addition, despite the predictive value of FC level at week 8 to identify endoscopic responders as demonstrated in this study, it remains challenging how to differentiate early between a delayed endoscopic response and non-response. To answer this question, a study with prolonged treatment and standardized endoscopy after several months of treatment is necessary. Such a study may be regarded as both unethical and unrealistic in this era with expanding treatment options for IBD31–33 due to the possible harm to non-responders and possible need for prolonged steroid use.7,8
Previous publications have focused on the development of a prediction tool based on baseline parameters to identify patients with higher response rates to VDZ at week 26.34,35 In these prediction models disease characteristics including anti-TNF exposure and longer disease duration in UC and anti-TNF exposure, bowel surgery and fistulizing phenotype in CD were associated with a lower response to VDZ. Unfortunately, the size of our study is too small to analyze the predictive value of early FC within these subgroups. The development of an integrated (clinical) algorithm, which accounts for adequate selection of therapy, and early objective response evaluation to timely optimize therapy, is a high clinical need. New potential predictors of response to VDZ may further improve (early) optimization strategies. For instance, patients with increased gene expression of PIWIL1, MAATS1, DCH2 and RGS12 might have higher response rates to VDZ therapy, suggesting that upregulation of these genes interferes with leukocyte extravasation and trafficking.36 Specific microbial genera may be associated with response to VDZ therapy; however, further quantification and validation is warranted.37
Important strengths of our study are the prospective inclusion of a real-world cohort of anti-TNF exposed patients with follow-up according to a standardized protocol with serial FC measurements and endoscopy. In this protocol, the decision on endoscopy was not influenced by the observed FC results, which reduced the risk of differential bias. Nevertheless, a few limitations need to be considered. Firstly, FC levels may be influenced by factors such as non-steroidal anti-inflammatory drugs, stool composition, blood admixture, storage temperature, infections and bowel movements. These factors were not recorded in our study and may have influenced the FC levels. Secondly, in the majority of patients in our study (80%), FC was analyzed with the same test at all time points. We assume that no bias has been introduced due to the used (different) tests, since a good correlation between the QoC and other ELISA laboratory tests (Bühlmann Turbo) has been reported in the literature, as well as a similar accuracy for mucosal inflammation.38–40 Thirdly, the sample size of our study is relatively small, and therefore subgroup analyses, including the separate analysis in UC and CD, might have been underpowered to detect differences. It is unlikely that the combined analysis of CD and UC has significantly influenced our results, since all patients with FC <250 µg/g at week 8 had endoscopic response at week 16; this value may serve as a cut-off to identify responders both in UC and in CD. In addition, the diagnostic accuracy of this FC cut-off was comparable in UC and CD. Fourthly, this study concerns an observational prospective study which lacks standardization of endoscopic and histologic examination. Although the endoscopies were performed by or under supervision of IBD specialists, intra- and inter-observer variation has not been eliminated since the endoscopists were not explicitly blinded to the FC levels, nor were the endoscopies read centrally. In addition, a pragmatic histology scoring as proposed in previous literature was used.41
Finally, the study population concerns a therapy refractory IBD population, which may limit external validity of the results to anti-TNF refractory patients.
Conclusion
In conclusion, this study demonstrates that the onset of a decline in FC levels as early as week 2 is associated with endoscopic response to VDZ induction therapy in IBD patients. FC level at week 8 may categorize patients with endoscopic response and non-response at week 16 with adequate diagnostic accuracy. FC level <250 µg/g at week 8 after the initiation of VDZ therapy corresponds with endoscopic response at week 16, whereas absence of a decline in FC at week 8 corresponds with absence of both endoscopic response in CD and histologic remission at week 16 in UC and CD. Therefore, FC levels 8 weeks after the start of VDZ could be used to guide clinical decisions and optimization of therapy, and might be an attractive alternative to invasive endoscopic evaluation.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-1-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-2-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-3-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-4-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Acknowledgments
Conference presentations: oral presentation at the Digestive Disease Week (DDW) San Diego 2019 (18–21 May) “Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab”. Selected at best of Digestive Disease Week (DDW) San Diego 2019, presented at the United European Gastroenterology Week (UEGW) Barcelona 2019 (19–23 October). Oral presentation at the Dutch Digestive Days (DDD) Veldhoven 2019 (20–21 March) “Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab”. Poster presentation at the 14th congress of European Crohn’s and Colitis Organisation (ECCO) Copenhagen 2019 (March 6–9) “Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab”.
Footnotes
Author contributions: ACV, CJW: study concept and design, critical revisions of the manuscript for important intellectual content. RWMP: patient recruitment, prospective follow-up of patients, acquisition of data, statistical analysis, drafting of the manuscript. NSE: statistical analysis (corrections) and interpretation of the data, revision of the manuscript.
Conflict of interest statement: R. W. M. Pauwels has nothing to disclose.
A. C. de Vries has participated in advisory board and/or received financial compensation from the following companies: Janssen, Takeda, Abbvie and Tramedico.
N. S. Erler has nothing to disclose.
C. J. van der Woude received grant support from Falk Benelux and Pfizer; received speaker fees from AbbVie, Takeda, Ferring, Dr. Falk Pharma, Hospira, Pfizer; and served as a consultant for AbbVie, MSD, Takeda, Celgene, Mundipharma and Janssen.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by QuantOn Cal home-based FC tests through Truvion Healthcare / Tramedico (Preventis).
ORCID iD: Renske W. M. Pauwels  https://orcid.org/0000-0002-2118-5687
https://orcid.org/0000-0002-2118-5687
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Renske W. M. Pauwels, Erasmus MC, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands
Christien J. van der Woude, Erasmus MC, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands
Nicole S. Erler, Erasmus MC, Department of Biostatistics, Rotterdam, The Netherlands
Annemarie C. de Vries, Erasmus MC, Department of Gastroenterology and Hepatology, Rotterdam, The Netherlands, PO Box 2040, Rotterdam, 3000 CA, The Netherlands.
References
- 1. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 3. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53: 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD-lessons from real-world experience; a systematic review and pooled analysis. J Crohns Colitis 2018; 12: 245–257. [DOI] [PubMed] [Google Scholar]
- 5. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Vedolizumab for inflammatory bowel disease: two-year results of the Initiative on Crohn and Colitis (ICC) Registry, a nationwide prospective observational cohort study: ICC Registry–vedolizumab. Clin Pharmacol Ther. Epub ahead of print 11 December 2019. DOI: 10.1002/cpt.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands BE, Van Assche G, Tudor D, et al. Vedolizumab in combination with corticosteroids for induction therapy in Crohn’s disease: a post hoc analysis of GEMINI 2 and 3. Inflamm Bowel Dis 2019; 25: 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology 2019; 157: 997–1006.e6. [DOI] [PubMed] [Google Scholar]
- 8. Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019; 157: 1007–1018.e7. [DOI] [PubMed] [Google Scholar]
- 9. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 10. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 11. Walsh AJ, Bryant RV, Travis SPL. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016; 13: 567–579. [DOI] [PubMed] [Google Scholar]
- 12. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the lichtiger index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013; 19: 332–341. [DOI] [PubMed] [Google Scholar]
- 13. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7: 982–1018. [DOI] [PubMed] [Google Scholar]
- 14. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 15. Sipponen T, Savilahti E, Kolho K-L, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2007; 14: 40–46. [DOI] [PubMed] [Google Scholar]
- 16. Mooiweer E, Severs M, Schipper MEI, et al. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. J Crohns Colitis 2014; 9: 50–55. [DOI] [PubMed] [Google Scholar]
- 17. Mao R, Xiao Y-l, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012; 18: 1894–1899. [DOI] [PubMed] [Google Scholar]
- 18. Molander P, Färkkilä M, Ristimäki A, et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2014; 9: 33–40. [DOI] [PubMed] [Google Scholar]
- 19. Reinisch W, Bressler B, Curtis R, et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a post hoc analysis of GEMINI 1. Inflamm Bowel Dis 2018; 25: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002; 122: 512–530. [DOI] [PubMed] [Google Scholar]
- 21. Walsh AJ, Ghosh A, Brain AO, et al. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis 2014; 8: 318–325. [DOI] [PubMed] [Google Scholar]
- 22. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 23. Moskovitz DN, Daperno M, Assche GA. Defining and validating cut-offs for the simple endocopic score for Crohn’s disease. Gastroenterology 2007; 132: S1097. [Google Scholar]
- 24. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018; 16: 1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 25. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med 2019; 17: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018; 47: 906–912. [DOI] [PubMed] [Google Scholar]
- 27. Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017; 15: 1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 28. Waljee AK, Liu B, Sauder K, et al. Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn’s disease. Inflamm Bowel Dis 2017; 23: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 30. Allocca M, Danese S, Laurent V, et al. Use of cross-sectional imaging for tight monitoring of inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020; 18: 1309–1323.e4. [DOI] [PubMed] [Google Scholar]
- 31. Macaluso FS, Maida M, Ventimiglia M, et al. Effectiveness and safety of ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther 2020; 20: 193–203. [DOI] [PubMed] [Google Scholar]
- 32. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 33. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 34. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology 2018; 155: 687–695.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dulai PS, Singh S, Casteele NV, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020; 18: 2952–2961.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verstockt B, Verstockt S, Veny M, et al. Expression levels of 4 genes in colon tissue might be used to predict which patients will enter endoscopic remission after vedolizumab therapy for inflammatory bowel diseases. Clin Gastroenterol Hepatol. Epub ahead of print 22 August 2019. DOI: 10.1016/j.cgh.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caenepeel C, Vieira-Silva S, Verstockt B, et al. The predictive role of gut microbiota in treatment response to vedolizumab and ustekinumab in inflammatory bowel disease. J Crohns Colitis 2019; 13: S542. [Google Scholar]
- 38. Sutton RT, Prosser C, Dhami N, et al. A139 agreement of IBDOC® and QUANTON CAL® rapid lateral flow-based fecal calprotectin tests with accepted lab-based assays for monitoring inflammatory bowel disease. J Can Assoc Gastroenterol 2018; 1(Suppl. 2): 208–209. [Google Scholar]
- 39. de Jong MJ, Roosen D, Degens J, et al. Development and validation of a patient-reported score to screen for mucosal inflammation in inflammatory bowel disease. J Crohns Colitis 2019; 13: 555–563. [DOI] [PubMed] [Google Scholar]
- 40. Ashorov O, Hamouda D, Dickman R, et al. Clinical accuracy of a new rapid assay for fecal calprotectin measurement. Clin Lab. Epub ahead of print 1 April 2020. DOI: 10.7754/Clin.Lab.2020.200126. [DOI] [PubMed] [Google Scholar]
- 41. Baars JE, Nuij VJAA, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis 2011; 18: 1634–1640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-1-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-2-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-3-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-4-tam-10.1177_1756284820979765 for Fecal calprotectin is an early predictor of endoscopic response and histologic remission after the start of vedolizumab in inflammatory bowel disease by Renske W. M. Pauwels, Christien J. van der Woude, Nicole S. Erler and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology