Abstract
Background:
Protracted withdrawal syndrome (PWS) after stopping antidepressants (frequently also referred to as post-acute withdrawal syndrome or PAWS) has been described in a few case reports. However, a detailed quantitative analysis of specific symptom manifestations in antidepressant PWS is still lacking.
Methods:
We extracted patient narratives from a large English-language internet forum SurvivingAntidepressants.org, a peer support site concerned about withdrawal from antidepressants. PWS was ascertained based on diagnostic criteria proposed by Chouinard and Chouinard, specifically ⩾6 months of continuous antidepressant use, with emergence of new and/or more intense symptoms after discontinuation that last beyond the initial 6 weeks of acute withdrawal. We assessed medication history, outcome of PWS, and the prevalence of specific symptoms.
Results:
In total, n = 69 individual reports of protracted withdrawal were selected for analysis. At time of the subjects’ most recent reports, duration of PWS ranged from 5 to 166 months, mean = 37 months, median = 26 months. Length of time on the antidepressant causing protracted withdrawal ranged from 6 to 278 months, mean = 96 months, and median = 79 months. Throughout the withdrawal experience, affective symptoms, mostly anxiety, depression, emerging suicidality and agitation, were reported by 81%. Somatic symptoms, mostly headache, fatigue, dizziness, brain zaps, visual changes, muscle aches, tremor, diarrhea, and nausea were reported by 75%. Sleep problems (44%) and cognitive impairments (32%) were mentioned less frequently. These broad symptom domains were largely uncorrelated.
Conclusion:
PWS or PAWS from antidepressants can be severe and long-lasting, and its manifestations clinically heterogeneous. Long-term antidepressant exposure may cause multiple body system impairments. Although both somatic and affective symptoms are frequent, they are mostly unrelated in terms of occurrence. Proper recognition and detection of PWS thus requires a comprehensive assessment of medication history, duration of the withdrawal syndrome, and its various somatic, affective, sleep, and cognitive symptoms.
Keywords: antidepressant, discontinuation, PAWS, persistent post-withdrawal disorder, protracted
Introduction
Antidepressants are the most widely prescribed psychiatric drugs worldwide, with prescription rates ranging from about 6% to 16% in European countries and North America.1–4 Prescription rates and the average duration people are on antidepressants have been rising constantly since the 1990s, so that an increasing proportion of the general population is now on (indefinite) long-term medication.5–8 Research further indicates that many people in receipt of long-term antidepressant medication do not necessarily require maintenance treatment.7,9,10 A possible driver of unnecessary long-term medication could be the propensity of antidepressants to cause physical dependence (i.e., pharmacodynamic neurophysiological adaptations) and withdrawal symptoms upon dose reduction or cessation discouraging discontinuation.11–18 In this respect, antidepressants do not differ from other dependence-forming central nervous system (CNS) drugs like benzodiazepines, opioids, gabapentinoids, or psychostimulants.3,19–22
Severe and persistent withdrawal syndromes from antidepressants have long been neglected or minimised. Obscuring a potentially serious risk, the pharmaceutical industry coined the term “discontinuation syndrome” to avoid association of antidepressants with psychotropic dependence. This term is unnecessary and misleading, suggesting antidepressants cannot cause dependence and withdrawal.11,16 Thus, patients and prescribers may misattribute withdrawal symptoms as relapse or emergence of new mental disorders.11,16,21 It is now suspected that antidepressant withdrawal syndrome is more common and severe than earlier presumed, affecting roughly 30–50% of those who attempt to stop their treatment.12,13,23,24 To avoid the costs and risks associated with unnecessary long-term antidepressant treatment and misdiagnosis of withdrawal,25–28 a better understanding and proper recognition of antidepressant withdrawal syndromes are required.15,29,30
Two main phases of withdrawal can be differentiated. Across psychotropics, acute withdrawal may last 1–8 weeks after complete discontinuation of the drug. Acute withdrawal sometimes transitions into a protracted withdrawal syndrome (PWS), which lasts much longer, from months to years.19,20,22 These persistent forms of withdrawal have also been termed post-acute withdrawal syndrome (PAWS), protracted abstinence syndrome, chronic/persistent withdrawal, or persistent post-withdrawal disorder.19,20 For the sake of simplicity, we will use the term PWS consistently throughout this manuscript, as this is the preferred term in addiction medicine.20,31
As with other psychotropics, the potential for antidepressant PWS is embedded in pharmacodynamic adaptations and possibly irreversible neurophysiological alterations following prolonged drug exposure.19,20,31 These processes, including receptor downregulation and desensitisation, are best understood as physical dependence,20 and were also subsumed under the oppositional model of tolerance by Fava.30 An extended amount of time may thus be required to re-adapt to the absence of the drug after physical dependence has developed (not to be confused with addiction).20,32 Some patients may recover spontaneously or after re-instatement of the antidepressant, others require a much longer time: months or years. However, hypothetically, prolonged antidepressant exposure could also cause permanent neurophysiological alterations, comparable with tardive dyskinesia after long-term antipsychotic use,33 with unremitting, chronic, withdrawal symptoms.30,34 In any case, the neurophysiology of protracted withdrawal is poorly understood.
Long known in addiction medicine and from benzodiazepines,20,31 protracted withdrawal from antidepressants has been recognized only recently.35 The scientific literature’s foundation of case reports documenting withdrawal syndrome thus far contains only a few studies qualitatively describing protracted withdrawal,19,20,35,36 and one small cohort study including three cases of PWS.37 To the best of our knowledge, the largest study specifically examining PWS comprised only seven case reports.38 That is, no quantitative analysis of the manifestation of PWS based on strict diagnostic criteria has been published thus far. Given the considerable burden of severe protracted antidepressant withdrawal for both the individual and society,12,15,29,34 this poses a significant gap in the scientific literature. The aim of this study was hence to quantitatively examine the manifestation of PWS from antidepressants in a relatively large sample.
Methods
Database and procedure
SurvivingAntidepressants.org is a large peer-led internet forum for people tapering psychiatric drugs or recovering from antidepressant withdrawal syndromes. From the founder and administrator of this website (AF), we received a link to reports that were tagged by moderators of the website as potential PWS. On SurvivingAntidepressants.org, these cases of protracted withdrawal are classified as PAWS (results available at https://www.survivingantidepressants.org/tags/paws/). These reports were accessed on 9 January 2020 and comprised 94 individual pseudonymous patient narratives, many over years, of varying length and level of detail. These texts were exported in MAXQDA – a software developed for the qualitative analysis of writings. One author (LS) then conducted a content analysis and coded the relevant variables required for the quantitative synthesis. These variables included (1) treatment duration and types of antidepressants and other psychotropic medications used; (2) persistence of withdrawal symptoms beyond the 6-week acute phase; and (3) the presence and quality of protracted withdrawal symptoms according to the classification system proposed by Fava et al.,13 that is, general, balance, sensory, visual, neuromotor, vasomotor, sleep, gastrointestinal, affective, psychotic, cognitive, and sexual symptoms. We were not able to assess age and gender, as these were not disclosed consistently. The coding and assessment of variables was cross validated by a second author (MPH). Discrepancies were resolved by consensus after re-evaluation.
We then applied the diagnostic criteria for PWS proposed by Chouinard and Chouinard to our 94 cases extracted from the website.35 Therefore, we included only reports where antidepressants were taken continuously for at least 6 months and where withdrawal symptoms definitely persisted beyond 6 weeks. We further required that the reported withdrawal symptoms were perceived as more intense (qualitatively different) than the symptoms of the original treatment indication and/or that they were symptoms of a new emergent disorder never experienced before. By consequence, we excluded reports when antidepressants were not taken continuously for at least 6 months, when no specific withdrawal symptoms were mentioned, or when it could not be reasonably inferred from the narrative that withdrawal symptoms persisted beyond 6 weeks. This led to removal of 25 reports and to a final sample of 69 cases with withdrawal symptoms meeting diagnostic criteria of PWS. These data were then exported to SPSS (SPSS Inc., Chicago, IL, USA) for quantitative statistical analysis. Duration of withdrawal was calculated by one author (AF) for each individual, with date of PWS onset determined by subject recollection of start of withdrawal symptoms after discontinuing an antidepressant taken ⩾6 months. Withdrawal was considered to continue if, by patient report, withdrawal symptoms persisted despite any treatment or if reinstatement of the implicated drug did not resolve the withdrawal symptoms.39 End date for PWS was established by reported date of recovery, effective reinstatement or drug treatment, or death. If no other information was available, the most recent report of symptom status by 30 September 2020 served as the end date for calculation of PWS duration.
As this was a secondary analysis of anonymous reports in the public domain, according to Swiss law this study was exempt from approval from an ethics committee and informed consent was not required.
Statistical analysis
We performed descriptive analyses to examine the frequency of specific withdrawal symptoms. Associations between symptoms were examined with Pearson correlations and contingency tables. The Pearson correlation coefficient for two binary variables is identical to the non-parametric phi coefficient based on a 2 × 2 contingency table; 95% confidence intervals (CI) for the Pearson correlation coefficients were computed with a bias-corrected and accelerated bootstrapping method based on 5000 samples. Due to the modest sample size and resulting limited statistical power, we chose not to apply a correction for multiple testing. This decision is also defensible on the grounds that all analyses were exploratory and hence an increased risk of false-positive findings was unavoidable. All analyses were conducted with SPSS version 26 for Windows.
Results
Medication history
Study subjects tended to have complex psychiatric drug histories. Due to medication switches (one person using several antidepressants over time) and comedication, the count of individual drugs is higher than the total count for a given drug class. Altogether 55 people (79.7%) took, at least once, a selective serotonin reuptake inhibitor (SSRI), of which 16 (23.2%) used sertraline, 9 (13.0%) paroxetine, 13 (18.8%) fluoxetine, 2 (2.9%) fluvoxamine, 15 (21.7%) escitalopram, and 18 (26.1%) citalopram. Serotonin and norepinephrine reuptake inhibitors (SNRI) were used by 20 people (29.0%), of which 2 (2.9%) used duloxetine, 17 (24.6%) venlafaxine, and 1 person (1.4%) desvenlafaxine. Atypical antidepressants were used by 12 people (17.4%), which included 1 person (1.4%) on trazodone, 4 (5.8%) on mirtazapine, 6 (8.7%) on bupropion, and 1 (1.4%) on agomelatine. Atypical antidepressants were used mostly (91.7% of cases) as comedication along with other antidepressants. Finally, three people (4.3%) used a first-generation antidepressant [one on a monoamine oxidase inhibitor (MAOI) and two on a tricyclic]. All users of first-generation antidepressants switched to second-generation antidepressants over time.
Altogether 46 people (66.7%) had used only one antidepressant over their medication history, 12 (17.4%) had used two different antidepressants, 6 (8.7%) had used three different antidepressants, 4 (5.8%) had used four different antidepressants, and 1 person (1.4%) had a medication history of five different antidepressants. Of 69 subjects, 19 reported having gone off the drug abruptly (the rest have done some sort of taper), 21 had withdrawal symptoms during tapering, and 33 had prior failed withdrawal attempts or other adverse drug reactions. In the aggregate, one or more of these incidents appeared in the histories of 53 of 69 subjects (77%). In total, 12 people (17.9%) also used other psychotropic drugs, including 4 people (6.0%) on mood-stabilizers, 1 (1.5%) on neuroleptics, 8 (11.9%) on benzodiazepines, and 2 (3.0%) on Z-drugs.
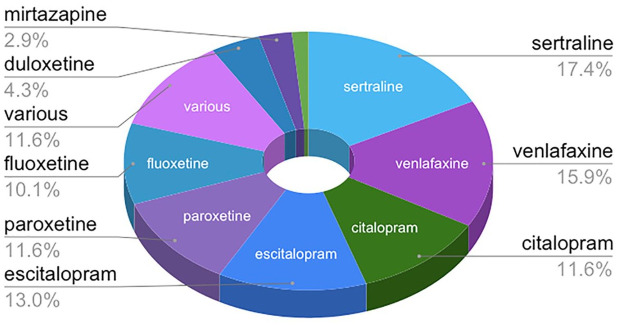
Subjects had taken the antidepressant suspected of precipitating PWS for 6–278 months, with an average of 96 months and a median of 79 months. Nine common antidepressants were suspected to have precipitated PWS in 61 subjects; the remaining subjects were taking various drug combinations including antidepressants. Sertraline was suspected as the cause of PWS for 12 subjects (17.4%); venlafaxine for 11 (15.9%); citalopram for 8 (11.6%); escitalopram for 9 (13%); paroxetine for 8 (11.6%); fluoxetine for 7 (10.1%); duloxetine for 3 (4.3%); mirtazapine for 2 (2.9%); fluvoxamine for 1 (1.4%); and various for 8 (11.6%). The top six drugs – sertraline, venlafaxine, citalopram, escitalopram, paroxetine, and fluoxetine – were implicated in 80% of PWS cases (see Figure 1).
Figure 1.
Antidepressants suspected to precipitate PWS.
PWS, protracted withdrawal syndrome.
Duration and outcome of PWS
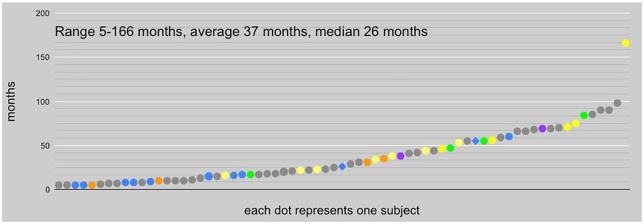
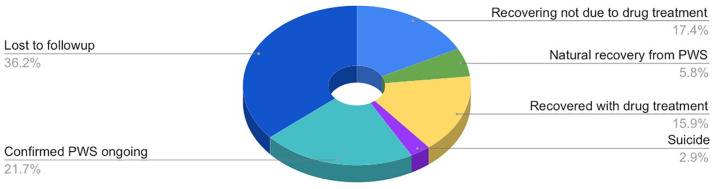
At time of the 69 subjects’ most recent reports, duration of PWS ranged from 5 to 166 months, with a mean of 37 months and a median of 26 months (see Figure 2). Some subjects attempted drug treatment of their protracted withdrawal symptoms. In our study population, reinstatement of the drug suspected to have precipitated PWS resolved the condition in 9 of the 19 individuals who attempted it (in months 5–60 of PWS; mean = 16 months; median = 9 months, successful reinstatement at 60 months being an outlier). Treatment with drugs other than the one implicated in PWS was attempted by 33 subjects; 4 reported some benefit and 2 substantial benefit: For one subject, subsequent escitalopram in month 12 of PWS resolved most venlafaxine withdrawal symptoms; the other recovered in month 55 with the support of pregabalin in approximately months 6–24. Other subjects reported sporadic use of drugs such as benzodiazepines, trazodone, propranolol, and cannabidiol oil for transitory symptomatic relief, usually of insomnia or anxiety, without significant progress in resolution of PWS. Otherwise, 4 persons reported natural recovery from PWS without reinstatement or drug treatment, 12 reported improvement short of full recovery; 2 persons died by suicide. More than one-third of the sample was lost to follow up, thus their outcome was unknown: PWS may be ongoing, resolved, or they may be deceased (see Figure 3).
Figure 2.
Recorded duration of PWS among subjects at time of last report. Blue dots indicate the 9 who resolved with reinstatement of the antidepressant precipitating PWS. Blue diamonds indicate the 2 who benefited significantly from other subsequent psychotropic treatment; orange dots the 4 who experienced only some benefit. Green dots indicate the 4 who resolved naturally without subsequent drug treatment; yellow, the 12 who, at last report, were partially recovered; violet, 2 suicides. The gray dots indicate the remaining 36 individuals for whom resolution of PWS has not been reported.
PWS, protracted withdrawal syndrome.
Figure 3.
Outcomes for subjects with PWS as recorded at time of last report.
PWS, protracted withdrawal syndrome.
The two suicides reported among our subjects were verified by public records. Both were women who had been active on several Internet sites dealing with psychiatric drug withdrawal and were explicit about their extreme distress. Both had medical treatment subsequent to developing PWS from antidepressants. One initiated sertraline at age 16; after 16 months on the drug, her 1-month medically supervised taper was uneventful, except for post-SSRI sexual dysfunction (PSSD), which persisted, despite treatment with mianserin, for 38 months, until her suicide. In multiple Internet support groups, she had expressed distress about her ongoing sexual dysfunction. Her last post on SurvivingAntidepressants.org was in October 2019: “To be honest, now I’m in absolute hell. I don’t see much hope in this situation anymore. It’s been 3+ years and I’m still in pretty much the same situation. I don’t know how much longer I’ll be here honestly”. The next day, she killed herself. She had recently turned 21 years old. The second woman took 15 mg mirtazapine for 69 months despite initial and ongoing adverse effects from the drug. Her lengthy and difficult taper brought no relief of symptoms. In her sixth year of PWS, she saw some improvement, but in May 2020 had an adverse reaction to an antibiotic-steroid ear drop that revived her most severe withdrawal symptoms, one of them akathisia. In Internet forums, she clearly expressed anguish over her iatrogenic condition. After 69 months of PWS, in August 2020, she killed herself.
Prevalence of protracted withdrawal symptoms
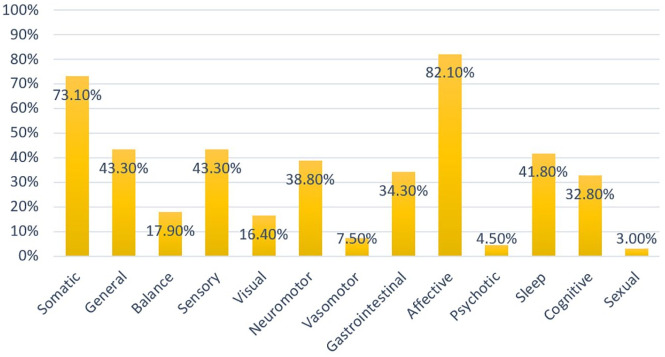
The prevalence rates of the various symptom domains persisting beyond the acute withdrawal phase (>6 weeks) are depicted graphically in Figure 4. General symptoms were reported by 30 people (43.5%). The most common symptoms from this group were headache (50.0% of all people with general symptoms) and fatigue (43.3%). Balance symptoms were reported by 12 people (17.4%), of which the most common specifically disclosed was dizziness (66.7%). Sensory symptoms were reported by 30 people (43.5%), of which 15 (50.0%) disclosed electric-shock-sensations (“brain zaps”). Visual symptoms were reported by 11 people (15.9%). These comprised visual changes in all cases (100%). Neuromotor symptoms were disclosed by 27 people (39.1%). The most common symptoms from this group were muscle aches (44.4%) and tremor (40.7%). Vasomotor symptoms were reported by five people (7.2%) and comprised mostly sweating (80.0%). Gastrointestinal symptoms were reported by 23 people (33.3%) and the most common symptoms specifically mentioned were nausea (47.8%) and diarrhea (43.5%). Affective symptoms were reported by 57 people (82.6%). The symptoms divulged most frequently in this group were anxiety (64.9%), depression (43.9%), and emerging suicidality (24.6%). Psychotic symptoms, specifically hallucinations, were reported by three people (4.3%). Sleep symptoms were reported by 30 people (43.5%) and in most cases (83.3%) comprised insomnia. Cognitive symptoms were stated by 22 people (31.9%) and comprised mostly decreased concentration (81.8%), which was frequently described as “brain fog”. Finally, persistent sexual symptoms were mentioned by two people (2.9%).
Figure 4.
Prevalence rates of protracted withdrawal symptoms. Somatic is a higher-order domain and comprises general, balance, sensory, visual, neuromotor, vasomotor, and gastrointestinal symptoms.
Associations between protracted withdrawal symptoms
The correlations between different symptom domains are shown in Table 1. Psychotic and sexual symptoms were excluded from this analysis because they were reported by three and two people only. Three main patterns emerged. First, somatic symptoms, including general, balance, sensory, visual, neuromotor, vasomotor, and gastrointestinal symptoms were moderately inter-correlated. Second, sleep symptoms correlated moderately with neuromotor symptoms only. Third, affective and cognitive symptoms were mostly unrelated to other symptom domains and correlated weakly with few symptom domains.
Table 1.
Pearson correlations (with 95% CI) between withdrawal symptoms.
| Balance | Sensory | Visual | Neuromotor | Vasomotor | Gastrointestinal | Affective | Sleep | Cognitive | |
|---|---|---|---|---|---|---|---|---|---|
| General | 0.29* (0.05–0.50) | 0.29* (0.06–0.51) | 0.34** (0.12–0.53) | 0.20 (−0.03 to 0.41) | 0.21 (−0.04 to 0.39) | 0.50** (0.28–0.69) | 0.25* (0.04–0.43) | 0.17 (−0.05 to 0.40) | 0.22 (−0.02 to 0.43) |
| Balance | 0.37** (0.15–0.56) | 0.22 (−0.08 to 0.52) | 0.26* (0.01–0.50) | 0.31** (−0.06 to 0.59) | 0.49** (0.25–0.69) | 0.11 (−0.13 to 0.27) | 0.22 (−0.03 to 0.43) | 0.01 (−0.22 to 0.26) | |
| Sensory | 0.26* (0.02–0.46) | 0.26* (0.03–0.47) | 0.21 (−0.04 to 0.39) | 0.43** (0.21–0.64) | 0.09 (−0.14 to 0.31) | 0.12 (−0.13 to 0.36) | 0.15 (−0.08 to 0.38) | ||
| Visual | 0.38** (0.12–0.60) | 0.03 (−0.14 to 0.29) | 0.20 (−0.07 to 0.46) | 0.20 (0.14–0.27) | −0.06 (−0.28 to 0.18) | 0.21 (−0.04 to 0.44) | |||
| Neuromotor | 0.12 (−0.14 to 0.35) | 0.19 (−0.05 to 0.43) | 0.29* (0.09–0.45) | 0.32** (0.09–0.53) | 0.15 (−0.08 to 0.37) | ||||
| Vasomotor | 0.40** (0.23–0.55) | 0.13 (0.07–0.19) | 0.21 (−0.05 to 0.40) | 0.17 (−0.10 to 0.40) | |||||
| Gastrointestinal | 0.16 (−0.08 to 0.35) | 0.12 (−0.11 to 0.35) | 0.04 (−0.19 to 0.28) | ||||||
| Affective | 0.17 (−0.08 to 0.38) | −0.01 (−0.25 to 0.20) | |||||||
| Sleep | −0.04 (−0.26 to 0.19) |
p <0.05 **p <0.01.
CI, confidence interval.
Due to their substantial inter-correlations, we grouped all somatic symptoms together. The prevalence of this somatic syndrome was 73.9% and it was uncorrelated with affective symptoms, sleep symptoms, and cognitive symptoms (all r ⩽ 0.16; p >0.18). Altogether, 44 people (63.8%) had co-occurring somatic and affective symptoms, whereas 25 people (36.2%) had either somatic or affective symptoms or other symptoms (i.e., sleep, sexual or cognitive symptoms). Among the 51 participants with somatic symptoms, 44 (86.3%) also reported affective symptoms and 7 (13.7%) did not. Among the 57 participants with affective symptoms, 44 (77.2%) also reported somatic symptoms and 13 (22.8%) did not. As detailed above, the association between somatic and affective symptoms was weak and statistically not significant (r = 0.16, p =0.18).
Discussion
Summary and interpretation
To the best of our knowledge, this is the first quantitative study of the manifestation of persistent post-acute antidepressant withdrawal symptoms in a sample of people meeting proposed diagnostic criteria for PWS,35 with many patient narratives amounting to naturalistic longitudinal case studies over years. Our findings show that antidepressants of all types may incur protracted withdrawal potentially lasting months to many years, as has been observed for other psychotropics.20 While distribution of cases among antidepressants is probably related to their popularity, that fluoxetine was implicated in 10% of our sample is worthy of remark, as the drug’s long half-life has been supposed to be protective against acute withdrawal symptoms.24,40 But, as others have suspected,11,36 an antidepressant’s half-life probably is not a primary risk factor for PWS. Rather, characteristics of the individual may be more important; posited, among other potentially predisposing factors, are lengthy drug treatment, medication switches, abrupt discontinuation, inadequate tapering, prior difficulty in withdrawal or other adverse drug events and physiological vulnerability.20,23,30,41–43 Of our 69 subjects, 77% reported drug mishaps in their histories, having gone off the drug abruptly, had prior failed discontinuation attempts or adverse drug reactions, or experienced withdrawal symptoms during tapering.
As PWS from antidepressants has been so poorly recognized, the literature is largely silent about its treatment.35,39 When withdrawal symptoms emerge in the acute phase of antidepressant withdrawal, reinstatement of the drug to treat them is recommended throughout the scientific literature and in many new-generation antidepressant package inserts.11,13,23,28,44 However, this recommendation is not supported by controlled data, and a few authors even suggested that reinstating the drug may be detrimental and lead to more severe withdrawal.39,45 Most importantly, success of reinstatement has never been documented in protracted antidepressant withdrawal, though reduction of withdrawal symptoms via reinstatement is a cornerstone of self-medication in post-acute addiction relapse.46,47 Attempted by about a quarter of our study population, reinstatement was successful in fewer than half. Exclusive of reinstatement, although half of the subjects attempted treatment with other drugs, only two experienced significant success. As the sample was small and selective, the results must be interpreted cautiously. Nevertheless, this observation is consistent with Chouinard and Chouinard: protracted withdrawal does not seem very amenable to reinstatement or other conventional drug treatment.35 This raises the possibility it may be mistaken for a “treatment-resistant” psychiatric condition.34
In our study sample, subjects struggled with a wide range of symptoms; affective symptoms, particularly anxiety, depression and emerging suicidality were the most prevalent, which is also in line with the literature.35,38 Our sample included two people who suicided and who attributed their motivation to protracted withdrawal symptoms (in one case, persistent sexual dysfunction).48,49 Affective withdrawal symptoms can be the very same symptoms for which the antidepressant was initially prescribed, implying that some patients may have confused a genuine relapse with a withdrawal syndrome. However, it has been shown that most antidepressant users can distinguish accurately between symptoms of the original disorder and adverse drug effects.50 The narratives analyzed in this study likewise indicate that consumers are able to distinguish drug withdrawal from the symptoms of the original illness, since withdrawal symptoms are experienced as more intense, urgent, and often unprecedented, that is, qualitatively different.28,30,35 This important qualitative component to the assessment and diagnosis of medical conditions underscores the necessity of incorporating the subjective patient perspective in antidepressant research,51 as well as in general medical research.52,53 Moreover, the frequent co-occurrence of somatic withdrawal symptoms with affective symptoms argues against relapse. Emotional states cannot be isolated as a separate phenomenon apart from the neurophysiological condition; the individual may be reacting in a habitual way emotionally to the distressing physical and neurological states of PWS. Due to these various reasons, we suggest that the symptoms reported in the patient narratives were indeed most likely a consequence of antidepressant withdrawal and not relapse or emergence of a new psychiatric disorder.
Focus on affective symptoms alone may also lead to misdiagnosis of PWS. Somatic symptoms, including general, balance, sensory, visual, neuromotor, vasomotor, and gastrointestinal symptoms, were moderately inter-correlated, and, when grouped together, this syndrome was also frequent. However, somatic and affective symptoms were largely unrelated. Although most people reported co-occurring somatic and affective symptoms, some had either of them, and some mentioned none of them but did mention sleep problems or cognitive impairments. A quarter of subjects reported affective symptoms but not somatic symptoms.
The substantial correlation between various somatic symptoms speaks to a neurophysiological basis for PWS, possibly autonomic nervous system dysfunction.25,54,55 According to our study, the most common somatic symptoms were headache, fatigue, dizziness, electric-shock-sensations (“brain zaps”), visual changes, muscle aches, tremor, diarrhea, and nausea. Other common symptoms were decreased concentration (“brain fog”) and insomnia (the latter proposed as a universal psychotropic withdrawal symptom).56 These findings agree with case reports of PWS previously published.38 Moreover, these symptoms are also frequent acute withdrawal symptoms,11,13,14,28 suggesting that persistent and acute withdrawal symptoms may have a common etiopathology that is most likely rooted in neurophysiological adaptations to prolonged antidepressant exposure, for example serotonin receptor downregulation and hypothalamic-pituitary-adrenal (HPA) axis sensitisation.11,18,41,57 According to the oppositional model of tolerance, PWS results from oppositional pharmacodynamic processes that no longer encounter resistance when drug treatment ends.30,40 Our data thus demonstrate that PWS from antidepressants is a heterogeneous condition that may manifest in diverse forms, duration rather than symptomology differentiating protracted from the acute phase. The various manifestations also confirm that protracted antidepressant withdrawal is very much like protracted withdrawal syndromes from other psychotropics.20
Finally, some of the diagnostic criteria proposed by Chouinard and Chouinard are not supported by robust scientific evidence due to a lack of research on PWS and hence are debatable, for example, the prerequisites of at least 6 months of continuous drug use and peak of symptom onset between 1 day and 6 weeks.35 Across psychotropics, neurophysiological adaptation, which is the prerequisite for withdrawal, occurs in 1 to 8 weeks.20,58,59 With new-generation antidepressants, it has been widely observed that risk of withdrawal emerges after the drugs have been taken for 1 to 2 months.24,39 Theoretically, severe (and protracted) withdrawal may also occur after short antidepressant exposure. For instance, Bloch et al. present two cases that developed a severe withdrawal syndrome lasting 2 to 4 weeks after just 6 weeks of treatment with paroxetine (one after abrupt discontinuation and the other after a 12-day taper).60 It is thus very likely that protracted withdrawal may also develop after treatment exposure of less than 6 months and there are reports on SurvivingAntidepressants.org that lend credence to this possibility.
With respect to peak of onset within 6 weeks, in our patient narratives, various symptoms reached their highest intensity or first developed several months after discontinuation. For instance, one participant wrote after coming off paroxetine in May: “The next few weeks I experienced mainly brain zaps and flu-like symptoms, but nothing unbearable and I had an idea that was to be expected. These went away and for the next 3 months, aside from heavy dreaming at night, I was totally fine. Then, in September, I got hit like a truck with crazy unexplainable symptoms. These symptoms are: waves of panic that feel different from my original anxiety/panic, OCD about symptoms and thoughts, feeling disconnected from the world around me, phantom smells (it is always the same burning smell), insomnia, vivid and whacky dreams, horrible ringing in my ears and head (almost feels like an electric current), weird burning/shivering of my brain, and more. These were so debilitating to me I left my job and am currently unable to function”. Another person came off citalopram in March and wrote “I took 2 weeks leave from work in anticipation of the withdrawal. At first there was only brain zaps and some agitation that in itself was manageable, then came the gastro intestinal issues about 1 month after stopping the meds, mostly heartburn and IBS (irritable bowel syndrome) type symptoms. I did not link these issues with withdrawal and went to a GI specialist that performed multiple tests (Colonoscopy, Gastroscopy, CT scan and blood tests) all tests came back reporting no issues. after about 2 months off the meds I started having severe panic attacks and anxiety as well as pressure in my head (behind my eyes), severe dizziness and a lot more symptoms that I cannot even recall as they disappeared and reappeared seemingly at random.”
Due to vague descriptions and no specification of exact time period, we cannot quantify how many people experienced late-onset withdrawal symptoms, but it appears to be sufficiently common to question the peak onset within 6 weeks criterion.
Limitations
We acknowledge several limitations of the present study. Despite being based on the largest sample of diagnostically established PWS cases thus far, with 69 people this sample was still of modest size. Due to lack of statistical power we were not able to analyse individual drugs or drug classes. Our data were derived from spontaneous self-reports posted in a large public internet forum. That is, symptoms were neither assessed systematically nor validated clinically. Consumers may have reported only the most disturbing or debilitating symptoms and may have omitted to mention milder or sensitive symptoms, for example sexual problems and psychotic phenomena. The estimated rates are thus conservative and likely underestimations of the true prevalence of specific withdrawal symptoms in people with PWS. However, it could also be that persistent sexual dysfunction and post-acute psychotic symptoms are truly rare events and that their prevalence among protracted withdrawal symptoms is indeed low. Only large prospective cohort studies with systematic assessment of symptoms will be able to answer these fundamental questions.
In our study sample there were eight cases (11.6%) where an antidepressant was discontinued among other drugs, for example, a neuroleptic or a z-drug, sometimes in an unclear timeline. One came off “Celexa and Ambien”, another off “Anafranil, Abilify, and Luvox”. For these eight subjects, the suspected drug was designated as “various”. None of the 69 had recently come off a benzodiazepine.
Finally, some people may have misattributed symptoms to antidepressant withdrawal that were caused by a mental disorder or a (undiagnosed) general medical condition. To minimize misattribution bias, we relied on the diagnostic criteria proposed by Chouinard and Chouinard,35 which require that symptoms are described as novel or more intense than ever experienced before. This selection process was facilitated as most participants described established withdrawal symptoms (e.g., brain zaps, dizziness, visual changes, muscle aches) that could not be attributed to the original treatment indication (mostly depression and anxiety). Yet, our semantic analysis was unable to distinguish between affective symptoms that might have been induced by the neurological dysregulation of PWS, individuals’ emotional reaction to their debilitated state and lack of medical support, and what might be true relapse of prior psychiatric disorder.
Conclusions and outlook
Patient experiences or narratives are considered an important part of evidence-based medicine, but are insufficiently incorporated in both research and practice.52,53,61 Antidepressant users have reported severe and protracted withdrawal symptoms for decades via user surveys, help hotlines, or internet forums.38,62–67 Unfortunately, until very recently, medical associations, pharmaceutical companies, drug regulators, psychiatric academics, and practitioners have largely neglected or minimized this serious public health issue.15,16,68,69 Therefore, we hope that our study of PWS from antidepressants, which is the largest conducted to date, may prompt more research on this largely unexplored syndrome. We further hope that our findings may help general practitioners (GPs) and mental health professionals to better recognize and take steps to avoid this severe and debilitating condition,15,29,30,70 which can lead even to suicide. Large prospective cohort studies and clinical trials should attempt to replicate our exploratory findings and could further examine associations between patient characteristics, medication histories, antidepressant drugs and classes, duration of treatment, and the varying manifestations of protracted antidepressant withdrawal.
Footnotes
Author contributions: MPH designed the study, participated in data preparation, conducted the statistical analysis, and drafted the manuscript. LS and AF participated in data preparation, analysis and interpretation of the data, and writing of the manuscript. AS participated in writing and interpretation of the data. All authors critically revised the manuscript and approved the final version.
Conflict of interest statement: Adele Framer is the founder and administrator of SurvivingAntidepressants.org, a website offering tapering information and peer support for withdrawal from psychiatric drugs. SurvivingAntidepressants.org does not provide medical care, psychiatric diagnosis, or psychotherapy. Donations underwrite site expenses. The author does not receive any compensation, monetary or otherwise, for serving in this capacity.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by Mr. Stefan Hearst. The sponsor had had no further role in the experimental design; collection, analysis, and interpretation of data; writing of this report; or the decision to submit this paper for publication.
ORCID iDs: Michael P. Hengartner  https://orcid.org/0000-0002-2956-2969
https://orcid.org/0000-0002-2956-2969
Adele Framer  https://orcid.org/0000-0002-4094-6744
https://orcid.org/0000-0002-4094-6744
Supplemental material: Data sources are freely available in the public domain (https://www.survivingantidepressants.org/tags/paws/). All statistical data and code are available from the first author upon request.
Contributor Information
Michael P. Hengartner, Department of Applied Psychology, Zurich University of Applied Sciences (ZHAW), PO Box 707, Zurich, 8037, Switzerland; Medical Faculty, University of Zurich, Switzerland.
Lukas Schulthess, Department of Applied Psychology, Zurich University of Applied Sciences, Switzerland.
Anders Sorensen, Nordic Cochrane Centre, Rigshospitalet, Copenhagen, Denmark.
Adele Framer, https://www.survivingantidepressants.org/.
References
- 1. Haller E, Watzke B, Blozik E, et al. Antidepressant prescription practice and related factors in Switzerland: a cross-sectional analysis of health claims data. BMC Psychiatry 2019; 19: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewer D, O’Reilly C, Mojtabai R, et al. Antidepressant use in 27 European countries: associations with sociodemographic, cultural and economic factors. Br J Psychiatry 2015; 207: 221–226. [DOI] [PubMed] [Google Scholar]
- 3. Marsden J, White M, Annand F, et al. Medicines associated with dependence or withdrawal: a mixed-methods public health review and national database study in England. Lancet Psychiatry 2019; 6: 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore TJ, Mattison DR. Adult utilization of psychiatric drugs and differences by sex, age, and race. JAMA Intern Med 2017; 177: 274–275. [DOI] [PubMed] [Google Scholar]
- 5. Huijbregts KM, Hoogendoorn A, Slottje P, et al. Long-term and short-term antidepressant use in general practice: data from a large cohort in the Netherlands. Psychother Psychosom 2017; 86: 362–369. [DOI] [PubMed] [Google Scholar]
- 6. Mars B, Heron J, Kessler D, et al. Influences on antidepressant prescribing trends in the UK: 1995-2011. Soc Psychiatry Psychiatr Epidemiol 2017; 52: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry 2014; 75: 169–177. [DOI] [PubMed] [Google Scholar]
- 8. Pratt LA, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and over: United States, 2011-2014. Hyattsville, MD: National Center for Health Statistics, 2017. [Google Scholar]
- 9. Johnson CF, Macdonald HJ, Atkinson P, et al. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br J Gen Pract 2012; 62: e773–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verhaak PFM, de Beurs D, Spreeuwenberg P. What proportion of initially prescribed antidepressants is still being prescribed chronically after 5 years in general practice? A longitudinal cohort analysis. BMJ Open 2019; 9: e024051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotzalidis GD, Patrizi B, Caltagirone SS, et al. The adult SSRI/SNRI withdrawal syndrome: a clinically heterogeneous entity. Clin Neuropsychiatry 2007; 4: 61–75. [Google Scholar]
- 12. Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav 2019; 97: 111–121. [DOI] [PubMed] [Google Scholar]
- 13. Fava GA, Gatti A, Belaise C, et al. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom 2015; 84: 72–81. [DOI] [PubMed] [Google Scholar]
- 14. Fava GA, Benasi G, Lucente M, et al. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom 2018; 87: 195–203. [DOI] [PubMed] [Google Scholar]
- 15. Hengartner MP, Davies J, Read J. Antidepressant withdrawal - the tide is finally turning. Epidemiol Psychiatr Sci 2019; 29: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massabki I, Abi-Jaoude E. Selective serotonin reuptake inhibitor ‘discontinuation syndrome’ or withdrawal. Br J Psychiatry. Epub ahead of print 6 January 2020. DOI: 10.1192/bjp.2019.269. [DOI] [PubMed] [Google Scholar]
- 17. Read J, Cartwright C, Gibson K. How many of 1829 antidepressant users report withdrawal effects or addiction? Int J Ment Health Nurs 2018; 27: 1805–1815. [DOI] [PubMed] [Google Scholar]
- 18. Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019; 6: 538–546. [DOI] [PubMed] [Google Scholar]
- 19. Cosci F, Chouinard G. Acute and persistent withdrawal syndromes following discontinuation of psychotropic medications. Psychother Psychosom 2020; 89: 283–306. [DOI] [PubMed] [Google Scholar]
- 20. Lerner A, Klein M. Dependence, withdrawal and rebound of CNS drugs: an update and regulatory considerations for new drugs development. Brain Commun 2019; 1: fcz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen M, Hansen EH, Gotzsche PC. What is the difference between dependence and withdrawal reactions? A comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction 2012; 107: 900–908. [DOI] [PubMed] [Google Scholar]
- 22. Baldessarini RJ, Tondo L. Effects of treatment discontinuation in clinical psychopharmacology. Psychother Psychosom 2019; 88: 65–70. [DOI] [PubMed] [Google Scholar]
- 23. Jha MK, Rush AJ, Trivedi MH. When discontinuing SSRI antidepressants is a challenge: management tips. Am J Psychiatry 2018; 175: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 24. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf 2001; 24: 183–197. [DOI] [PubMed] [Google Scholar]
- 25. Andrews PW, Thomson JA, Jr, Amstadter A, et al. Primum non nocere: an evolutionary analysis of whether antidepressants do more harm than good. Front Psychol 2012; 3: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom 2016; 85: 270–288. [DOI] [PubMed] [Google Scholar]
- 27. Hengartner MP. Methodological flaws, conflicts of interest, and scientific fallacies: implications for the evaluation of antidepressants’ efficacy and harm. Front Psychiatry 2017; 8: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haddad PM, Anderson IM. Recognising and managing antidepressant discontinuation symptoms. Adv Psychiatr Treat 2007; 13: 447–457. [Google Scholar]
- 29. Davies J, Read J, Hengartner MP, et al. Clinical guidelines on antidepressant withdrawal urgently need updating. BMJ 2019; 365: l2238. [DOI] [PubMed] [Google Scholar]
- 30. Fava GA, Cosci F. Understanding and managing withdrawal syndromes after discontinuation of antidepressant drugs. J Clin Psychiatry 2019; 80: 19com12794. [DOI] [PubMed] [Google Scholar]
- 31. Ashton H. Protracted withdrawal syndromes from benzodiazepines. J Subs Abuse Treat 1991; 8: 19–28. [DOI] [PubMed] [Google Scholar]
- 32. O’Brien C. Addiction and dependence in DSM-V. Addiction 2011; 106: 866–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): current concept and modern approaches to its management. Psychiatry Clin Neurosci 2015; 69: 321–334. [DOI] [PubMed] [Google Scholar]
- 34. Fava GA, Cosci F, Guidi J, et al. The deceptive manifestations of treatment resistance in depression: a new look at the problem. Psychother Psychosom 2020; 89: 265–273. [DOI] [PubMed] [Google Scholar]
- 35. Chouinard G, Chouinard VA. New classification of selective serotonin reuptake inhibitor withdrawal. Psychother Psychosom 2015; 84: 63–71. [DOI] [PubMed] [Google Scholar]
- 36. Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol 2013; 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fava GA, Bernardi M, Tomba E, et al. Effects of gradual discontinuation of selective serotonin reuptake inhibitors in panic disorder with agoraphobia. Int J Neuropsychopharmacol 2007; 10: 835–838. [DOI] [PubMed] [Google Scholar]
- 38. Belaise C, Gatti A, Chouinard VA, et al. Patient online report of selective serotonin reuptake inhibitor-induced persistent postwithdrawal anxiety and mood disorders. Psychother Psychosom 2012; 81: 386–388. [DOI] [PubMed] [Google Scholar]
- 39. Wilson E, Lader M. A review of the management of antidepressant discontinuation symptoms. Ther Adv Psychopharmacol 2015; 5: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry 1997; 58(Suppl. 7): 5–10. [PubMed] [Google Scholar]
- 41. Fava GA, Offidani E. The mechanisms of tolerance in antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1593–1602. [DOI] [PubMed] [Google Scholar]
- 42. Fava GA, Rafanelli C. Iatrogenic factors in psychopathology. Psychother Psychosom 2019; 88: 129–140. [DOI] [PubMed] [Google Scholar]
- 43. Ruhe HG, Horikx A, Van Avendonk MJP, et al. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry 2019; 6: 561–562. [DOI] [PubMed] [Google Scholar]
- 44. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract 2013; 26: 389–396. [DOI] [PubMed] [Google Scholar]
- 45. Fava GA, Belaise C. Discontinuing antidepressant drugs: lesson from a failed trial and extensive clinical experience. Psychother Psychosom 2018; 87: 257–267. [DOI] [PubMed] [Google Scholar]
- 46. Heilig M, Egli M, Crabbe JC, et al. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 2010; 15: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Becker HC. Alcohol dependence, withdrawal, and relapse. Alcohol Res Health 2008; 31: 348–361. [PMC free article] [PubMed] [Google Scholar]
- 48. Healy D. Post-SSRI sexual dysfunction & other enduring sexual dysfunctions. Epidemiol Psychiatr Sci 2019; 29: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bala A, Nguyen HMT, Hellstrom WJG. Post-SSRI sexual dysfunction: a literature review. Sex Med Rev 2018; 6: 29–34. [DOI] [PubMed] [Google Scholar]
- 50. Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 2009; 195: 211–217. [DOI] [PubMed] [Google Scholar]
- 51. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence 2016; 10: 1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cognetta-Rieke C, Guney S. Analytical insights from patient narratives: the next step for better patient experience. J Patient Exp 2014; 1: 20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greenhalgh T. Narrative based medicine: narrative based medicine in an evidence based world. BMJ 1999; 318: 323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koschke M, Boettger MK, Schulz S, et al. Autonomy of autonomic dysfunction in major depression. Psychosom Med 2009; 71: 852–860. [DOI] [PubMed] [Google Scholar]
- 55. Maslej MM, Bolker BM, Russell MJ, et al. The mortality and myocardial effects of antidepressants are moderated by preexisting cardiovascular disease: a meta-analysis. Psychother Psychosom 2017; 86: 268–282. [DOI] [PubMed] [Google Scholar]
- 56. Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses 2010; 74: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harvey BH, McEwen BS, Stein DJ. Neurobiology of antidepressant withdrawal: implications for the longitudinal outcome of depression. Biol Psychiatry 2003; 54: 1105–1117. [DOI] [PubMed] [Google Scholar]
- 58. Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry 1996; 153: 151–162. [DOI] [PubMed] [Google Scholar]
- 59. Haahr ME, Fisher PM, Jensen CG, et al. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry 2014; 19: 427–432. [DOI] [PubMed] [Google Scholar]
- 60. Bloch M, Stager SV, Braun AR, et al. Severe psychiatric symptoms associated with paroxetine withdrawal. Lancet 1995; 346: 57. [DOI] [PubMed] [Google Scholar]
- 61. Rocca E, Anjum RL. Causal evidence and dispositions in medicine and public health. Int J Environ Res Public Health 2020; 17: 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kessing LV, Hansen HV, Demyttenaere K, et al. Depressive and bipolar disorders: patients’ attitudes and beliefs towards depression and antidepressants. Psychol Med 2005; 35: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 63. Ostrow L, Jessell L, Hurd M, et al. Discontinuing psychiatric medications: a survey of long-term users. Psychiatr Serv 2017; 68: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 64. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res 2014; 216: 67–73. [DOI] [PubMed] [Google Scholar]
- 65. Stockmann T, Odegbaro D, Timimi S, et al. SSRI and SNRI withdrawal symptoms reported on an internet forum. Int J Risk Saf Med 2018; 29: 175–180. [DOI] [PubMed] [Google Scholar]
- 66. Taylor D, Stewart S, Connolly A. Antidepressant withdrawal symptoms-telephone calls to a national medication helpline. J Affect Disord 2006; 95: 129–133. [DOI] [PubMed] [Google Scholar]
- 67. van Geffen ECG, Brugman M, van Hulten R, et al. Patients’ concerns about and problems experienced with discontinuation of antidepressants. Int J Pharm Pract 2007; 15: 291–293. [Google Scholar]
- 68. Hengartner MP, Plöderl M. False beliefs in academic psychiatry: the case of antidepressant drugs. Ethical Hum Psychol Psychiatry 2018; 20: 6–16. [Google Scholar]
- 69. Nielsen M, Hansen EH, Gotzsche PC. Dependence and withdrawal reactions to benzodiazepines and selective serotonin reuptake inhibitors. How did the health authorities react? Int J Risk Saf Med 2013; 25: 155–168. [DOI] [PubMed] [Google Scholar]
- 70. Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol 2020; 10: 2045125320932452. [DOI] [PMC free article] [PubMed] [Google Scholar]