Abstract
Objects:
To evaluate the performance of preoperative magnetic resonance imaging (MRI) in evaluating diagnoses, operation methods and recurrence of meningiomas according to the World health organization (WHO) pathological classification.
Methods:
MRI characteristics of 127 meningioma patients were retrospectively analysed according to pathological results (WHO grade) and their association with Simpson’s grades (resection) and recurrence.
Results:
The T1-weighted imaging (T1WI) signal intensity of WHO grade I meningiomas was slightly hypointense or isointense gray, while the T2-weighted imaging (T2WI) signal intensity was isointense or slightly hyperintense. The T1WI and T2WI signal intensity in WHO grade II and III meningiomas was isointense gray. The enhancement degree and patterns, lobulation, flowing voids, dural tail, maximum diameter, peritumoural oedema, ADC values and margin were significantly different between any 2 grades (P < 0.05). The ADC values were higher for WHO grade I tumors than for WHO grade II and III tumors (P < 0.001). Among all the analyzed characteriscs, ADC values, peritumoural oedema, and margin effectively predicted the diagnosis according to the WHO classification. The operation method and surgical resection were different between WHO grade Ⅰ and WHO grade Ⅱ/Ⅲ meningiomas (P < 0.05). The recurrence rate increased with tumor grade, but there was no statistical difference among the 3 types(P> 0.05).
Conclusions:
WHO grades and pathological subtypes of meningiomas can generally be determined based on their MRI characteristics. In addition, MRI provides significant guidance for the grading of surgical success and prognosis.
Keywords: meningioma, who classification, pathological subtype, magnetic resonance imaging, Simpson grade, recurrence
Introduction
Meningiomas represent one of the most common brain tumors and account for 37% of primary intracranial tumors1 According to the latest classification criteria published in The 2016 World Health Organization (WHO) Central Nervous System (CNS) Tumour Classification, meningiomas can be classified as grade I, II and III as well as 15 pathological subtypes.2
Tumors of different pathological subtypes and grades have different biological characteristics, and the tumor recurrence rate is closely correlated with WHO grades and the extent of surgical resection.
WHO grade I tumors show lower rates of proliferative activity and are less aggressive than has been found in the other 2 types. It has been reported that only 20% of WHO grade I meningiomas recur in 20 years and that these tumors result in very few cases of malignancy and metastasis.3 Most are cured by Simpson grade 0, I or II resection.4 The new WHO classification incorporated cerebral invasion into the diagnostic criteria for WHO grade Ⅱ meningiomas. This modification has led to an increase in the prevalence of high-grade meningiomas, including WHO grade II and WHO grade III meningiomas. The proportion of high-grade meningiomas is now estimated to reach one-fifth to one-third of newly diagnosed meningiomas.5 Importantly, these tumors are more likely to display invasive behaviors and result in post-treatment recurrence, increased morbidity and a decreased survival rate. According to a statistical analysis of anaplastic meningioma, the recurrence rate of these tumors is as high as 94%, the median survival duration is approximately 3-6 years, and the prognosis is very poor even with complete resection.6 The preoperative evaluation according to WHO classification is instructive to the development of personalized treatment plans, surgical resection and assessing prognosis. Magnetic resonance imaging (MRI) is recommended by the 2016 NCCN Guidelines for assessing the conditions of the tissues inside and around the tumors with a sensitivity and specificity of 75.0% and 93.5%, respectively.7
Therefore, the purpose of this study was to perform a retrospective statistical analysis to explore the correlations between preoperative MRI characteristics and pathological subtypes of meningioma and thereby provide guidance for the selection of treatment options and prognosis evaluation.
Materials and Methods
Patients
A retrospective analysis was conducted in 127 patients with meningiomas who underwent MRI in the Second People’s Hospital of Shenzhen from 2015 to 2017. The patients included 71 males and 56 females ranging in age from 16 to 67 years, with an average age of 47.37 ± 14.00 years. Because the present study was a retrospective clinical study, informed consent was waived.
Protocols of Conventional MRI
Scans were performed on a 3.0 Tesla magnetic resonance imaging scanner (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) and a 1.5T MRI scanner (MAGNETOM Avanto; Siemens Healthcare, Erlangen, Germany) with 20 and 8-channel encoding head coils, respectively. General sequences (T1WI, T2WI, FLAIR, DWI were conducted before contrast enhancement T1(CE-T1). Finally, contrast enhancement scans were performed following Gd-DTPA (dose: 0.1 mmol·kg−1) injection. MRV scans was performed after CE-T1 if needed.
Determination of MRI Characteristics of Tumors
Two doctors with more than 10 years of experience in neuroradiology reviewed the MRI characteristics(intensity signal of T1WI and T2WI, enhancement degree and patterns (homogeneous/heterogeneous), lobulation, flowing voids, dural tail, maximum diameter, edema and ADC values)in the collected images while blinded to pathology results. The scoring system used for T1WI, T2WI and CE-T1 signal intensity was followed by Elster standards. 8 Original DWI data were uploaded into a Workstation (Syngo. via Siemens Healthcare, Erlangen, Germany) and used to obtain ADC values. Regions of interest (ROI) from the tumor parenchyma have to avoid cystic degeneration and necrosis areas, and each ROI was set as 5 cm2 (∼200 pixels2).
MRI Signal Elster Standards
The scoring of system used for T1-weighted imaging (T1WI) signal intensity was as follows: significantly hypointense compared to the gray matter and isointense compared to the cerebrospinal fluid (CSF) was given a score of 1, slightly hypointense compared to the gray matter was given a score of 2, isointense compared to the gray matter was given a score of 3, slightly hyperintense compared to the gray matter was given a score of 4, and significantly hyperintense compared to the gray matter and isointense compared to fat was given a score of 5.
Scoring of T2-weighted imaging (T2WI) signal intensity: the signal significantly hypointense to that of gray matter and isointense to that of bone cortex was given 1 score; the signal hypointense to that of gray matter was given 2 scores; the signal isointense to that of gray matter was given 3 scores; the signal slightly hyperintense to that of gray matter was given 4 scores; the signal significantly hyperintense to that of gray matter and isointense to that of CSF was given 5 scores.
Classification of T1WI signal intensity with Gd-DTPA injection: grade Ⅰ describes enhancement isointense to that of fat; grade Ⅱ describes moderate enhancement which is hypointense to that of fat; grade Ⅲ is for mild enhancement which is significantly hypointense to the that of fat but hyperintense to the gray matter and white matter signals. Enhancement patterns are catogerized into 2 groups, homogeneous and heterogeneous.
Morphological Characteristics
The maximum diameter of tumors was used to describe tumor size and the median diameter of 4cm was select as cut-off to separate big from small tumors(4cm was the median of this cases). Lobulation sign refers to the shape of the tumor, that is not round or oval, which is more common in malignant tumors. Tumor cerebral invasion was determined based on whether there is a clear boundary between tumor and normal brain tissue, and was further confirmed by pathological examination. These features can be clearly observed on the T1WI and T2WI sequences. Flow voids are detected because MRI cannot collect the signal of the blood flowing in the tumor blood vessels and they appear as a low signal in the form of a string on the T1WI and T2WI sequences, which is easy to observe. Corresponding pathological results found thick and thin-walled blood vessels in the tumor tissues.9 Dural tail sign represents a thickening of the dura adjacent to the tumor on CE-T1, which reflects the invasion of meninges by the attached tumors.10 Edema are generally observed on T2WI and Flair sequences, and the maximum diameter is measured. Venous sinus invasion is generally observed on MRV and can also be evaluated by T2WI,11 which is recognized based on that the tumor tissues are compressing or growing into the sinus.
Simpson’s Grade and Recurrence
The guidelines proposed by Simpson in 1957 were used for the classification of resection degree in meningioma.12 Grade I indicates that the tumor was completely removed with the attached dura mater, venous sinus and abnormal skull by fingertip surgery. A surgical removal grade of Ⅱ indicates that tumor tissues were visible to the naked eye, and parts of the dura were cauterized in place. A grade of Ⅲ indicates the presence of residual tumor tissue residues according to processing in the pathology department. A grade of Ⅳ indicates an operation that achieved only tumor resection. Grade Ⅰ and Ⅱ indicate complete, while grade Ⅲ and Ⅳ indicate incomplete tumor resection. The time from the first surgical resection to recurrence was defined as progression-free survival (PFS) and was used as the cut-off value.
Statistics
The evaluation results from the 2 radiologists were analyzed by Coherence Analysis. All pathological results were interpreted by 2 or more pathologists using a combination of microscopy and immunohistochemistry and according to the new classification and grading indexes for meningiomas to determine the pathological subtypes of the meningiomas.13
SPSS software (v. 22.0, IBM Inc., USA) and EmpowerStats(http:www. empowerstats.com, X & Y Solutions, INC., Boston,MA) was used for statistical analysis. MRI characteristics, Simpson grade and recurrence rate of meningiomas with different WHO classification were compared. ROC curve was generated for each MRI sign according to WHO classification and identify the most valuable characteristics. P < 0.05 was considered statistically significant.
Results
Classification of Meningiomas According to the WHO Guidelines
Of the 127 meningioma patients, the 90 cases in the WHO grade I group (70.9%) included 21 cases of transitional meningiomas, 17 of fibroplastic meningiomas, 28 of meningothelial meningiomas, 7 of psammomatous meningiomas, 16 of angiomatous meningiomas, and 1 of the microcystic subtype (Figure 1). Of the 21 cases classified into WHO grade II group (16.5%), there were19 atypical cases (Figure 2) and 2 chordoid meningiomas. The remaining 16 cases were classified as WHO grade III (12.6%), and all of these cases were anaplastic meningiomas (Table 1). Two doctors conducted a consistency test on the evaluation of MRI signs, and the degree of conformity was good(Kappa=0.84, P=0.000).
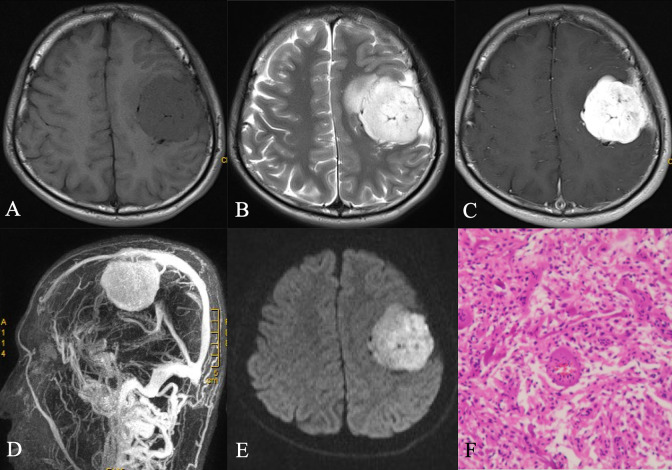
Figure 1.
A. Axial T1-weighted sequence in a 53-year-old woman with a 3-month history of confusion demonstrates a a well-circumscribed, lobulated mass located in the frontal and parietal lobe, to be isointense to gray matter and to invade the inner table base. B. Axial T2-weighted sequence in the same patient as A demonstrates to be hyperintense to gray matter. A number of flow voids are visualized around the periphery of the mass and a peripheral high signal intensity rim represents surrounding cortical gray matter and mild vasogenic edema. C. Post-contrast axial T1-weighted sequence in the same patient as A demonstrates homogeneous enhancement with broad dural tails that inferomedially contact the inner table. D. MRV shows rich blood vessels in the tumor, which are closely related to the sagittal sinus but not invasion. E. Diffusion-weighted image demonstrates a hyperintense signal mass. F.The histological diagnosis at surgery was an WHO grade I angiomatous meningiomas with microcapsule formation.The tissue was mainly composed of capillaries of varying sizes and irregular lumen and foamy interstitial cells containing phagocytosing lipids.
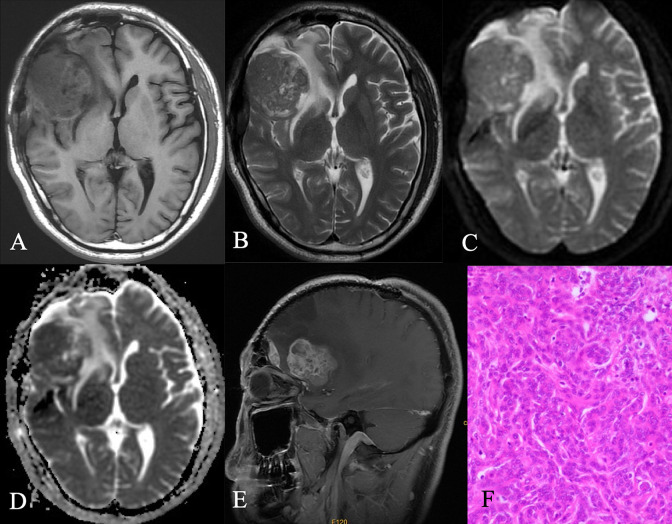
Figure 2.
A. Axial T1-weighted sequence in a 66-year-old man with headache and dizziness demonstrates a lobulated, heterogeneously isointense signal mass over the right lateral convexity. B. Axial T2-weighted sequence in the same patient as A demonstrates the mass to be mildly, homogeneously isointense to gray matter, marked peritumoral edema. C. D. Axial Diffusion-weighted sequence and ADC map show the mass is isointense signal. E. Post-contrast axial T1-weighted sequencen demonstrates a large, homogeneously enhanced extra axial mass over the inferolateral right frontal lobe and anterior temporal lobe with the adjacent meninges thickened. F. Histology at surgery was an atypical meningioma (WHO grade II). A thin layer of fibrous envelope was found locally in the tumor. The tumor demonstrated a small nest-like and strand-like arrangement. The tumor cells were crowded and the nucleus was oval, small nucleoli were visible, mitotic images were 3-10 /10HPF, and patchy necrosis was visible.
Table 1.
The Signal Intensity of WHO GradeⅠ, Ⅱ and Ⅲ Tumors in T1WI, T2WI.
| Subtypes | n | 1 score | 2 scores | 3 scores | 4 scores | 5 scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1WI | T2WI | T1WI | T2WI | T1WI | T2WI | T1WI | T2WI | T1WI | T2WI | |||
| Ⅰ | 90 | 2 | - | 23 | 9 | 51 | 37 | 11 | 32 | 3 | 12 | |
| Transitional meningioma | 21 | - | - | 5 | - | 10 | 13 | 6 | 8 | - | - | |
| Fibroblastic meningioma | 17 | - | - | 3 | 4 | 9 | 3 | 2 | 8 | 3 | 2 | |
| Meningothelial meningioma | 28 | 1 | - | 6 | 5 | 21 | 14 | - | 6 | - | 3 | |
| Psammomatous meningioma | 7 | - | - | - | - | 4 | 5 | 3 | 2 | - | - | |
| Angiomatous meningioma | 16 | 1 | - | 8 | - | 7 | 2 | - | 7 | - | 7 | |
| Microcystic meningioma | 1 | - | - | 1 | - | - | - | - | 1 | - | - | |
| Ⅱ | 21 | - | - | 2 | 5 | 17 | 9 | 2 | 7 | - | - | |
| Atypical meningioma | 19 | - | - | 2 | 5 | 15 | 7 | 2 | 7 | - | - | |
| Chordoid meningioma | 2 | - | - | - | - | 2 | 2 | - | - | - | - | |
| Ⅲ | 16 | - | - | - | - | 10 | 13 | 6 | 3 | - | - | |
| Anaplastic meningioma | 16 | - | - | - | - | 10 | 13 | 6 | 3 | - | - | |
WHO, World health organization;
T1WI, T1-weighted imaging-T2WI, T2-weighted imaging.
Comparison of MRI T1WI and T2WI Intensity of Different Subtypes of Meningiomas
For meningiomas with WHO grade I, the T1WI signal intensity was isointense or slightly hypointense to the gray matter, while the T2WI signal intensity was usually isointense to or slightly hyperintense to the gray matter. In WHO grade II and III cases, both T1WI and T2WI signal intensity was generally isointense to the gray matter.
However, various pathological subtypes of meningiomas also exhibit differences in signal intensity. The statistical results obtained in these experiments are summarized in Table 1.
Comparison of Morphology of Meningiomas of Different WHO Grades
Morphological characteristics, including the enhancement degree and patterns (homogeneous/heterogeneous), lobulation, flow voids and dural tail, of the meningiomas were recorded and then analyzed by chi-square test. The results are shown in Tables 2-4.
Table 2.
Comparison of MRI Morphological Characteristics of WHO Grade Ⅰ, Ⅱ and Ⅲ Meningiomas[n (%)].
| WHO grade | n | Enhancement degree | Enhancement homogeneity | Lobulation | Flowing voids | Dural tail | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Ⅰ | 90 | 44(48.8) | 31(34.5) | 15(16.7) | 45(50.0) | 45(50.0) | 54(60.0) | 36(40.0) | 41(45.6) | 49(54.4) | 82(91.1) | 8(8.9) |
| Ⅱ | 21 | 4(19.0)c | 14(66.7)c | 3(14.3)c | 8(38.1) a | 13(61.9) a | 18(85.7) a | 3(14.3)a | 10(47.6) | 11(52.4) | 18(85.7)a | 3(14.3)a |
| Ⅲ | 16 | 7(43.8) | 6(37.5) | 3(18.7) | 2(12.5) a | 14(87.5)a | 16(100.0)ab | 0(0.0)ab | 14(81.3)ab | 2(18.7)ab | 8(50.0)ab | 8(50.0)ab |
Compared with WHO grade Ⅰ, a P < 0.001, c P < 0.05;
Compared with WHO grade Ⅱ, b P < 0.001, d P < 0.01.
Table 4.
Comparison of Invasion, Operation Method and Prognosis of Meningiomas in Different WHO Types[n (%)].
| WHO grade | n | Venous sinuses invasion |
Clear
boundary |
Simpson’s grade | Recurrence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Yes | No | ||
| Ⅰ | 90 | 50(55.6) | 40(44.4) | 87(96.7) | 3(3.3) | 33(26.0) | 46(36.2) | 9(7.0) | 2(1.6) | 18(20.0) | 72(80.0) |
| Ⅱ | 21 | 13(61.9) | 8(38.1) | 2(9.5) a | 19(90.5) a | 2(9.5) c | 10(47.6) c | 7(33.3) c | 2(9.5) c | 6(28.6) | 15(71.4) |
| Ⅲ | 16 | 8(50.0) | 8(50.0) | 0(0.0) a | 16(100.0) a | 0(0.0) a | 5(31.3) a | 7(43.7) a | 4(25.0) a | 6(37.5) | 10(62.5) |
Compared with WHO grade Ⅰ, a P < 0.001, c P < 0.05;
Compared with WHO grade Ⅱ, b P < 0.001, d P < 0.01.
Statistical analysis of the enhancement degree in all cases showed that overall, WHO grade I was always given priority when it showed obvious enhancement, WHO grade II was given priority when it showed moderate enhancement, and WHO grade III was given priority when there was obvious and moderate enhancement. The differences in the degree of tumor enhancement between grade I and grade II tumors were statistically significant (P < 0.05). Among these tumors, all 16 cases of angiomatous meningiomas were characterized by obvious enhancement (Table 3). The proportion of tumors with heterogeneous enhancement was significantly different between WHO grade I and WHO grade II and III tumors (P < 0.001 for both).
Table 3.
The MRI Morphological Characteristics of the Meningioma Subtypes(n).
| WHO grade | Subtypes | n | Enhancement degree | Enhancement uniformity | Lobulation sign | Flowing void effect | Dural tail sign | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Yes | No | Yes | No | Yes | No | Yes | No | |||
| Ⅰ (n = 90) |
Transitional meningioma | 21 | 8 | 9 | 4 | 13 | 8 | 11 | 10 | 11 | 10 | 17 | 4 |
| Fibroblastic meningioma | 17 | 5 | 10 | 2 | 13 | 4 | 8 | 9 | 5 | 12 | 15 | 2 | |
| Meningothelial meningioma | 28 | 10 | 9 | 9 | 10 | 18 | 21 | 7 | 7 | 21 | 27 | 1 | |
| Psammomatous meningioma | 7 | 4 | 3 | 0 | 1 | 6 | 0 | 7 | 2 | 5 | 6 | 1 | |
| Angiomatous meningioma | 16 | 16 | 0 | 0 | 8 | 8 | 13 | 3 | 15 | 1 | 16 | 0 | |
| Microcystic meningioma | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | |
| Ⅱ (n = 21) |
Atypical meningioma | 19 | 4 | 12 | 3 | 8 | 11 | 16 | 3 | 10 | 9 | 16 | 3 |
| Chordoid meningioma | 2 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | |
| Ⅲ (n = 16) |
Anaplastic meningioma | 16 | 7 | 6 | 3 | 2 | 14 | 16 | 0 | 14 | 2 | 8 | 8 |
WHO, World health organization.
Lobulation was evaluated in all cases by MRI. In the WHO grade I group, lobulation was more common in meningothelial and angiomatous meningiomas than in other pathological subtypes, and a higher WHO grade indicated more obvious foliation (P < 0.001). The proportion of tumors with flow voids was significantly different between grade III tumors and tumors with other 2 grades (P < 0.001). In angioblastic meningiomas, the occurrence of flow voids was as high as 93.8%. The proportion of meningiomas with a characteristic “dural tail” sign was significantly different among the 3 tumor grades (P < 0.001). Our evaluation of venous sinus invasion found no significant difference between the 3 types of WHO (P> 0.05). And the evaluation of tumor boundary revealed that most of the WHO grade Ⅱ and Ⅲ tumors had no clear boundary, which is significantly different from WHO grade Ⅰ tumors (P < 0.001).
Comparison of Maximum Tumor Diameter, Edema, and ADC Values Among Meningiomas with Different Pathological Subtypes
The maximum diameters, edema grades and ADC values of tumors each fitted a Gaussian distribution, and homogeneity of variance was therefore assumed. One-way analysis of variance (ANOVA) and intra-group pair-to-peer Student-Newman-Keuls (SNK) tests were performed, as shown in Table 5.
Table 5.
The MRI Characteristics of the Different WHO Grades Meningioma(n = 127, , ).
| WHO grade | n | Maximum diameter/cm | Peri-tumor edema /cm |
ADC value
/×10-5 mm 2 ·s − 1 |
|---|---|---|---|---|
| Ⅰ | 90 | 3.97 ± 1.52 | 1.48 ± 1.63 | 93.74 ± 11.49 |
| Ⅱ | 21 | 4.06 ± 1.16 | 2.85 ± 1.70a | 82.13 ± 14.52a |
| Ⅲ | 16 | 4.86 ± 0.96ad | 4.27 ± 1.96ab | 80.75 ± 11.47a |
Compared with WHO grade Ⅰ, a P < 0.001, c P < 0.05;
Compared with WHO grade Ⅱ, b P < 0.001, d P < 0.01.
(i) With regard for maximum tumor diameter, WHO grade III tumors were larger than either WHO grade I (P < 0.01) or WHO grade II (P < 0.01) tumors. (ii) The area of peritumoural edema was smallest for WHO grade I meningiomas (P < 0.001) and larger for WHO grade III meningiomas than for WHO grade II meningiomas (P < 0.01). (ⅲ) ADC values were higher for WHO grade I tumors than for WHO grade II and III tumors (P < 0.001), but there was no significant difference between WHO grade II and III meningiomas (P> 0.05).
MRI Features on WHO Grading Diagnostic Efficacy of Meningiomas
ROC curve was used to analyze the diagnostic efficacy of MRI features on WHO classification, and the results were shown in Table 6. Among all the analyzed characteriscs, peri-tumor edema, ADC value and tumor boundary appear to be more effective for WHO classification. The combination of all MRI features formed a new variable and performed ROC curve. The result is: AUC = 0.964, PPV = 92.1%, NPV = 97.8%, Se = 75.7%, Sp = 68.9%, Ac=96.1%.
Table 6.
MRI Features on WHO Grading Diagnostic Efficacy of Meningiomas.
| AUC | Se(%) | Sp(%) | Ppv(%) | Npv(%) | Ac(%) | Cut off | |
|---|---|---|---|---|---|---|---|
| T1WI | 0.647 | 94.59 | 31.11 | 36.08 | 93.33 | 0.4961 | |
| T2WI | 0.636 | 70.27 | 51.11 | 37.14 | 80.70 | 0.5669 | |
| Enhancement degree | 0.606 | 54.05 | 64.44 | 38.46 | 77.33 | 0.6142 | |
| Enhancement homogeneity | 0.615 | 72.97 | 50.00 | 37.50 | 81.82 | 56.69 | |
| ADC value* | 0.754 | 75.68 | 68.89 | 50.00 | 87.32 | 70.87 | 88.45/×10−5 mm2·s−1 |
| Maximum diameter | 0.597 | 72.97 | 48.89 | 36.99 | 81.48 | 55.91 | 3.85/cm |
| Lobulation | 0.695 | 91.89 | 40.00 | 38.64 | 92.31 | 55.12 | |
| Peritumoral edema* | 0.791 | 85.56 | 64.86 | 64.86 | 85.56 | 79.53 | 3.25/cm |
| Flowing voids | 0.585 | 64.86 | 52.22 | 35.82 | 78.33 | 55.91 | |
| Dural tail | 0.604 | 70.27 | 80.89 | 24.07 | 42.11 | 26.77 | |
| Smooth boundary * | 0.956 | 94.59 | 96.67 | 92.11 | 97.75 | 96.06 | |
| Venous sinuses invasion | 0.550 | 56.76 | 53.33 | 33.33 | 75.00 | 54.33 | |
| Combination Variable* | 0.964 | 94.59 | 96.67 | 92.11 | 97.75 | 96.06 |
* Good diagnostic efficacy.
Simpson Grade and Recurrence Rate
Our statistical analysis showed that the surgical resections were different for different WHO grades of meningiomas (P < 0.05). The WHO grade Ⅰ tumors were removed mainly by Simpson grade Ⅰ and Ⅱ resections, with a recurrence rate of 20.0%. The WHO grade Ⅱ meningiomas were removed mainly by Simpson grade Ⅱ and Ⅲ resections, some of them were by grade Ⅰ resection, and the recurrence rate is 28.6%. The WHO grade Ⅲ meningiomas are removed mainly by Simpson grade Ⅱ and Ⅲ resections, some of them were by grade Ⅳ resection, and the recurrence rate is 37.5%. (Table 4)
Discussion
MRI is one of the imaging methods recommended by the NCCN Guidelines published in 2016 for the diagnosis, follow-up and recurrence detection of meningiomas. MRI can generally be used to predict the WHO classification of meningiomas. Surgeons choose postoperative treatment options and conduct postoperative follow-up according to tumor classifications and MRI characteristics.14
The imaging features of high-grade tumors included isointensity on T1WI and T2WI or slight hyperintensity on T2WI, and most showed heterogeneous moderate enhancement, were more lobulated, displayed a dural tail sign, had a larger area of peritumor edema and fuzzier tumor margins. Most of them also invaded venous sinuses.
In general, the signal characteristic of meningiomas is mainly determined by the composition of its tissues.14,15 For example, fibroblastic and angiomatous meningiomas showed distinctive signal intensities on T1WI and T2WI. The characteristic pathological feature of fibroblastic meningiomas is spindle cell proliferation, which exhibits a bundle-shaped, mat-shaped, and dense arrangement. It therefore mainly appears as isointense on T1WI and hyperintense on T2WI. Angioblastic meningiomas are mainly composed of foamy interstitial cells, which have a loose arrangement. Therefore, their T1WI signal are characteristically hypointense or isointense, while their T2WI signal is usually hyperintense.14 In terms of the degree of enhancement, there was no significant difference among WHO grades though angioblastic meningiomas showed characteristic obvious enhancement. In terms of the enhancement patterns, a higher tumor grade was associated with less homogeneous tumor enhancement. Most of the atypical meningiomas showed moderate and heterogeneous enhancement. Anaplastic tumors usually show moderate heterogeneous enhancement.16
Our statistical analysis showed that WHO grade Ⅲ tumors were larger than WHO grade I and II tumors, with a best threshold of 3.85 cm. A survey of the available literature showed that the average diameter of WHO grade III tumor is larger than 5 cm.17
The lobulation of high grade meningiomas was more common than that of low grade tumors. WHO grade III meningiomas, especially anaplastic meningiomas, were often lobulated and grew across the midline or tentorium cerebelli.17 And we found that a higher tumor grade indicated a higher likelihood that flow voids will be found on MRI images. However, there was one exception to this finding: 93.8% of angioblastic meningiomas classified as WHO grade I exhibited this feature. This is because angioblastic meningiomas contain capillaries with varying sizes and irregular lumens, as shown by pathological analysis, and they therefore characteristically show a markedly hyperintense signal on T2WI and significant enhancement on contrast enhancement sequences.14
In general, the dural tail sign is a characteristic manifestation of meningiomas that distinguishes these from other tumors.14 In our study, this sign was more common in benign tumors. However, Rokni-Yazdi et al proposed that the observation of a dural tail on MRI is not an effective indicator for differentiating meningiomas types. The dura mater should be resected as completely as possible because of the frequent presence of tumor nests in these structures.18
About peritumoral edema: Many WHO grade I meningiomas do not show peritumoral edema, but high-grade meningiomas will show obvious edema, which will often spread along white matter fiber bundles. We propose that the region of edema may be closely related to tumor volume and tumor angiogenesis and may also be related to higher expression levels of vascular endothelial growth factor (VEGF).19,20
ADC values are significantly higher for WHO grade I meningiomas ((93.74 ± 11.49) × 10−3 mm2 · s−1 ) than for WHO grade II and III tumors((82.13 ± 14.52) × 10−3 mm2 · s−1 and (80.75 ± 11.47) × 10−3 mm2 · s−1), with a cut off at 0.885 × 10−3 mm2 · s−1. The result is not very different from a recent meta-analysis ( the calculated mean ADC value of the benign grade I tumors was 0.93 × 10−3 mm2 · s−1 [95%-Confidence interval 0.84;1.03] and the mean value of the high-grade tumors was 0.77 × 10−3 mm2 · s−1 [95%-Confidence interval 0.73-0.80])21 The ADC value indicates the apparent diffusion coefficient of water molecules in the tumor and is related to cell density, nuclear atypia and the highly enhanced mitotic activity of tumors. Recent evidence demonstrates that diffusion MRI techniques are efficient at differentiating high-grade meningiomas from-low grade meningiomas.12 The results of this study show that this method is generally efficiency (AUC 0.745).
It is important to evaluate whether the venous sinus is invaded to determine the extent of surgical resection, reduce complications and the recurrence rate.22 In our study, there was no significant difference in the proportion of cases with sinus invasion among the WHO grades. CE-T1 and T2WI sequences were mainly used to evaluate the venous sinus, and few cases were evaluated with MRV; this is therefore a limitation. However, in Niu J et al.’s study, they used a radiomics method based on MRI CE-T1 and T2WI, which showed good efficacy in the evaluation of pituitary tumor sinus invasion.11
The evaluation of cerebral invasion mainly depends on pathology. In diagnoses based on MRI, we mainly observed that tumors had unclear edges and whether there was a clear boundary between tumor and brain tissues. In the new WHO guidelines for the classification of meningioma, cerebral invasion is an indicator of WHO grade Ⅱ meningioma. This change led to an increase in the proportion of high grade tumors, especially grade Ⅱ tumors.5 In the statistical analyses presented in this article, most of the WHO grade Ⅱ and Ⅲ tumors were characterized by blurry tumor margins.
Many studies have proposed that the WHO classification and Simpson’s surgical resection classification are closely related to tumors recurrence.23 The results of this paper support this view. In WHO grade I tumors with Simpson’s grade Ⅱ resection, the postoperative recurrence rate was 20.0%, which is higher than that reported in Chen et al. (4% to 9%).4 WHO Ⅱ meningiomas with a Simpson’s grade of Ⅱ or Ⅲ that were given priority had a recurrence rate of 28.6%, while WHO grade Ⅲ meningiomas with Simpson’s grade Ⅱ, Ⅲ or Ⅳ excision that were given priority had a recurrence rate of 37.5%, which is lower than the rates in previous reports6 potentially due to the length of our follow-up time (5 years).
Conclusions
MRI characteristics can be used to determine the WHO classification and pathological subtypes of tumors, and this information can provide detailed guidance for preoperative decision-making regarding operations and also help in assessing prognosis. Unfortunately, the number of cases in our study was not big enough to include all the pathological subtypes of meningiomas.
Footnotes
Authors’ Note: Juan Yu, Fan-fan Chen, and Han-wen Zhang contributed equally to this study.
Author Contribution: Juan Yu, Fan-fan Chen, Han-wen Zhang Equal contributors. Yi Lei and Liangping Luo Equal contributors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by a grant from Basic Plan Program of Shenzhen, China (No. JCYJ20180228163333734).
ORCID iD: Han-wen Zhang  https://orcid.org/0000-0001-5731-7429
https://orcid.org/0000-0001-5731-7429
References
- 1. Morin O, Chen WC, Nassiri F, et al. Integrated models incorporating, radiologic and radiomic features predict meningioma grade local failure, and overall, survival. Neuro Oncol Adv. 2019;1(1):vdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Ryu HS, Moon KS, Lee KH, et al. Recurred intracranial meningioma: a retrospective analysis for treatment outcome and prognostic factor. Brain Tumor Res Treat. 2017;l5(2):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L, Zou X, Wang Y, Mao Y, Zhou L. Central nervous system tumors: a single center pathology review of 34,140 cases over 60 years. BMC Clin Pathol. 2013;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bulleid LS, James Z, Lammie A, Hayhurst C, Leach PA. The effect of the revised WHO classification on the incidence of grade II meningioma. Brit J Neurosurg. 2020;34(5):584–586. [DOI] [PubMed] [Google Scholar]
- 6. Hwang KL, Hwang WL, Bussière MR, Shih HA. The role of radiotherapy in the management of high-grade meningiomas. Chin Clin Oncol. 2017;6(1):S5. [DOI] [PubMed] [Google Scholar]
- 7. Park YW, Oh J, You SC, et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol. 2019;29(8):4068–4076. [DOI] [PubMed] [Google Scholar]
- 8. Elster AD, Challa VR, Gilbert TH, Richardson DN, Contento JC. Meningiomas: MR and histopathologic features. Radiology. 1989;170(3):857–862. [DOI] [PubMed] [Google Scholar]
- 9. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14(21):2161–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doddamani RS, Meena RK, Sawarkar D. Ambiguity in the dural tail sign on MRI. Surg Neurol Int. 2018;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niu J, Zhang S, Ma S, et al. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur Radiol. 2019;29(3):1625–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slot KM, Verbaan D, Bosscher L, Sanchez E, Vandertop WP, Peerdeman SM. Agreement between extent of meningioma resection based on surgical Simpson Grade and based on postoperative magnetic resonance imaging findings. World Neurosurg. 2018;111:e856–e862. [DOI] [PubMed] [Google Scholar]
- 13. Villa C, Miquel C, Mosses D, Bernier M, Di Stefano AL. The 2016 World Health Organization classification of tumours of the central nervous system. Presse Med. 2018;47(11-12):e187–e200. [DOI] [PubMed] [Google Scholar]
- 14. Krivoshapkin AL, Sergeev GS, Kalneus LE, et al. New software for preoperative diagnostics of meningeal tumor histologic types. World Neurosurg. 2016;90:123–132. [DOI] [PubMed] [Google Scholar]
- 15. Butts AM, Weigand S, Brown PD, et al. Neurocognition in individuals with incidentally-identified meningioma. J Neuro Oncol. 2017;134(1):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masalha W, Heiland DH, Delev D, et al. Survival and prognostic predictors of anaplastic meningiomas. World Neurosurg. 2019;131:e321–e328. [DOI] [PubMed] [Google Scholar]
- 17. Lemée JM, Joswig H, Da Broi M, et al. WHO grade I meningiomas: classification-tree for prognostic factors of survival. Neurosurg Rev. 2020;43(2):749–758. [DOI] [PubMed] [Google Scholar]
- 18. Pavelin S, Becic K, Forempoher G, et al. Expression of Ki-67 and p53 in meningiomas. Neoplasma. 2013;60(5):480–485. [DOI] [PubMed] [Google Scholar]
- 19. Manrique-Carmona LP, Pérez-Neri I. Pathophysiology and treatment of peritumoral brain edema: possible effect of lidocaine. Neurochem J. 2018;12(1):9–14. [Google Scholar]
- 20. Ke C, Chen H, Lv X, et al. Differentiation between benign and nonbenign meningiomas by using texture analysis from multiparametric MRI. J Magn Reson Imaging. 2019;51(6):1810–1820. [DOI] [PubMed] [Google Scholar]
- 21. Meyer HJ, Wienke A, Surov A. ADC values of benign and high grade meningiomas and associations with tumor cellularity and proliferation—a systematic review and meta-analysis. J Neurol Sci. 2020;415:116975. [DOI] [PubMed] [Google Scholar]
- 22. Maiuri F, Donzelli R, Pagano S, Mariniello G. The management of the venous sinuses during surgery for posterior fossa meningiomas. World Neurosurg. 2019;125:357–363. [DOI] [PubMed] [Google Scholar]
- 23. Anthofer J, Seidel-Schulz R, Proescholdt M, Brawanski A, Schebesch KM. Meningiomas adjacent to major venous sinuses-clinical outcome and recurrence. World Neurosurg. 2017;104:560–566. [DOI] [PubMed] [Google Scholar]