Figure 2.
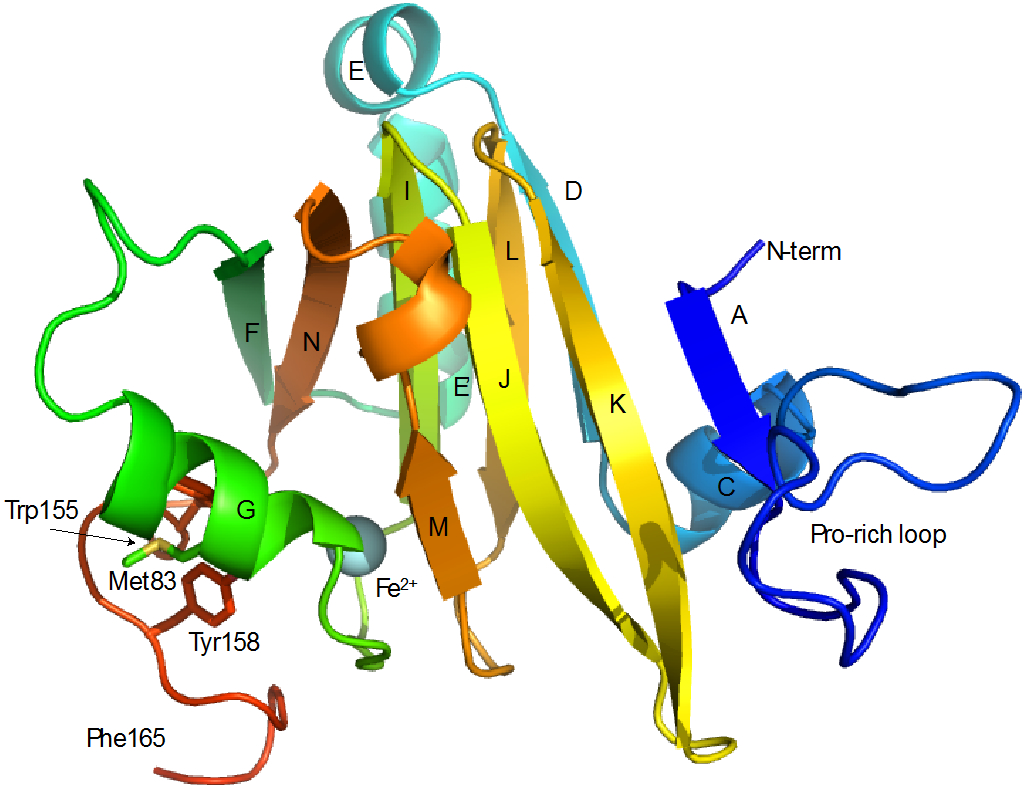
Secondary structure in Fe-HsARD. A: Ala 4-Tyr 6, C: Leu 28-Leu 34, D: Val 36-Leu 41, E: Ala 43-Asn 48, E’: Pro 50-Arg 59, F: Met 64-Ile 69, G: Tyr 77-Tyr 85, I: Glu 94-Asp 100, J: Ser 102-Asp 109, K: Asp 112-Met 119, L: Gly 122-Leu 127, M: Arg 134-Thr 136, N: Tyr 142-Leu 148. Secondary structures are labeled for direct comparison with Ni-KoARD (Figure 11). C-terminal residues (Glu 166-Ala 179) are not shown, as they are disordered in solution. Also indicated are positions of the proline-rich loop (Met7-Pro25) Met83, Trp155 and Tyr158 that are discussed in the text.
