Abstract
Background
Lung cancer screening (LCS) is an important secondary prevention measure to reduce lung cancer mortality. The goal of this study was to assess state-level variations in LCS among the US elderly during the first 3 years since Medicare began its LCS reimbursement policy in 2015.
Methods
This ecological study examined the relations between LCS utilization density, defined as the number of low-dose CT (LDCT) or shared decision-making and counseling (SDMC) services per 1,000 Medicare fee-for-service (FFS) beneficiaries derived from the Medicare Provider Utilization and Payment Data: Physician and Other Supplier public use file, and state-level factors from several publicly available data sources. The study included Kruskal-Wallis tests and a cluster analysis.
Results
In 2017, the median utilization density per 1,000 Medicare FFS beneficiaries was 3.32 for LDCT and 0.46 for SDMC, which was 24 and 13 times the 2015 level, respectively. From 2015 to 2017, the total number of unique providers billed for LCS increased from 222 to 3,444 for LDCT imaging and from 20 to 523 for SDMC. Higher utilizations for both LDCT and SDMC services tended to concentrate in the northeastern and upper Midwest states than in the southwest states. The cluster of states with high utilization density did not include those states with the most lung cancer mortality and/or smoking prevalence.
Conclusions
A steady increase was noted in LCS utilization since Medicare began its reimbursement policy. The utilization and its growth varied across the United States and differed between LDCT imaging and SDMC, indicating large growth potentials for LCS and for states with high lung cancer mortality and smoking prevalence.
Key Words: geographic variations, Medicare, low-dose CT, lung cancer screening, shared decision-making and counseling
Abbreviations: FFS, fee-for-service; LDCT, low-dose CT; PUF, public use file; SDMC, shared decision-making and counseling; SES, socioeconomic status
In December 2013, the United States Preventive Services Task Force recommended annual lung cancer screening (LCS) with low-dose CT (LDCT) imaging for high-risk people after evaluating evidence from large randomized clinical trials such as the National Lung Screening Trial,1,2 as well as simulation and observational studies.3, 4, 5, 6, 7 Asymptomatic people aged 55 to 80 years who have a ≥ 30 pack per-year smoking history and currently smoke or have quit within the past 15 years are defined as at high risk and thus eligible for screening.2 Starting effectively February 5, 2015, the Centers for Medicare & Medicaid Services have been providing Medicare coverage through Part B (for those aged 55-77 years) for LCS with LDCT imaging for eligible people.8 Additional preventive services that must accompany the initial LCS process and prior to an LDCT scan include shared decision-making and counseling (SDMC) on issues such as the benefit and harm of LCS, adherence to annual LCS, and smoking cessation counseling.8, 9, 10
Despite these endorsements, the overall adoption of LCS remains low. At the national level, the LDCT utilization rate ranged between 2% and 5% from 2010 to 2015; it was 14% in 2017 among the general population11, 12, 13, 14 and 0.44% among Medicare fee-for-service (FFS) enrollees.15 The low and slow adoption of LDCT is associated with multiple factors, such as the awareness of and belief in LCS guidelines among both providers and patients, familiarity with insurance reimbursement policies among providers, barriers in assessing the SDMC process, and insurance coverage.11, 12, 13, 14, 15, 16 Access to LDCT facilities presents another challenge, as the availability of these facilities varies across the United States.17, 18, 19 We found that geographic variations in the availability and the growth of LDCT facilities across the United States were interconnected with issues such as lung cancer incidence and mortality burden, socioeconomic factors, and smoking prevalence.20 However, to our knowledge, no studies have investigated the geographic variations of actual LCS use among older adults in the United States.
The goal of the current study was to evaluate the variations in the state-level utilization of both LDCT and SDMC based on the most recent Medicare claim summaries. We hypothesized that areas with high lung cancer mortality would also be high LCS utilization areas. The analysis based on the receipts of LCS services may offer a direct assessment of LDCT and SDMC utilizations, and provide evidence that can help identify the barriers and evaluate the progress of LCS among older adults in the United States.
Materials and Methods
Utilization Density
The primary outcomes of interests were LDCT utilization density and SDMC utilization density, defined as the number of services per 1,000 Medicare FFS beneficiaries aggregated at the state level. Two datasets were used: the Medicare Provider Utilization and Payment Data: Physician and Other Supplier public use file (PUF) and the Medicare FFS enrollment PUF data for the calendar year 2015 to 2017.21,22 The provider PUF includes summaries of service and procedures provided by physicians and other health-care professionals to the Medicare FFS beneficiaries, who are not enrolled in Medicare Advantage plans, with specific information on the following: (1) provider, such as their unique national provider identifier, address, and specialty type; (2) utilization, such as the Health Care Common Procedure Coding System codes and total provider services; and (3) payment, such as the Medicare-allowed amount for the specific service and procedure. The enrollment PUF provides data on Medicare enrollment, such as the counts of Medicare FFS beneficiaries aggregated at varied geographic scales.
LDCT and SDMC services were identified by using their corresponding Health Care Common Procedure Coding System codes (G0297and G0296, respectively) and summarized the total number of LDCT and SDMC utilizations at the provider level. Although LDCT imaging is recommended as an annual scan, our data could not differentiate a new LDCT scan from a follow-up LDCT scan. We geocoded the address of individual providers to obtain their latitude and longitude.23 We aggregated the total utilization numbers of LDCT scans and SDMC at the state level, which were subsequently divided by the total number of Medicare FFS beneficiaries at the state level.
Area-Level Factors
The state-level age-adjusted lung cancer-specific mortality rate was obtained for the age group 50 to 79 years during 2012 to 2015 using SEER*Stat software24 and the prevalence of ever smokers and current smokers among adults aged ≥ 65 years from the 2012 to 2014 Behavioral Risk Factor Surveillance System.25 State-level median household income, as an indicator of socioeconomic status (SES), was determined by using the 2010 to 2014 American Community Survey.26
Statistical Analysis
Descriptive statistics of LDCT and SDMC utilizations were summarized and mapped. Using Kruskal-Wallis tests, the four state-level factors were compared by the quartiles of the utilization density of LDCT and SDMC services in each year, respectively. The statistically significant alpha level was set at 0.002 with Bonferroni correction (0.05/24 = 0.002) for multiple testing. A cluster analysis was performed on a complete dataset, including three utilization variables (the total number of LDCT/SDMC utilizations, the number of Medicare FFS beneficiaries, and the number of unique providers) and the four state-level factors. The number of the final cluster was selected by using the largest silhouette width, and the cluster partition was also visually examined (e-Fig 1).27, 28, 29 We also compared the four state-level factors across the clusters using the Kruskal-Wallis test. All analyses were conducted by using SAS version 9.4 (SAS Institute, Inc.) and R version 3.5 (R Foundation for Statistical Computing).
Results
A sharp increase was found in the claims for both LDCT imaging and SDMC from 2015 to 2017 (Table 1). In 2017, there were 107,309 LDCT services billed by 3,444 unique providers and 14,962 SDMC services billed by 523 unique providers. In comparison, there were 4,530 LDCT services billed by 222 unique providers and 392 SDMC billed by 20 unique providers in 2015. The 2017 median state-level utilization density of LDCT imaging and SDMC per 1,000 Medicare FFS beneficiaries was 3.32 and 0.46, respectively, which was 24 times and 13 times the 2015 level and ∼2 times the 2016 level.
Table 1.
Summary Statistics of the Utilizations of LDCT Imaging and SDMC Based on Data Aggregated at the Provider Level, 2015 to 2017
| Year | Variable | Mean ± SD | Median | Min-Max | Sample N |
|---|---|---|---|---|---|
| 2015 | SDMC per provider | 19.6 ± 8.5 | 16 | 11-40 | 392 |
| 2016 | SDMC per provider | 27.4 ± 22.6 | 19 | 11-143 | 7,638 |
| 2017 | SDMC per provider | 28.1 ± 23.8 | 20 | 11-194 | 14,962 |
| 2015 | LDCT scans per provider | 20.2 ± 11.5 | 16 | 11-101 | 4,530 |
| 2016 | LDCT scans per provider | 26.2 ± 23.0 | 18 | 11-302 | 50,915 |
| 2017 | LDCT scans per provider | 29.0 ± 26.9 | 20 | 11-447 | 107,309 |
| 2015 | Unique providers per state | 2.5 ± 3.9 | 1 | 1-12 | 8 |
| SDMC per state | 49 ± 70.6 | 24.5 | 13-222 | 8 | |
| SDMC per 1,000 FFS beneficiaries per state | 0.05 ± 0.06 | 0.04 | 0.01-0.19 | 8 | |
| 2016 | Unique providers per state | 7.7 ± 7.3 | 5 | 1-36 | 36 |
| SDMC per state | 212.2 ± 211.4 | 156.5 | 11-990 | 36 | |
| SDMC per 1,000 FFS beneficiaries per state | 0.31 ± 0.28 | 0.20 | 0.07-1.41 | 36 | |
| 2017 | Unique providers per state | 12.2 ± 11.0 | 8 | 1-45 | 43 |
| SDMC per state | 348 ± 334.5 | 244 | 13-1452 | 43 | |
| SDMC per 1,000 FFS beneficiaries | 0.55 ± 0.46 | 0.46 | 0.04-2.64 | 43 | |
| 2015 | Unique providers per state | 6.3 ± 6.9 | 4 | 1-30 | 35 |
| LDCT scans per state | 129.4 ± 161.3 | 68 | 11-684 | 35 | |
| LDCT scans per 1,000 FFS beneficiaries per state | 0.18 ± 0.17 | 0.14 | 0.01-0.68 | 35 | |
| 2016 | Unique providers per state | 38 ± 36.4 | 22 | 1-157 | 49 |
| LDCT scans per state | 1,039 ± 1,085 | 738 | 12-5,120 | 49 | |
| LDCT scans per 1,000 FFS beneficiaries per state | 1.64 ± 1.33 | 1.36 | 0.04-7.09 | 49 | |
| 2017 | Unique providers per state | 70.3 ± 62.4 | 55 | 2-238 | 49 |
| LDCT scans per state | 2,190 ± 2,135.8 | 1257 | 66-9,486 | 49 | |
| LDCT scans per 1,000 FFS beneficiaries per state | 3.44 ± 2.17 | 3.32 | 0.49-8.74 | 49 | |
| 2015 | SDMC per unique provider | 19.6 ± 8.5 | 16 | 11-40 | 20 |
| 2016 | SDMC per unique provider | 27.7 ± 22.7 | 20 | 11-143 | 276 |
| 2017 | SDMC per unique provider | 28.6 ± 26.1 | 20 | 11-209 | 523 |
| 2015 | LDCT scans per unique provider | 20.4 ± 12 | 16 | 11-101 | 222 |
| 2016 | LDCT scans per unique provider | 27.4 ± 24.6 | 19 | 11-302 | 1,860 |
| 2017 | LDCT scans per unique provider | 31.2 ± 29.2 | 21 | 11-447 | 3,444 |
Estimates were based on data from the Medicare Provider Utilization and Payment Data: Physician and Other Supplier PUF file and the Medicare FFS enrollment PUF data for calendar years 2015 to 2017. The provider PUF includes summaries of service and procedures provided by physicians and other health-care professionals to Medicare FFS beneficiaries; thus, the data are at the individual provider level instead of the individual patient level. Only providers with > 11 services were available from the provider PUF data. Data were for Medicare FFS enrollees only. The summary statistics at the national, state, and provider levels were based on the different unit of analysis used to aggregate the data. FFS = fee-for-service; LDCT = low-dose CT; PUF = public use file; SDMC = shared decision-making and counseling.
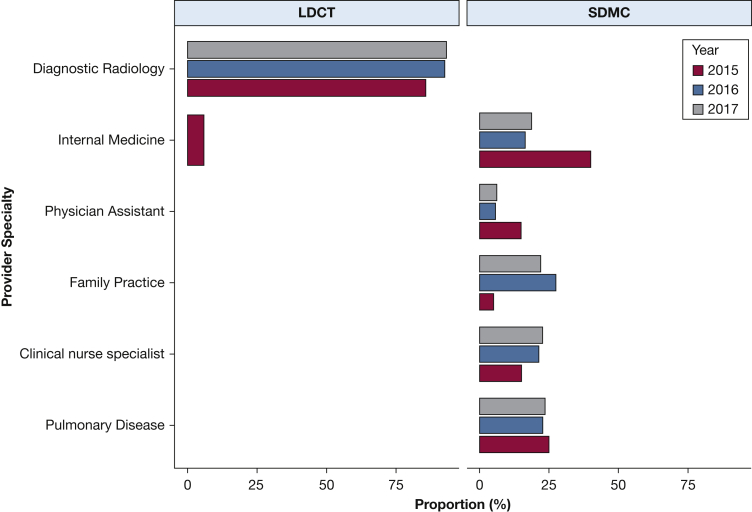
The average LDCT and SDMC services at the individual provider level in 2017 were similar (Table 1): 31.2 ± 29.2 (median, 21) for LDCT imaging and 28.6 ± 26.1 (median, 20) for SDMC, which were 1.3 to 1.5 times the 2015 level, respectively. The type of providers who billed for LDCT and SDMC services also changed (Fig 1). In 2017, 93% of the LDCT services were billed by providers with a “Diagnostic Radiology” specialty, which is an increase from 85% in 2015. For SDMC, the most frequently billed provider specialty was “Internal Medicine” in 2015 (40%), “Family Practice” in 2016 (27.2%), and “Pulmonary Diseases” in 2017 (23.5%). Approximately 59% of all the providers (58% for LDCT services and 35% for SDMC) were located in metropolitan statistical areas, within which there are at least one urbanized area of ≥ 50,000 inhabitants.
Figure 1.
Utilizations of LDCT and SDMC services according to provider specialties. Note: Estimates were based on 2015 to 2017 data from the Medicare Provider Utilization and Payment Data: Physician and Other Supplier public use file, which included only providers with > 11 services and were for Medicare fee-for-service enrollees only. Utilizations < 5% were not included in the graphic. LDCT = low-dose LDCT; SDMC = shared decision-making and counseling.
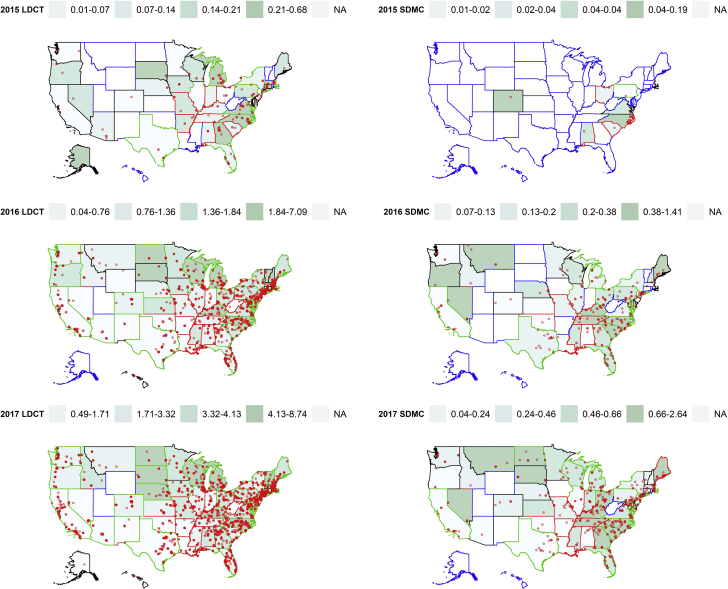
Large variations in the utilization of LDCT and SDMC services (Fig 2) were observed across the United States. High utilization regions for both LDCT images and SDMC were generally concentrated in the northeastern and upper Midwest states, whereas low utilization was found in the southwest states. In 2015, states with high median household income can be found in both the upper and lower quartiles of LDCT utilization density, displaying a U-shaped relation (P = .0036) (e-Fig 2). States with above the national average prevalence of former smokers also tended to have above the national median LDCT utilization density in 2016 (P = .0056) and 2017 (P = .031). However, these differences were not statistically significant at the alpha level of 0.002 after adjusting for multiple testing.
Figure 2.
Variations of the state-level utilization density of LDCT imaging and SDMC in quartiles, with an overlay of individual provider locations. Utilization density was defined as utilizations per 1,000 Medicare fee-for-service beneficiaries. States were grouped into three clusters, shown as three different colors (black, red, and green) for the state boundaries; those with blue color boundaries were states with no data. Clusters were generated based on three utilization variables (numbers of utilizations, Medicare fee-for-service beneficiaries, and unique providers) and four state-level factors (lung cancer mortality rate, prevalence of current smokers aged ≥ 65 years and former smokers, and median household income). See Figure 1 legend for expansion of abbreviations.
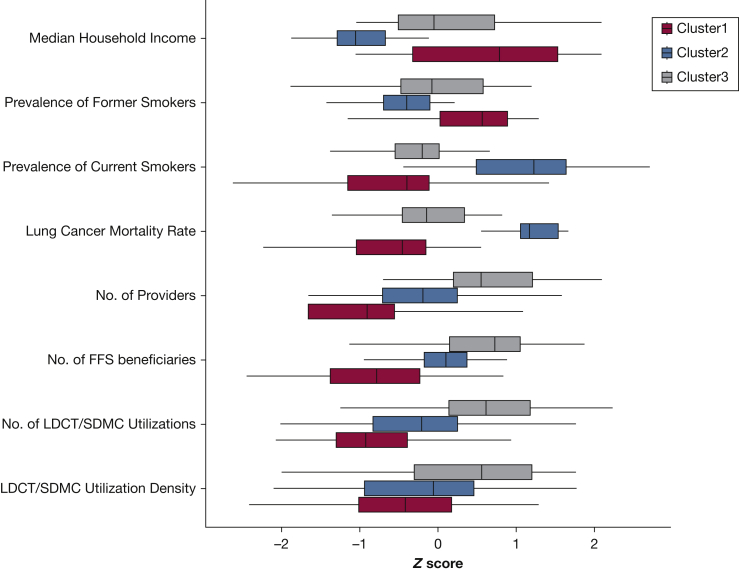
We found three clusters that differed significantly (P < .001) in their LCS utilization and area level factors (Fig 3). Cluster 1 had the highest SES and prevalence of former smokers; cluster 2 had the highest lung cancer mortality and prevalence of current smokers aged ≥ 65 years but lowest SES; and cluster 3 had the highest number of utilization, Medicare FFS beneficiaries, and providers. There were seven states only found in cluster 1 (Alaska, Connecticut, District of Columbia, Hawaii, Minnesota, Montana, and Wyoming), nine states only found in cluster 2 (Arkansas, Indiana, Kentucky, Louisiana, Missouri, Mississippi, Oklahoma, Tennessee, and West Virginia); and 10 states only in cluster 3 (Florida, Georgia, Iowa, Illinois, Michigan, North Dakota, New York, Pennsylvania, Rhode Island, and Texas). The cluster of states with the highest utilization density (cluster 3) did not include those (ie, cluster 2) with the most lung cancer burden in terms of mortality and smoking prevalence. In fact, a majority of the cluster 2 states had LDCT utilization density below the national median and continued to remain at that level up to 2017. Some noticeable exceptions were seen in Kentucky, which ranked in the top utilization quartiles in 2016 and 2017, and in Alabama and Missouri, where the LDCT utilizations were in the 50th to 75th percentiles by 2017. Both Tennessee and Mississippi had above the national median utilization of SDMC in 2016 and 2017.
Figure 3.
Variations in the utilization of LDCT and SDMC services and state-level factors according to the three identified clusters. Cluster analysis was conducted on a complete dataset including three utilization variables (numbers of utilizations, Medicare FFS beneficiaries, and unique providers) and four state-level factors (lung cancer mortality rate, prevalence of current smokers aged ≥ 65 years and former smokers, and median household income). All the variables were log transformed and normalized by using z scores; as such, a (positive/negative) z score indicates that a state level factor is z score times (above/below) the overall average. Statistically significant differences were found for all variables across the three clusters (Kruskal-Wallis tests, P < .001). States included in cluster 1 were Alaska, Arizona, California, Colorado, Connecticut, District of Columbia, Delaware, Hawaii, Idaho, Kansas, Massachusetts, Maryland, Maine, Minnesota, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, Nevada, Oregon, South Dakota, Virginia, Vermont, Washington, Wisconsin, and Wyoming; cluster 2, Alabama, Arkansas, Idaho, Indiana, Kansas, Kentucky, Louisiana, Maine, Missouri, Mississippi, North Carolina, New Mexico, Nevada, Ohio, Oklahoma, South Carolina, Tennessee, and West Virginia; and cluster 3, Arizona, California, Colorado, Delaware, Florida, Georgia, Iowa, Idaho, Illinois, Kansas, Massachusetts, Maryland, Maine, Michigan, North Carolina, North Dakota, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Virginia, Vermont, Washington, and Wisconsin. (Bolded states were unique states in each cluster.) FFS = fee-for-service. See Figure 1 legend for expansion of other abbreviations.
Discussion
We presented a first look at LCS utilization among older adults in the United States since Medicare started to reimburse for this secondary cancer preventive service. A steady increase was found in LCS overall, although the uptake and growth exhibited considerable geographic variations. The results provide a baseline level for future evaluations of LCS utilization among the US elderly and assessment of capacity building for SDMC and LDCT imaging at the state level. With the annual data release schedule, we will be able to continue tracking and examining the spatiotemporal variations of LCS.
Our estimate of the LDCT utilization density at the state level was in line with previous estimates for the Medicare population at the national level.15 Although we were not able to estimate screening-eligible people from our data, we provided temporal and geographic estimates of LDCT use at the national, state, and provider levels. We also provided state-level estimates of SDMC utilization, which have not been previously reported. If 5% to 12.5% of Medicare enrollees are eligible for LCS,15,30,31 we could postulate that the overall median state-level utilizations per 1,000 eligible Medicare FFS beneficiaries would be 9.9 to 24.7 for LDCT services and 3.3 to 8.2 for SDMC, which increased expectedly from 1.3 LDCT scans and 0.3 SDMC utilization per 1,000 Medicare FFS beneficiaries. Currently, there are 40 million Medicare FFS enrollees, which represent 67% of all Medicare beneficiaries or 83% of the 47.8 million people aged ≥ 65 years in the United States.
The rapidly increasing utilization of both LDCT imaging and SDMC from 2015 to 2017 is encouraging, especially for a few states (eg, Kentucky, Alabama, Missouri) with a high lung cancer mortality rate and high smoking prevalence. The trend that high LDCT utilizations tended to occur in states with high prevalence of former smokers was also positive and consistent with the LCS recommendation, which includes previous smokers who meet the specified conditions. However, we also found many states in cluster 2 (eg, Arkansas, Indiana, Louisiana, Oklahoma, West Virginia) that had both high lung cancer mortality and smoking prevalence but low LDCT and SDMC utilizations, suggesting large growth potentials. Because LCS is recommended for high-risk people, including both former and current smokers with specified smoking exposures, increasing the utilization of LDCT imaging and SDMC in these states is needed and crucial for the success of the LCS program.
We found that low utilizations and low growth of utilization tended to occur in the southwest regions. Multiple reasons may explain the observed patterns. For example, although high burdens of lung cancer mortality and screening-eligible populations may have driven the high utilization density in some states, there are also other contributing factors. Previous studies of the diffusion of digital mammography adoption and distribution in New York showed that affluent areas were more likely to be among the early adopters of the technology.32 In the current study, we found that in 2015, high LDCT utilization seemed to have occurred in both states with high SES and those with high lung cancer burden, whereas during 2016 to 2017, increasing quartiles of the LDCT utilization density tended to increase with median household income. A different pattern was found for SDMC utilization quartiles, which tended to have a negative relation with median household income, as has also been found for longer existing breast and colon cancer screening.
These trends suggest a greater role of SES in affecting the utilization of LDCT imaging than SDMC, as the capacity of providing LDCT scans may require substantial economic investment of equipment as well as training and retention of the provider force. For example, both the radiology imaging facility and the reading radiologist have to meet eligibility criteria.8,10 Studies have also shown that the availability, accessibility, and growth of certified LDCT facilities vary across the United States,17, 18, 19 and the limited number of LDCT facilities may be a contributing factor in the low number of people screened. We also found that although the absolute numbers of providers billed for LDCT and SDMC services in 2017 were 16 times and 26 times the 2015 level, respectively, the median services billed per provider in 2017 were only 1.3 to 1.5 times the 2015 levels, suggesting that to meet the LCS demand, more capacity building (which is closely tied to SES) for both LCS facilities and providers is needed.
Although SDMC was only required for the initial LDCT scan,8,9 we found that SDMC utilizations were 8.7% of the LDCT services in 2015. Possible explanations include, first, that SDMC might be occurring in a less centralized fashion than LDCT imaging; providers may therefore be more likely to have < 11 SDMC, and thus are suppressed from the public data release. However, we found similar SDMC and LDCT services billed per provider. Second, there may be large discrepancies in billing practices between SDMC and LDCT imaging. For example, providers may be more incentivized to bill for LDCT imaging than for SDMC, as the average Medicare-allowed amount for SDMC was ∼20% to 26% of the LDCT payment (median, 21%-53% for 2015-2017; data not shown). Finally, there may exist a true low utilization of SDMC, as has been found in other studies for LCS and other major cancer screenings.10,33,34 For example, only 9% of Medicare FFS beneficiaries who underwent LDCT imaging in 2016 also had a SDMC, and only 60.8% of those who had SDMC underwent an LDCT scan.34 Using SDMC as a proxy for a new LDCT scan (because our data could not differentiate a new LDCT scan from a follow-up LDCT scan), we found that 15.0% to 13.9% of all 2016 to 2017 LDCT utilizations were for a new LDCT scan, assuming all SDMC led to an LDCT scan (9.1%-8.5%, assuming 60.8% of the SDMC led to an LDCT scan). The combined new and follow-up LDCT scans were only 15.8% to 21.4% of the total LDCT utilizations in 2016 to 2017, indicating lower-than-expected SDMC utilizations.
Although LDCT imaging is an effective secondary prevention effort that can contribute to decrease lung cancer mortality by increasing early diagnosis, smoking cessation, which is part of SDMC, is still the foundation for reducing smoking-related mortality and is a necessary component of LCS. The current study adds evidence to the ongoing debates on the effectiveness and challenges in implementing SDMC as part of LCS and argues for further studies to understand the reasons and modifiable factors of low SDMC utilization.
We acknowledge a few limitations of this study. First, the absolute number of utilizations overall is likely underreported, due to unavailable data when the number of beneficiaries was < 11 to protect the privacy of Medicare beneficiaries. Second, because our data were for Medicare FFS beneficiaries, our results may not be representative of the LDCT services prescribed for those enrolled in Medicare Advantage and commercial insurances and for those aged < 65 years. Third, we did not explore other factors (eg, racial composition, education level) that may influence the diffusion of LCS, although many of these variables may highly correlate with the four state-level factors included in the current analysis. Finally, covariates included in the analysis came from different publicly available data sources in which the specific age cutoffs for each age category, while with substantial overlaps, were not always consistent.
Conclusions
The first 3 years following Medicare’s approval for LCS reimbursement have witnessed a large increase in LDCT and SDMC utilizations among the Medicare FFS population. The lower utilization of SDMC compared with LDCT imaging highlights challenges in implementing SDMC and warrants further investigation. The observed geographic disparities in LCS utilization and its growth suggest that multiple factors, such as lung cancer mortality, smoking prevalence, and SES, are at play in influencing the pace of LCS adoption across the United States.
Acknowledgments
Author contributions: B. L. was responsible for study concept and design, acquisition of data, statistical analysis, result interpretation, and drafting of the manuscript. B. L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. I. H. is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the foundation, which was established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. Recipients include the International Early Lung and Cardiac Action Program, among others. The funding comes from a variety of sources, including philanthropic donations, grants and contracts with agencies (federal and nonfederal), imaging, and pharmaceutical companies relating to image processing assessments. The various sources of funding exclude any funding from tobacco companies or tobacco-related sources. None declared (B. L., K. D., E. T.).
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.National Lung Screening Trial Research T. Aberle D.R., Adams A.M. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer V.A., US Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 3.Henschke C.I., McCauley D.I., Yankelevitz D.F. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 4.Henschke C.I. CT screening for lung cancer: principles and results. Lung Cancer-J Iaslc. 2004;46:S16–S19. [Google Scholar]
- 5.de Koning H.J., Meza R., Plevritis S.K. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the US Preventive Services Task Force. Ann Intern Med. 2014;160(5):311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphrey L.L., Deffebach M., Pappas M., Zakher B., Slatore C.G. Screening for lung cancer with low-dose computed tomography. Ann Intern Med. 2014;160(3):212. doi: 10.7326/L14-5003-3. [DOI] [PubMed] [Google Scholar]
- 7.International Early Lung Cancer Action Program I. Henschke C.I., Yankelevitz D.F. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 8.CMS Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 9.CMS Medicare Coverage of Screening for Lung Cancer with Low Dose Computed Tomography (LDCT). Centers for Medicare & Medicaid Services. MLN Matters® Number: MM9246. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM9246.pdf Accessed April 3, 2018.
- 10.Shieh Y., Bohnenkamp M. Low-dose CT scan for lung cancer screening: clinical and coding considerations. Chest. 2017;152(1):204–209. doi: 10.1016/j.chest.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Richards T.B., Doria-Rose V.P., Soman A. Lung cancer screening inconsistent with US Preventive Services Task Force Recommendations. Am J Prev Med. 2019;56(1):66–73. doi: 10.1016/j.amepre.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria-Rose V.P., White M.C., Klabunde C.N. Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1049–1059. doi: 10.1158/1055-9965.EPI-12-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahnd W.E., Eberth J.M. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med. 2019;57(2):250–255. doi: 10.1016/j.amepre.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Nishi S., Zhou J., Kuo Y.F., Goodwin J.S. Use of lung cancer screening with low-dose computed tomography in the Medicare population. Mayo Clinic Proc Innov Qual Outcomes. 2019;3(1):70–77. doi: 10.1016/j.mayocpiqo.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner A.T., Malo T.L., Margolis M. Evaluating shared decision making for lung cancer screening. JAMA Intern Med. 2018;178(10):1311–1316. doi: 10.1001/jamainternmed.2018.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberth J.M., Bozorgi P., Lebron L.M. Geographic availability of low-dose computed tomography for lung cancer screening in the United States, 2017. Prev Chronic Dis. 2018;15:E119. doi: 10.5888/pcd15.180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberth J.M., Qiu R., Adams S.A. Lung cancer screening using low-dose CT: the current national landscape. Lung Cancer-J Iaslc. 2014;85(3):379–384. doi: 10.1016/j.lungcan.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Charkhchi P., Kolenic G.E., Carlos R.C. Access to lung cancer screening services: preliminary analysis of geographic service distribution using the ACR Lung Cancer Screening Registry. J Am Coll Radiol. 2017;14(11):1388–1395. doi: 10.1016/j.jacr.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kale M.S., Wisnivesky J., Taioli E., Liu B. The landscape of US lung cancer screening services. Chest. 2019;155(5):900–907. doi: 10.1016/j.chest.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CMS Medicare Provider Utilization and Payment Data: Physician and Other Supplier. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/physician-and-other-supplier.html
- 22.CMS The Geographic Variation Public Use File. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Geographic-Variation/GV_PUF.html
- 23.TAM Texas A&M Geocoding Services. http://geoservices.tamu.edu/Services/Geocode/
- 24.SEER Surveillance, Epidemiology, and End Results (SEER) Program. www.cdc.gov/nchs www.seer.cancer.gov. SEER*Stat Database: Mortality—All COD, Aggregated With State, Total US (1990-2015) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2017. Underlying mortality data provided by NCHS.
- 25.BRFSS Behavioral Risk Factor Data: Tobacco Use (2011 to present) https://chronicdata.cdc.gov/Survey-Data/Behavioral-Risk-Factor-Data-Tobacco-Use-2011-to-pr/wsas-xwh5
- 26.American Community Survey. US Census Bureau, 2010-2014 American Community Survey 5-Year Estimates. GCT1901. Median Household Income (in 2014 Inflation-Adjusted Dollars)-State-County/County Equivalent. Accessed April 8, 2018.
- 27.Rousseeuw P.J. Silhouettes—a graphical aid to the interpretation and validation of cluster-analysis. J Comput Appl Math. 1987;20:53–65. [Google Scholar]
- 28.Maechler M. Package ‘cluster’. https://cran.r-project.org/web/packages/cluster/cluster.pdf
- 29.Krijthe J., van der Maaten L. Package ‘Rtsne’. https://cran.r-project.org/web/packages/Rtsne/Rtsne.pdf
- 30.Roth J.A., Sullivan S.D., Goulart B.H.L., Ravelo A., Sanderson J.C., Ramsey S.D. Projected clinical, resource use, and fiscal impacts of implementing low-dose computed tomography lung cancer screening in Medicare. J Oncol Pract. 2015;11(4):267–272. doi: 10.1200/JOP.2014.002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pyenson B.S., Henschke C.I., Yankelevitz D.F., Yip R., Dec E. Offering lung cancer screening to high-risk Medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272–282. [PMC free article] [PubMed] [Google Scholar]
- 32.Boscoe F.P., Zhang X. Visualizing the diffusion of digital mammography in New York State. Cancer Epidemiol Biomarkers Prev. 2017;26(4):490–494. doi: 10.1158/1055-9965.EPI-16-0928. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman R.M., Elmore J.G., Fairfield K.M., Gerstein B.S., Levin C.A., Pignone M.P. Lack of shared decision making in cancer screening discussions: results from a national survey. Am J Prev Med. 2014;47(3):251–259. doi: 10.1016/j.amepre.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin J.S., Nishi S., Zhou J., Kuo Y.F. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179(5):716–718. doi: 10.1001/jamainternmed.2018.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.