Abstract
The objective of the study was to examine the risk of other morbidities among patients with systemic lupus erythematosus (SLE). A total of 1006 adult new-onset SLE patients were identified during 1.1.2000- 31.12.2014 from the register of Social Insurance Institution. For each case three general population controls matched according to age, sex and place of residence at the index day were sampled from the population register. Both groups were followed up from the index date until the end of 2017 or until death. The national register on specialized care was explored to gather broadly their 12 organ-specific morbidities, which were found among 91.2% of SLE patients and 66.7% of comparators. The rate ratio (RR) was elevated in almost all disease groups. Musculoskeletal, cardiovascular and genitourinary conditions were the most common comorbidities with RRs of 1.82 (1.68 to 1.97), 1.91 (1.76 to 2.08) and 1.91 (1.73 to 2.09), respectively. Men with SLE had a significantly higher risk for diseases of the genitourinary system and endocrine, nutritional and metabolic diseases compared to women with SLE. The risk of concurrent morbidities is essential to note in the care of SLE patients.
Keywords: Systemic lupus erythematosus, morbidity, cardiovascular disease, gender, comorbidity
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that can affect almost all organs and tissues. The clinical picture can vary greatly, and it is influenced by gender, age, ethnicity, residence and medication. Due to the heterogeneity of the disease, symptoms can be diverse. Most patients are female.1–3
Patients diagnosed with SLE have a considerable burden due to multi-organic involvement of the disease and the treatment chosen for it.4,5 Previous studies have shown a significant risk for various diseases, such as cardiovascular diseases (CVDs), renal diseases, psychiatric disorders, infections and osteoporosis. For example, the risk for myocardial infarct has been estimated to be 2 to 9 times higher in SLE patients than in the general population.6–11
The European League Against Rheumatism (EULAR) recommendations from the year 2008 advise to carefully monitor certain comorbidities, and in recent years, the presence of concurrent disorders in SLE has slowly gained more attention among health care professionals.12 Despite the recent publicity, reports on the occurrence of concomitant diseases among patients with SLE are far from ample, and only a few of them depict the wide spectrum of the comorbidities. In this study, we examine broadly the relations between SLE and concurrent diseases.
Patients and methods
In Finland every resident has national health insurance, and the Social Insurance Institution (SII) keeps a register of them. Patients with chronic inflammatory rheumatic disorders are entitled to a special (higher than basic) reimbursement for the cost of anti-rheumatic drugs. Identification of SLE patients was based on new special reimbursement decisions with the 10th International Classification of Diseases code (ICD-10) of M32 in the register of SII during 1.1.2000 – 31.12.2014. The date of acceptance of reimbursement was defined as the date of diagnosis (index date). For every SLE patient, three individually matched (age, gender and residence at the index date) population controls were selected from the Population Register Centre. Only adults (age >17 years) were included. Rate ratios (RR) were standardized by education level at baseline (basic, middle, lower high and upper high level), and information about education level was acquired from Statistics Finland.
The Finnish law on personal register obligates the service providers to produce information to the Care Register of the National Institute for Health and Welfare (NIHW). The Care Register covers all hospitalizations since 1969. Outpatient visits in specialized care are included since 1998. The data contains, among others, each patient's personal identification code (PIC) and diagnoses of medical disorders according to the codes of the ICD-10.
We retrieved data on 12 organ-specific disease groups and examined some subgroups of special interest as well (Table 1). Systemic connective tissue disorders M30-M36 were excluded from the study and only disease groups of M00-M25 and M40-M99 were included to study from the group of the diseases of the musculoskeletal system and connective tissue. Infectious diseases were not included in this study because the diagnoses made in primary health care would have been missed.
Table 1.
The number of comorbidities in systemic lupus erythematosus patients and population controls and the rate ratio during 2000–2017 according to 10th revision of the International Classification of Diseases codes.
| ICD-10 disease codes | SLE patientsN = 1006 | ControlsN = 3005 | RRa (95% CI) | P-valueb | |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Malignant neoplasms | C00-D09 | 117 (11.6) | 268 (8.9) | 1.29 (1.05 to 1.59) | 0.057 |
| Bening neoplasms | D10-D49 | 201 (20.0) | 356 (11.8) | 1.68 (1.44 to 1.97) | <0.001 |
| Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | D50-D89 | 178 (17.7) | 103 (3.4) | 5.15 (4.08 to 6.49) | <0.001 |
| Endocrine, nutritional and metabolic diseases | E00-E90 | 254 (25.2) | 388 (12.9) | 1.90 (1.65 to 2.18) | <0.001 |
| Disorders of thyroid gland | E00-E07 | 101 (10.0) | 120 (4.0) | 2.49 (1.93 to 3.22) | <0.001 |
| Other hypothyroidism | E03 | 80 (8.0) | 70 (2.3) | 3.39 (2.48 to 4.63) | <0.001 |
| Diabetes mellitus | E10-E14 | 83 (8.3) | 138 (4.6) | 1.74 (1.34 to 2.27) | <0.001 |
| Disorders of lipoprotein metabolism and other lipidemias | E78 | 53 (5.3) | 98 (3.3) | 1.56 (1.13 to 2.16) | 0.036 |
| Mental and behavioral diseases | F00-F99 | 199 (19.8) | 399 (13.3) | 1.46 (1.25 to 1.70) | <0.001 |
| Dementia in Alzheimer`s disease, vascular dementia, dementia in other diseases classified elsewhere and unspecified dementia | F00-F03 | 24 (2.4) | 72 (2.4) | 0.96 (0.61 to 1.52) | 0.88 |
| Schizophrenia, schizotypal and delusional disorders | F20-F29 | 15 (1.5) | 40 (1.3) | 1.07 (0.60 to 1.93) | 0.82 |
| Mood (affective) disorders | F30-F39 | 102 (10.1) | 177 (5.9) | 1.71 (1.36 to 2.16) | <0.001 |
| Diseases of the nervous system | G00-G99 | 313 (31.1) | 511 (17.0) | 1.78 (1.58 to 2.01) | <0.001 |
| Other degenerative diseases of the nervous system | G30-G32 | 16 (1.6) | 79 (2.6) | 0.58 (0.34 to 0.99) | 0.14 |
| Epilepsy and status epilepticus | G40-G41 | 33 (3.3) | 34 (1.1) | 2.88 (1.79 to 4.63) | <0.001 |
| Diseases of the eye and adnexa | H00-H59 | 322 (32.0) | 499 (16.6) | 1.88 (1.67 to 2.12) | <0.001 |
| Iridocyclitis | H20 | 18 (1.8) | 21 (0.7) | 2.62 (1.40 to 4.90) | 0.015 |
| Diseases of the circulatory system | I00-I99 | 511 (50.8) | 761 (25.3) | 1.91 (1.76 to 2.08) | <0.001 |
| Hypertensive diseases | I10-I15 | 237 (23.6) | 351 (11.7) | 1.93 (1.67 to 2.24) | <0.001 |
| Ischemic heart diseases | I20-I25 | 100 (9.9) | 177 (5.9) | 1.62 (1.29 to 2.04) | <0.001 |
| Cerebrovascular diseases | I60-I69 | 78 (7.8) | 117 (3.9) | 1.92 (1.46 to 2.53) | <0.001 |
| Other chronic obstructive pulmonary disease, asthma and status asthmaticus | J44-J46 | 112 (11.1) | 142 (4.7) | 2.32 (1.83 to 2.94) | <0.001 |
| Noninfective enteritis and colitis | K50-K52 | 28 (2.8) | 42 (1.4) | 2.02 (1.26 to 3.24) | 0.021 |
| Disease of the musculoskeletal system and connective tissue | M00-M99c | 532 (52.9) | 863 (28.7) | 1.82 (1.68 to 1.97) | <0.001 |
| Osteoporosis with pathological fracture and osteoporosis without pathological fracture | M80-M81 | 61 (6.1) | 35 (1.2) | 5.08 (3.38 to 7.64) | <0.001 |
| Diseases of the genitourinary system | N00-N99 | 456 (45.3) | 708 (23.6) | 1.91 (1.73 to 2.09) | <0.001 |
| Renal tubulointerstitial diseases | N00-N16 | 197 (19.6) | 94 (3.1) | 6.15 (4.86 to 7.78) | <0.001 |
| Renal failure | N17-N19 | 53 (5.3) | 34 (1.1) | 4.53 (2.96 to 6.92) | <0.001 |
aAdjusted for education level.
bThe significance were correct for multiplicity using Hommel’s multiple comparison procedure.
cExcluding systemic connective tissue disorders M30–M36; ICD-10 code = 10th International Classification of Diseases code; SLE=Systemic lupus erythematosus; RR = Rate ratio.
The follow-up started from the index date of the each SLE patient and ended when the patient died or at end of the year 2017, whichever occurred first. Permission to use the databases were obtained from the SII and the NIHW. By Finnish law, no approval of an ethical committee nor the patient's informed consent are required for register-based studies done without contacting study subjects.
Statistical methods
Data are presented as means with standard deviation (SD) and as counts with percentages. Adjusted RRs of comorbidities were calculated using generalized linear models with log link and binomial distribution. Penalized maximum likelihood logistic regression (Firthlogit) was used if the event of interest was rare. Models included education level as a covariate. A possible nonlinear relationship between age at the index day and RR for cardiovascular diseases was assessed by using four-knot-restricted generalized linear models. The length of the distribution (age at the index day) of knots was located at the 5th, 35th, 65th, and 95th percentiles. Knot locations were based on Harrell's recommended percentiles.13 Hommel’s adjustment was used to correct levels of significance for multiple testing, because it is more powerful than alternative procedures, including the Bonferroni, Holm's, and Hochberg's procedures.13 Stata 16.1 (StataCorp LP; College Station, Texas, USA) statistical package was used for the analyses.
Results
A total of 1006 patients with newly-diagnosed SLE (mean age 45.5 years, SD 16 years, females 84,0%) and 3005 controls were included. The females were younger than the males: 44.9 years (SD 15.9 years) and 48.6 years (SD 16.4 years), respectively. The cumulative follow-up time was 8631 person years in SLE patients and 26382 person years in controls, with a mean follow-up of 8.6 years and 8.8 years, respectively.
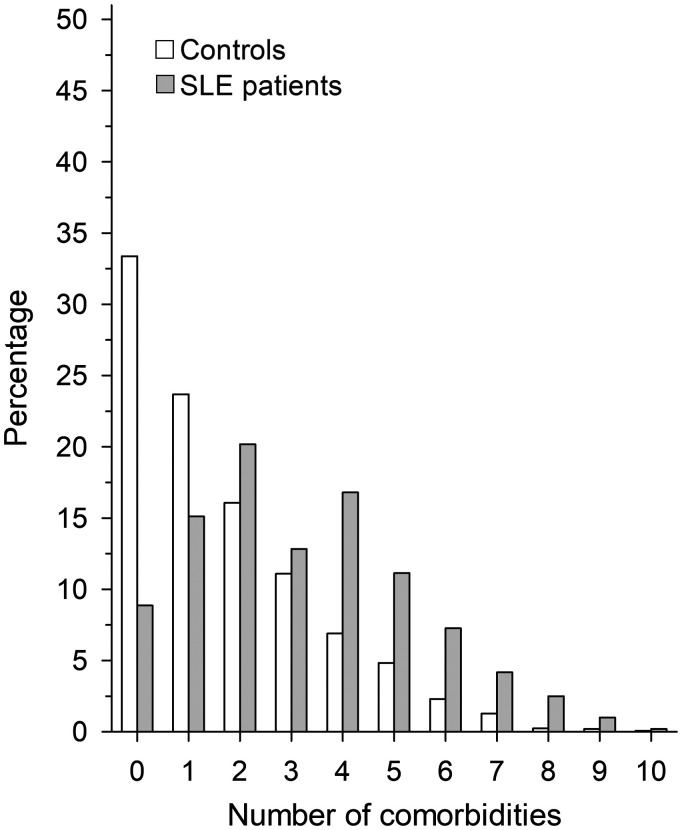
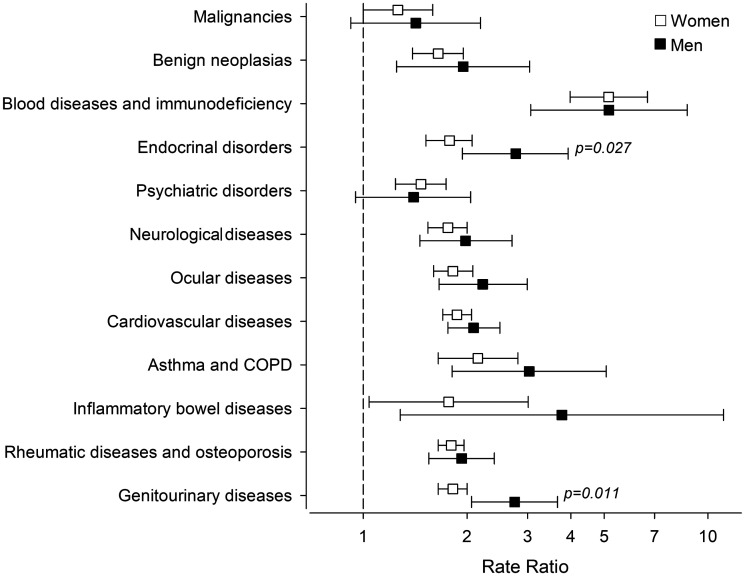
Morbidities of interest were found among 91.2% of SLE patients and among 66.7% of comparators. Musculoskeletal, cardiovascular and genitourinary conditions were the three most common comorbidities in both groups. Number of comorbid conditions per individual was higher among SLE patients (Figure 1). Table 1 displays the numbers of the selected comorbid diseases and the respective RRs. Compared to the general population, SLE patients had elevated RRs for most of the diseases studied. Only schizophrenia, dementia, degenerative diseases of the nervous system and malignant neoplasms were not more frequent in the patient population. Men with SLE had a higher risk for diseases of the genitourinary system and endocrine, nutritional and metabolic diseases (Figure 2). After controlling confounders, no difference was found between genders in the number of comorbidities: men with SLE 3.5 (95% CI: 3.1 to 3.8) and women with SLE 3.2 (95% CI: 3.0 to 3.3); p = 0.10.
Figure 1.
Cumulative number of comorbidities among new-onset systemic lupus erythematosus patients diagnosed between 2000–2014 and their controls at the end of the follow-up 2000–2017. Infections and systemic connective tissue diseases are not included.
Figure 2.
Education level adjusted rate ratios of comorbid diseases of systemic lupus erythematosus patients and controls by gender during 2000–2017 and according to 10th revision of the International Classification of Diseases codes.
Malignancies = malignant neoplasms C00-D09, Benign neoplasias = benign neoplasms D10–D49, Blood diseases and immune deficiency = disease of the blood and blood-forming organs and certain disorders involving the immune mechanism D50–D89, Endocrinal diseases = endocrine, nutritional and metabolic diseases E00-E90, Psychiatric disorders = mental and behavioural diseases F00–F99, Neurological diseases = diseases of the nervous system G00-G99, Ocular diseases = diseases of the eye and adnexa H00–H59, Cardiovascular diseases = diseases of the circulatory system I00-I99, Asthma and COPD = other chronic obstructive pulmonary disease, asthma and status asthmaticus J44–J46, Inflammatory bowel diseases = noninfective enteritis and colitis K50–K52, Rheumatic diseases and osteoporosis = disease of the musculoskeletal system and connective tissue M00–M99 (excluding systemic connective tissue disorders M30–M36), Genitourinary diseases = diseases of the genitourinary system N00–N99.
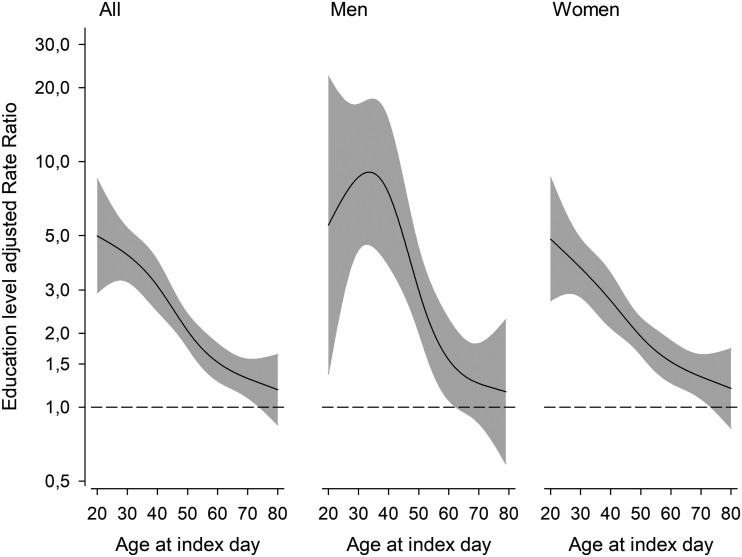
The relative risk of CVDs depended on age. The RR was highest among patients, who were diagnosed with SLE in the young age groups (Figure 3).
Figure 3.
Education level adjusted rate ratios of cardiovascular diseases between systemic lupus erythematosus patients and controls during 2000–2014 according to age at index day. The curves were derived from a four-knot-restricted cubic splines generalized linear models. The models were adjusted for education levels. The grey area represents a 95% confidence interval.
Discussion
In our study, the rate of morbidities was higher in Finnish patients with newly diagnosed SLE than their population controls. Moreover, the number of morbidities per individual was higher.
Our findings are mostly in line with previous research, although some variation in morbidities has been reported depending on study design and ethnicity.14–16 In a case-control study from UK SLE patients were compared using general practice records. The risk of comorbidities was elevated in almost all disease groups studied, but especially renal diseases and CVDs were more frequent, as it was in our cohort.14 In a South African case-control study including mostly young black women, approximately 80% of SLE patients had more than one comorbidity after a six-year follow-up. However, CVDs, except hypertension, were rare compared to our study or studies performed in other industrial countries.11,15,17
SLE itself predisposes to CVDs due to endothelial dysfunction.18,19 Again, metabolic syndrome is more frequent in SLE patients, contributing to the CVD burden.20 In our study the relative CVD burden was substantial in young men, but young women had increased RR for CVDs as well. With advancing age the CVD risk approached but did not reach that of the general population. This age-related relative risk has also been reported from the UK.6
Kidney disease in SLE patients is mostly due to lupus nephritis (LN). The prevalence of LN varies depending on ethnicity, and LN is more common among non-white people.21,22 Male SLE patients seem to have a greater risk for kidney diseases, and end stage renal disease and renal failure are common among SLE patients.23,24 Our study results were in line with the aforementioned, although RRs were not so high. Hydroxychloroquinine has been shown to decrease prevalence of chronic kidney disease, and at least in Finland it is often used as the primary medicine in SLE treatment.25,26 Another explanation for our result may be that almost all of our patients were native Finnish.
SLE affects nervous system inducing neuropsychiatric disorders (neuropsychiatric SLE, NPSLE).5,27–33 Mood disorders, and especially depression, are considered to be one of the most prevalent neuropsychiatric comorbidities in SLE.5,34 Our study results support the aforementioned even though a considerable number of the mild cases are treated in the primary health care and never reach specialized care. However, we found no significant RR for Alzheimer’s disease, vascular dementia, dementia in other diseases classified elsewhere and unspecified dementia or other degenerative diseases of the nervous system.
We found a definite risk for osteoporosis. SLE patients have been shown to be prone to osteoporosis due to systemic inflammation and glucocorticoid treatment.4 In addition, vulnerability to osteoporosis may result from sensitiveness to sunlight, lupus nephritis and low D-vitamin levels.35,36 SLE patients may also be screened for osteoporosis and followed more carefully than other populations.37
According to our study, there is a positive correlation between SLE and obstructive pulmonary disease. However, we could not differentiate between asthma and chronic obstructive lung disease (COPD). Conflicting results have been published about the risk of these conditions, and the matter needs more investigation.38–42
Men with SLE tend to have more severe disease course than women, at least among white and African-American patients followed in the Hopkins Lupus Cohort. Men more often had cardiovascular, renal and hematological manifestations, whereas women experienced more malar rash, photosensitivity, alopecia, oral ulcers and arthralgia at the end of the follow-up.43 In our study, no difference was found between genders in the number of comorbidities, but men with SLE had a higher risk in genitourinary and in endocrine and metabolic diseases.
It is not clear, why SLE seems to be harsher in males. One possible explanation is that men might seek medical care later, which could delay the diagnosis and the start of proper treatment.
A weakness of this register-based study is the lack of clinical data. Therefore, we could not determine the severity of SLE and evaluate whether a harsher disease course was related to a higher morbidity rate. A major limitation in this study is the lack of infectious diseases. In addition, some diagnoses made by general practitioners may be missing. Many SLE patients are regularly monitored in rheumatology outpatient clinics and are more prone to be diagnosed with morbidities than individuals in the general population.
The strengths of this study are the relatively long follow-up time, the case-control study design and the linkage of extensive nationwide information from different official registers. In addition, the diagnoses of morbidities were made in specialized care, strengthening the reliability of the diagnosis. Our study consisted of only new-onset SLE patients and their morbidities manifesting in the following years. This nationwide study included practically all patients using medication for SLE, but some mild forms of the disease without need for disease-modifying anti-rheumatic drugs might have been left out.
In conclusion, our study shows that SLE patients have a considerable burden of various morbidities. Particularly, CVDs are more frequent in SLE patients than in the rest of the population, but vulnerability to other morbidities is also notable.
Acknowledgements
The authors wish to thank Dr. David Laaksonen from Kuopio University Hospital for help with the language revision.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the Finnish Rheumatic Disease Research Foundation and the Maire Lisko Foundation.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
S Kariniemi https://orcid.org/0000-0001-8355-0531
P Elfving https://orcid.org/0000-0002-9885-7242
References
- 1.Tamirou F, Arnaud L, Talarico R, et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open 2019; 4: e000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz N, Goilav B, Putterman C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol 2014; 26: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervera R, Abarca-Costalago M, Abramovicz D, et al. ; European Working Party on Systemic Lupus Erythematosus. Systemic lupus erythematosus in Europe at the change of the millennium: lessons from the “Euro-Lupus project”. Autoimmun Rev 2006; 5: 180–186. [DOI] [PubMed] [Google Scholar]
- 4.Sarkissian A, Sivaraman V, Bout-Tabaku S, et al. Bone turnover markers in relation to vitamin D status and disease activity in adults with systemic lupus erythematosus. Lupus 2019; 28: 156–162. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Magder LS, Petri M. Predictors of incident depression in systemic lupus erythematosus. J Rheumatol 2014; 41: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 6.Rees F, Doherty M, Grainge M, Lanyon P, Davenport G, Zhang W. Burden of comorbidity in systemic lupus erythematosus in the UK, 1999-2012. Arthritis Care Res (Hoboken) 2016; 68: 819–827. [DOI] [PubMed] [Google Scholar]
- 7.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses's health study’. Arthritis Rheum 2009; 61: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson H, Nived O, Sturfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine (Baltimore) 1989; 68: 141–150. [PubMed] [Google Scholar]
- 9.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum 1999; 42: 338–346. [DOI] [PubMed] [Google Scholar]
- 10.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol 2009; 38: 362–368. [DOI] [PubMed] [Google Scholar]
- 11.Bengtsson C, Ohman ML, Nived O, Rantapää-Dahlqvist S. Cardiovascular event in systemic lupus erythematosus in Northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 2012; 21: 452–459. [DOI] [PubMed] [Google Scholar]
- 12.Bertsias G, Ioannidis JPA, Boletis J, et al. ; Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis 2008; 67: 195–205. [DOI] [PubMed] [Google Scholar]
- 13.Wright SP. Adjusted p-values for simultaneous inference. Biometrics 1992; 48: 1005–1013. [Google Scholar]
- 14.Kuo CF, Chou IJ, Rees F, et al. Temporal relationships between systemic lupus erythematosus and comorbidities. Rheumatology (Oxford) 2019; 58: 840–848. [DOI] [PubMed] [Google Scholar]
- 15.Greenstein L, Makan K, Tikly M. Burden of comorbidities in South Africans with systemic lupus erythematosus. Clin Rheumatol 2019; 38: 2077–2082. [DOI] [PubMed] [Google Scholar]
- 16.Fernández M, Alarcón GS, Calvo-Alén J, et al. ; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57: 576–584. [DOI] [PubMed] [Google Scholar]
- 17.Thorburn CM, Ward MM. Hospitalizations for coronary artery disease among patients with systemic lupus erythematosus. Arthritis Rheum 2003; 48: 2519–2523. [DOI] [PubMed] [Google Scholar]
- 18.Sciatti E, Cavazzana I, Vizzardi E, et al. Systemic lupus erythematosus and endothelial dysfunction: a close relationship. Curr Rheumatol Rev 2019; 15: 177–188. [DOI] [PubMed] [Google Scholar]
- 19.Lim SY, Bae EH, Han KD, et al. Systemic lupus erythematosus is a risk factor for cardiovascular disease: a nationwide, population-based study in Korea. Lupus 2018; 27: 2050–2056. [DOI] [PubMed] [Google Scholar]
- 20.Parker B, Urowitz MB, Gladman DD, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis 2013; 72: 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seligman VA, Lum RF, Olson JL, Hongzhe L, Criswell LA. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med 2002; 112: 726–729. [DOI] [PubMed] [Google Scholar]
- 22.Patel M, Clarke AM, Bruce IN, Symmons DPM. The prevalence and incidence of biopsy-proven lupus nephritis in the UK: Evidence of an ethnic gradient. Arthritis Rheum 2006; 54: 2963–2969. [DOI] [PubMed] [Google Scholar]
- 23.Crosslin KL, Wiginton KL. Sex differences in disease severity among patients with systemic lupus erythematosus. Gend Med 2011; 8: 365–371. [DOI] [PubMed] [Google Scholar]
- 24.Reppe Moe SE, Molberg Ø, Strøm EH, Lerang K. Assessing the relative impact of lupus nephritis on mortality in a population-based systemic lupus erythematosus cohort. Lupus 2019; 28: 818–825. [DOI] [PubMed] [Google Scholar]
- 25.Pokroy-Shapira E, Gelernter I, Molad Y. Evolution of chronic kidney disease in patients with systemic lupus erythematosus over a long-period follow-up: a single-center inception cohort study. Clin Rheumatol 2014; 33: 649–657. [DOI] [PubMed] [Google Scholar]
- 26.Elfving P, Puolakka K, Kautiainen H, Virta LJ, Pohjolainen T, Kaipiainen-Seppänen O. Drugs used in incident systemic lupus erythematosus – results from the Finnish nationwide register 2000-2007. Lupus 2016; 25: 666–670. [DOI] [PubMed] [Google Scholar]
- 27.Hesselvig JH, Egeberg A, Kofoed K, Gislason G, Dreyer L. Increased risk of depression in patients with cutaneous lupus erythematosus and systemic lupus erythematosus: a Danish nationwide cohort study. Br J Dermatol 2018; 179: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 28.Tisseverasinghe A, Peschken C, Hitchon C. Anxiety and mood disorders in systemic lupus erythematosus: Current insights and future directions. Curr Rheumatol Rep 2018; 20: 85. [DOI] [PubMed] [Google Scholar]
- 29.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol 2003; 30: 985–992. [PubMed] [Google Scholar]
- 30.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol 2004; 31: 2156–2162. [PubMed] [Google Scholar]
- 31. The American college of rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. ACR ad hoc committee on neuropsychiatric lupus nomenclature. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed] [Google Scholar]
- 32.Lin YR, Chou LC, Chen HC, Liou TH, Huang SW, Lin HW. Increased risk of dementia in patients with systemic lupus erythematosus: a nationwide population‐based cohort study. Arthritis Care Res (Hoboken) 2016; 68: 1774–1779. [DOI] [PubMed] [Google Scholar]
- 33.Gendelman O, Tiosano S, Shoenfeld Y, et al. High proportions of dementia among SLE patients: a big data analysis. Int J Geriatr Psychiatry 2018; 33: 531–536. [DOI] [PubMed] [Google Scholar]
- 34.Hanly JG, Su L, Urowitz MB, et al. Mood disorders in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Rheumatol 2015; 67: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol 1988; 124: 1802–1804. [PubMed] [Google Scholar]
- 36.Bultink IE. Osteoporosis and fractures in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012; 64: 2–8. [DOI] [PubMed] [Google Scholar]
- 37.Felten R, Sagez F, Gavand PE, et al. 10 Most important contemporary challenges in the management of SLE. Lupus Sci Med 2019; 6: e000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 2010; 34: 258–265. [DOI] [PubMed] [Google Scholar]
- 39.Bieber V, Cohen AD, Freud T, Agmon-Levin N, Gertel S, Amital H. Autoimmune smoke and fire–coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross-sectional analysis. Immunol Res 2013; 56: 261–266. [DOI] [PubMed] [Google Scholar]
- 40.Shen TC, Lin CL, Chen CH, et al. Increased risk of chronic obstructive pulmonary disease in patients with systemic lupus erythematosus: a population-based cohort study. PLoS One 2014; 9: e91821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen TC, Tu CY, Lin CL, Wei CC, Li YF. Increased risk of asthma in patients with systemic lupus erythematosus. Am J Respir Crit Care Med 2014; 189: 496–499. [DOI] [PubMed] [Google Scholar]
- 42.Sekigawa I, Yoshiike T, Iida N, Hashimoto H, Ogawa H. Allergic disorders in systemic lupus erythematosus: prevalence and family history. Lupus 2002; 11: 426–429. [DOI] [PubMed] [Google Scholar]
- 43.Tan TC, Fang H, Magder LS, Petri MA. Differences between male and female systemic lupus erythematosus in a multiethnic population. J Rheumatol 2012; 39: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]