Adult mammalian hearts cannot regenerate after myocardial infarction (MI): cardiomyocytes (CM) that die after MI are replaced with a fibrotic scar that compromises heart function and induces heart failure. Gene delivery via modified mRNA (modRNA) is a safe, transient, non-immunogenic, local, controlled gene delivery platform that rapidly translates genes in heart cells post MI1. We recently demonstrated that our specific modRNA translation system (SMRTs) delivers potent intracellular genes (e.g., cell cycle-promoting Pkm2), which are beneficial when expressed in one cell type (CM), but not others (non-CM), exclusively to CM2. Here we show how SMRTs allow modRNA translation only in CM or non-CM both in culture and after MI.
SMRTs may reduce several CM-specific microRNA (cmsmiRs) that are detrimental post MI. miR208a encodes using the same intron as the CM-specific marker Myh6 and induces hypertrophy by upregulating βMHC3. Elevated muscle-specific miR-1 increases cell death4. CM-specific miR-199a impairs autophagy and promotes cardiac hypertrophy through mTOR activation5. We created four inactive human CD25 (reporter gene ihCD25) modRNA with or without recognition elements for these cardiac-detrimental cmsmiRs (A). We transfected these modRNA into neonatal rat heart cells and immunostained them for hCD25 1 day later in vitro (B). In parallel, we delivered these modRNA to adult mouse hearts 4 days post MI in vivo and collected the hearts 2 days later (C). All animal procedures accorded with institutional guidelines. Our results (D-F) show that CD25 modRNA carrying miR1 or miR208 recognition elements were translated only in non-CM, while CD25 modRNA with or without miR199 recognition elements were translated in both CM and non-CM, in vitro (D, upper level) and in vivo (D, lower level). These data suggest that miR1 and miR208 recognition elements can significantly inhibit modRNA translation in CM, in vitro (E), and in vivo (F); however, our results do not necessarily mean that CM expresses or function more miR1/miR208 than other cmsmiRs.
To test if using miR1 / miR208 recognition sites on SMRTs would alter cmsmiRs-targeted gene expression immediately after MI, we administered modRNA with an L7AE gene intended for CM-specific modRNA, with or without miR1 / miR208 recognition elements. We collected heart apexes 2 days later to evaluate the expression of several miR1 and miR208 target genes (G). Our data show that both miR1 (H) and miR208 (I) recognition sites behave as “sponges” for their cognate cmsmiRs and upregulate their target genes as compared to no-miR modRNA. In addition, we demonstrate that modRNA carrying miR1 and miR208 (miR1-208) raises both cmsmiRs target genes (H&I), making this an ideal cmsmiRs recognition element for SMRTs.
To investigate miR1-208 in non-CM-specific modRNA, we designed nGFP with or without miR1-208 recognition element (J) for cell culture transfection, similar to the experiment in B. We show that nGFP modRNA without cmsmiRs recognition sites translates in both CMs and non-CMs; however, nGFP modRNA carrying miR1-208 recognition sites significantly and exclusively translates in non-CM but not CM. Similarly, Cre recombinase modRNA with or without miR1-208 recognition (M) resulted in Cre excising a stop codon between the two loxp sites, allowing significant GFP gene activation in non-CM but not CM 28 days post MI in an R26mTmG mouse MI model (M&N). We concluded that adding the miR1-208 recognition element to the 3’UTR of modRNA enables translation only in non-CM in vitro and in vivo.
We used two distinct modRNA to enact CM specificity (P). modRNA carrying L7Ae, which regulates Cre or nGFP modRNA translation by targeting a specific L7Ae binding site (kink-turn motif), suppresses either Cre or nGFP modRNA translation when co-transfected. Adding the miR1-208 recognition element to L7Ae modRNA prevents L7Ae translation in CMs that abundantly and nearly exclusively express those miRs (“suppress the suppressor” approach), so that the gene-of-interest modRNA translates strictly in CMs. Using an R26mTmG mouse MI model, we show that delivering Cre carrying kink-turn motif (Cre K) alone activated GFP expression in both CM and non-CM (Q); however, delivering both Cre K and L7Ae modRNA carrying miR1-208 exclusively transfects CM and not non-CM (Q), which can cover >20% of the transfected area 28 days post MI2. This system has limitations, specifically that the expression efficiency remains low and warrants improvement. In parallel, using our adult mouse MI model, we show that transfecting nGFP k-motif generated nGFP translation in both CM and non-CM, yet nGFP k-motif co-transfection with L7Ae modRNA carrying miR1-208 (1:0.5 ratio) produced sufficient nGFP for exclusive translation in CMs (R).
Importantly, miR1 / miR208 sequence alignment in rat, mouse and human show high similarity (S), suggesting that SMRTs can also be used in humans. Accordingly, we designed two efficient SMRTs that translate mRNA exclusively in CM or non-CM in vitro and in vivo. Our SMRTs also reduced disadvantageous cmsmiRs in the heart post MI. This new system will help researchers evaluate function or tailor gene-of-interest treatment in different cell types post MI.
Development of CM- and non-CM-specific modRNA expression platform.
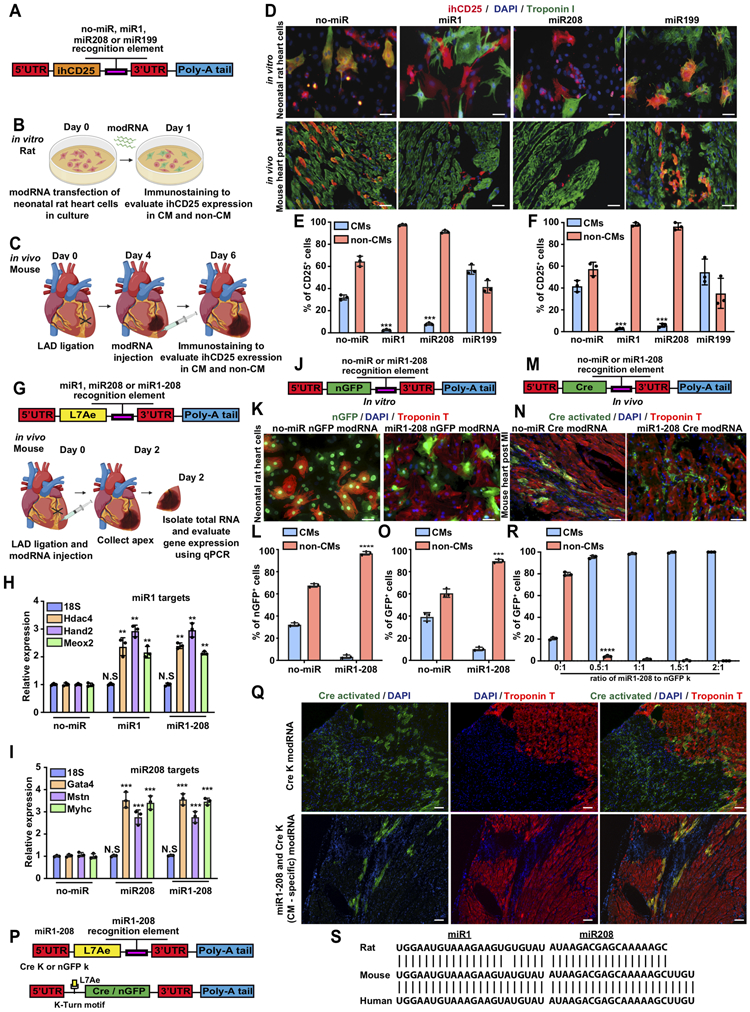
A. Construction design for identifying CM-specific miRs (cmsmiRs) using inactive human CD25 (ihCD25) modRNA with or without recognition elements for known cmsmiRs post transfection. B&C. Experimental timeline for evaluating cmsmiRs expression in neonatal rat heart cells in vitro (B) and adult mouse MI model in vivo (C). D. Representative images of CD25 immunostaining (red) after delivery of ihCD25 modRNA with or without recognition elements for different miRs post transfection in neonatal rat heart cells in vitro (upper panel) and mouse MI model in vivo (lower panel). E&F. Quantification of the in vitro (E) and in vivo (F) experiments (n=3). G. Construct design and experimental timeline to analyze the inhibition of miR1 and miR208 target genes in mouse hearts 2 days after MI and delivery of modRNA with or without recognition elements for miR1 or/and miR208. H&I. qPCR evaluation of miR1 (H) and miR208 (I) target genes, 2 days post MI and delivery of modRNA with or without recognition elements for miR1 or/and miR208 (n=3). J. Construct design for SMRTs GFP non-CM-specific modRNA to be evaluated in neonatal rat heart cells in vitro. K. GFP immunostaining to evaluate nGFP modRNA expression (green) in neonatal rat CM (red) 1 day after delivery of nGFP modRNA with or without CM-specific recognition elements (miR1-208) in vitro (similar timeline as in B). L. Quantification of the experiment described in K (n=3). M. Construct design for SMRTs Cre non-CM-specific modRNA to be evaluated in vivo (similar timeline as in C) using Rosa26mTmG reporter mice in MI model (upon Cre expression, cells express GFP). N. GFP immunostaining to evaluate Cre modRNA expression (green) in non-CM and CM (red) 28 days post delivery of Cre modRNA with or without CM-specific recognition elements (miR1-208) in vivo. O. Quantification of the experiment described in N (n=3). P. SMRTs CM-specific GFP/Cre based on two modRNAs: one modRNA is a suppressor gene (L7AE) with miR1-208 recognition element upstream of its 3’UTR, and in the second modRNA the gene of interest is regulated by a K-motif downstream of its 5’UTR. Upon L7AE translation, the circuit modRNA binds to the K-motif and prevents translation of the gene of interest (Cre or nGFP). Upon transfection into CMs, L7AE is suppressed by endogenous miR1 or/and miR208. Lack of L7AE results in Cre or nGFP translation; however, in non-CM cells, L7AE is expressed and suppresses Cre or nGFP expression. Q. Rosa26mTmG reporter mice co-transfected with Cre-K alone or circuit modRNA (Cre-K+miR1-208, (CM-SMRTs Cre)) and analyzed 28 days post MI. R. Evaluation of different ratios of miR1-208 and nGFP k modRNA for delivery in an MI setting in vivo (similar timeline as in C). S. Sequence alignment of miR1 or miR208 in rat, mouse and human. Unpaired two-tailed t-test for E&F, L, O and S. One-way ANOVA, Tukey's Multiple Comparison Test for H&I. ****, P<0.0001, ***, P<0.001, **, P<0.01, Scale bar = 20μm (D (upper panel) and K), 50μm (D (lower panel) and N) and 100μm (Q).
ACKNOWLEDGEMENTS
The authors acknowledge Neha Singh, Magdalena M. Żak, Keerat Kaur, Yoav Hadas and Nishat Sultana for their help with this manuscript.
FUNDING SOURCES
This work was funded by a cardiology start-up grant awarded to the Zangi laboratory and also by a National Institutes of Health grant (R01 HL142768-01).
Footnotes
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure presented in this article.
DISCLOSURES
L.Z. and A.M. are inventors of a Provisional Patent (Cell-specific expression of modRNA, WO2018053414A1), which covers the results in this manuscript.
REFERENCES
- 1.Sultana N, Magadum A, Hadas Y, Kondrat J, Singh N, Youssef E, Calderon D, Chepurko E, Dubois N, Hajjar RJ, et al. Optimizing Cardiac Delivery of Modified mRNA. Molecular therapy. 2017;25:1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K, Sharkar MTK, Chepurko E, Sassi Y, Oh JG, et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation. 2020; 14;141(15):1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. The Journal of clinical investigation. 2009;119:2772–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z and Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. Journal of cell science. 2007;120:3045–52. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Song Y, Liu L, Hou N, An X, Zhan D, Li Y, Zhou L, Li P, Yu L, et al. miR-199a impairs autophagy and induces cardiac hypertrophy through mTOR activation. Cell death and differentiation. 2015; 24(7):1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]


