Abstract
Background
The recent pandemic highlights the essential nature of optimizing the use of invasive mechanical ventilation (IMV) in complex critical care settings. This review of reviews maps evidence-based practices (EBPs) that are associated with better outcomes among adult patients with acute respiratory failure or ARDS on the continuum of care, from intubation to liberation.
Research Question
What EPBs are recommended to reduce the duration of IMV and mortality rate among patients with acute respiratory failure/ARDS?
Study Design and Methods
We identified an initial set of reports that links EBPs to mortality rates and/or duration of IMV. We conducted a review of reviews, focusing on preappraised guidelines, meta-analyses, and systematic reviews. We searched Scopus, CINAHL, and PubMed from January 2016 to January 2019 for additional evidence that has not yet been incorporated into current guidelines.
Results
Our initial search produced 61 publications that contained 42 EBPs. We excluded 42 manuscripts during the data extraction process, primarily because they were not associated with improved patient outcomes. The remaining 19 preappraised guidelines, meta-analyses, and systematic reviews met our full inclusion criteria and spanned the continuum of IMV care from intubation to liberation. These contained 20 EBPs, a majority of which were supported with moderate levels of evidence. Of these, six EBPs focused on intubation and escalation of care, such as ventilator management and synchrony; ten EBPs reduced complications associated with IMV, which included spontaneous awakening and breathing trials and early mobility protocols; and four EBPs promoted timely extubation and postextubation recovery.
Interpretation
This review describes EBPs that are associated with fewer ventilator days and/or lower mortality rates among patients who received IMV for acute respiratory failure/ARDS. Many of these EBPs are connected across the care continuum, which indicates the need to promote and assess effective implementation jointly, rather than individually.
Key Words: acute respiratory failure, ARDS, evidence-based practice, invasive mechanical ventilation
Abbreviations: ARF, respiratory failure; EBP, evidence-based practices; IMV, invasive mechanical ventilation
Millions of adults are admitted to ICUs to receive invasive mechanical ventilation (IMV) for acute respiratory failure (ARF) in the United States each year. Approximately 200,000 of these patients will experience ARDS, with morbidity rates remaining high at 40% to 50% and annual costs exceeding $27 billion.1, 2, 3 A number of evidence-based practices (EBPs) are associated with improved patient outcomes, but overall adherence to these EBPs is variable. For example, lung protective ventilation can reduce morbidity and mortality rates among patients with ARDS. Yet, 30% to 60% of eligible patients receive a lung protective strategy.4, 5, 6, 7
One challenge is that IMV care is not a single, discrete event, but a series of linked, sequential and nonsequential events or practices over a period of time that range from a few hours to days or weeks. Our focus is on EBPs that cover the sequences of practices, or continuum, that are required to provide care from intubation to liberation (Fig 1). We are interested particularly in those that optimize the provision of IMV by reducing ventilator days and/or deaths. The empiric support for these EBPs can be found in published reviews, guidelines, and meta-analyses. Therefore, to assist clinicians, we conducted a systematic review of reviews to synthesize this literature in one place.
Figure 1.
Evidence-based practices in the provision of invasive mechanical ventilation for acute respiratory failure/ARDS across the care continuum. HFNC = high-flow nasal cannula; IMV = mechanical ventilation; NIV = noninvasive mechanical ventilation; PEEP = positive-end expiratory pressure.
Methods
Search Strategy
We conducted a systematic review of reviews, focusing on the quality and quantity of data evaluating key outcomes of patients who received IMV for ARF or ARDS.8 This approach is helpful for reviewing, comparing, and contrasting findings from separate reviews. Thus, our source materials include preappraised reviews, guidelines, and meta-analyses. To begin our systematic search of the secondary literature, clinician coauthors identified already appraised and synthesized guidelines or meta-analyses focused on care of adult patients who received IMV for ARF and/or ARDS in an ICU in late 2018.4,9, 10, 11, 12, 13, 14, 15, 16 All coauthors discussed these materials and agreed that they reflect the most current EBPs across the continuum of IMV care at the time of the search. To ensure that we included the most updated synthesized and appraised evidence, we then searched Scopus, CINAHL, and PubMed (which includes Medline and The Cochrane Database of Systematic Reviews) for additional systematic reviews and meta-analyses that provided EBPs across the continuum of care that were linked to reductions in mortality rates and/or IMV duration. e-Appendix 1 provides an example of our search strategy.
We did not include grey literature, because we restricted our search to preappraised and synthesized reports. Our search dates ranged from January 1, 2016, to January 31, 2019, to identify additional synthesized evidence not yet incorporated into current guidelines. For our search strategy, we included combinations of the following terms: mechanical ventilation, artificial respiration, ARF, acute respiratory distress syndrome, guideline, consensus, meta-analysis, and systematic review. Once the search was conducted, the list of EBPs and associated evidence was discussed among the research team until agreement was reached that the selected EBPs represented the spectrum of care for patients with ARF and/or ARDS. We relied on our inclusion criteria of preappraised guidelines, meta-analyses, or systematic reviews that contained evidence that linked the EBP to fewer ventilator days and/or improved mortality rates to resolve disagreements.
Title and Abstract Review
The title and abstract review process was conducted by four authors (J. N. E., V. C. R., E. R. D., A. E. S.). Our inclusion criteria were guidelines, reviews, and meta-analyses that include studies of invasively mechanically ventilated acutely ill adult patients in the ICU that were published between 2016 and 2019 with direct evaluation of the association between EBPs and outcomes of interest (IMV duration and/or deaths). We excluded all primary studies, because they describe information that has not been appraised or synthesized. We excluded articles that were published in languages other than English and those articles that focused on clinical conditions other than ARF or ARDS. During the title and abstract review process, we noticed that some EBPs had limited support or lacked clinical consensus. The research team decided to add these as additional exclusion criteria to be applied for the remainder of the screening process.
Full-Text Review
Full-text review and data extraction was conducted by two authors (J. N. E. and V. C. R.). They focused on the description of the EBP, the nature of the recommendation, the level of evidence as identified by the source authors, the justification, the outcomes, the implementation notes, the measurement notes, and the study design. We then mapped this final set of EBPs along the continuum of care for ARF/ARDS, which we divided into three phases: (1) initiation and optimization of IMV, (2) prevention of IMV complications, and (3) deescalation of interventions to prevent unnecessary prolongation of IMV and extubation (ie, discontinuation of IMV). This final step was an iterative process that took place over several discussions until consensus was reached.
Results
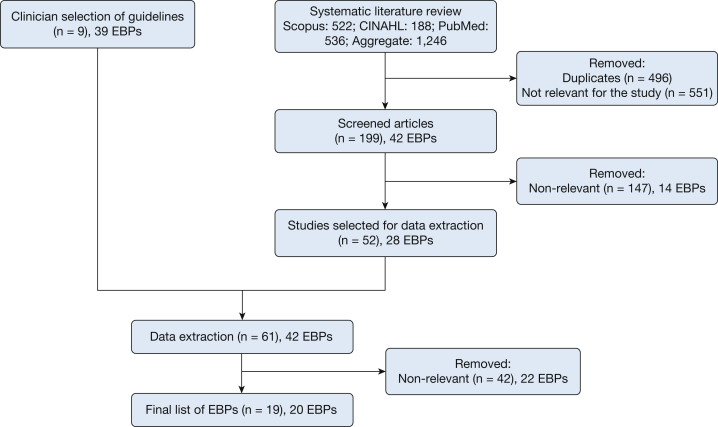
Our clinical experts recommended nine guidelines that contained 39 EBPs (Fig 2). Some of these EBPs were not specific to patients with ARF or ARDS but were selected because of their overall importance to most or all patients with IMVs, for example extubation to non-IMV. After applying our inclusion and exclusion criteria, our literature search produced an additional 52 articles that contained a total of 28 EBPs. Considering that there was significant redundancy with the original 39 EBPs, only three additional EBPs were identified, all through systematic reviews and/or meta-analyses. We did not identify any additional, more current guidelines. Thus, 61 unique manuscripts that contained 42 EBPs were eligible for title and abstract review. During the data extraction process, 42 of those manuscripts that contained 22 EBPs were excluded, primarily because the EBPs were not associated with improved mortality rates or IMV duration outcomes for patients with ARF or ARDS. Some reports contained multiple EBPs but not all included the specific outcomes for our search.
Figure 2.
Flow diagram of literature search results. EBP = evidence-based practice.
Our final synthesis included 19 manuscripts that contained 20 EPBs, which were based on more than 117 studies of more than 30,000 patients (Table 1).4,10, 11, 12, 13, 14,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 We accounted for the fact that guidelines, systematic reviews, and meta-analyses often report multiple, sometimes overlapping EBPs. The extracted EBP recommendations can be found in Table 2. We included whether the EBP is recommended for use (Yes/No) and the level of evidence that was based on the quality assessments that had been conducted in the original review or meta-analysis. Most of the authors of the source materials used the Grading of Recommendations Assessment, Development, and Evaluation criteria or a similar assessment guide; evidence was rated low or moderate in quality based on risk of bias, directness and consistency of the findings, and measurement precision, which is similar to the process reported by Fan et al.4 None of the evidence was deemed to be high quality by the authors of these sources. In fact, we found it striking that most of the evidence was moderate at best.
Table 1.
Summary of the Systematic Reviews, Meta-Analyses, and Guidelines That Were Included
| Review | Type of Study | Aim | Patient Population | Studies Included, No. | Participants, No. | Assessed Publication Bias | Assessed Heterogeneity |
|---|---|---|---|---|---|---|---|
| Aggarwal et al13 (2018) | Reanalysis of randomized controlled trial data | Evaluate oxygen exposure and Pao2 | Patients diagnosed with ARDS | 10 | 4,361 | No | No |
| Aoyama et al17 (2018) | Systematic review and meta-analysis | Evaluate evidence that links higher vs lower driving pressure and patient outcomes | Patients diagnosed with ARDS | 7 | 6,062 | Yes | Yes |
| Devlin et al10 (2018) | Systematic review and guidelines | Evaluate evidence and update and expand the 2013 clinical guidelines for pain prevention and management, delirium, mobility, and sleep | Critically ill patients | Varied across topic | Varied across topic | Yes | Yes |
| Fan et al4 (2017) | Systematic review and guidelines | To provide clinical practice guidelines and review supportive evidence | Patients diagnosed with ARDS | Varied across evidence-based practices | Varied across evidence-based practices | Yes | Yes |
| Girard et al11 (2017) | Systematic review and guidelines | Evaluate evidence that links rehabilitation protocols, ventilator liberation protocols, and cuff leak tests to patient outcomes | Critically ill patients | Varied across topic | Varied across topic | Yes | Yes |
| Goligher et al18 (2017) | Systematic review and meta-analysis | Evaluate evidence that links lung recruitment maneuvers to patient outcomes | Patients diagnosed with ARDS | 6 | 1,423 | Yes | Yes |
| Guo et al19 (2018) | Systematic review and meta-analysis | Evaluate evidence that links positive-end expiratory pressure to patient outcomes | Patients diagnosed with ARDS | 9 | 3,612 | Yes | Yes |
| Huang et al20 (2018) | Systematic review and meta-analysis | Evaluate evidence that links noninvasive ventilation on patient outcomes | Critically ill patients | 7 | 2,781 | Yes | Yes |
| Maitra et al21 (2016) | Systematic review and meta-analysis | Evaluate evidence that links noninvasive ventilation on patient outcomes | Patients diagnosed with acute hypoxic respiratory failure | 7 | Varied across analyses | Yes | Yes |
| Meduri et al22 (2016) | Meta-analysis | Evaluate evidence that links glucocorticoid treatment to patient outcomes | Patients diagnosed with ARDS | 4 | 322 | Yes | Yes |
| Meduri et al23 (2018) | Meta-analysis | Updated evaluation of evidence that links glucocorticoid treatment to patient outcomes | Patients diagnosed with ARDS | 9 | 816 | Yes | Yes |
| Munshi et al24 (2017) | Systematic review and meta-analysis | Evaluate evidence that links prone position to patient outcomes | Patients diagnosed with ARDS | 8 | 2,129 | Yes | Yes |
| Murray et al25 (2016) | Systematic review and guidelines | Update 2002 clinical practice guidelines for sustained neuromuscular blockade | Critically ill patients | Varied across topic | Varied across topic | Yes | Yes |
| Ni et al26 (2017) | Systematic review and meta-analysis | Evaluate evidence that links conventional oxygen therapy to non-invasive ventilation to patient outcomes | Patients diagnosed with acute respiratory failure | 18 | 3,881 | Yes | Yes |
| Ouellette et al12 (2017) | Systematic review and guidelines | Evaluate evidence that supports liberation from mechanical ventilation | Critically ill patients | Varied across topic | Varied across topics | Yes | Yes |
| Schmidt et al27 (2017) | Systematic review and guidelines | Evaluate evidence that links rehabilitation protocols, ventilator liberation protocols, and cuff leak tests to patient outcomes | Critically ill patients | Varied across topic | Varied across topic | Yes | Yes |
| Silversides et al14 (2017) | Systematic review and meta-analysis | Evaluate the evidence that links fluid management to patient outcomes | Patients diagnosed with ARDS or sepsis | 11 | 2,051 | Yes | Yes |
| Walkey et al28 (2017) | Systematic review and meta-analysis | Review evidence that links low tidal volumes and inspiratory pressure with clinical outcomes | Patients diagnosed with ARDS | 7 Randomized controlled trials | 1,481 | Yes | Yes |
| Yang et al29 (2017) | Meta-analysis | Evaluate evidence that links glucocorticoid treatment to patient outcomes | Patients diagnosed with ARDS | 14 | 1,441 | Yes | Yes |
Table 2.
Summary of Recommendations and Results
| Review | Care Continuum Phase | Evidence-Based Practice | Evidence-Based Practice Description | Impact on Outcome |
Recommended for Use | Level of Evidence | |
|---|---|---|---|---|---|---|---|
| Mechanical Duration | Death | ||||||
| Aggarwal et al13 (2018) | 1 | Conservative oxygen therapy | The goal of Po2 in arterial blood is 55-80 mm Hg; oxygen exposure >80 mm Hg is associated with worse patient outcomes, irrespective of the severity of ARDS. | Yes | Yes | Yes | Moderate |
| Aoyama et al17 (2018) | 1 | Driving pressure | Higher driving pressure is associated with death. | … | Yes | No | Moderate |
| Fan et al4 (2017) | 1 | Lung protective ventilation | Invasive mechanical ventilation with the use of lower tidal volumes (4-8 mL/kg predicted body weight). | … | Yes | Yes | Moderate |
| Walkey et al28 (2017) | 1 | Lung protective ventilation | Invasive mechanical ventilation with the use of lower tidal volumes (4-8 mL/kg predicted body weight). | … | Yes | Yes, conditional on higher positive-end expiratory pressure among patients with moderate-to-severe ARDS | Moderate |
| Murray et al25 (2016) | 1 | Neuromuscular blocking agent | Conditional recommendation to administer a neuromuscular blocking agent by continuous IV infusion early in the course of ARDS. | Yes | Yes | Yes, conditional, for patients with moderate-to-severe ARDS | Moderate |
| Fan et al4 (2017) | 1 | Positive-end expiratory pressure | Conditional recommendation for higher positive-end expiratory pressure, of approximately 15 cm water. | … | Yes | Yes, conditional, for patients with moderate-to-severe ARDS | Moderate |
| Goligher et al18 (2017) | 1 | Positive-end expiratory pressure | Conditional recommendation for higher positive-end expiratory pressure, of approximately 15 cm water. | … | Yes | Yes, conditional, in combination with lung recruitment maneuvers for patients with moderate-to-severe ARDS | Low |
| Guo et al19 (2018) | 1 | Positive-end expiratory pressure | Conditional recommendation for higher positive-end expiratory pressure, of approximately 15 cm water. | … | Yes | Yes, conditional on patients who have better oxygenation in response to positive-end expiratory pressure | Low |
| Walkey et al28 (2017) | 1 | Positive-end expiratory pressure | Conditional recommendation for higher positive-end expiratory pressure, of approximately 15 cm water. | … | Yes | Yes, conditional when used in combination with lung protective ventilation when compared to high tidal volumes low positive-end expiratory pressure | Low |
| Fan et al4 (2017) | 1 | Prone position | Conditional recommendation for prone positioning | … | Yes | Yes, conditional, when used for >12 h/d for patients with moderate-to-severe ARDS | Moderate |
| Munshi et al24 (2017) | 1 | Prone position | Conditional recommendation for prone positioning | … | Yes | Yes, conditional, when used for >12 h/d for patients with moderate-to-severe ARDS | Moderate |
| Devlin et al10 (2018) | 2 | Analgesia-first approach to sedation and pain management | Minimize the use of opioids and sedatives; administer pharmacologic adjuvants to opioid therapy or nonpharmacologic interventions to reduce pain. | Yes | … | Yes | Moderate |
| Silversides et al14 (2017) | 2 | Conservative fluid management | Use protocols for patients who are diuresing, and/or monitor extravascular lung water, pulse pressure variation, or intrathoracic blood volume index, while restricting or minimizing fluid. | Yes | … | Yes | Moderate |
| Devlin et al10 (2018) | 2 | Delirium assessment, prevention, and management | Use screening tools to regularly assess delirium; avoid benzodiazepine; only short-term use of antipsychotic agents should be used while patients are in distress; consider multicomponent, nonpharmacologic interventions that reduce modifiable risk factors. | … | Yes | Yes | Low |
| Devlin et al10 (2018) | 2 | Early mobility protocols | Protocolized rehabilitation directed toward early mobilization for patients receiving invasive mechanical ventilation for >24 h. | Yes | … | Yes | Low |
| Girard et al11 (2017) | 2 | Early mobility protocols | Protocolized rehabilitation directed toward early mobilization for patients receiving invasive mechanical ventilation for >24 h. | Yes | … | Yes | Low |
| Schmidt et al27 (2017) | 2 | Early mobility protocols | Protocolized rehabilitation directed toward early mobilization for patients receiving invasive mechanical ventilation for >24 h. | Yes | … | Yes | Low |
| Meduri et al22 (2016) | 2 | Glucocorticoid treatment | Early- and low-dose methylprednisolone treatment helps resolve ARDS symptoms. | Yes | Yes | Yes | Moderate |
| Meduri et al23 (2018) | 2 | Glucocorticoid treatment | Conditional recommendation to provide methylprednisolone. | Yes | Yes | Yes, conditional on early moderate-to-severe and late persistent ARDS | Moderate |
| Yang et al29 (2017) | 2 | Glucocorticoid treatment | Early- and low-dose methylprednisolone treatment helps resolve ARDS symptoms. | Yes | Yes | Yes | Low |
| Fan et al4 (2017) | 2 | High-frequency oscillatory ventilation | Conditional recommendation regarding routine use high-frequency oscillatory ventilation. | Yes | Yes | No, conditional, not to be used for patients with moderate-to-severe ARDS | Strong |
| Devlin et al10 (2018) | 2 | Sedation protocols | Monitor sedation; use protocols that attempt to minimize sedation in patients who are not receiving neuromuscular blockades by interrupting sedation daily or continuously titrating sedatives to maintain a light level of sedation (ie, use a targeted sedation strategy). | Yes | Yes | Yes | Low |
| Ouellette et al12 (2017) | 2 | Sedation protocols | Monitor sedation; use protocols that attempt to minimize sedation. | Yes | … | Yes | Low |
| Schmidt et al27 (2017) | 2 | Sedation protocols | Monitor sedation; use protocols that attempt to minimize sedation. | Yes | … | Yes | Low |
| Devlin et al10 (2018) | 2 | Sleep management | Do not administer propofol to assist in sleep. | Yes | … | No | Low |
| Schmidt et al27 (2017) | 2 | Spontaneous awakening trial | Lighten or discontinue sedation for a period of time each day to wake the patient up and evaluate alertness. | Yes | … | Yes | Low |
| Schmidt et al27 (2017) | 2 | Spontaneous breathing trial | Turn ventilator support down or off with pressure augmentation to exercise the lungs and assess readiness for extubation. | Yes | … | Yes | Moderate |
| Girard et al11 (2017) | 3 | Cuff leak test | Perform cuff leak test for patients who meet extubation criteria and are deemed high risk for postextubation stridor; if failed but are ready for extubation, administer systemic steroids at least 4 hours before extubation; repeat cuff test not required. | Yes | … | Yes | Low |
| Schmidt et al27 (2017) | 3 | Cuff leak test | Perform cuff leak test for patients who meet extubation criteria and are deemed high risk for postextubation stridor; if failed but are ready for extubation, administer systemic steroids at least 4 hours before extubation; repeat cuff test not required. | Yes | … | Yes | Low |
| Huang et al20 (2018) | 3 | Extubation to high-flow nasal cannula | Extubate to high-flow nasal cannula is an effective alternative to patients who cannot tolerate noninvasive mechanical ventilation. | Yes | Yes | Yes | Low |
| Maitra et al21 (2016) | 3 | Extubation to high-flow nasal cannula | Extubate to high-flow nasal cannula is an effective alternative to patients who cannot tolerate noninvasive mechanical ventilation. | Yes | Yes | Yes | Low |
| Ni et al26 (2017) | 3 | Extubation to high-flow nasal cannula | Extubate to high-flow nasal cannula is an effective alternative to patients who cannot tolerate noninvasive mechanical ventilation. | Yes | Yes | Yes | Moderate |
| Huang et al20 (2018) | 3 | Extubation to noninvasive mechanical ventilation | Noninvasive mechanical ventilation appears to be comparable with high-flow nasal oxygen. | Yes | Yes | Yes | Low |
| Maitra et al21 (2016) | 3 | Extubation to noninvasive mechanical ventilation | Noninvasive mechanical ventilation appears to be comparable with high-flow nasal oxygen. | Yes | Yes | Yes | Low |
| Ni et al26 (2017) | 3 | Extubation to noninvasive mechanical ventilation | Noninvasive mechanical ventilation appears to be comparable with high-flow nasal oxygen. | Yes | Yes | Yes | Moderate |
| Ouellette et al12 (2017) | 3 | Extubation to noninvasive mechanical ventilation | For patients at risk for extubation failure who have received invasive mechanical ventilation >24 h and who have passed a spontaneous breathing trial, extubate to preventive noninvasive mechanical ventilation. | Yes | Yes | Yes | Moderate |
| Schmidt et al27 (2017) | 3 | Extubation to noninvasive mechanical ventilation | For patients at risk for extubation failure who have received invasive mechanical ventilation >24 h and who have passed a spontaneous breathing trial, extubate to preventive noninvasive mechanical ventilation. | Yes | Yes | Yes | Moderate |
| Girard et al11 (2017) | 3 | Ventilator liberation protocol | Treat patients who have received invasive mechanical ventilation for >24 h with a ventilator liberation protocol; however, insufficient evidence to recommend any single protocol over another. | Yes | … | Yes | Low |
| Schmidt et al27 (2017) | 3 | Ventilator liberation protocol | Treat patients who have received invasive mechanical ventilation for >24 h with a ventilator liberation protocol; however, insufficient evidence to recommend any single protocol over another. | Yes | … | Yes | Low |
Extracted Data Across the Care Continuum
We placed the extracted EBPs across the continuum of care so that we could consider their role in the cycle of escalation/deescalation to enable clinicians to contextualize them in the process of providing care. We identified six EBPs as phase 1 (initiation of IMV and intubation): conservative oxygen therapy, avoidance of high driving pressure, lung protective ventilation, neuromuscular blockades, positive-end expiratory pressure, and prone positioning. All six EPBs were associated with improved mortality rates, although the recent ROSE trial changes the level of evidence for neuromuscular blockades.30
We identified ten EBPs as phase 2 (prevention of complications during IMV). These include analgesia-first approach to sedation and pain management; conservative fluid management; delirium assessment, prevention, and management; early mobility protocols; glucocorticoid treatment; high-frequency oscillatory ventilation; sedation protocols; sleep management; spontaneous awakening trials; and spontaneous breathing trials. Most EBPs were associated with duration of IMV (with the exception of delirium management and spontaneous awakening trials).
There are four EBPs in phase 3 (extubation and discontinuation of IMV): cuff leak tests, extubation to high-flow nasal cannula, extubation to non-IMV, and use of a ventilator liberation protocol. One challenge is that, although ventilator liberation protocols are recommended, there is no evidence yet on which protocols are most effective in decreasing IMV duration or mortality rates.
Discussion
This is the first comprehensive review of EBPs to address care across the full continuum, from intubation to liberation, for patients who received IMV for ARF and/or ARDS. We assessed EBPs across a body of synthesized evidence, with a long-term goal of supporting critical care clinicians in the identification and implementation of best practices for treatment of ARF or ARDS. Of a total of 20 EBPs that indicated shorter duration of IMV and/or a mortality rate benefit, six of them focus on intubation and escalation of care. Ten EBPs reduce complications. We also identified four EBPs that address deescalation of interventions and the promotion of extubation. For each report, we provide a brief overview of the focus of the guideline, systematic review, or meta-analysis; the recommendations that we included, and the citations for source materials for further exploration.
Clinically, we brought all of these recommendations, which were previously in separate guidelines, systematic reviews, and meta-analyses, into a single set and identified where they fit on the continuum from intubation to liberation. This focuses attention on the need to address the entire continuum, not isolated components. Efforts at quality improvement related to IMV often have focused on a single EBP, such as lung protective ventilation,7,31 or have bundled recommendations together, such as the prominent, widely disseminated ICU Liberation bundle,32,33 but without full attention to the entire continuum of care. Although lung protective ventilation plays an important role in the provision of optimal care throughout the period of mechanical ventilation, it is not included in the ICU Liberation DEF bundle. This gap may account for less than optimal outcomes when efforts are made to improve the quality of IMV care.
Furthermore, the three phases that represent the continuum of care are not intended to be mutually exclusive or linear, because patients may cycle through phases. We also recognize that certain EBPs are connected directly within and across the continuum. Daily interruptions in sedation, for example, reduce complications such as delirium. Patients whose sedation is interrupted are also more likely to receive early mobility exercises and spontaneous breathing trials, which further reduces the duration of IMV, shortens ICU and hospital lengths of stay, and improves short-term mortality rates.34,35 The point that we wish to emphasize is that some EBPs perhaps should happen earlier in the process of care, either to support and facilitate the use of other EPBs or to promote safe but timely extubation in general. Thus, we propose a unified approach to the implementation of EBPs along the continuum of ARF and/or ARDS and suggest that we assess effectiveness of EBPs implemented jointly, rather than individually.
The use of a review of reviews approach, by its nature, excludes primary reports, such as important, multisite trial evidence that may recast the utility of specific practices, and change the evidence base. It is inherently a conservative approach and does not admit rapidly changing evidence. Given the difficulties inherent in the implementation EBPs in complex care settings such as ICUs, we feel it appropriate to be more conservative. The literature is growing, and the evidence-base changes in critical care. Our goal is not to be the most up to date but to provide a relatively stable platform of relevant EBPs that cover the care continuum and have the potential for implementation. In addition, we excluded non-English language systematic reviews, meta-analyses, and guidelines that were lacking sufficient resources for translation. We found few non-English language reports in our search.
This review adds to the literature by capturing all EPBs across the care continuum that are supported by evidence of improved patient outcomes. However, it does not help prioritize the EBPs. Our next steps include the systematic prioritization to focus our implementation efforts and the development of methods to measure performance with the use of digitally extracted data. As part of this larger project, we are exploring the feasibility of extracting and using electronic health record data to improve adherence to recommended practices for ARF and ARDS treatment. Although we have specific goals for future work, which includes supporting implementation of linked EBPs to cover the continuum of ARF/ARDS care, this work can provide an integrated view of the evidence for best practices in IMV and support optimization of care across a broad range of ICU settings.
Acknowledgments
Author contributions: J. E., V. R., E. D., M. S., T. I., C. H., M. N. G., and A. S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. A. S. served as the principal author. J. E., V. R., E. D., M. S., T. I., C. H., M. N. G., and A. S. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers U01HL143453 and K12HL138039.
Supplementary Data
References
- 1.Wunsch H., Linde-Zwirble W.T., Angus D.C., Hartman M.E., Milbrandt E.B., Kahn J.M. The epidemiology of mechanical ventilation use in the United States. Criti Care Med. 2010;38(10):1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld G.D., Caldwell E., Peabody E. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Stefan M.S., Shieh M.S., Pekow P.S. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8(2):76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan E., Del Sorbo L., Goligher E.C. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 5.Petrucci N., De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;(2):CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 7.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Smith V., Devane D., Begley C.M., Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt G.A., Girard T.D., Kress J.P. Official executive summary of an American Thoracic Society/American College of Chest Physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med. 2017;195(1):115–119. doi: 10.1164/rccm.201610-2076ST. [DOI] [PubMed] [Google Scholar]
- 10.Devlin J.W., Skrobik Y., Gélinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 11.Girard T.D., Alhazzani W., Kress J.P. An official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med. 2017;195(1):120–133. doi: 10.1164/rccm.201610-2075ST. [DOI] [PubMed] [Google Scholar]
- 12.Ouellette D.R., Patel S., Girard T.D. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal N.R., Brower R.G., Hager D.N. Oxygen exposure resulting in arterial oxygen tensions above the protocol goal was associated with worse clinical outcomes in acute respiratory distress syndrome. Crit Care Med. 2018;46(4):517. doi: 10.1097/CCM.0000000000002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silversides J.A., Major E., Ferguson A.J. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–170. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez G., Vaquero C., Colinas L. Effect of postextubation high-flow nasal cannula vs noninvasive ventialtion on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2017;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 16.Strom T., Martinussen T., Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama H., Pettenuzzo T., Aoyama K., Pinto R., Englesakis M., Fan E. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Criti Care Med. 2018;46(2):300–306. doi: 10.1097/CCM.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 18.Goligher E.C., Hodgson C.L., Adhikari N.K. Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl4):S304–S311. doi: 10.1513/AnnalsATS.201704-340OT. [DOI] [PubMed] [Google Scholar]
- 19.Guo L., Xie J., Huang Y. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18(1):172. doi: 10.1186/s12871-018-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H.-W., Sun X.-M., Shi Z.-H. Effect of high-flow nasal cannula oxygen therapy versus conventional oxygen therapy and noninvasive ventilation on reintubation rate in adult patients after extubation: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care Med. 2018;33(11):609–623. doi: 10.1177/0885066617705118. [DOI] [PubMed] [Google Scholar]
- 21.Maitra S., Som A., Bhattacharjee S., Arora M.K., Baidya D.K. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. J Crit Care. 2016;35:138–144. doi: 10.1016/j.jcrc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Meduri G.U., Bridges L., Shih M.C. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;1:42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 23.Meduri G.U., Bridges L., Siemieniuk R.A., Kocak M. An exploratory reanalysis of the randomized trial on efficacy of corticosteroids as rescue therapy for the late phase of acute respiratory distress syndrome. Crit Care Med. 2018;46(6):884–891. doi: 10.1097/CCM.0000000000003021. [DOI] [PubMed] [Google Scholar]
- 24.Munshi L., Del Sorbo L., Adhikari N.K. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl4):S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 25.Murray M.J., DeBlock H., Erstad B. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44(11):2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 26.Ni Y.-N., Luo J., Yu H. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation? A systematic review and meta-analysis. Chest. 2017;151(4):764–775. doi: 10.1016/j.chest.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt G.A., Girard T.D., Kress J.P. Official executive summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med. 2017;195(1):115–119. doi: 10.1164/rccm.201610-2076ST. [DOI] [PubMed] [Google Scholar]
- 28.Walkey A.J., Goligher E.C., Del Sorbo L. Low tidal volume versus non–volume-limited strategies for patients with acute respiratory distress syndrome. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl4):S271–S279. doi: 10.1513/AnnalsATS.201704-337OT. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z.G., Lei X.L., Li X.L. Early application of low-dose glucocorticoid improves acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Exp Ther Med. 2017;13(4):1215–1224. doi: 10.3892/etm.2017.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Heart, Lung and Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato M.B.P., Barbas C.S.V., Medeiros D.M. Effect of a protective-ventilationstrategy on monrtality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 32.Ely E.W. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017;45(2):321. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ICU Liberation. Society of Critical Care Medicine website. https://www.sccm.org/ICULiberation/Home. Accessed March 1, 2020.
- 34.Ely E.W., Baker A.M., Dunagan D.P. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 35.Girard T.D., Kress J.P., Fuchs B.D. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.