Abstract
Objective:
Preterm labor and birth are strongly associated with sterile intra-amniotic inflammation, a clinical condition that is proposed to be initiated by danger signals or alarmins. The aim of this study was to investigate whether the intra-amniotic administration of the alarmin heat shock protein 70 (HSP70) induces preterm labor/birth and adverse neonatal outcomes.
Methods:
Pregnant mice received an intra-amniotic injection of 200 ng (n = 8), 400 ng (n = 6), 500 ng (n = 10) or 1 μg of HSP70 (n = 6). Control mice were injected with saline (n = 10). Following injection, the rate of preterm labor/birth and neonatal mortality were recorded. Neonatal weights at weeks 1, 2, and 3 were also recorded.
Results:
The intra-amniotic injection of 400 ng [late preterm birth 16.7 ± 16.7% (1/6)], 500 ng [early and late preterm birth 10 ± 10% (1/10) each], or 1 μg [early preterm birth 16.7 ± 16.7% (1/6)] of HSP70 induced low rates of preterm/birth. However, the intra-amniotic injection of 500 ng or 1 μg of HSP70 induced significantly higher rates of neonatal mortality compared to controls [saline 14.2% (10/74), 200 ng 9.8% (6/61), 400 ng 17.9% (9/45), 500 ng 28.8% (23/78), and 1 μg 21.4% (13/49)]. Neonates born to dams injected with 200 ng, 500 ng, or 1 μg HSP70 were leaner than controls (p ≤ 0.05).
Conclusion:
Intra-amniotic administration of the alarmin HSP70 did not induce high rates of preterm labor/birth; yet, it did indeed result in adverse neonatal outcomes.
Keywords: Acute histologic chorioamnionitis, sterile intra-amniotic inflammation, DAMPs, inflammation, parturition, pregnancy, prematurity, funisitis, fetal inflammatory response, amniotic fluid, mouse
Introduction
Preterm birth, a leading cause of perinatal morbidity and mortality worldwide [1, 2, 3, 4], is preceded by spontaneous preterm labor, a syndrome arising from multiple distinct etiologies [5]. Currently, the only established causal link to preterm labor is intra-amniotic infection and inflammation [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]. The majority of women who undergo spontaneous preterm labor with intra-amniotic inflammation do not have detectable microorganisms in amniotic fluid (using both cultivation and molecular microbiology techniques), referred to as sterile intra-amniotic inflammation [18, 19, 20, 21, 22]. This clinical condition is also frequently observed in women with a short cervix [23] and those with clinical chorioamnionitis at term [24]. Patients with preterm labor and sterile intra-amniotic inflammation present similar rates of preterm birth and adverse neonatal outcomes compared to those with intra-amniotic infection [19], highlighting the importance of identifying the initiators of sterile intra-amniotic inflammation, as well as the mechanisms implicated in this clinical condition.
The process of sterile inflammation is initiated by endogenous danger signals, termed damage-associated molecular patterns (DAMPs) [25, 26], or alarmins [27] derived from damaged and necrotic cells. Multiple alarmins are elevated in amniotic fluid of women with preterm labor and intra-amniotic inflammation [28], including interleukin (IL)-1α [29], high-mobility group box-1 (HMGB1) [30], S100B [31], and heat shock protein 70 (HSP70) [32]. Indeed, the administration of IL-1α [33, 34], HMGB1 [35], or S100B [36] induces preterm birth in mice, providing further evidence that alarmins are implicated in the mechanisms that lead to preterm parturition in the context of sterile intra-amniotic inflammation. Yet, whether the exogenous administration of HSP70 can also induce preterm birth has not been shown.
Heat shock proteins (HSPs) are highly conserved molecules [37, 38] that function as molecular chaperones [39, 40, 41, 42] to maintain cellular homeostasis. They are found in almost all subcellular compartments of all cell types and are upregulated in response to a range of physiological, pathological, and environmental stressors including hormonal stimulation, hypoxia, ischemia, and high temperature [43, 44, 45]. Moreover, HSPs participate in innate and adaptive immune responses and their extracellular presence reflects tissue damage or “danger signals” [46]. It has been proposed that extracellular HSPs, released either through non-classical pathways or from necrotic cells [47, 48], act like alarmins, activating monocytes [49, 50, 51] and inducing the secretion of pro-inflammatory cytokines [52, 53, 54]. Specifically, HSP70 concentrations are increased in amniotic fluid of women with spontaneous preterm labor/birth and microbial invasion of the amniotic cavity (MIAC) (i.e. intra-amniotic infection) [32]. In the current study, we investigated whether the ultrasound-guided intra-amniotic administration of HSP70 induces preterm labor/birth and adverse neonatal outcomes.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Sacramento, CA) and bred in the animal care facility at C.S. Mott Center for Human Growth and Development at Wayne State University (Detroit, MI). All mice were kept under a circadian cycle (light:dark = 12:12 h). Females, 8-12 weeks old, were bred with males of proven fertility. Female mice were checked daily between 8:00 a.m. and 9:00 a.m. for the appearance of a vaginal plug, which indicated 0.5 days post coitum (dpc). Females were then housed separately from the males, their weights were monitored daily, and a gain of two or more grams by 12.5 dpc confirmed pregnancy. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Wayne State University (Protocol No. A 07-03-15 and 18-03-0584).
Intra-amniotic administration of HSP70
Pregnant C57BL/6 mice were anesthetized on 16.5 dpc by inhalation of 2-3% isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL, USA) and 1-2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.75-2% isoflurane and 2.0 L/min of oxygen during the ultrasound procedure, which was performed using the Vevo® 2100 Imaging System (VisualSonics Inc., Toronto, Ontario, Canada). Mice were positioned on a heating pad and stabilized with adhesive tape. Fur removal from the abdomen was accomplished by applying Nair depilatory cream (Church & Dwight Co., Inc., Ewing, NJ, USA) to that area. Body temperature was maintained at 37±1°C and detected using a rectal probe (VisualSonics Inc.). Respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe was anchored and mobilized with a mechanical holder, and the transducer was slowly moved toward the Aquasonic CLEAR® ultrasound gel (Parker Laboratories, Inc, Fairfield, NJ, USA) applied on the abdomen. Ultrasound-guided intra-amniotic injection of recombinant human heat shock protein 70 (HSP70; abcam, Cambridge, UK) at concentrations of 200 ng, 400 ng, 500 ng or 1 μg/25 μL of sterile 1X phosphate-buffered saline (PBS; Fisher Scientific Bioreagents, Fair Lawn, NJ, USA) was performed in each amniotic sac using a 30-gauge needle (BD PrecisionGlide Needle, Becton Dickinson, Franklin Lakes, NJ, USA) (n = 6-10). Controls were injected with 25 μL of PBS (n = 10). The syringe was stabilized with a mechanical holder (VisualSonics Inc.). Following the ultrasound, mice were placed under a heat lamp until they regained full motor function, which occurred 5-10 min after heating.
Video monitoring of pregnancy outcomes
Immediately after intra-amniotic injection of HSP70 or PBS, dams were monitored until delivery using a video camera and infrared light (Sony Corporation, Tokyo, Japan). Gestational length was defined as the time elapsed from the detection of the vaginal plug (0.5 dpc) through the delivery of the first pup. Preterm labor/birth was defined as delivery occurring before 18.5 dpc, and its rate was represented by the percentage of females delivering preterm among the total number of mice. We classified preterm birth in two distinct groups; early preterm birth which was defined as delivery before day 18.0 of gestation, and late preterm birth which was defined as delivery between day 18.0 and 18.5 of gestation. The rate of neonatal mortality for each litter was defined as the proportion of delivered pups found dead among the total litter size. Neonatal weights at weeks 1, 2, and 3 were also recorded.
Statistical analysis
Data analysis was completed using SPSS version 19.0 software (IBM Corp, Armonk, NY, USA). Differences in the rate of preterm birth between the control group (i.e. PBS) and study groups, as well as the rate of pup mortality at birth, were analyzed using the Fisher’s exact test. The neonatal weights at weeks 1, 2, and 3 were compared between control and study groups using the Mann-Whitney U test. A p-value ≤0.05 was regarded as statistically significant for all tests.
Results
Intra-amniotic administration of high concentrations of HSP70 induces low rates of preterm birth
Pathophysiological concentrations of HSP70 in the amniotic fluid range from 80 ng to 500 ng [32]. First, we intra-amniotically injected pregnant mice with elevated HSP70 concentrations ranging from 200 ng to 500 ng (Figure 1A). Dams injected with PBS (controls) or 200 ng of HSP70 delivered at term (Figure 1B). However, a small proportion of dams injected with 400 ng of HSP70 delivered late preterm [16.7 ± 16.7% (1/6)] (Figure 1B). Higher concentrations of HSP70 (500 ng) induced both early and late preterm birth [10 ± 10% (1/10) each] (Figure 1B); yet, the effect was still minimal. Given that we have previously shown that mice require double the pathological amniotic fluid concentration of LPS [55] and S100B [31] to deliver preterm [36, 56], we decided to double the concentration of HSP70 to 1 μg. However, the intra-amniotic injection of 1 μg of HSP70 also induced low rates of early preterm birth [16.7 ± 16.7% (1/6)] (Figure 1B). These results show that the intra-amniotic administration of the alarmin HSP70 induces low rates of preterm birth, even at supraphysiological concentrations.
Figure 1. Intra-amniotic administration of the alarmin HSP70.
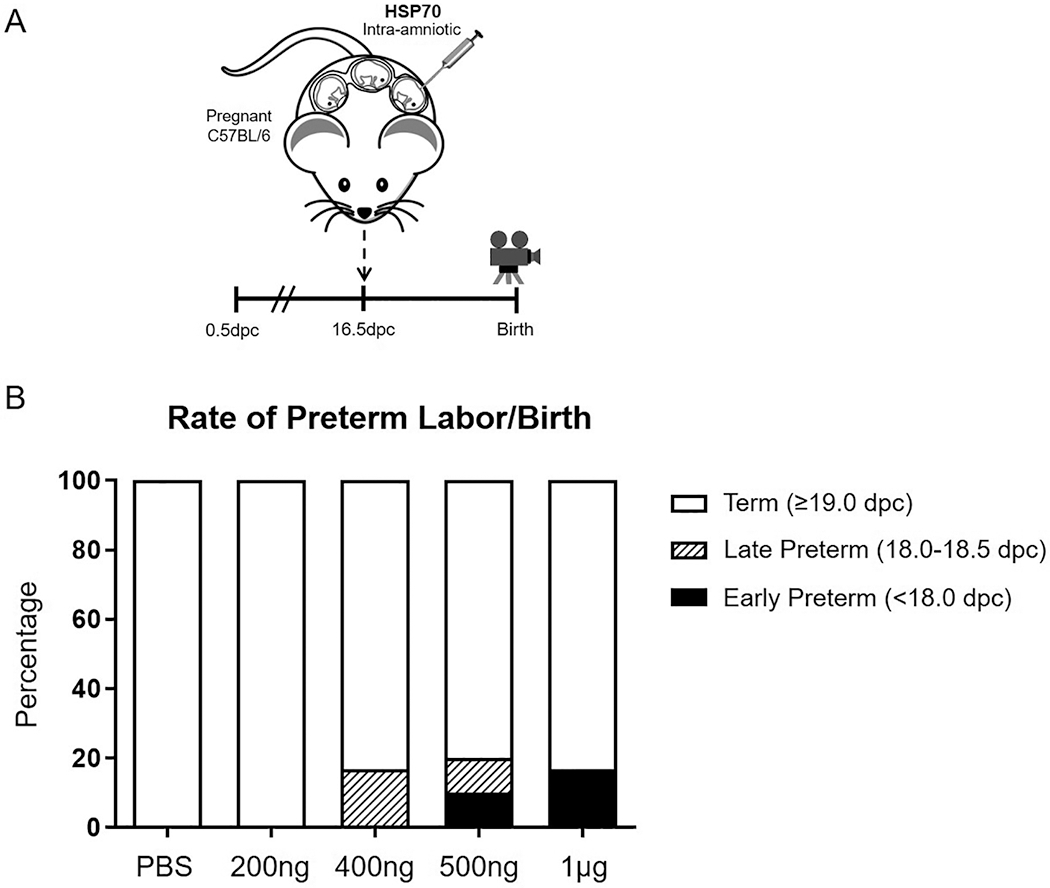
(A) Pregnant C57BL/6 dams were intra-amniotically injected with heat shock protein 70 (HSP70) or saline (1X phosphate-buffered saline; PBS) on 16.5 days post coitum (dpc). (B) The rate of preterm birth of mice injected intra-amniotically with 200 ng/25 μL, 400 ng/25 μL, 500 ng/25 μL, 1 μg/25 μL, or saline. The black bars represent early preterm birth (<18.0 dpc) and the striped bars represent late preterm birth (18.0-18.5 dpc). N = 6-10 dams per group.
Intra-amniotic administration of HSP70 causes adverse neonatal outcomes
The intra-amniotic injection of HSP70 did not induce high rates of preterm birth; however, elevated intra-amniotic concentrations of this alarmin may be inducing fetal damage. Therefore, we evaluated the rate of neonatal mortality after intra-amniotic administration of HSP70. Most neonates born to dams injected with PBS (controls), 200 ng, or 400 ng of HSP70, delivered at term and thrived up to 3 weeks of life [14.2 ± 4.8% (10/74), 9.8 ± 5.4% (6/61), and 17.9 ± 8.9% (9/45), respectively] (Figure 2). However, some of the neonates born to dams injected with 500 ng or 1 μg of HSP70 died at birth [28.8 ± 12.9% (23/78) and 21.4 ± 16.0% (13/49), respectively] (Figure 2).
Figure 2. The rate of neonatal mortality.
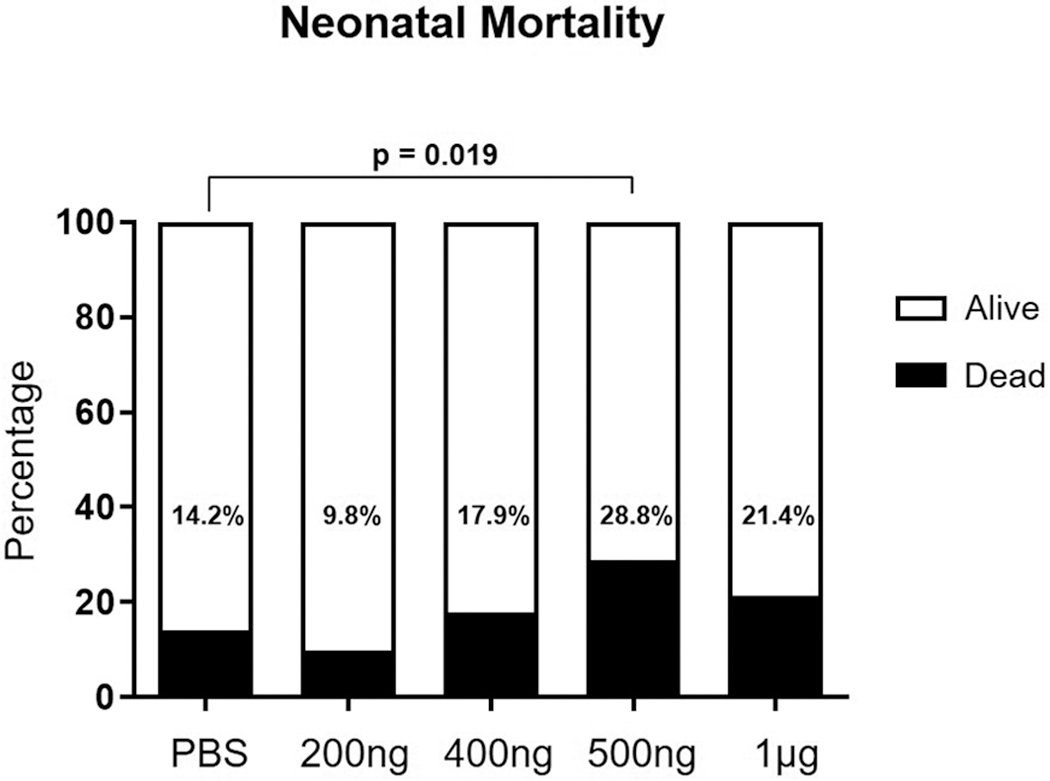
Pregnant mice were injected intra-amniotically with 200 ng/25 μL, 400 ng/25 μL, 500 ng/25 μL, 1 μg/25 μL, or saline (1X phosphate-buffered saline; PBS) and the rate of neonatal mortality was recorded at birth. N = 6-10 dams per group.
Lastly, we assessed the wellbeing of the fetus by determining the neonatal weight at weeks 1, 2, and 3. At one week of age, no differences in weight were found between the HSP70 groups (regardless of the concentration) and controls (Figure 3). However, neonates born to dams injected with 200 ng, 500 ng, or 1 μg of HSP70 were leaner than those born to control dams at two weeks of age [200 ng HSP70: median 6.4 g (IQR = 5.65–7.1 g) versus PBS: median 7.1 g (IQR = 6.6–7.65 g); p = 0.017], [500 ng HSP70: median 6.7 g (IQR = 6.2–7.2) versus PBS: median 7.1 g (IQR = 6.6–7.65 g); p = 0.05] or [1 μg HSP70: median 6.6 g (IQR = 5.8–7.1) versus PBS: median 7.1 g (IQR = 6.6–7.65 g); p = 0.006] (Figure 3). No differences were observed in the neonatal weights among the study groups at the third week of age (Figure 3), suggesting that thriving neonates recover from the effects of HSP70 by the third week.
Figure 3. Neonatal weights at weeks 1, 2, and 3 after birth.
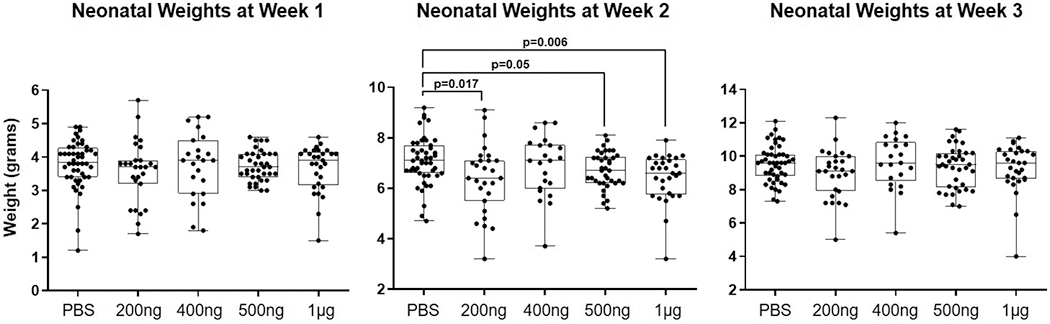
Neonates were weighed at the ages of 1 week, 2 weeks, and 3 weeks. N = 4-9 litters per group.
Together, these data indicate that the intra-amniotic administration of HSP70 at supraphysiological concentrations impacts neonatal life.
Discussion
Herein, we report that, while intra-amniotic injection of HSP70 did not induce preterm birth to a significant degree, it did indeed result in adverse neonatal outcomes. These findings show that HSP70 has a milder intra-amniotic effect than previously studied alarmins (i.e. HMGB1 [35] and S100B [36]).
The alarmin HSP70 can induce adverse pregnancy outcomes in the context of infection, sterile inflammation, and oxidative stress, among others [57, 58]. HSP70 is recognized by the pattern recognition receptors (PRRs) TLR2 and TLR4 [59, 60], which are expressed in the chorioamniotic membranes [61], placenta [62, 63, 64], and other fetal tissues [65]. The engagement of TLR2 and TLR4 by HSP70 leads to activation of the nuclear factor kappa B (NF-κB) pathway, resulting in the release of pro-inflammatory cytokines such as TNF-α, IL1-β, and IL-6 [52]. NF-κB also serves as an inhibitor of autophagy [66], and the expression of proteins associated with this mechanism of cell death are altered in the placenta and chorioamniotic membranes of women who underwent spontaneous preterm labor [67, 68], suggesting that HSP70 is implicated in the inflammatory process of preterm parturition. In addition to triggering the NF-κB pathway, HSP70 can lead to the activation of mTORC1 [69], which is a major inhibitor of autophagy [70, 71, 72]. The combined inhibition of autophagy by NF-κB and mTORC1 results in an increase of intracellular reactive oxygen species (ROS) and pro-inflammatory cytokines [58], which suggests that HSP70 may induce intra-amniotic inflammation through the inhibition of autophagy.
In the current study, low rates of preterm birth were observed after intra-amniotic injection of pathological concentrations of HSP70 [32] (400 ng to 1 μg). A potential explanation for this finding is that the sole administration of HSP70 induces a mild inflammatory response that is not severe enough to cause high rates of preterm birth. This is in contrast to what occurs in the clinical scenario of intra-amniotic infection, where microorganisms invading the amniotic cavity initiate strong inflammatory responses [73, 74, 75, 76], resulting in high amniotic fluid concentrations of HSP70 [32]. This concept is supported by previous studies showing that the chorioamniotic membranes displayed increased mRNA expression of HSP70 upon incubation with endotoxin [77]. In this setting, alarmins such as HSP70 could be released by necrotic and apoptotic cells [48] that are generated upon treatment with endotoxin or other microbial products [78, 79].
Nonetheless, our findings showed that adverse neonatal outcomes did occur upon intra-amniotic injection of HSP70. This suggests that, while HSP70 alone is not sufficient to induce high rates of preterm birth, the fetal inflammatory response initiated by this alarmin could result in adverse neonatal outcomes, which are similar to those observed in neonates born to dams injected with HMGB1 or S100B [35, 36]. Such a fetal inflammatory response is more severe in cases of intra-amniotic infection, causing adverse neonatal outcomes [80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97]. In line with this concept, elevated systemic concentrations of extracellular HSP70 have been associated with other inflammatory diseases [98, 99, 100]. Although more investigation is required to investigate the fetal inflammatory responses induced by HSP70, it is tempting to suggest that this alarmin is sensed by PRRs expressed on innate and adaptive immune cells present in the amniotic cavity [101, 102, 103, 104, 105, 106].
In summary, the findings herein provide evidence that the alarmin HSP70 can induce adverse neonatal outcomes without necessarily inducing premature labor and birth. These data show that not all alarmins in the amniotic cavity are sufficient to cause preterm labor/birth, yet these inflammatory mediators may still impact neonatal quality of life. Further research is needed in order to investigate the mechanisms whereby HSP70 in the amniotic cavity induces adverse neonatal outcomes.
Acknowledgements
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health
Footnotes
Conflict of Interests
The authors declare no potential conflicts of interest.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. Epub 2012/06/12. [DOI] [PubMed] [Google Scholar]
- 2.Monier I, Ancel PY, Ego A, Jarreau PH, Lebeaux C, Kaminski M, Goffinet F, Zeitlin J. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. Am J Obstet Gynecol. 2017;216:516.e1–.e10. Epub 2017/02/12. [DOI] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, Gulmezoglu AM. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2018. Epub 2018/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travers CP, Carlo WA, McDonald SA, Das A, Bell EF, Ambalavanan N, Jobe AH, Goldberg RN, D'Angio CT, Stoll BJ, Shankaran S, Laptook AR, Schmidt B, Walsh MC, Sanchez PJ, Ball MB, Hale EC, Newman NS, Higgins RD. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018;218:130.e1–.e13. Epub 2017/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. Epub 2014/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. Epub 1989/09/01. [DOI] [PubMed] [Google Scholar]
- 7.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. Epub 1994/12/01. [DOI] [PubMed] [Google Scholar]
- 8.Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol. 1994;171:1668–72. Epub 1994/12/01. [DOI] [PubMed] [Google Scholar]
- 9.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–6. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 10.Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. Am J Obstet Gynecol. 1996;174:1725–31; discussion 31–3. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 11.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–89. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 12.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–7. Epub 2008/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigsby PL, Novy MJ, Adams Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17:85–94. Epub 2009/10/22. [DOI] [PubMed] [Google Scholar]
- 14.Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA, Kallapur SG. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92:56. Epub 2014/12/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaemsaithong P, Romero R, Docheva N, Chaiyasit N, Bhatti G, Pacora P, Hassan SS, Yeo L, Erez O. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2018;31:228–44. Epub 2017/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusanovic JP, Romero R, Martinovic C, Silva K, Erez O, Maymon E, Diaz F, Ferrer F, Valdes R, Cordova V, Vargas P, Nilo ME, Le Cerf P. Transabdominal collection of amniotic fluid “sludge” and identification of Candida albicans intra-amniotic infection. J Matern Fetal Neonatal Med. 2018;31:1279–84. Epub 2017/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, Gomez-Lopez N. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasome. Biol Reprod. 2019;100:1290–305. Epub 2018/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, Heyborne K, ProteoGenix/Obstetrix Collaborative Research N. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125 e1–e15. Epub 2013/11/28. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80:e13049. Epub 2018/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2015;28:1343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matzinger P An innate sense of danger. Semin Immunol. 1998;10:399–415. [DOI] [PubMed] [Google Scholar]
- 26.Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, Romero R. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449–61. Epub 2008/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–71. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–5. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, Panaitescu B. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardwell JC, Craig EA. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984;81:848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert J Evolution of heat shock protein and immunity. Dev Comp Immunol. 2003;27:449–64. [DOI] [PubMed] [Google Scholar]
- 39.Ellis J Proteins as molecular chaperones. Nature. 1987;328:378–9. [DOI] [PubMed] [Google Scholar]
- 40.Pelham HR. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986;46:959–61. [DOI] [PubMed] [Google Scholar]
- 41.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–9. [DOI] [PubMed] [Google Scholar]
- 42.Haslbeck M sHsps and their role in the chaperone network. Cell Mol Life Sci. 2002;59:1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelham HR. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984;3:3095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–81. [DOI] [PubMed] [Google Scholar]
- 45.Prohaszka Z, Fust G. Immunological aspects of heat-shock proteins-the optimum stress of life. Mol Immunol. 2004;41:29–44. [DOI] [PubMed] [Google Scholar]
- 46.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. [DOI] [PubMed] [Google Scholar]
- 48.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 49.Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–5. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava P Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–94. [DOI] [PubMed] [Google Scholar]
- 51.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–76. [DOI] [PubMed] [Google Scholar]
- 52.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. [DOI] [PubMed] [Google Scholar]
- 53.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–12. [DOI] [PubMed] [Google Scholar]
- 54.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–84. [DOI] [PubMed] [Google Scholar]
- 55.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, Gould MJ, Hobbins JC. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–9. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, Hassan SS. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018;31:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanninen TT, Sisti G, Witkin SS. Induction of the 70 kDa heat shock protein stress response inhibits autophagy: possible consequences for pregnancy outcome. J Matern Fetal Neonatal Med. 2016;29:159–62. Epub 2014/11/28. [DOI] [PubMed] [Google Scholar]
- 58.Witkin SS, Kanninen TT, Sisti G. The Role of Hsp70 in the Regulation of Autophagy in Gametogenesis, Pregnancy, and Parturition. Adv Anat Embryol Cell Biol. 2017;222:117–27. [DOI] [PubMed] [Google Scholar]
- 59.Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int J Hyperthermia. 2009;25:610–6. [DOI] [PubMed] [Google Scholar]
- 60.Sevin M, Girodon F, Garrido C, de Thonel A. HSP90 and HSP70: Implication in Inflammation Processes and Therapeutic Approaches for Myeloproliferative Neoplasms. Mediators Inflamm. 2015;2015:970242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, Nien JK, Gomez R, Mazor M, Saito S, Abrahams VM, Mor G. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:921–7. [DOI] [PubMed] [Google Scholar]
- 62.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar N, Nandula P, Menden H, Jarzembowski J, Sampath V. Placental TLR/NLR expression signatures are altered with gestational age and inflammation. J Matern Fetal Neonatal Med. 2017;30:1588–95. Epub 2016/07/22. [DOI] [PubMed] [Google Scholar]
- 64.Strauss JF 3rd, Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, Pearson LN, York TP, Schenkein HA. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol. 2018;218:294–314 e2. Epub 2017/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrikin JE, Gaedigk R, Leeder JS, Truog WE. Selective Toll-like receptor expression in human fetal lung. Pediatr Res. 2010;68:335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373–82. [DOI] [PubMed] [Google Scholar]
- 67.Brickle A, Tran HT, Lim R, Liong S, Lappas M. Autophagy, which is decreased in labouring fetal membranes, regulates IL-1beta production via the inflammasome. Placenta. 2015;36:1393–404. [DOI] [PubMed] [Google Scholar]
- 68.Avagliano L, Massa V, Zullino S, Doi P, Marconi AM, Ferrazzi E, Bulfamante GP. Inflammation modulates LC3 expression in human preterm delivery. J Matern Fetal Neonatal Med. 2017;30:698–704. Epub 2016/04/30. [DOI] [PubMed] [Google Scholar]
- 69.Chou SD, Prince T, Gong J, Calderwood SK. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7:e39679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–91. Epub 2009/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. Epub 2009/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–60. Epub 1998/11/20. [DOI] [PubMed] [Google Scholar]
- 74.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA, Roberts CT Jr., Nagalla SR. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. Epub 2004/07/29. [DOI] [PubMed] [Google Scholar]
- 75.Cobo T, Kacerovsky M, Palacio M, Hornychova H, Hougaard DM, Skogstrand K, Jacobsson B. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PLoS One. 2012;7:e43677. Epub 2012/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Docheva N, Martinez-Varea A, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menon R, Gerber S, Fortunato SJ, Witkin SS. Lipopolysaccharide stimulation of 70 kilo Dalton heat shock protein messenger ribonucleic acid production in cultured human fetal membranes. J Perinat Med. 2001;29:133–6. [DOI] [PubMed] [Google Scholar]
- 78.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- 79.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol. 2013;191:5702–13. Epub 2013/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. Epub 1998/08/15. [DOI] [PubMed] [Google Scholar]
- 81.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, Berry SM. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–7. Epub 2000/11/21. [DOI] [PubMed] [Google Scholar]
- 82.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–9. Epub 2000/11/21. [DOI] [PubMed] [Google Scholar]
- 83.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, Kim JC, Kadar N, Romero R. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. Epub 2002/10/17. [DOI] [PubMed] [Google Scholar]
- 84.Rounioja S, Rasanen J, Glumoff V, Ojaniemi M, Makikallio K, Hallman M. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction. A mouse model for fetal inflammatory response. Cardiovasc Res. 2003;60:156–64. Epub 2003/10/03. [DOI] [PubMed] [Google Scholar]
- 85.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. Epub 2007/09/01. [DOI] [PubMed] [Google Scholar]
- 86.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1–6. Epub 2007/09/11. [DOI] [PubMed] [Google Scholar]
- 87.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, Erez O, Vaisbuch E, Gotsch F, Pacora P, Yeo L, Gervasi MT, Lamont RF, Yoon BH, Hassan SS. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–52. Epub 2009/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stampalija T, Romero R, Korzeniewski SJ, Chaemsaithong P, Miranda J, Yeo L, Dong Z, Hassan SS, Chaiworapongsa T. Soluble ST2 in the fetal inflammatory response syndrome: in vivo evidence of activation of the anti-inflammatory limb of the immune response. J Matern Fetal Neonatal Med. 2013;26:1384–93. Epub 2013/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kemp MW, Molloy TJ, Usuda H, Woodward E, Miura Y, Payne MS, Ireland DJ, Jobe AH, Kallapur SG, Stock SJ, Spiller OB, Newnham JP, Saito M. Outside-in? Acute fetal systemic inflammation in very preterm chronically catheterized sheep fetuses is not driven by cells in the fetal blood. Am J Obstet Gynecol. 2016;214:281.e1–.e10. Epub 2015/09/27. [DOI] [PubMed] [Google Scholar]
- 90.Lee J, Romero R, Lee KA, Kim EN, Korzeniewski SJ, Chaemsaithong P, Yoon BH. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol. 2016;214:366.e1–9. Epub 2015/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozalkaya E, Karatekin G, Topcuoglu S, Gursoy T, Ovali F. Morbidity in preterm infants with fetal inflammatory response syndrome. Pediatr Int. 2016;58:850–4. Epub 2015/12/31. [DOI] [PubMed] [Google Scholar]
- 92.Rueda CM, Presicce P, Jackson CM, Miller LA, Kallapur SG, Jobe AH, Chougnet CA. Lipopolysaccharide-Induced Chorioamnionitis Promotes IL-1-Dependent Inflammatory FOXP3+ CD4+ T Cells in the Fetal Rhesus Macaque. J Immunol. 2016;196:3706–15. Epub 2016/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Musilova I, Andrys C, Drahosova M, Soucek O, Stepan M, Bestvina T, Spacek R, Jacobsson B, Cobo T, Kacerovsky M. Intraamniotic inflammation and umbilical cord blood interleukin-6 concentrations in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017;30:900–10. Epub 2016/06/07. [DOI] [PubMed] [Google Scholar]
- 94.Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM, Gomez-Lopez N. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol. 2018;9:1291. Epub 2018/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SM, Kim BJ, Park JS, Norwitz ER, Oh JW, Oh S, Vixay C, Kim SM, Park CW, Jun JK. Risk of intra-amniotic infection/inflammation and respiratory distress syndrome according to the birth order in twin preterm neonates. J Matern Fetal Neonatal Med. 2018:1–6. Epub 2018/09/21. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell T, MacDonald JW, Srinouanpranchanh S, Bammler TK, Merillat S, Boldenow E, Coleman M, Agnew K, Baldessari A, Stencel-Baerenwald JE, Tisoncik-Go J, Green RR, Gale MJ Jr., Rajagopal L, Adams Waldorf KM. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1–.e16. Epub 2018/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Francis F, Bhat V, Mondal N, Adhisivam B, Jacob S, Dorairajan G, Harish BN. Fetal inflammatory response syndrome (FIRS) and outcome of preterm neonates - a prospective analytical study. J Matern Fetal Neonatal Med. 2019;32:488–92. Epub 2017/09/26. [DOI] [PubMed] [Google Scholar]
- 98.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. [DOI] [PubMed] [Google Scholar]
- 99.Njemini R, Lambert M, Demanet C, Vanden Abeele M, Vandebosch S, Mets T. The induction of heat shock protein 70 in peripheral mononuclear blood cells in elderly patients: a role for inflammatory markers. Hum Immunol. 2003;64:575–85. [DOI] [PubMed] [Google Scholar]
- 100.Mrkaic A, Rosenn B, Stojanovic I, Tivari S. Troponins, heat shock proteins and glycogen phosphorylase BB in umbilical cord blood of complicated pregnancies. J Matern Fetal Neonatal Med. 2017;30:2978–84. Epub 2016/12/13. [DOI] [PubMed] [Google Scholar]
- 101.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, Jack RM. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 102.Macias AE, Wong SW, Sadowsky DW, Luetjens CM, Axthelm MK, Gravett MG, Haluska GJ, Novy MJ. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol. 2001;55:159–70. Epub 2001/12/18. [DOI] [PubMed] [Google Scholar]
- 103.Marquardt N, Ivarsson MA, Sundstrom E, Akesson E, Martini E, Eidsmo L, Mjosberg J, Friberg D, Kublickas M, Ek S, Tegerstedt G, Seiger A, Westgren M, Michaelsson J. Fetal CD103+ IL-17-Producing Group 3 Innate Lymphoid Cells Represent the Dominant Lymphocyte Subset in Human Amniotic Fluid. J Immunol. 2016;197:3069–75. Epub 2016/09/04. [DOI] [PubMed] [Google Scholar]
- 104.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, Panaitescu B. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017;78. Epub 2017/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, Maymon E. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017;217:693.e1–.e16. Epub 2017/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, Hsu CD. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79:e12827. Epub 2018/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]


